Abstract
In living systems, iron is found in many different structures, including Fe/S clusters, hemes and nonheme centers, magnetically interacting aggregates, etc. Understanding Fe metabolism and trafficking will require biophysical spectroscopic tools that can evaluate the types of Fe centers within entire cells and isolated organelles. Mössbauer spectroscopy will play an important role in such analyses, as it has perhaps the best combination of resolution, sensitivity, coverage and quantifying abilities. Other spectroscopic techniques, with particular strengths, will be used in combination with Mossbauer, and results will be integrated to assess the “ironome” of such complex samples. This integrative biophysical approach is illustrated by a discussion of iron trafficking in yeast cells.
Introduction
Molecular geneticists have uncovered many molecular-level details of iron metabolism in eukaryotic cells, mainly using yeast as a model system. Analysis of the phenotypes resulting from e.g. deleting a gene often involves measuring the iron concentration in the mutated cell or in an isolated organelle. Such measurements are certainly informative, but determining the types of Fe centers involved and how they change due to the mutation would contribute significantly to deciphering the role of the affected gene. This is where biophysical spectroscopy can help. Many spectroscopies are sensitive to Fe, but each has strengths and weaknesses that dictate its utility in characterizing Fe in complex samples such as organelles, whole cells or tissues. These characterizations are generally at a Systems Biology level in which hundreds or thousands of Fe-containing species contribute, rather than at the level of individual proteins in which only a few Fe atoms are involved. For such “ironomic” studies, the most important considerations for selecting biophysical probes are:
Resolution: How well can the probe resolve the spectral contributions of different Fe species?
Sensitivity: Can the probe detect Fe species present at low concentrations? The concentrations of many Fe species in complex samples are in the nM or μM range.
Coverage: Can the probe detect all states of Fe, or are some states spectroscopically silent? If only particular states of Fe can be detected, it becomes difficult to know the fate of an Fe species that has vanished.
Quantification: Can the probe quantify the level of Fe species present in a complex sample? One often wants to know changes in the levels of Fe species, not simply whether a species is or is not present in a sample.
Mössbauer Spectroscopy as a Biophysical Probe
Mössbauer spectroscopy is a rather esoteric technique that has not been on the radar screen of most molecular biologists. However, relative to other spectroscopic probes, Mössbauer scores reasonably well with regard to each criterion listed above. It cannot resolve the contributions of individual Fe species in complex samples, but it can resolve groups of such species, e.g. high-spin and low-spin hemes, reduced and oxidized Fe/S clusters, FeII and FeIII mononuclear nonheme species, and ferric phosphate nanoparticles. The technique is sufficiently sensitive such that species with concentrations of 10 – 20 μM can be observed (if one is willing to collect spectra for 100 – 200 hours). Coverage is excellent, in that all oxidation and spin states of Fe in a sample are detected; there are no “Mossbauer silent” forms. Quantification is also very good; the relative intensities of various features in a Mössbauer spectrum are essentially equal to the percentage of the corresponding Fe species in the sample. For example, if 10% of the spectral intensity is a particular quadrupole doublet, ~ 10% of the iron in the sample will give rise to that doublet.
There are of course disadvantages of Mössbauer spectroscopy. For biological systems, Mössbauer is useful only for Fe; other transition metals (Mn, Cu or Zn) are not Mössbauer active, at least in practical terms. Spectral analysis is nontrivial, as it requires some background in quantum mechanics and inorganic coordination chemistry. Operating Mössbauer spectrometers requires some practical knowledge of experimental physics. Maintenance is expensive, requiring a new radioactive source annually and (for most instruments) daily additions of liquid helium. The technique requires that samples be enriched in a particular and expensive isotope of iron – namely 57Fe (I = ½). Even with full isotopic enrichment, data collection is slow. We typically collect data for 50 – 150 hours to obtain a single spectrum! That being said, studies from the past few years demonstrate that the rewards of Mössbauer spectroscopy outweigh the pains of obtaining and interpreting spectra.
A Mössbauer centered biophysical analysis
Lesuisse and coworkers were the first to use Mössbauer to study the Fe in intact mitochondria [1,2]. Mitochondria play a major role in cellular Fe metabolism, as they house the respiratory complexes, most of which contain Fe prosthetic groups (namely Fe/S cluster and heme centers). These groups are also biosynthesized in mitochondria. These authors initially examined a genetic strain of yeast in which the gene YFH1 was deleted. Yfh1p is a mitochondrial protein that either delivers Fe to the Fe/S assembly complex or activates the complex’s activity [3]. In humans, the lack of the homologous protein frataxin is responsible for Friedreich’s Ataxia. This disease is characterized by the accumulation of Fe in brain and heart tissues. Deletion of YFH1 in yeast also causes the accumulation of Fe, in the form of ferric phosphate nanoparticles within mitochondria [1].
In collaboration with Eckard Münck (Carneige Mellon University), our group has explored the possibility of using Mössbauer spectroscopy more generally to investigate the distribution of Fe in mitochondria from wild-type (WT) yeast cells in which there is no abnormal accumulation of Fe. This corresponds to Lesuisse et al.’s control sample in which no Mössbauer signals were detected [1]. By using high levels of enrichment and by packing purified mitochondrial samples tightly into Mössbauer sample holders, we were able to detect signals from WT cells and isolated mitochondria. Many years and scores of samples later, we have a fairly good understanding of the ironome in yeast mitochondria. Our approach uses Mössbauer spectroscopy in conjunction with electron paramagnetic resonance (EPR), electronic absorption spectroscopy, electron microscopy and X-ray absorption spectroscopy (XAS).
Isolated yeast mitochondria contain 700 – 800 μM Fe. However, the distribution of that Fe depends on whether the cells from which the mitochondria are isolated have been grown under respiring or fermenting conditions [4,5]. Mössbauer spectra show that the Fe in mitochondria isolated from respiring cells is present as [Fe4S4]2+ clusters and heme centers - the prosthetic groups of the respiratory complexes (Figure 1A). Collectively, these groups account for ~ 70% of mitochondrial Fe. About 13% of this Fe is present as S = 1/2 [Fe2S2]1+ clusters (probably from the clusters in the Rieske protein and in succinate dehydrogenase). Much of the remaining Fe cannot be identified but ~ 2–3% is present as nonheme high-spin FeII ions. In mitochondria isolated from fermenting cells, the Fe due to respiratory complexes accounts for only ~ 30% of total Fe, consistent with the reduced need for respiratory complexes under these growth conditions. About 20% of the Fe in fermenting mitochondria is present as nonheme HS FeII species, 15% as mononuclear HS FeIII and ~ 30% as ferric phosphate nanoparticles (Figure 1B). These last three forms of Fe may be in redox equilibrium, but this is not certain. The nonheme HS FeII species probably represents a metabolic pool used as feedstock for Fe/S cluster and heme biosynthesis.
Figure 1.

5 K, 0.05 T Mössbauer spectra of A) respiring mitochondria, B) fermenting mitochondria, C) Atm1-depleted mitochondria, and D) whole fermenting yeast cells. Red lines represent simulations of spectral features. See [18] and [18] for analysis of the respective spectra.
The depletion of other proteins also causes Fe to accumulate in mitochondria, and we characterized this type of Fe using our integrative biophysical approach [6,7]. Yah1p is a [Fe2S2]-containing ferredoxin located in the mitochondrial matrix that donates electrons used in Fe/S cluster and heme biosynthesis. Lack of Yah1p causes Fe to accumulate in mitochondria. Atm1p is an ABC transporter on the mitochondrial inner membrane that exports an unknown species from the matrix into the cytosol. This species appears to be required by the cytosolic Fe/S cluster assembly (CIA) machinery to generate Fe/S clusters in the cytosol. Lack of this protein causes Fe to accumulate in the mitochondria and an Fe/S cluster defect to develop in the cytosol. Previous reports [8] concluded that there was no Fe/S cluster defect in the mitochondria of Atm1p-depleted cells.
Using our integrated biophysical approach, we found that the Fe that accumulates in Yah1p-depleted and Atm1p-depleted cells was ferric phosphate nanoparticles in both cases. The 5 K low-field Mössbauer spectrum of isolated mitochondria from such cells (Figure 1C) exhibited a quadrupole doublet similar to that observed in spectra of YFH1-deleted samples. EPR and High-field Mössbauer spectra indicated superparamagnetic behavior. This indicates that the ferric ions are magnetically interacting and thus are very close to each other as would be the case in precipitated aggregates. A magnetically isolated ferric complex would not exhibit this behavior. We also used Energy Dispersive X-ray Spectroscopy to probe the elemental composition of the nanoparticles; they contain Fe, P, O but not C, S or N. In collaboration with Robert Scott (University of Georgia) we also characterized the nanoparticles by XAS. Taken collectively, our data suggest a structure similar to that in Figure 2. The Fe/S cluster defect in the mitochondria is probably caused by reactive oxygen species (ROS) generated by the formation of the nanoparticles. Others have reported a close connection between ROS formation and Fe/S cluster defects e.g. [9,10].
Figure 2.
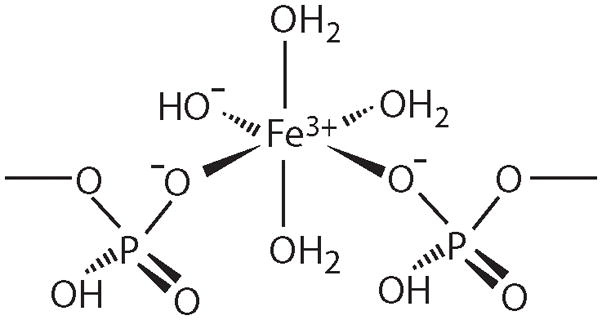
Proposed structure of FeIII phosphate nanoparticles found in Atm1p-depleted cells and probably certain other strains of yeast in which mitochondria accumulate Fe. This is a slight modification of a previous proposal [18] that is perhaps more consistent with the known coordination chemistry of FeIII species.
We have also examined the Mössbauer spectra of whole yeast cells grown under different conditions (Figure 1D). The Fe in these cells can be divided into two major groups. In fermenting cells, ca. 75% of the Fe is present as magnetically isolated high-spin FeIII. There is some evidence that this Fe is located in vacuoles, organelles known to store Fe [11–13]. The remaining Fe exhibits spectral features of isolated mitochondria. There are undoubtedly Fe species located in other cellular compartments, but in quantitative terms, they play minor roles. Crudely considered, the Fe in yeast cells appears to be divisible into vacuolar and mitochondrial fractions, with these two organelles serving as major “hubs” for cellular Fe traffic (Figure 3).
Figure 3.
A simplified depiction of Fe trafficking in yeast highlighting the proteins discussed here. Fe is imported into the cell by the plasma membrane permease Ftr1p which utilizes a multicopper oxidase Fet3p. Expression of these proteins is under control of the transcriptional regulator Aft1p. Under high cytosolic Fe conditions, Aft1p is located predominately in the cytosol, while under low cytosolic Fe, it is located in the nucleus where it promotes expression of the “iron regulon” genes, including FTR1 and FET3. Cytosolic Fe can be imported into the mitochondria via the inner membrane proteins Mrs3p/Mrs4p, or into the vacuole via the membrane-bound protein Ccc1p. “L” in the figure refers to an undefined ligand. There are also identified pathways for the export of Fe from the vacuole. Once inside mitochondrial matrix, Fe is utilized in the production of Fe/S clusters and heme groups. The import flow of Fe into the mitochondria is regulated by an unidentified mechanism.
Other biophysical methods have also been used to address questions of Fe metabolism in cells. George and coworkers used XAS to probe the Fe content of mitochondria from human fibroblasts cultured from normal individuals and those affected with Friedreich’s Ataxia [14]. The organelles from both groups were rich in ferrihydrite (~ 80% of the Fe in the samples) while those from the diseased cells were concluded to contain more mitoferritin. Such distinctions are difficult to establish because the XAS probe cannot resolve one feature from another; only average composition can be determined. Penner-Hahn and Culotta used XAS to evaluate the Fe content of mitochondria isolated from yeast cells lacking Mtm1p [15]. The function of this protein is unknown, but its absence causes the accumulation of Fe in mitochondria and the mis-incorporation of Fe into the apo form of Mn Superoxide Dismutase (Sod2p). Their XAS study suggested that the Sod2p-reactive Fe pool is undetectably small, in contrast to an earlier conclusion [16]. No changes in the bulk mitochondrial Fe could be detected using mitochondria isolated from genetic strains that inactivated SOD2 vs. strains that did not. The bulk Fe in these samples indicated a mixture of FeII and FeIII species, with O/N donor ligands and Fe-L distances of ~ 2.0 Å. However, accurate ratios of different species could not be determined, again due to the low resolving power of XAS. Future studies in which XAS spectra are obtained on complex samples that have been cross-characterized by Mössbauer spectroscopy may provide a synergistic advantage for both methods.
Electronic absorption spectroscopy has played an important role in characterizing and quantifying heme centers in mitochondria, e.g. see [17]. Its major drawback is poor coverage, as some Fe species will not contribute noticeably to such spectra. EPR will be somewhat useful for metallomic studies of complex samples [18], but it can only detect Fe species with unpaired electrons. Another promising biophysical probe for cell metallomic studies is Electron Nuclear Double Resonance (ENDOR) spectroscopy. The resolving ability of this technique is outstanding for EPR-active species, as evidenced by the recent study of Hoffman, Culotta and Valentine to explore EPR-active Mn species in cells [19]. The major difficulty in using this technique is again coverage; EPR-silent species cannot be studied.
Conclusions
In summary, although Mössbauer spectroscopy has serious limitations of resolution and sensitivity, it is better suited overall than other biophysical probes for the study of intracellular ironomics; those of us working in this field are simply lucky to have it available as a probe. Although the spectral resolution of Mössbauer does not allow characterization of individual metal-containing components of the cell, it does allow novel insights into the “big picture” – namely trafficking and regulation of metal ions in cells. In our opinion, the best approach in future ironomic studies will be an integrative biophysical approach centered on Mössbauer spectroscopy but supplemented by the other spectroscopic probes listed above to strengthen particular aspects of the analysis. Understanding how traffic patterns are regulated in cells has important implications for understanding the molecular details of Fe-associated diseases, which generally involve Fe overload or under-load (anemia). The associated alterations in traffic patterns will need to be better understood before treatments of these diseases can be improved.
Acknowledgments
These studies were supported by the National Institutes of Health (GM084266, to PAL) and by the CBI training program (T32GM008523, to GPHH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Articles of special interest (•) or outstanding interest (••) for this current review article are indicated.
- •1.Lesuisse E, Santos R, Matzanke BF, Knight SAB, Camadro JM, Dancis A. Iron use for haeme synthesis is under control of the yeast frataxin homologue (Yfh1) Human Molecular Genetics. 2003;12:879–889. doi: 10.1093/hmg/ddg096. [DOI] [PubMed] [Google Scholar]
- 2.Seguin A, Sutak R, Bulteau AL, Garcia-Serres R, Oddou JL, Lefevre S, Santos R, Dancis A, Camadro JM, Latour JM, et al. Evidence that yeast frataxin is not an iron storage protein in vivo. Biochimica Et Biophysica Acta-Molecular Basis of Disease. 2010;1802:531–538. doi: 10.1016/j.bbadis.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Tsai C-L, Barondeau DP. Human Frataxin Is an Allosteric Switch That Activates the Fe S Cluster Biosynthetic Complex. Biochemistry. 2010;49:9132–9139. doi: 10.1021/bi1013062. [DOI] [PubMed] [Google Scholar]
- ••4.Holmes-Hampton GP, Miao R, Morales JG, Guo YS, Munck E, Lindahl PA. A Nonheme High-Spin Ferrous Pool in Mitochondria Isolated from Fermenting Saccharomyces cerevisiae. Biochemistry. 2010;49:4227–4234. doi: 10.1021/bi1001823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••5.Morales JG, Holmes-Hampton GP, Miao R, Guo YS, Munck E, Lindahl PA. Biophysical Characterization of Iron in Mitochondria Isolated from Respiring and Fermenting Yeast. Biochemistry. 2010;49:5436–5444. doi: 10.1021/bi100558z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••6.Miao R, Martinho M, Morales JG, Kim H, Ellis EA, Lill R, Hendrich MP, Munck E, Lindahl PA. EPR and Mossbauer spectroscopy of intact mitochondria isolated from Yah1p-depleted Saccharomyces cerevisiae. Biochemistry. 2008;47:9888–9899. doi: 10.1021/bi801047q. [DOI] [PubMed] [Google Scholar]
- ••7.Miao R, Kim H, Koppolu UMK, Ellis EA, Scott RA, Lindahl PA. Biophysical Characterization of the Iron in Mitochondria from Atm1p-Depleted Saccharomyces cerevisiae. Biochemistry. 2009;48:9556–9568. doi: 10.1021/bi901110n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kispal G, Csere P, Guiard B, Lill R. The ABC transporter Atm1p is required for mitochondrial iron homeostasis. Febs Letters. 1997;418:346–350. doi: 10.1016/s0014-5793(97)01414-2. [DOI] [PubMed] [Google Scholar]
- 9.Napoli E, Taroni F, Cortopassi GA. Frataxin, iron-sulfur clusters, heme, ROS, and aging. Antioxidants & Redox Signaling. 2006;8:506–516. doi: 10.1089/ars.2006.8.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JH, Sedlak M, Gao Q, Riley CP, Regnier FE, Adamec J. Oxidative Stress Studies in Yeast with a Frataxin Mutant: A Proteomics Perspective. Journal of Proteome Research. 2010;9:730–736. doi: 10.1021/pr900538e. [DOI] [PubMed] [Google Scholar]
- 11.Li LT, Murdock G, Bagley D, Jia XA, Ward DM, Kaplan J. Genetic Dissection of a Mitochondria-Vacuole Signaling Pathway in Yeast Reveals a Link between Chronic Oxidative Stress and Vacuolar Iron Transport. Journal of Biological Chemistry. 2010;285:10232–10242. doi: 10.1074/jbc.M109.096859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eide DJ, Clark S, Nair TM, Gehl M, Gribskov M, Guerinot ML, Harper JF. Characterization of the yeast ionome: a genome-wide analysis of nutrient mineral and trace element homeostasis in Saccharomyces cerevisiae. Genome Biology. 2005:6. doi: 10.1186/gb-2005-6-9-r77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L, Bagley D, Ward DA, Kaplan J. Yap5 is an iron-responsive transcriptional activator that regulates vacuolar iron storage in yeast. Molecular and Cellular Biology. 2008;28:1326–1337. doi: 10.1128/MCB.01219-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Popescu BFG, Pickering IJ, George GN, Nichol H. The chemical form of mitochondrial iron in Friedreich's ataxia. Journal of Inorganic Biochemistry. 2007;101:957–966. doi: 10.1016/j.jinorgbio.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Naranuntarat A, Jensen LT, Pazicni S, Penner-Hahn JE, Culotta VC. The Interaction of Mitochondrial Iron with Manganese Superoxide Dismutase. Journal of Biological Chemistry. 2009;284:22633–22640. doi: 10.1074/jbc.M109.026773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang M, Cobine PA, Molik S, Naranuntarat A, Lill R, Winge DR, Culotta VC. The effects of mitochondrial iron homeostasis on cofactor specificity of superoxide dismutase 2. Embo Journal. 2006;25:1775–1783. doi: 10.1038/sj.emboj.7601064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glerum DM, Muroff I, Jin C, Tzagoloff A. COX15 codes for a mitochondrial protein essential for the assembly of yeast cytochrome oxidase. Journal of Biological Chemistry. 1997;272:19088–19094. doi: 10.1074/jbc.272.30.19088. [DOI] [PubMed] [Google Scholar]
- 18.Hagen WR. Metallomic EPR spectroscopy. Metallomics. 2009;1:384–391. doi: 10.1039/b907919j. [DOI] [PubMed] [Google Scholar]
- 19.McNaughton RL, Reddi AR, Clement MHS, Sharma A, Barnese K, Rosenfeld L, Gralla EB, Valentine JS, Culotta VC, Hoffman BM. Probing in vivo Mn2+ speciation and oxidative stress resistance in yeast cells with electron-nuclear double resonance spectroscopy. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15335–15339. doi: 10.1073/pnas.1009648107. [DOI] [PMC free article] [PubMed] [Google Scholar]



