Abstract
Objectives
We characterized contemporary utilization of oral targeted therapies (i.e., sunitinib, sorafenib) among patients with renal cell carcinoma (RCC), and assessed factors associated with short-term and sequential treatment.
Methods
We used an administrative claims database of privately-insured patients to evaluate oral targeted therapy use among RCC patients from 2006–2007. After identifying RCC patients treated with sunitinib and/or sorafenib, we determined the prevalence of patients treated with short-term and/or sequential therapy. We performed bivariate and multivariate analyses to estimate associations between patient characteristics and receipt of short-term and/or sequential treatment regimens.
Results
We identified 938 RCC patients initially treated with sunitinib (n=554) or sorafenib (n=384). Among this group, 36% and 23% of patients received short-term or sequential therapy, respectively. A majority of patients (61%) who received sequential therapy received short-term treatment with one or more drugs, with second-line sorafenib more likely to be short-term than sunitinib (63% vs 34%, p<0.001). Short-term therapy was more common among female patients (OR 1.53, 95% CI 1.12–2.09) and patients in the Southern U.S. (OR 1.71, 95% CI 1.05–2.80). Sequential therapy was more common among patients receiving sorafenib first (OR 2.30, 95% CI 1.64–3.21).
Conclusions
Short-term and sequential oral targeted therapy use was relatively prevalent among patients with RCC. For patients treated with sunitinib and sorafenib, patterns of short-term use varied by the sequence of medications, suggesting differences in effectiveness or tolerability of each regimen. These findings highlight the need for future studies to characterize “real-world” clinical outcomes and economic impact associated with these treatment courses.
Keywords: renal cell carcinoma, sunitinib, sorafenib, physician practice patterns, health services research
Introduction
Historical survival statistics for patients with metastatic renal cell carcinoma (RCC) are dismal, reflecting the nearly-ubiquitous resistance of this tumor to conventional chemo- and immunotherapy.1–3 More recently, however, new medications that target the molecular pathogenesis of RCC have been shown to significantly improve survival among patients with disseminated disease. Based on these data, the oral tyrosine kinase inhibitors (TKI) sunitinib (January 2006)4 and sorafenib (December 2005)5 received Food and Drug Administration (FDA) approval and emerged as first-line therapies for most patients with metastatic RCC.
Although the progression-free (sunitinib, sorafenib)6, 7 and overall (sunitinib)8 survival benefits of oral targeted therapies are indisputable, available clinical trial data indicate that these gains are modest and vary across individual medications and patient populations. Given this limited therapeutic margin, there is a need to better understand whether results of clinical trials are being translated into everyday clinical practice. Moreover, given the recent controversy surrounding coverage of these agents in the United Kingdom,9 data describing the utilization of oral TKIs is immediately relevant to several high-profile policy debates including mandated versus evidence-based coverage decisions for novel biological therapies,10 episode-based payments for cancer care, and reforms aimed at controlling Medicare spending for new cancer drugs.11
In this context, we sought to evaluate initial patterns of treatment with the oral TKIs among a large and diverse sample of patients with RCC. We specifically assessed the average duration of treatment with sunitinib and/or sorafenib, as well as the frequency of short-term and sequential use of the oral TKIs. Once available, these data will clarify initial practice patterns with the oral TKIs and provide context for efforts aimed at understanding the real-world clinical and cost-effectiveness of these important medications.
Material and Methods
Analytic cohort
The MarketScan® Commercial Claims and Encounters (CCE) Research Database is comprised of inpatient, outpatient, and pharmaceutical insurance claims from nearly 29 million individuals covered by employer-sponsored private health insurance plans.12 Using CCE data from 2003 through 2007, we employed a four-step process to identify a cohort of patients with both a diagnosis of RCC and pharmacy claims specifying treatment with sunitinib and/or sorafenib. First, we identified all patients who had either an inpatient or outpatient insurance claim that included an ICD-9-DM diagnosis code corresponding to RCC (list available in online Appendix). Next, we excluded from this group patients with fewer than 90 days of benefit plan enrollment following the date of their first claim with an RCC diagnosis, as well as patients with missing or incomplete outpatient pharmacy claims data. This step ensured that we included only those patients with longitudinal pharmacy coverage and the corresponding claims data. For this group of subjects, we then used FDA National Drug Codes to identify all patients with outpatient pharmacy claims for sunitinib and/or sorafenib (list available in online Appendix). Finally, we excluded patients who only had claims specifying a diagnosis of gastrointestinal stromal tumor (ICD-9-CM 171.5 or 238.1, n = 34), the only other condition where use of sunitinib is FDA-approved.
Study variables
Next, we used patient-level information included in the CCE database to ascertain age (at the time of the first claim with an RCC diagnosis), gender, geographic residence, and insurance benefit plan (e.g., health maintenance organization) for each case in our analytic cohort. We also used inpatient or outpatient procedure claims to determine whether each of these patients had received kidney cancer surgery and/or RCC-directed immunotherapy (i.e., interleukin-2 (IL-2) or interferon alpha (IFN-α)) prior to their first pharmacy claim for oral TKI therapy (list available in online Appendix). The CCE Database does not provide information regarding cancer severity (e.g., stage, grade).
Outcomes
We defined the duration of therapy for each medication as the total number of days supplied by all consecutive outpatient prescriptions. For instance, if a patient had two pharmacy claims for sunitinib that both specified a 28-day course, we defined the treatment duration as 56 days (regardless of the time span between the dates for the two claims). Our primary outcomes of interest were the prevalence of short-term and/or sequential use of oral TKIs. We classified as short-term therapy any treatment regimen that lasted fewer than 60 days (a time period roughly equivalent to two cycles of sunitinib or sorafenib). We defined sequential therapy as the presence of outpatient drug claims for both sunitinib and sorafenib; we considered the initial and subsequent drugs as “first-line” and “second-line” therapy, respectively. In order to better understand the relationships between sequential and short-term TKI therapy, we also categorized patients receiving sequential therapy into the following four groups: 1) short-term use of first-line TKI only; 2) short-term use of second-line TKI only; c) short-term of both TKIs; and d) no short-term use.
Statistical Analysis
Chi-square tests were used to determine whether differences existed for patient factors by the first TKI prescribed (sunitinib or sorafenib), whether subjects had short-(<60 days) or long-term (≥60 days) therapy, and whether subjects had monotherapy or sequential therapy. Among those receiving sequential therapy, the duration of each therapy was compared by first TKI prescribed using a chi-square test. Finally, multivariable logistic regression models were used to determine the independent effect of initial TKI (sunitinib vs sorafenib) on both short-term and sequential TKI use, after adjusting for age, gender, type of benefit plan, geographic region, year of RCC diagnosis, prior kidney surgery (yes/no), prior immunotherapy (yes/no), and year of first TKI therapy. All statistical analyses were performed with two-sided tests at the 5% significance level using SAS version 9.2 (Cary, NC).
Results
We identified 938 patients with a diagnosis of RCC and at least one pharmacy claim for sunitinib and/or sorafenib. The median duration of first-line TKI treatment was 90 (range 2–690) and 120 days (range 14–690) for sunitinib and sorafenib, respectively. Table 1 summarizes the characteristics of patients with RCC treated with oral TKIs, stratified by the initial therapeutic agent. Overall, nearly three out of four (73%) patients treated with sunitinib and/or sorafenib were men, 57% were between 51–60 years of age, and 43% had claims for prior kidney cancer surgery. Forty-seven percent of patients treated with oral TKIs lived in the Southern United States. Compared with those treated in 2006, patients starting treatment in 2007 were significantly more likely to receive first-line sunitinib (73% vs 49%, p<0.001).
Table 1.
Characteristics of RCC patients initially treated with sunitinib and/or sorafenib
| Total (n = 938) |
Sunitinib (n = 554) |
Sorafenib (n = 384) |
||
|---|---|---|---|---|
| N | N (%*) | N (%*) | p | |
| Initial Treatment Date | <0.001 | |||
| 2006a | 537 | 261 (49) | 276 (51) | |
| 2007 | 401 | 293 (73) | 108 (27) | |
| Initial RCC Diagnosis Date | <0.001 | |||
| 2003 | 96 | 53 (55) | 43 (45) | |
| 2004 | 111 | 51 (46) | 60 (54) | |
| 2005 | 173 | 89 (51) | 84 (49) | |
| 2006 | 366 | 216 (59) | 150 (41) | |
| 2007 | 192 | 145 (76) | 47 (24) | |
| Age | 0.905 | |||
| 19–40 years | 51 | 28 (55) | 23 (45) | |
| 41–50 years | 184 | 111 (60) | 73 (40) | |
| 51–60 years | 531 | 315 (59) | 216 (41) | |
| 61–65 years | 172 | 100 (58) | 72 (42) | |
| Gender | 0.783 | |||
| Male | 686 | 407 (59) | 279 (41) | |
| Female | 252 | 147 (58) | 105 (42) | |
| Benefit Planb | 0.632 | |||
| Basic/Comprehensive | 94 | 55 (59) | 39 (41) | |
| HMO | 136 | 75 (55) | 61 (45) | |
| Otherc | 685 | 408 (60) | 277 (40) | |
| Geographic Regiond | 0.260 | |||
| Northeast | 97 | 52 (54) | 45 (46) | |
| North-Central | 233 | 147 (63) | 86 (37) | |
| South | 444 | 267 (60) | 177 (40) | |
| West | 153 | 83 (54) | 70 (46) | |
| Prior Kidney Surgery | 0.092 | |||
| Yes | 402 | 250 (62) | 152 (38) | |
| No | 536 | 304 (57) | 232 (43) | |
| Prior Immunotherapy | 0.592 | |||
| Yes | 47 | 26 (55) | 21 (45) | |
| No | 891 | 528 (59) | 363 (41) |
Abbreviations: RCC = renal cell carcinoma; HMO = health maintenance organization;
Row percentages;
includes one case from 2005;
unknown in 23 cases;
Includes preferred provider organizations, exclusive provider organizations, point-of-service, and consumer-driven health care plans;
Unknown in 3 cases
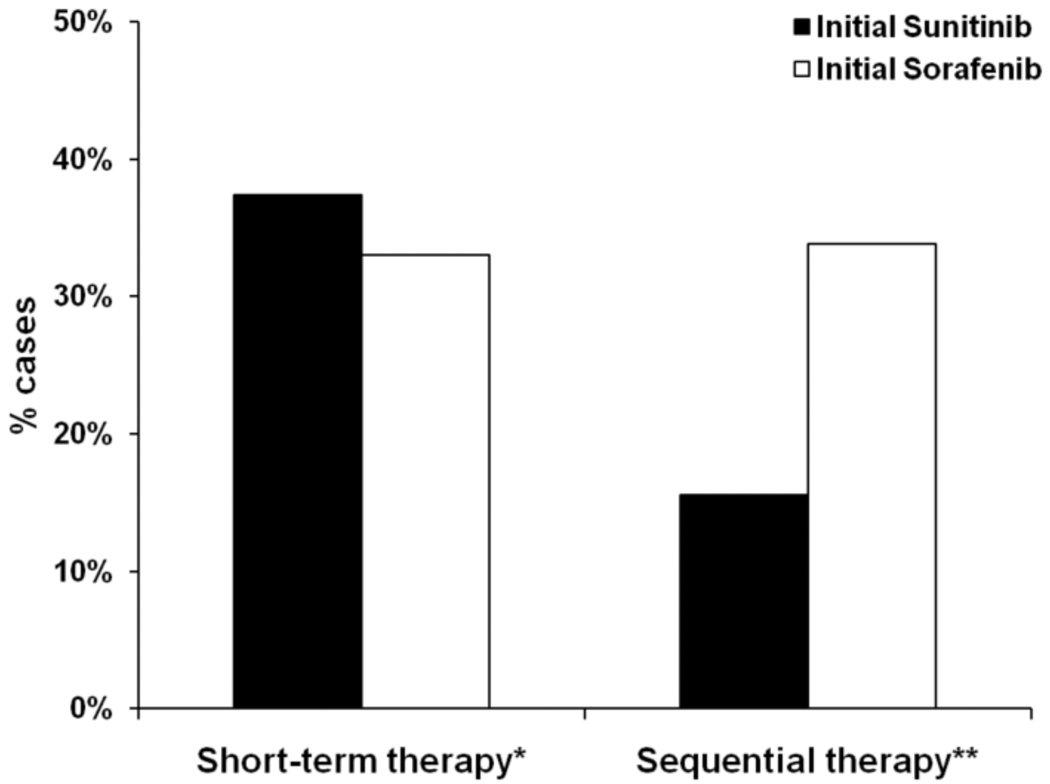
Figure 1 presents the prevalence of short-term and sequential therapy among RCC patients treated with oral TKIs. Short-term use of oral TKIs was common, with nearly four out of ten patients (36%) receiving first-line treatment lasting fewer than 60 days; the prevalence of initial short-term therapy did not differ based on initial drug prescribed (37% sunitinib vs 33% sorafenib, p = 0.177). Overall, 23% of patients received sequential therapy, and this treatment pattern was more common among patients who received first-line sorafenib (34% first-line sorafenib vs 16% first-line sunitinib, p<0.001).
Figure 1.
Initial patterns of use of oral TKIs among RCC patients.
*p=0.177 for comparison between patients receiving initial sunitinib versus initial sorafenib.
**p<0.001 for comparison between patients receiving initial sunitinib versus initial sorafenib
This figure demonstrates the proportion of patients who received the treatment courses of interest (i.e., short-term or sequential therapy). The x-axis corresponds to groups of patients in our analytic cohort who received either short-term therapy (i.e., < 60 days) or sequential therapy (i.e., both sunitinib and sorafenib). The y-axis corresponds to the proportion of cases that received the treatment courses of interest. The black bars represent the proportion of cases who were treated with sunitinib first, and the white bars correspond to those in the analytic cohort who received sorafenib first.
Table 2 presents the distribution of short-term and sequential therapy according to patient characteristics and summarizes results from multivariable analyses evaluating independent associations between patient characteristics and receipt of short-term or sequential TKI therapy. Compared to sorafenib, patients started on sunitinib were not more likely to receive short-term therapy (OR 0.90, 95% CI 0.67–1.21). Short-term therapy was more common among female patients (OR 1.53, 95% CI 1.12–2.09) and patients who resided in the South (OR 1.71, 95% CI 1.05–2.80). Patients aged 51–60 years were significantly less likely to receive short-term therapy (OR 0.52, 0.28–0.95), compared to patients aged 19–40 years. Sequential therapy was more common among patients treated with sorafenib first (OR 2.30, 95% CI 1.64–3.21) and among those between the ages of 51–60 years (OR 2.52, 95% CI 1.07–5.91).
Table 2.
Characteristics of patients receiving short-term and sequential oral TKI therapy.
| Total (n = 938) |
Short-term use of TKIs (n = 334) |
Sequential use of TKIs (n = 216) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| N | N (%*) | p | Adjusted OR |
95% CI | N (%*) | p | Adjusted OR |
95% CI | |
| Initial Treatment Date | 0.011 | <0.001 | |||||||
| 2006a,** | 537 | 170 (32) | 1.00 | - | 170 (32) | 1.00 | - | ||
| 2007 | 401 | 164 (41) | 1.27 | 0.89–1.81 | 46 (11) | 0.34 | 0.21–0.54 | ||
| Initial RCC Diagnosis Date | 0.005 | <0.001 | |||||||
| 2003–2005** | 380 | 122 (32) | 1.00 | - | 99 (26) | 1.00 | - | ||
| 2006 | 366 | 125 (34) | 1.12 | 0.81–1.54 | 97 (27) | 1.26 | 0.88–1.80 | ||
| 2007 | 192 | 87 (45) | 1.51 | 0.95–2.37 | 20 (10) | 1.02 | 0.52–1.99 | ||
| Age | 0.124 | 0.338 | |||||||
| 19–40 years** | 51 | 24 (47) | 1.00 | - | 7 (14) | 1.00 | - | ||
| 41–50 years | 184 | 67 (36) | 0.64 | 0.33–1.23 | 39 (21) | 1.96 | 0.79–4.85 | ||
| 51–60 years | 531 | 175 (33) | 0.52 | 0.28–0.95 | 128 (24) | 2.52 | 1.07–5.91 | ||
| 61–65 years | 172 | 68 (40) | 0.73 | 0.38–1.41 | 42 (24) | 2.42 | 0.98–6.00 | ||
| Gender | 0.008 | 0.723 | |||||||
| Male** | 686 | 227 (33) | 1.00 | - | 160 (23) | 1.00 | - | ||
| Female | 252 | 107 (42) | 1.53 | 1.12–2.09 | 56 (22) | 1.01 | 0.70–1.47 | ||
| Benefit Planb | 0.140 | 0.866 | |||||||
| Basic/Comprehensive** | 94 | 34 (36) | 1.00 | - | 20 (21) | 1.00 | - | ||
| HMO | 136 | 59 (43) | 1.34 | 0.75–2.37 | 33 (24) | 1.47 | 0.74–2.93 | ||
| Otherc | 685 | 236 (34) | 0.86 | 0.53–1.39 | 160 (23) | 1.57 | 0.88–2.79 | ||
| Geographic Regiond | 0.092 | 0.326 | |||||||
| Northeast** | 97 | 29 (30) | 1.00 | - | 21 (22) | 1.00 | - | ||
| North-Central | 233 | 77 (33) | 1.22 | 0.72–2.09 | 61 (26) | 1.27 | 0.69–2.33 | ||
| South | 444 | 178 (40) | 1.71 | 1.05–2.80 | 91 (20) | 0.91 | 0.52–1.62 | ||
| West | 153 | 46 (30) | 1.07 | 0.60–1.89 | 39 (25) | 1.12 | 0.59–2.14 | ||
| Prior Kidney Surgery | 0.503 | 0.140 | |||||||
| Yes** | 402 | 148 (37) | 1.00 | - | 102 (25) | 1.00 | - | ||
| No | 536 | 186 (35) | 0.92 | 0.70–1.22 | 114 (21) | 0.74 | 0.53–1.02 | ||
| Prior Immunotherapy | 0.693 | 0.028 | |||||||
| Yes** | 47 | 18 (38) | 1.00 | - | 17 (36) | 1.00 | - | ||
| No | 891 | 316 (35) | 0.59 | 0.31–1.12 | 199 (22) | 0.53 | 0.26–1.06 | ||
Abbreviations: TKI = tyrosine kinase inhibitor; OR = odds ratio; CI = confidence interval; RCC = renal cell carcinoma; HMO = health maintenance organization;
Row percentages;
Reference group;
includes one case from 2005;
unknown in 23 cases;
Includes preferred provider organizations, exclusive provider organizations, point-of-service, and consumer-driven health care plans;
Unknown in 3 cases
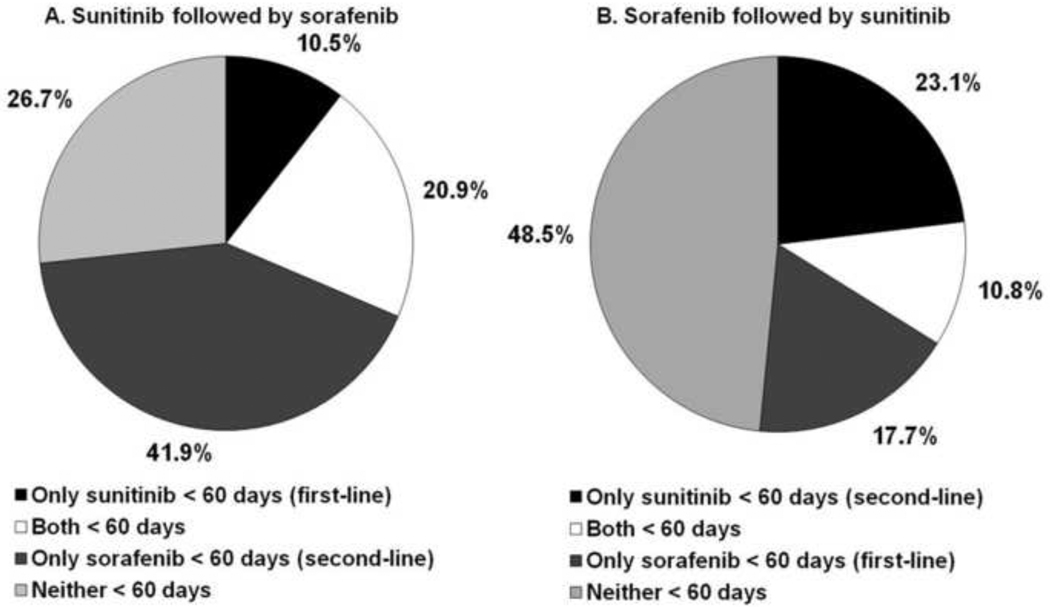
In analyses that jointly assessed sequential and short-term therapy, we observed that 61% of patients treated with sequential oral TKIs had a short-term regimen with at least one of the two medications. As illustrated in Figure 2, patients who received first-line sunitinib were significantly more likely to experience a short-term regimen with one or both oral TKIs (73% vs 52%, p<0.001). Compared to second-line sunitinib, second-line sorafenib was significantly more likely to be prescribed for less than 60 days (63% vs 34%, p<0.001). Furthermore, among patients who received first-line sunitinib for >60 days, 61% (36/59) were treated with second-line sorafenib for ≤ 60 days. On the other hand, only 32% (30/93) of patients receiving first-line sorafenib for more than 60 days received short-term second-line sunitinib.
Figure 2.
Patterns of short-term therapy among patients treated with sunitinib and sorafenib.
Figure 2 describes patterns of short-term use among patients receiving sequential therapy (i.e., both sunitinib and sorafenib). Figure 2A represents patients who received sunitinib followed by sorafenib, and Figure 2B describes patients treated with sorafenib followed by sunitinib. The black portions of the pie charts represent the proportions of patients who received short-term sunitinib (first-line for figure 2A vs second-line for figure 2B) but received sorafenib for more than 60 days. The white segments represent patients who received both medications for less than 60 days. The dark gray areas represent patients who received short-term sorafenib (second-line for figure 2A and first-line for figure 2B) but received sunitinib for more than 60 days. Finally, the light gray portions of the charts represent patients who received both drugs for more than 60 days.
Comment
This study has several findings that clarify initial utilization patterns for the oral tyrosine kinase inhibitors among patients with RCC. Because it highlights a potentially significant disconnect between the efficacy and true clinical benefit of sunitinib and sorafenib, our most consequential finding may be the relatively-high prevalence (36%) of short-term treatment regimens. The most likely explanations for a short course of oral TKI therapy include adverse drug reactions, disease progression, and/or death. In the large clinical trials of sunitinib and sorafenib, where patient eligibility and accrual is strictly controlled, adverse event-related discontinuation of these TKIs occurred in only 6–10% of patients.6,7,13 Moreover, only 15% of patients in these trials stopped treatment within two months of initiating oral TKI therapy.6,7 Our observation that nearly 40% of patients continued their first-line TKI for fewer than 60 days—and that the median treatment course was only 90–120 days—suggests that the results from these landmark trials are not being translated into everyday clinical practice.
In light of these data, there is a need to understand the basis for this disconnect between clinical trial results and routine clinical practice. Both patient and provider factors may explain the observed discrepancy in average length of treatment between our analytic cohort and patients in the published clinical trials. Although not evaluated in this study, one immediate explanation is the potential liberalization of treatment indications to include patients who would have been excluded from clinical trials based on the severity of either their cancer or their comorbidity (and who are therefore less likely to respond to and/or tolerate these medications). In fact, there are several factors that may promote this trend, including the relative ease of oral TKI administration (compared to intravenous chemotherapy) and an understandable “therapeutic imperative” for high-risk patients with few existing treatment options.14 Furthermore, it is likely that some patients in our analytic cohort had renal cell carcinomas with non-clear cell histology. Although small retrospective studies have suggested similar efficacy for the targeted therapies among patients with papillary and chromophobe renal tumors, the data remain inconclusive.15,16 Because the clinical trials for sunitinib and sorafenib were limited to patients with clear cell RCC, the observed discrepancy between our results and the reported clinical trial findings may be explained partially by the inclusion of patients with non-clear cell RCC.
The relatively high prevalence of short-term therapy might also reflect lower provider thresholds for discontinuation of the oral TKIs due to unexpected and/or difficult-to-manage side effects, and/or greater patient preferences for stopping the medications in the absence of the intensive follow-up and support provided in most clinical trials. Although we could not address this question empirically, the high prevalence of short term therapy also suggests infrequent use of dose reduction as a strategy for managing adverse events and prolonging therapy. In fact, this approach was implemented as a means for continuing therapy in 32% of patients in the treatment arm of the phase III sunitinib trial.6 Further investigation of these factors via multi-institutional and population-based studies (including qualitative research) will be necessary to confirm the validity of our findings, reduce the prevalence of short-term treatment regimens, and thereby optimize the implementation of oral TKIs in “real-world” clinical practice.17
At the same time, the not-infrequent use of sequential therapy (a practice pattern more prevalent among patients initially treated with sorafenib) also highlights important issues concerning real-world utilization of the oral TKIs. In theory, patients might receive both sunitinib and sorafenib because they experienced either intolerable side effects or disease progression while on the first-line TKI. Although small retrospective case series16,17 and a recent prospective phase II trial18 suggest that sequential therapy with sunitinib and sorafenib is safe and possibly beneficial, there are no randomized phase III trials supporting the efficacy of sequential oral TKI treatment algorithms compared with best supportive care. Indeed, our data indicate that six of every ten sequential therapy patients who tolerated first-line sunitinib (i.e., those patients whose initial treatment course with sunitinib exceeded 60 days) received only a short course of second-line sorafenib, a finding that is consistent with sorafenib’s limited effectiveness in this clinical setting.19
Taken together, our findings highlight the need for in-depth and prospective studies that can accurately assess patient outcomes and better characterize the clinical and “real-world” cost-effectiveness of short-term and sequential treatment regimens among patients with metastatic RCC. In reality, however, such studies are exceedingly unlikely given the increasingly rapid introduction of newer targeted therapies and the availability of established clinical trials for patients who fail treatment with sunitinib or sorafenib. Nonetheless, we believe it of interest to patients, physicians and policymakers that these unproven sequential practice patterns not only exist, but are actually fairly common among patients with advanced RCC.
Several additional findings raise important hypotheses for future research in this area. For instance, we observed that the prevalence of short-term and sequential therapy varied according to patient age, gender, and geographic region. These associations may be explained by a number of factors including, for instance, unmeasured differences in patient comorbidity, performance status, and/or cancer-severity; these and other hypotheses could be tested in future studies based on more comprehensive clinical datasets. Finally, the prevalence of prior of kidney surgery among our analytic cohort (43%) is far lower than the nephrectomy rates (90%) among patients in the TKI clinical trials. Although measurement error may contribute to this discrepancy (e.g., some patients underwent a nephrectomy in the years prior to our available claims data), it is also possible that some clinicians are prescribing sunitinib and/or sorafenib for patients with intact primary tumors. While, at present, implications of this practice pattern remain unclear,20 the survival benefits of cytoreductive nephrectomy prior to targeted therapy are being investigated in an active clinical trial (i.e., the CARMENA trial).21
This study has several limitations. First, as stated above, we cannot measure tumor characteristics, patient performance status, and other risk-related covariates. Thus, our analyses may not adequately characterize or adjust for important patient-specific determinants of utilization of oral TKIs. Second, our findings may not be generalizable to older patients (e.g., Medicare-eligible) with RCC who were not included in this analysis. Third, we recognize that patients may have switched benefit plans or dropped coverage during the study interval, potentially leading to measurement error in our estimates of short-term and sequential therapy. Fourth, we recognize that some patients may have received therapeutic kidney cancer surgery that was not identified by this claims database, either because the operation took place prior to 2003, or occurred while a patient was enrolled in a different health insurance plan. Fifth, we did not assess the frequency of dose reduction among patients in our analytic cohort. Future studies evaluating the prevalence of dose reduction of TKIs among RCC patients may help explain the discrepancies between published clinical trials and our findings. Sixth, as mentioned above, we did not have information on tumor histology, which can affect response to treatment with the oral TKIs. Finally, our findings may not reflect practice patterns for more-recently-introduced oral medications for patients with RCC (e.g., everolimus). Despite these limitations, our findings provide useful insight regarding initial practice patterns with the oral targeted therapies for patients with renal cell carcinoma.
Conclusions
After their introduction, the initial use of sorafenib and sunitinib was characterized by relatively prevalent short-term and sequential treatment regimens among patients with RCC. For patients treated with both sunitinib and sorafenib, patterns of short-term use varied by the sequence of medications, suggesting differences in effectiveness or tolerability of each regimen. These findings highlight the need for future studies to characterize “real-world” clinical outcomes and economic impact associated with these treatment courses.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health Clinical Training Grant in Urology (NIH-T32-DK007782) to C.P.F.; the Edwin Beer Research Fellowship in Urology and Urology-Related Fields from the New York Academy of Medicine and the University of Michigan Cancer Center Munn Idea Grant to D.C.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Eng J Med. 1996;335:865–875. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Russo P. Systemic therapy for renal cell carcinoma. J Urol. 2000;163:408–417. [PubMed] [Google Scholar]
- 3.Gupta K, Miller J, Li J, et al. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev. 2008;34:193–205. doi: 10.1016/j.ctrv.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Goodman V, Rock E, Dagher R, et al. Approval summary: sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clin Cancer Res. 2007;13:1367–1373. doi: 10.1158/1078-0432.CCR-06-2328. [DOI] [PubMed] [Google Scholar]
- 5.Kane R, Farrell A, Saber H, et al. Sorafenib for the treatment of advanced renal cell carcinoma. Clin Cancer Res. 2006;12:7271–7278. doi: 10.1158/1078-0432.CCR-06-1249. [DOI] [PubMed] [Google Scholar]
- 6.Motzer R, Hutson T, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Eng J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 7.Escudier B, Eisen T, Stadler W, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Eng J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 8.Motzer R, Hutson T, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinbrook R. Saying no isn't NICE - the travails of Britain's National Institute for Health and Clinical Excellence. N Eng J Med. 2008;359:1977–1981. doi: 10.1056/NEJMp0806862. [DOI] [PubMed] [Google Scholar]
- 10.Bach PB. Limits on Medicare's ability to control rising spending on cancer drugs. N Eng J Med. 2009;360:626–633. doi: 10.1056/NEJMhpr0807774. [DOI] [PubMed] [Google Scholar]
- 11.Pollack A. As Pills Treat Cancer, Insurance Lags Behind. [Accessed July 3rd, 2010];New York Times. In: nytimes.com. Available at: http://www.nytimes.com/2009/04/15/business/15pill.html. [Google Scholar]
- 12.Health Research Data for the Real World: The MarketScan Databases. [Accessed on July 3rd, 2010]; In: thomsonhealthcare.com. Available at: http://support.thomsonhealthcare.com/uploadedFiles/docs/2008HealthResearchDatafortheRealWorldThe%20MarketScanDatabases(1).pdf.
- 13.Gore M, Szczylik C, Porta C, et al. Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. Lancet Oncol. 2009;10:757–763. doi: 10.1016/S1470-2045(09)70162-7. [DOI] [PubMed] [Google Scholar]
- 14.Kocs D, Fendrick AM. Effect of off-label use of oncology drugs on pharmaceutical costs: the rituximab experience. Am J Manag Care. 2003;9:393–400. [PubMed] [Google Scholar]
- 15.Choueiri TK, Plantade A, Elson P, et al. Efficacy of sunitinib and sorafenib in metastatic papillary and chromophobe renal cell carcinoma. J Clin Oncol. 2008;26:127–131. doi: 10.1200/JCO.2007.13.3223. [DOI] [PubMed] [Google Scholar]
- 16.Strumburg D. Efficacy of sunitinib and sorafenib in non-clear cell renal cell carcinoma: results from expanded access studies. J Clin Oncol. 2008;26:3469. doi: 10.1200/JCO.2008.17.7410. [DOI] [PubMed] [Google Scholar]
- 17.Dougherty D, Conway PH. The "3T's" road map to transform US health care: the "how" of high-quality care. JAMA. 2008;299:2319–2321. doi: 10.1001/jama.299.19.2319. [DOI] [PubMed] [Google Scholar]
- 18.Dudek AZ, Zolniernek J, Dham A, et al. Sequential therapy with sorafenib and sunitinib in renal cell carcinoma. J Clin Oncol. 2009;115:61–67. doi: 10.1002/cncr.24009. [DOI] [PubMed] [Google Scholar]
- 19.Eichelberg C, Heuer R, Chun FK, et al. Sequential use of the tyrosine kinase inhibitors sorafenib and sunitinib in metastatic renal cell carcinoma: a retrospective outcome analysis. Eur Urol. 2008;54:1373–1378. doi: 10.1016/j.eururo.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 20.Di Lorenzo G, Carteni G, Autorino R, et al. Phase II Study of Sorafenib in Patients With Sunitinib-Refractory Metastatic Renal Cell Cancer. J Clin Oncol. 2009;27:4469–4474. doi: 10.1200/JCO.2009.22.6480. [DOI] [PubMed] [Google Scholar]
- 21.Sablin MP, Negrier S, Ravaud A, et al. Sequential sorafenib and sunitinib for renal cell carcinoma. J Urol. 2009;182:29–34. doi: 10.1016/j.juro.2009.02.119. [DOI] [PubMed] [Google Scholar]
- 22.Pantuck AJ, Belldegrun AS, Figlin RA. Cytoreductive nephrectomy for metastatic renal cell carcinoma: is it still imperative in the era of targeted therapy? Clin Cancer Res. 2007;13:693s–696s. doi: 10.1158/1078-0432.CCR-06-1916. [DOI] [PubMed] [Google Scholar]
- 23.Clinical Trial to Assess the Importance of Nephrectomy (CARMENA) [Accessed July 3rd, 2010]; In: Clinicaltrials.gov. Available at: http://clinicaltrials.gov/ct2/show/NCT00930033.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.