Abstract
JCV causes the CNS demyelinating disease progressive multifocal leukoencephalopathy (PML). After primary infection, JCV persists in a latent state, where viral protein expression and replication are not detectable. NF-κB and C/EBPβ regulate the JCV promoter via a control element, κB, suggesting proinflammatory cytokines may reactivate JCV to cause PML, e.g., in HIV-1/AIDS. Since HIV-1 induces cytokines in brain, including TNF-α, we examined a role for TNF-α in JCV regulation. TNF-α stimulated both early and late JCV transcription. Further, the κB element conferred TNF-α response to a heterologous promoter. Immunohistochemistry of HIV+/PML revealed robust labeling for TNF-α and TNFR-1. These data suggest TNF-α stimulation of κB may contribute to JCV reactivation in HIV+/PML.
Keywords: TNF-α, JC Virus, Progressive Multifocal Leukoencephalopathy, Cytokines, Viral Latency
1. Introduction
The human neurotropic polyomavirus JC (JCV) is the etiological of the fatal CNS demyelinating disease, progressive multifocal leukoencephalopathy, PML (Berger, 2003; Khalili et al., 2006, 2008). While this disease is very rare, the occurrence of JCV infection is common throughout the population worldwide as judged by the high prevalence of antibodies to JCV in human sera (Padgett and Walker, 1973; Walker and Padgett, 1983). JCV infects most people in childhood and is usually subclinical and asymptomatic, after which the virus is thought to continue to persist in the body in a latent state, where viral protein expression cannot be detected and replication occurs only episodically and at low levels (Hou and Major, 2000; Khalili et al., 2006). The nature of the latent state is not well understood but it is known that viral DNA can be detected by PCR but expression of the viral proteins is not detectable. Sites where latent JCV has been reported include the kidneys, tonsils, GI tract and brain (reviewed in Del Valle et al., 2008). Interestingly, the genomic arrangement of the JCV non-coding control region (NCCR) reported in the brain has the hallmark rearrangements typical of strains of JCV isolated from PML, e.g., Mad-1 (Perez-Liz et al., 2008; Elsner and Dorries, 1992; Greenlee et al., 2005; Mori et al., 1991, 1992; White et al., 1992). The cells that harbor the latent JCV DNA are the oligodendrocytes and astrocytes but not neurons as determined by laser capture microdissection followed by PCR (Perez-Liz et al., 2008). On the other hand, it has been argued that the probability that the brain harbors JCV DNA as a functional site of latency may be small and that B lymphoid cells are the real site of functional latency (Major, 2010). Recently, Tan et al (2010) demonstrated the presence of JCV DNA in non-PML brain using three different approaches: quantitative PCR, cloning and sequencing of PCR products and in situ hybridization.
In most individuals, JCV replication is either undetectable or occurs episodically at a low level, as evidenced by the intermittent appearance of virus in the urine of normal individuals, and infection is asymptomatic. However in the context of severe immunosuppression, especially AIDS, JCV can be reactivated in the CNS leading to PML. The high incidence of PML in patients infected with HIV-1 makes it an AIDS-defining disease (Holman et al., 1998). The clinical signs that are observed in patients with PML vary depending on the location of the demyelinated lesions but common symptoms include headaches, limb weakness and cognitive impairments (Berger et al., 2008; Brooks and Walker, 1984). In PML lesions, expression of the JCV early proteins, e.g., large T-antigen, and late proteins, e.g. VP1 capsid protein, is clearly observable by immunohistochemical labeling in the nuclei of both oligodendrocytes and astrocytes (Del Valle et al., 2008). Replication of virus occurs in both of these cell types as judged by the presence of virions observed by electron microscopy (Mázló et al., 2001). Lytic destruction of oligodendrocytes, which produce myelin, is thought to account for the demyelinated phenotype of the PML lesion.
Since the presence of latent PML-type JCV DNA is detectable in the oligodendrocytes and astrocytes of some human brains but viral protein expression is not, it is possible that, in at least some cases of PML, reactivation of the transcriptional status of JCV in the brain might be an initiating event in the pathogenesis of PML. The mechanism of reactivation of JCV from latency is unknown but likely involves molecular events that upregulate the expression of viral genes including the viral early protein T-antigen, which is necessary for viral DNA replication. The genome of JCV is a circular, closed and supercoiled DNA, is small in size (5,130 base pairs for the Mad-1 strain) and is comprised of two regions, early and late, which are transcribed in opposite directions from a bidirectional promoter (Imperiale and Major, 2007). This bidirectional promoter is also known as the non-coding regulatory region (NCCR) and contains the sites from which early and late transcription are initiated and the origin of viral DNA replication. The NCCR contains binding sites for many transcription factors that regulate early and late gene expression and some of these transcription factors are regulated by signal transduction pathways that lie downstream of extracellular growth factors and immunomodulators such as proinflammatory cytokines (reviewed in White et al., 2009). In particular, earlier work indicated the involvement of the NF-κB signaling pathway in the activation of JCV transcription (Mayreddy et al., 1996; Ranganathan and Khalili, 1993; Safak et al., 1999; Romagnoli et al., 2009). The unique binding site for NF-κB (the κB element) is located in the JCV NCCR on the early side of the origin of replication (Ranganathan and Khalili, 1993). Previous studies demonstrated that this site is a functional NF-κB binding site and activates JCV gene expression (Mayreddy et al., 1996; Ranganathan and Khalili, 1993; Romagnoli et al., 2009). Recently, we have found that the κB element of JCV can also bind and be modulated by the transcription factor C/EBPβ (Romagnoli et al., 2009).
NF-κB is a transcription factor that is induced by a variety of proinflammatory cytokines, including TNF-α, and regulates the expression of many cellular and viral genes (Fiers, 1991; Nabel and Baltimore, 1987; West et al., 2001). NF-κB normally exists in the cytoplasm complexed with the inhibitor protein IκB (Ghosh and Karin, 2002; Phelps et al., 2000) but activated proinflammatory cytokine receptors trigger upstream protein kinases causing IκB to become phosphorylated and targeted for ubiquitination and degradation releasing active NF-κB to the nucleus (Karin and Ben-Neriah, 2000). Like NF-κB, C/EBPβ activity is controlled by cytokines, including TNFα, but its regulation is more complex and occurs at multiple levels including transcription, alternate translation initiation and phosphorylation (Buck et al., 2001; Cardinaux et al., 2000; Ramji and Foka, 2002).
Since JCV transcription is regulated by NF-κB and C/EBPβ via the κB site in the JCV and these two transcription factors are, in turn, regulated by proinflammatory cytokines, such as TNF-α, we have now investigated a role for TNF-α in the reactivation of latent JCV to cause PML. Most cases of PML occur in the context of HIV-1/AIDS. In this regard, the presence of HIV-1 and its associated proteins in the CNS can lead to the occurrence of cytokine cascades involving the production of pro-inflammatory cytokines, including TNF-α (Benveniste, 1994; Kaul et al., 2005; Yeung et al., 1995). We now report that TNF-α can stimulate JCV transcription measured using different reporter constructs in both the early and late orientations in several astrogilal and oligodendroglial cell types. Moreover, robust expression of TNF-α and its receptor TNFR1 was observed in JCV-infected astrocytes and oligodendrocytes in PML lesions from an HIV-positive patient relative to normal brain.
2. Materials and methods
2.1. Plasmids
The promoter reporter constructs, JCVE-CAT and JCVL-CAT contained the noncoding control region (NCCR) from the Mad-1 strain of JCV linked to the chloramphenicol acetyltransferase (CAT) gene in the early and late orientations respectively (Chen and Khalili, 1995). The JCVE-CAT promoter mutants m1 and m2 were made by site-directed mutagenesis of the κB site as previously described (Romagnoli et al., 2009). The reporter constructs, JCVE-LUC and JCVL-LUC were made by blunt end cloning of the full-length Mad-1 NCCR into the SmaI site immediately upstream of the luciferase gene in the plasmid pGL3 (Promega, Madison WI). The hygromycin-selectable luciferase reporter plasmids JCVE-LUC(4) and JCVL-LUC(4) were made by cutting out the JCV promoter elements from JCVE-LUC and JCVL-LUC respectively with KpnI and BglII, which flank the SmaI site, and cloning into the KpnI/BglII site of pGL4.14 (Promega). Heterologous reporter plasmids were made by cloning tandem wild-type or mutant JCV κB sites into pBLCAT2 to give pBLCAT2-wt-κB and pBLCAT2-mt-κB respectively. The CAT reporter plasmid pBLCAT2 contains the constitutive Herpes simplex virus thymidine kinase (tk) promoter driving CAT expression (Luckow and Schütz, 1987). The tandem κB elements were introduced by cloning double-stranded synthetic oligonucleotide into the HindIII/BamH1 site upstream of the pBLCAT2 tk promoter. For wt-κB, the following oligonucleotides were used: TOP: 5′-agc tta aaa caa ggg aat ttc cct ggc ctc aaa caa ggg aat ttc cct ggc ctc gct agc g-3′; BOTTOM: 5′-gat ccg cta gcg agg cca ggg aaa ttc cct tgt ttg agg cca ggg aaa ttc cct tgt ttt a-3′. The κB elements are shown underlined. For mt-κB, the following oligonucleotides were used: TOP: 5′-agc tta aaa caa CTC aat ttc cct ggc ctc aaa caa CTC aat ttc cct ggc ctc gct agc g-3′; BOTTOM: 5′-gat ccg cta gcg agg cca ggg aaa ttG AGt tgt ttg agg cca ggg aaa ttG AGt tgt ttt a-3′. The mutated nucleotides are show in capitals.
Lentivirus constructs containing the luciferase reporter gene under the control of the JCV early and late promoters were made with the HIV-1-based lentivirus expression vector pLVX-Puro (Clontech, Mountain View CA) as follows. The pCMV promoter was removed from the pLVX-Puro vector by digestion with ClaI followed by blunt-ending by fill-in with Klenow, digestion with BamHI and gel purification of the plasmid fragment now lacking the promoter. Similarly, fragments were produced containing the JCV early and late promoters from JCVE-LUC(4) and JCVL-LUC(4) respectively by digestion with KpnI, blunt-ending, digesting with BamHI and gel purification followed by ligation to the vector fragment. The resulting pLUX-puro early or late vectors were then introduced into 293T cells by transfection using fugene reagent (Roche, Basel, Schweiz) together with pCMV-VSVG, pRSV-REV and pMDLg/pRRE. After 48 hours, the lentivirus-containing supernatants were harvested, filtered through 0.45 μm filters and added to cells 36–48 hours prior to the cytokine stimulation assay.
2.2. Cell culture and transfection
The human TC620 oligodendroglioma cell line was obtained from Dr. Bassel Sawaya (Sawaya et al., 1996) and was cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin and 1% l-glutamine. Primary cultures of human fetal astrocytes were prepared as described previously (Radhakrishnan et al., 2003, 2004). Experiments involving co-transfection of reporter plasmids and C/EBPβ-LIP and NF-κB-p65 expression plasmids were performed as we have previously described (Romagnoli et al., 2009). Stable clonal cell lines expressing luciferase under the control of the JCV early and late promoters by transfecting TC620 with JCVE-LUC(4) and JCVL-LUC(4) respectively followed by hygromycin selection and dilution cloning as follows, Following transfection, TC620 cells were subcultured in complete DEM containing 400 μg/ml hygromycin B for two weeks. Clonal colonies were detached by trypsinization using cloning rings and expanded in culture in the presence of hygromycin. Clones were tested for integrity of the luciferase gene by luciferase assay and for integrity of the JCV promoters by measuring for inducibility by PMA. All transfections were performed using fugene according to the manufacturer’s instructions (Roche).
2.3. Cytokine stimulation reporter assays
Cell cultures were grown in 6-well plates and then incubated in serum-free DMEM for 24 hours prior to cytokine addition and were harvested for reporter assays after another 24 hours. For assays involving transient transfection with reporter plasmid, transfections were performed with 1 μg of plasmid/well using fugene reagent (Roche) at 24 hours prior to the serum starvation step. Phorbol 12-myristate 13-acetate (PMA) was used as a positive control (100 ng/ml). Cytokines were used at the following concentrations: TNF-α - 10ng/ml; IL-6 - 10ng/ml; IL-1β - 10ng/ml; TGFβ - 20 ng/ml. For, reporter assays the chloramphenicol acetyl transferase (CAT) activity of samples was determined as previously described (Romagnoli et al., 2008, 2009) and luciferase (LUC) activity was determined with the dual-luciferase assay kit according to the Manufacturer’s instructions (Promega).
2.4. Antibodies
Mouse monoclonal antibody to TNFR1 and rabbit polyclonal antibody to TNFR2 were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Mouse monoclonal antibody to TNF-α was obtained from Chemicon international (Temecula CA, clone 195). Rabbit polyclonal antibody against JCV capsid protein VP1 was provided by Dr. Walter Atwood (Brown University).
2.5. Western blots
Western blot assays were performed as previously described (White et al., 2006).
2.6. Immunohistochemistry
Immunohistochemical analysis was performed with the use of an avidin–biotin–peroxidase complex system according to the manufacturer’s instructions (Vectastain Elite ABC Peroxidase Kit; Vector Laboratories Inc., Burlingame, CA) as we have described previously (Del Valle et al., 2002).
3. Results
3.1. The JCV early and late promoters are modulated by NF-κB p65 and C/EBPβ LIP in TC620 oligodendroglioma cells
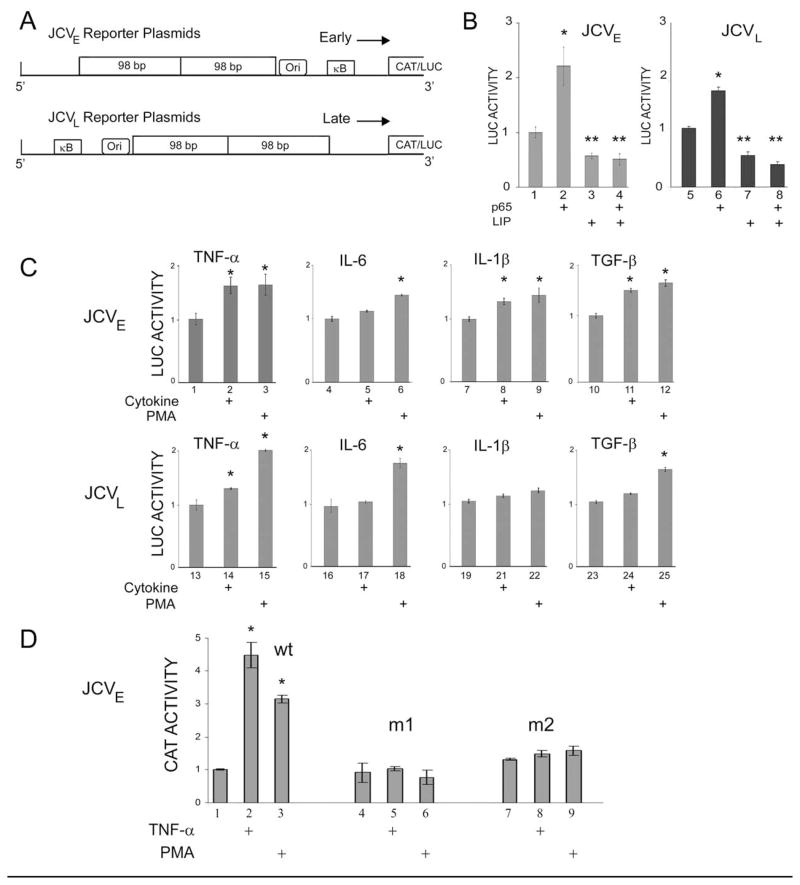
Previous studies had shown that the JCV early and late promoters were positively regulated by NF-κB and negatively regulated by C/EBPβ isoforms (especially LIP) in U-87 MG human glioblastoma cells (Romagnoli et al., 2009). In order to test whether this was also the case in cells of oligodendrocytic origin, we performed co-transfection experiments with the human oligodendroglioma cell line TC620. Note that in earlier studies, we employed JCV early and late reporter plasmids based on the chloramphenicol acetyltransferase (CAT) gene (Romagnoli et al., 2009). In most of the studies reported here, we use plasmids in which we have subcloned the JCV early and late promoters in front of the luciferase (LUC) reporter gene since the luciferase assay is simpler and less time-consuming to perform. The construction of these reporter plasmids is described in Materials and Methods and a schematic of the reporter plasmids used in the transient transfection experiments is shown in Fig. 1A indicating the location of the NF-κB binding site (labeled κB) relative to the early and late JCV transcription units and the origin of viral DNA replication (ORI). Co-transfection of early reporter plasmid, JCVE-LUC, with plasmid expressing NF-κB p65 stimulated transcription (Fig. 1B, compare lanes 1 and 2) while C/EBPβ LIP inhibited (lanes 3 and 4). The same results were obtained with the JCV late promoter (lanes 5–8). Thus NF-κB p65 and C/EBPβ modulate the JCV early and late promoters in human oligodendroglioma cells as we reported previously in human glioblastoma cells (Romagnoli et al., 2009).
Figure 1. Effects of NF-κB p65, C/EBPβ LIP and cytokines on JCV promoter reporter constructs transiently transfected into TC620 human oligodendroglioma cells.
A. Schematic representation of the JCV promoter reporter constructs showing the relative locations and orientations of the early (upper) and late (lower) promoters, the κB element, which contains binding sites for NF-κB and C/EBPβ, the origin of viral DNA replication (Ori) and the 98 base-pair tandem repeats. B. TC620 cells were transfected with the JCVE-LUC (left) or JCVL-LUC reporter plasmids in the presence or absence of pCMV-p65 and/or pCMV-LIP. In each assay, LUC activity was normalized to reporter alone (lanes 1 and 5) and presented as a histogram. The error bars encompass one standard deviation. The asterisks indicate lanes that are significantly different (P < .05) from the control in each panel (lanes 1 and 5). C. TC620 cells were transfected with JCVE-LUC (upper panels) or JCVL-LUC reporter plasmids (lower panels), serum-starved and then stimulated with cytokine (as indicated) or PMA and then harvested for luciferase assay as described in Materials and Methods. LUC activity was normalized to reporter plasmid alone (left-hand lane in each panel) and presented as a histogram. The error bars encompass one standard deviation. The asterisks indicate lanes that are significantly different (P < .05) from the control in each panel (left-hand lane). D. The effect of TNF-α was assessed for three CAT reporter plasmids: wild-type and two mutants m1 and m2, which have mutations in the κB element. Protocol was as for C. except that CAT assays were performed.
3.2. The JCV early and late promoters are modulated by TNF-α in transient transfection reporter assays with TC620 oligodendroglioma cells
Since the NF-κB pathway is activated by TNF-α, we next examined the effect of TNF-α on the JCV early and late transcription in transient reporter plasmid transfection assays. Also included in these experiments were IL-1β, another proinflammatory cytokine that activates the NF-κB pathway (Mercurio and Manning, 1999), and IL-6, which has been reported to be upregulated in PML (Nagano et al., 1994; Torre et al., 1992). TGF-β was included as a positive control, since we have previously found that JCV transcription can be activated by the Smad signaling pathway downstream of the TGF-β receptor (Enam et al., 2004). Each individual cytokine stimulation experiment included PMA as a positive control. TNF-α stimulated the early promoter as strongly as did PMA (Fig. 1C, compare lanes 1–3) as did TGF-β (lanes 10–12). IL-6 had a minimal effect (lanes 4–6) while IL-1β was intermediate (lanes 7–9). For the late promoter, there was a smaller but statistically significant effect for TNF-α (lanes 13–15) and a minimal effect for the other cytokines (lanes 16–25). Similar results were obtained with U-87 MG human glioblastoma cells (data not shown).
3.3. Mutation of the κB site in the JCV early promoter abrogates its induction by TNF-α and PMA
For our earlier studies of NF-Kβ/C/EBPβ interaction with the JCV promoter, we constructed mutant JCV early promoter CAT reporter constructs in which the κB site (where NF-κB and C/EBPβ bind) and these were designated m1 and m2 (Romagnoli et al., 2009). As shown in Fig. 1D, while wild-type early promoter (lane 1) responded to TNF-α (lane 2) and PMA (lane 3), neither of the κB site mutants responded (lanes 4–9) demonstrating the importance of the κB site for response of the JCV early promoter to TNF-α.
3.4. Stably transfected clonal cell lines expressing the luciferase reporter gene from the JCV early or late promoters are responsive to TNF-α
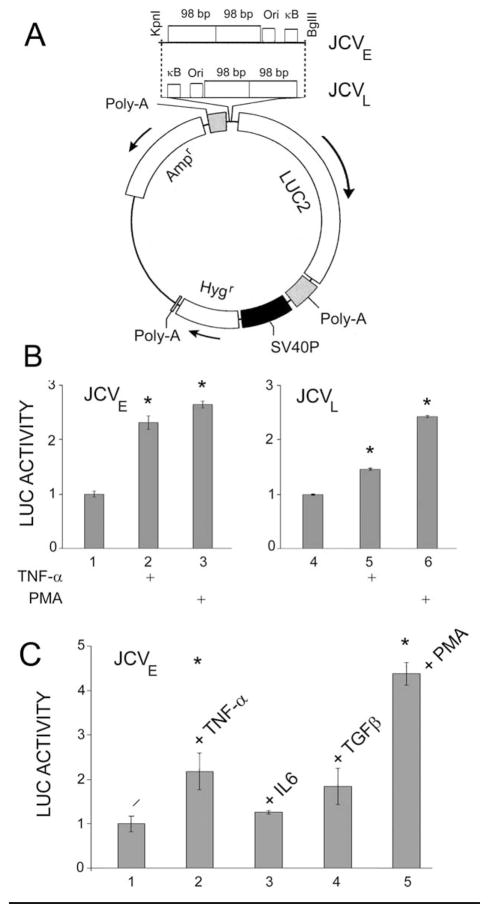
As well as the transient transfection experiments described above, we also produced clonal reporter cell lines by stable transfection. The JCV promoter was cloned into the pGL4 reporter plasmid in both the early and late orientations as described in Materials and Methods. The resulting plasmids, which have the hygromycin-resistance gene, are shown in Fig. 2A. After transfection into TC620, a series of clonal early and late reporter cell lines were generated. Clones were chosen that were positive for luciferase to make sure the luciferase structural gene was intact and positive for PMA-inducibility to make sure the JCV promoter was intact. Clones were treated with or without TNF-α and PMA. A representative experiment is shown in Fig. 2b. In agreement with the data obtained with the transient transfection assays, TNF-α stimulated both the early and later promoter with the early promoter showing the stronger stimulation. Similarly, PMA but not IL-6 stimulated the early promoter, while the stimulation by TGF-β was not as strong (Fig. 2C).
Figure 2. Effects of cytokines on JCV early and late promoter clonal reporter cell lines derived from TC620 human oligodendroglioma cells.
A. Clonal cell lines were derived by cloning the JCV early or late promoter into pGL4, which contains the hygromycin-resistance gene, transfecting into TC620, selecting with hygromycin and cell cloning as described in Materials and Methods. B. Clonal early reporter cells (left-hand panel) or late reporter cells (right-hand panel) were serum-starved and then stimulated with TNF-α or PMA as indicated and then harvested for luciferase assay as described in Materials and Methods. LUC activity was normalized to reporter plasmid alone (left-hand lane in each panel) and presented as a histogram. The error bars encompass one standard deviation. The asterisks indicate lanes that are significantly different (P < .05) from the control in each panel (left-hand lane). C. Clonal early reporter cells were serum-starved and then stimulated with cytokines or PMA as indicated and then harvested for LUC assay. LUC activity was normalized to reporter plasmid alone (lane 1) and presented as a histogram. The error bars encompass one standard deviation. The asterisks indicate lanes that are significantly different (P < .05) from the control (lane 1).
3.5. The JCV NCCR κB element confers NF-κB p65 and C/EBPβ LIP regulation to a heterologous promoter
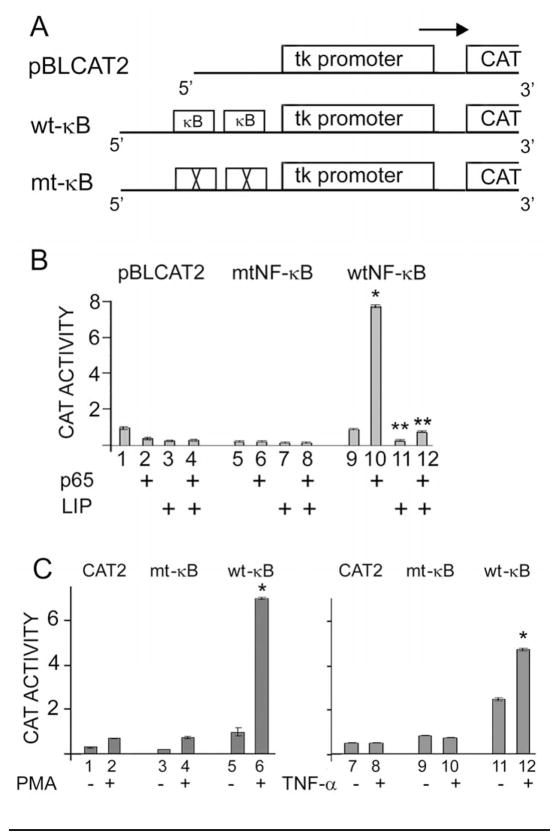
Since the introduction of mutations into κB site of the JCV early promoter abrogates its ability to respond to NF-κB (Romagnoli et al., 2009) and TNF-α (Fig. 1D), this suggests that the κB element is the site of action of TNF-α-stimulated NF-κB activation. To further investigate this, we constructed heterologous promoters based on the Herpes simplex thymidine kinase promoter (tk), which is not responsive to cytokine stimulation. Tandem wild-type or mutant κB elements were cloned upstream of tk in the reporter plasmid as described in Materials and Methods and shown schematically in Fig. 3A. As shown in Fig. 3B, reporter plasmid with wild-type κB elements was strongly stimulated by co-transfection of NF-κB whereas plasmid with mutant κB elements or no elements were not responsive. Further, this stimulation is blocked in the presence of C/EBPβ LIP (compare lane 10 to 12). These data indicate the JCV κB element is sufficient to confer responsiveness to both NF-κB and C/EBPβ and supports earlier studies that it κB binds both transcription factors and confers NF-κB and C/EBPβ responsiveness to the JCV promoters Romagnoli et al., 2009).
Figure 3. Introduction of the JCV κB element into a heterologous promoter confers responsiveness to NF-κB p65, C/EBPβ LIP, PMA and TNF-α.
A. Diagrammatic representations of the three constructs used in these assays are shown. The first contains the HSV tk promoter driving the CAT reporter. The second has a tandem repeat of the wild-type JCV κB element inserted as described in Materials and Methods, while in the third the κB element was mutated. B. TC620 cells were transfected with each of the reporter plasmids in the presence or absence of pCMV-p65 and/or pCMV-LIP. The error bars encompass one standard deviation. The asterisks indicate lanes that are significantly different (P < .05) from the control in each panel (lanes 1 and 5). C. TC620 cells were transfected with each of the reporter plasmids, serum-starved and then stimulated with TNF-α or PMA as indicated and then harvested for CAT assay as described in Materials and Methods. The error bars encompass one standard deviation. The asterisks indicate lanes that are significantly different (P < .05) from the control in each panel (left-hand lane).
3.6. The JCV NCCR κB element confers TNF-α regulation to a heterologous promoter
Next, we examined the effect of cytokine stimulation on the heterologous promoters using PMA as a positive control. TNF-α stimulated the wild-type κB promoter (Fig. 3C, compare lanes 11 and 12) but not the mutant κB or pBLCAT2 promoter. Neither IL-6 nor TGFβ had any effect on these three promoters (data not shown).
3.7. TNF-α regulated the JCV promoters in primary cultures of human astrocytes transduced by reporter lentiviruses
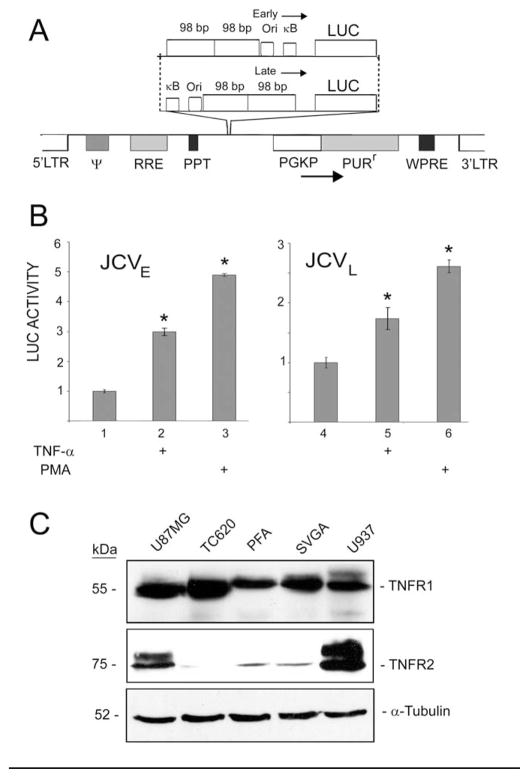
In order to introduce JCV reporters into a primary cell type that is capable of supporting productive JCV replication, we constructed lentiviral expression vectors for transduction of primary cultures of human fetal astrocytes (Fig. 4A). Luciferase activity was inducible by TNF-α in astrocytes transduced by both lentiviruses expressing the reporter gene from the JCV early and late promoter (Fig. 4B). Next, we evaluated the expression of the two types of TNF-α receptors (TNFR1 and TNFR2) in the human cells used in these studies, i.e., TC620 oligodendroglioma cells and primary fetal astrocytes (PFA) and in our earlier study, U87 MG human glioblastoma and the SV40 T-antigen transformed human glial cell line, SVGA (Romagnoli et al., 2009). U937 human monocytic cells were used as a positive control for TNFR2. As shown in Fig. 4C, all cell types expressed high levels of TNFR1. TNFR2 was expressed at a high level in U937 cells but was barely detectable in the other cell types except for U87 MG. Therefore, we conclude that the effects of TNF-α observed in primary astrocytes and TC620 cells are likely mediated by TNFR1.
Figure 4. Introduction of the JCV early and late reporters into primary human fetal astrocytes using a lentiviral vector.
A. Diagrammatic representation of two lentiviral vectors containing a JCVE-LUC or JCVL-LUC reporter cassette. B. Primary cultures of human fetal astrocytes were transduced with the lentiviruses, serum-starved and then stimulated with TNF-α or PMA as indicated and then harvested for luciferase assay as described in Materials and Methods. LUC activity was normalized to unstimulated transduced cells (left-hand lane in each panel) and presented as a histogram. The error bars encompass one standard deviation. The asterisks indicate lanes that are significantly different (P < .05) from the control in each panel (left-hand lane). C. Western blots were performed for the cell types used in these studies to determine which forms of the TNF-α receptor are expressed: TNFR1 and TNFR2. The control for protein loading was α-tubulin.
3.8. Immunohistochemistry of clinical samples of PML from an HIV+ patient reveals the robust presence of TNF-α and TNFR1
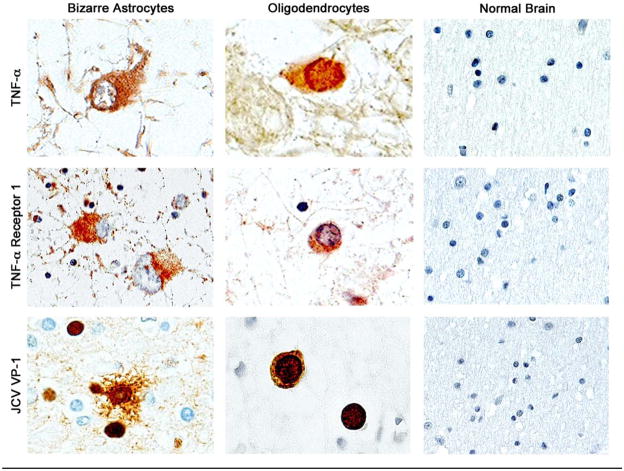
To investigate the relevance of our findings to PML, immunohistochemistry (IHC) was performed on tissue from a PML lesion and from a normal brain using primary antibodies to TNF-α, TNFR1 and TNFR2 (Fig. 5). IHC for TNF-α demonstrates robust expression in bizarre astrocytes within demyelinated plaques and in the inclusion body-harboring oligodendrocytes. TNF-R1 was found upregulated on the cell surface of bizarre astrocytes and in enlarged oligodendrocytes as indicated by a punctuate pattern. IHC for TNF-R2 gave only a weak labeling (data not shown). No labeling for TNF-α or TNF-R1 was observed in brain tissue that lay outside of the PML lesion or in IHC of normal brain tissue. Productive infection of the bizarre astrocytes and the inclusion body-harboring oligodendrocytes within the PML lesion was demonstrated by IHC with antibody to the VP1 capsid protein. These data suggest that the stimulation of the JCV promoters observed in cell culture may be important for the pathogenesis of PML.
Figure 5. Expression of TNF-α and TNF-α receptor 1 (TNFR1) in JCV-infected cells in PML brain tissue and normal brain.
Immunohistochemistry was performed in a sample of PML. The PML case was of a 29 year-old male, HIV+, with AIDS, exhibiting signs of dementia, confusion and belligerence. The lesions were located in the subcortical white matter of predominantly the frontal and temporal lobes, consistent with the symptomatology, i.e., frontal lobe lesions associated with dementia, and temporal lobe lesions associated with confusion and belligerence. The immunohistochemistry shows the presence of TNF-α and TNF-α receptor in reactive astrocytes and enlarged JCV-infected oligodendrocytes, while there is no evidence of TNF-α expression or its receptor in the control normal brain. The presence of JCV infection was demonstrated with an anti-capsid antibody (VP-1), which shows a robust labeling in both, bizarre astrocytes and enlarged oligodendrocytes. X1000.
4. Discussion
In earlier work, we described an NF-κB binding site (κB), which positively regulates JCV early and late transcription in response to NF-κB and lies between the JCV early region start codon and the origin of viral DNA replication (Ranganathan and Khalili, 1993; Mayreddy et al., 1996; Safak et al., 1999; Romagnoli et al., 2009), Since NF-κB can be activated by TNF-α (Fiers, 1991), this suggests a mechanism for JCV activation in glial cells similar to the stimulation of the HIV-1 promoter activity in glial elicited by TNF-α via NF-κB (Atwood et al., 1994). However, it has been reported that TNF-α does not stimulate transcription of JCV reporter constructs or the multiplication of JCV in human fetal glial cells (Atwood et al., 1995) although it should be noted that the JCV reporter construct used in this study did not contain the κB element. Since most cases of PML occur in the context of HIV-1/AIDS and HIV-1 can generate cytokine cascades in the CNS involving TNF-α (Wesselingh et al., 1993; Benveniste, 1994; Kaul et al., 2005; Yeung et al., 1995), this is an important issue. Accordingly we have carefully and thoroughly evaluated a role for TNF-α and the κB site in latent JCV transcriptional reactivation. We now report that TNF-α can stimulate JCV transcription measured using four different reporter constructs in both the early and late orientations in astroglial and oligodendroglial cell types. This stimulation was abrogated by mutations within the κB element. Moreover, the κB element was able to confer inducibility by NF-κB and TNF-α to a heterologous promoter. The relevance of these findings to the pathogenesis of HIV+/PML is indicated by the presence of robust levels of TNF-α and TNFR1 in clinical samples of PML lesions from an HIV+ patient. Note that is not clear whether TNF-α expression is involved in other disorders associated with PML, e.g., chronic lymphocytic leukemia where significant alterations in cytokine expression in the brain milieu would not be anticipated. Also it is not possible to exclude the possibility that TNF-α is induced as a consequence of PML itself rather than HIV-1 cytokine storms.
Other studies have also highlighted connections between proinflammatory cytokines and PML pathogenesis. A link between inflammatory cytokines and JCV infection was indicated by a study using the anti-inflammatory agent cyclosporine A which was found to block infection of glial cells by inhibition of the NFAT4 transcription factor (Manley et al., 2006). Using microarray analysis of glial cells resistant to JCV infection, Manley et al (2007) provided evidence for a correlation between viral infection and inflammatory cytokine gene expression. Enam et al (2004) provided evidence for the involvement of the TGFβ/Smad signaling pathway in PML. Other transcription factors that are modulated by signaling pathways downstream of cytokines have been found to bind and regulate JCV transcription such as Egr-1 (Romagnoli et al., 2008) and AP-1 (Kim et al., 2003; Sadowska et al., 2003). These cytokine-regulated signal transduction pathways and possibly other important cytokines and signaling pathways are likely to co-exist and interact with the TNF-α/NF-κB pathway in regulating JCV activation.
The finding that TNF-α activates JCV transcription and is present in PML lesions may be relevant to understanding the pathogenesis of PML. The life cycle of JCV is thought to involve transmission of the virus to an individual during childhood, establishment of a primary viremia, elimination by the immune system followed by entry into a “latent state”. Following the occurrence of severe immunosuppression, JCV then “reactivates” to cause PML. The nature and site(s) of viral latency within the body and the molecular events involved in viral reactivation have been much debated and are incompletely understood. Many tissues have been reported to harbor latent JCV including kidney, tonsil, peripheral blood leukocytes and the brain (reviewed by Del Valle et al., 2008). With regard to the brain, there have been many reports that JCV can be present in the brain of normal individuals who do not have PML (White et al., 1992; Elsner and Dorries, 1992; Greenlee et al., 2005; Mori et al., 1991, 1992; Perez-Liz et al., 2008). For example, the presence of subclinical areas of JCV replication presenting as tiny punched demyelinated foci can often be detected in the brains of elderly patients without PML (Mori et al., 1991). Perez-Liz et al (2008) detected JCV DNA in brain from some non-PML individuals and used laser-capture microdissection to show the presence of JCV DNA in oligodendrocytes and astrocytes, but not in neurons. On the other hand, it has been argued that functional JCV DNA is not found in the normal brain and that B cells represent the site of functional latency (Major, 2010). Recently, using quantitative PCR, cloning and sequencing of PCR products and in situ hybridization, Tan et al (2010) found JCV DNA to be present in non-PML brain. Taken together, these studies suggest that the normal brain may be a site of viral latency in at least some individuals and thus JCV reactivation events may involve cytokines such as TNF-α acting directly on glial cells. In this scenario, cytokine storms in the CNS activate JCV transcription, expression of T-antigen and onset of viral replication. In the context of a lack of immunosurveillance, virus could then spread and PML lesions form.
Other models of JCV latency and reactivation have been proposed. For example, it has been suggested that B-lymphocytes in the bone marrow may be sites of JCV latency and serve as a source of virus allowing JCV to circulate around the body and enter the brain (Berger et al., 2009; Major, 2010). This may be important in the induction of PML in patients receiving drugs that modulate the immune system such as natalizumab and rituximab. This model is not inconsistent with models proposing cytokine induction in the brain. Indeed, Berger et al (2009) have argued that the great rarity of PML compared to the ubiquity of JCV infection is indicative of the existence of multiple barriers to the development of PML and all of these barriers must be breached for PML to occur. Understanding and targeting these barriers may provide new routes for developing novel treatments for PML.
Acknowledgments
We thank past and present members of the Center for Neurovirology for their insightful discussion and sharing of ideas and reagents. This work was supported by a grant awarded by the NIH to MKW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atwood WJ, Tornatore CS, Traub R, Conant K, Drew PD, Major EO. Stimulation of HIV type 1 gene expression and induction of NF-kappa B (p50/p65)-binding activity in tumor necrosis factor alpha-treated human fetal glial cells. AIDS Res Hum Retroviruses. 1994;10:1207–1211. doi: 10.1089/aid.1994.10.1207. [DOI] [PubMed] [Google Scholar]
- Atwood WJ, Wang L, Durham LC, Amemiya K, Traub RG, Major EO. Evaluation of the role of cytokine activation in the multiplication of JC virus (JCV) in human fetal glial cells. J Neurovirol. 1995;1:40–49. doi: 10.3109/13550289509111009. [DOI] [PubMed] [Google Scholar]
- Benveniste EN. Cytokine circuits in brain. Implications for AIDS dementia complex. Res Publ Assoc Res Nerv Ment Dis. 1994;72:71–88. [PubMed] [Google Scholar]
- Berger JR. Progressive multifocal leukoencephalopathy in acquired immunodeficiency syndrome: explaining the high incidence and disproportionate frequency of the illness relative to other immunosuppressive conditions. J Neurovirol. 2003;9(Suppl 1):38–41. doi: 10.1080/13550280390195261. [DOI] [PubMed] [Google Scholar]
- Berger JR, Pall L, Lanska D, Whiteman M. Progressive multifocal leukoencephalopathy in patients with HIV infection. J Neurovirol. 1998;4:59–68. doi: 10.3109/13550289809113482. [DOI] [PubMed] [Google Scholar]
- Berger JR, Houff SA, Major EO. Monoclonal antibodies and progressive multifocal leukoencephalopathy. MAbs. 2009;1:583–589. doi: 10.4161/mabs.1.6.9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks BR, Walker DL. Progressive multifocal leukoencephalopathy. Neurol Clin. 1984;2:299–313. [PubMed] [Google Scholar]
- Buck M, Zhang L, Halasz NA, Hunter T, Chojkier M. Nuclear export of phosphorylated C/EBPbeta mediates the inhibition of albumin expression by TNF-alpha. EMBO J. 2001;20:6712–6723. doi: 10.1093/emboj/20.23.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinaux JR, Allaman I, Magistretti PJ. Pro-inflammatory cytokines induce the transcription factors C/EBPbeta and C/EBPdelta in astrocytes. Glia. 2000;29:91–97. [PubMed] [Google Scholar]
- Chen NN, Khalili K. Transcriptional regulation of human JC polyomavirus promoters by cellular proteins YB-1 and Pur alpha in glial cells. J Virol. 1995;69:5843–5848. doi: 10.1128/jvi.69.9.5843-5848.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Valle L, Gordon J, Enam S, Delbue S, Croul S, Abraham S, Radhakrishnan S, Assimakopoulou M, Katsetos CD, Khalili K. Expression of human neurotropic polyomavirus JCV late gene product agnoprotein in human medulloblastoma. J Natl Cancer Inst. 2002;94:267–273. doi: 10.1093/jnci/94.4.267. [DOI] [PubMed] [Google Scholar]
- Del Valle L, White MK, Khalili K. Potential mechanisms of the human polyomavirus JC in neural oncogenesis. J Neuropathol Exp Neurol. 2008;67:729–740. doi: 10.1097/NEN.0b013e318180e631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsner C, Dorries K. Evidence of human polyomavirus BK and JC infection in normal brain tissue. Virology. 1992;191:72–80. doi: 10.1016/0042-6822(92)90167-n. [DOI] [PubMed] [Google Scholar]
- Enam S, Sweet TM, Amini S, Khalili K, Del Valle L. Evidence for involvement of transforming growth factor beta1 signaling pathway in activation of JC virus in human immunodeficiency virus 1-associated progressive multifocal leukoencephalopathy. Arch Pathol Lab Med. 2004;128:282–291. doi: 10.5858/2004-128-282-EFIOTG. [DOI] [PubMed] [Google Scholar]
- Fiers W. Tumor necrosis factor. Characterization at the molecular, cellular and in vivo level. FEBS Lett. 1991;285:199–212. doi: 10.1016/0014-5793(91)80803-b. [DOI] [PubMed] [Google Scholar]
- Greenlee JE, Clawson SA, Carney HC, O-Neill FJ. Detection of JC virus early region sequences of brains of individuals without progressive multifocal leukoencephalopathy. Ann Neurol. 2005;28(S9):S61. [Google Scholar]
- Holman RC, Torok TJ, Belay ED, Janssen RS, Schonberger LB. Progressive multifocal leukoencephalopathy in the United States, 1979–1994: increased mortality associated with HIV infection. Neuroepidemiology. 1998;17:303–309. doi: 10.1159/000026184. [DOI] [PubMed] [Google Scholar]
- Hou J, Major EO. Progressive multifocal leukoencephalopathy: JC virus induced demyelination in the immune compromised host. J Neurovirol. 2000;6(Suppl 2):S98–S100. [PubMed] [Google Scholar]
- Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005;12(Suppl 1):878–892. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- Khalili K, Gordon J, White MK. The polyomavirus, JCV, and its involvement in human disease. Adv Exp Med Biol. 2006;577:274–287. doi: 10.1007/0-387-32957-9_20. [DOI] [PubMed] [Google Scholar]
- Khalili K, Safak M, Del Valle L, White MK. JC virus molecular biology and the human demyelinating disease, progressive multifocal leukoencephalopathy. In: Shoshkes Reiss C, editor. Neurotropic virus infections. Cambridge University Press; Cambridge, UK: 2008. pp. 190–211. [Google Scholar]
- Kim J, Woolridge S, Biffi R, Borghi E, Lassak A, Ferrante P, Amini S, Khalili K, Safak M. Members of the AP-1 family, c-Jun and c-Fos, functionally interact with JC virus early regulatory protein large T antigen. J Virol. 2003;77:5241–5252. doi: 10.1128/JVI.77.9.5241-5252.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckow B, Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987;15:5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major EO. Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu Rev Med. 2010;61:35–47. doi: 10.1146/annurev.med.080708.082655. [DOI] [PubMed] [Google Scholar]
- Manley K, O’Hara BA, Gee GV, Simkevich CP, Sedivy JM, Atwood WJ. NFAT4 is required for JC virus infection of glial cells. J Virol. 2006;80:12079–12085. doi: 10.1128/JVI.01456-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley K, Gee GV, Simkevich CP, Sedivy JM, Atwood WJ. Microarray analysis of glial cells resistant to JCV infection suggests a correlation between viral infection and inflammatory cytokine gene expression. Virology. 2007;366:394–404. doi: 10.1016/j.virol.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayreddy RP, Safak M, Razmara M, Zoltick P, Khalili K. Transcription of the JC virus archetype late genome: importance of the kappa B and the 23-base-pair motifs in late promoter activity in glial cells. J Virol. 1996;70:2387–2393. doi: 10.1128/jvi.70.4.2387-2393.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercurio F, Manning AM. Multiple signals converging on NF-kappaB. Curr Opin Cell Biol. 1999;11:226–232. doi: 10.1016/s0955-0674(99)80030-1. [DOI] [PubMed] [Google Scholar]
- Mázló M, Ressetar HG, Stoner GL. The neuropathology and pathogenesis of progressive multifocal leukoencephalopathy. In: Khalili K, Stoner GL, editors. Human polyomaviruses: molecular and clinical perspectives. Wiley-Liss, Inc; New York: 2001. pp. 257–335. [Google Scholar]
- Mori M, Kurata H, Tajima M, Shimada H. JC virus detection by in situ hybridization in brain tissue from elderly patients. Ann Neurol. 1991;29:428–432. doi: 10.1002/ana.410290414. [DOI] [PubMed] [Google Scholar]
- Mori M, Aoki N, Shimada H, Tajima M, Kato K. Detection of JC virus in the brains of aged patients without progressive multifocal leukoencephalopathy by the polymerase chain reaction and Southern hybridization analysis. Neurosci Lett. 1992;141:151–155. doi: 10.1016/0304-3940(92)90883-9. [DOI] [PubMed] [Google Scholar]
- Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326:711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Nagano I, Nakamura S, Yoshioka M, Onodera J, Kogure K, Itoyama Y. Expression of cytokines in brain lesions in subacute sclerosing panencephalitis. Neurology. 1994;44:710–715. doi: 10.1212/wnl.44.4.710. [DOI] [PubMed] [Google Scholar]
- Padgett BL, Walker DL. Prevalence of antibodies in human sera against JC virus, an isolate from a case of progressive multifocal leukoencephalopathy. J Infect Dis. 1973;127:467–470. doi: 10.1093/infdis/127.4.467. [DOI] [PubMed] [Google Scholar]
- Perez-Liz G, Del Valle L, Gentilella A, Croul S, Khalili K. Detection of JC virus DNA fragments but not proteins in normal brain tissue. Ann Neurol. 2008;64:379–387. doi: 10.1002/ana.21443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan S, Otte J, Enam S, Del Valle L, Khalili K, Gordon J. JC virus-induced changes in cellular gene expression in primary human astrocytes. J Virol. 2003;77:10638–10644. doi: 10.1128/JVI.77.19.10638-10644.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan S, Gordon J, Del Valle L, Cui J, Khalili K. Intracellular approach for blocking JCV gene expression using RNA interference during viral infection. J Virol. 2004;78:7264–7269. doi: 10.1128/JVI.78.13.7264-7269.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J. 2002;365:561–575. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan PN, Khalili K. The transcriptional enhancer element, kappa B, regulates promoter activity of the human neurotropic virus, JCV, in cells derived from the CNS. Nucleic Acids Res. 1993;21:1959–64. doi: 10.1093/nar/21.8.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnoli L, Sariyer IK, Tung J, Feliciano M, Sawaya BE, Del Valle L, Ferrante P, Khalili K, Safak M, White MK. Early growth response-1 protein is induced by JC virus infection and binds and regulates the JC virus promoter. Virology. 2008;375:331–341. doi: 10.1016/j.virol.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnoli L, Wollebo HS, Deshmane SL, Mukerjee R, Del Valle L, Safak M, Khalili K, White MK. Modulation of JC virus transcription by C/EBPβ. Virus Res. 2009;146:97–106. doi: 10.1016/j.virusres.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowska B, Barrucco R, Khalili K, Safak M. Regulation of human polyomavirus JC virus gene transcription by AP-1 in glial cells. J Virol. 2003;77:665–672. doi: 10.1128/JVI.77.1.665-672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safak M, Gallia GL, Khalili K. A 23-bp sequence element from human neurotrophic JC virus is responsive to NF-kappa B subunits. Virology. 1999;262:178–189. doi: 10.1006/viro.1999.9886. [DOI] [PubMed] [Google Scholar]
- Sawaya BE, Rohr O, Aunis D, Schaeffer E. Chicken ovalbumin upstream promoter transcription factor, a transcriptional activator of HIV-1 gene expression in human brain cells. J Biol Chem. 1996;271:23572–23576. doi: 10.1074/jbc.271.38.23572. [DOI] [PubMed] [Google Scholar]
- Tan CS, Ellis LC, Wüthrich C, Ngo L, Broge TA, Saint-Aubyn J, Miller JS, Koralnik IJ. JC virus latency in the brain and extraneural organs of patients with and without progressive multifocal leukoencephalopathy. J Virol. 2010;84:9200–9209. doi: 10.1128/JVI.00609-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre D, Zeroli C, Ferraro G, Speranza F, Tambini R, Martegani R, Fiori GP. Cerebrospinal fluid levels of IL-6 in patients with acute infections of the central nervous system. Scand J Infect Dis. 1992;24:787–791. doi: 10.3109/00365549209062465. [DOI] [PubMed] [Google Scholar]
- Walker DL, Padgett BL. The epidemiology of human polyomaviruses. Prog Clin Biol Res. 1983;105:99–106. [PubMed] [Google Scholar]
- Wesselingh SL, Power C, Glass JD, Tyor WR, McArthur JC, Farber JM, Griffin JW, Griffin DE. Intracerebral cytokine messenger RNA expression in acquired immunodeficiency syndrome dementia. Ann Neurol. 1993;33:576–582. doi: 10.1002/ana.410330604. [DOI] [PubMed] [Google Scholar]
- West MJ, Lowe AD, Karn J. Activation of human immunodeficiency virus transcription in T cells revisited: NFkB p65 stimulates transcriptional elongation. J Virol. 2001;75:8524–8537. doi: 10.1128/JVI.75.18.8524-8537.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Ishaq M, Stoner GL, Frisque RJ. JC virus DNA is present in many human brain samples from patients without progressive multifocal leukoencephalopathy. J Virol. 1992;66:5726–5734. doi: 10.1128/jvi.66.10.5726-5734.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MK, Skowronska A, Gordon J, Del Valle L, Deshmane SL, Giordano A, Khalili K. Analysis of a mutant p53 protein arising in a medulloblastoma from a mouse transgenic for the JC virus early region. Anticancer Res. 2006;26:4079–4092. [PubMed] [Google Scholar]
- White MK, Safak M, Khalili K. Regulation of gene expression in primate polyomaviruses. J Virol. 2009;83:10846–10856. doi: 10.1128/JVI.00542-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung MC, Pulliam L, Lau AS. The HIV envelope protein gp120 is toxic to human brain-cell cultures through the induction of interleukin-6 and tumor necrosis factor-alpha. AIDS. 1995;9:137–143. [PubMed] [Google Scholar]