Abstract
TRAIL (tumour necrosis factor-related apoptosis inducing ligand) is most often reported to induce apoptosis in tumour cells. It is expressed in artery walls but its role and regulation in vascular pathologies is little studied.
We aimed to measure the effect of genetic deletion of TRAIL on atherosclerosis in a mouse model. TRAIL was mainly expressed in endothelium, smooth muscle cells and macrophages within plaques. The absence of TRAIL in chow and in fat-fed mice led to greater lesion coverage in aortae (8 weeks, % area ± SEM), n = 7–8, 1.24 ± 0.2 (no TRAIL, chow diet) vs. 0.42 ± 0.1, p < 0.01 and 3.4 ± 0.8 (no TRAIL, Western diet) vs. 0.94 ± 0.2, p < 0.01 and larger, smooth muscle cell rich lesions at aortic roots than control mice (8 weeks, mean lesion area/total cross sectional area ± SEM, n = 7–8, 0.17 ± 0.01 (no TRAIL, chow diet) vs. 0.135 ± 0.006, p < 0.05 and 0.36 ± 0.03 (no TRAIL, Western diet) vs. 0.23 ± 0.02, p < 0.05) particularly at early time points. The larger early lesions appeared to be as a result of increased smooth muscle cells in lesions of TRAIL deficient, pro-atherosclerotic animals. We conclude that TRAIL attenuates plaque size at early stages of atherosclerosis.
Keywords: Apolipoprotein E-deficient mice, Atherosclerosis, TRAIL, Apoptosis
1. Introduction
The pathogenesis of atherosclerosis is a complex process involving inflammation, cell proliferation, apoptosis and necrosis. The tumour necrosis factor (TNF) superfamily of cytokines includes tumour necrosis factor-related apoptosis-inducing ligand (TRAIL/TNFSF10); the primary ligand involved in apoptosis signalling in this family. TRAIL is a 40 kDa type II transmembrane protein that induces apoptosis by interaction with two ‘death’ receptors (TRAIL R1 and R2) [1]. In addition, there are 3 ‘decoy’ receptors for TRAIL: TRAIL R3, R4 and the soluble receptor osteoprotegerin, which, when bound to the ligand, do not induce apoptosis [2,3]. TRAIL exists in soluble and membrane bound forms and its expression has been detected in a variety of human tissues [4]. Lymphocytes, monocytes and neutrophils have been shown to express TRAIL, and soluble TRAIL (sTRAIL) is detectable in humans in both health and disease [5,6].
Following TRAIL ligand–receptor binding, downstream signalling leads to apoptosis by the caspase-dependent and mitochondrial pathways [7]. However, TRAIL signalling can also promote cell survival and proliferation via induction of transcription factors NF-кB and c-jun, the mitogen-activated protein kinase ERK1 and the phosphatidylinositol 3-kinase P13K/serine/threonine kinase Akt pathway signalling which is of central importance to angiogenesis and endothelial cell survival [8,9]. Whilst TRAIL can induce apoptosis of many malignant and transformed cell lines, there are conflicting data surrounding the effects of TRAIL on normal human cells, including vascular smooth muscle cells (VSMCs) and endothelial cells. Published data have shown that TRAIL can induce apoptosis of human VSMC and endothelial cells [10,11] yet, under conditions of trophic factor withdrawal, TRAIL promotes their survival and proliferation [8,12,13]. These conflicting data have led to uncertainty regarding the role of TRAIL in vascular biology and disease.
TRAIL ligand is detectable in stable and unstable human atherosclerotic plaques and levels of sTRAIL are lower in patients with coronary disease compared to disease-free controls [14,15]. These findings have led to the suggestion that TRAIL may have a protective role in the atherosclerotic process.
Recent data indicate that TRAIL promotes VSMC proliferation and neointimal formation after arterial injury induced using a femoral cuff in mice [16]. Using the ApoE−/− mouse model of atherogenesis, Secchiero et al. showed that administration of recombinant human TRAIL to diabetic ApoE−/− mice led to a greater VSMC lesion content and a reduced overall lesion size [17]. These findings indicating that TRAIL is pro-proliferative contrast with those of Sato et al. who demonstrated induction of VSMC apoptosis by TRAIL-expressing T cells isolated from patients with ACS [11]. Given these conflicting data, we sought to further clarify the role of TRAIL in atherogenesis by genetic deletion of TRAIL in ApoE−/− mice, feeding of a Western diet and assessment of atherosclerosis.
2. Methods
2.1. Animals
TRAIL−/− mice, on a C57BL/6 background, were the kind gift of Mark Smyth, Peter MacCallum Cancer Institute, Australia under Amgen materials transfer agreement 200308096. ApoE−/− mice, also on a C57BL/6 background, were bred in house. All mice were used in accordance with UK legislation (1986 Animals (Scientific Procedures) Act) and were housed in a controlled environment with a 12 h light/dark cycle at 22 °C. All animal experiments were approved by the University of Sheffield Project Review Committee and carried out under a UK Home Office Project Licence. Following re-derivation, TRAIL−/− mice were crossed with ApoE−/− mice to create a double knockout mouse colony. Genotype was confirmed by polymerase chain reaction. All animals were fed standard chow until they reached 6 weeks of age (20 g). Male TRAIL−/−/ApoE−/− mice and TRAIL+/+/ApoE−/− controls were then fed chow or a cholate-free ‘Western’ diet (n = 6–8 for each group, Special Diet Services, Essex, UK). All mice were observed daily and weighed weekly to monitor their health. After 8 or 12 weeks of high fat diet, the animals were euthanized by pharmacological overdose of pentobarbitone. Blood was collected by cardiac puncture and the mice were perfusion-fixed with 10% (w/v) buffered formalin. The aortic sinus from each animal was dissected, embedded in paraffin and serially sectioned (5 μm). The thoracic aorta was removed and fixed in 4% (w/v) buffered formalin prior to en face preparation. Aortic sinus sections were stained with Alcian Blue/Elastic Van Gieson for atheromatous lesions. The thoracic aortae were stained with Oil Red O and pinned onto wax.
2.2. Immunohistochemistry
Immunohistochemistry was carried out on aortic sinus sections. Sections were dewaxed and incubated with VSMC α-smooth muscle actin primary antibody (Dako αSMA Clone 1A4, diluted 1:150) for 1 h, then with a secondary biotinylated goat anti-mouse antibody (Vector Labs), followed by ABC solution (Vector Labs). Immunogenicity was visualized with DAB (Sigma) and sections were counterstained with Carazzi's haematoxylin. Macrophages were detected using F480 and the Mac3 antibody (M3/84 monoclonal antibody 550292 BD Pharmingen). Sections were dewaxed and antigen retrieval was carried out using citrate buffer. Dako serum-free protein block was applied to the sections for 1 h, followed by incubation with the primary antibody (1:50 dilution) for 2 h. A secondary antibody (biotinylated anti-rat IgG, Vector Labs) was applied for 1 h, the slides were incubated in ABC-alkaline phosphatase (Vector labs) and the Vector Blue stain applied. For TRAIL staining an iso HC DAB kit (Biogenex) was used. Apoptotic cells were detected using Apoptag® Fluorescein in situ reagents (Millipore).
2.3. Morphometry
Total lesion area or area of positive staining was determined for each section/aorta, respectively, using computer assisted image analysis (NIS-elements/Lucia G software, Nikon UK). Data were generated for mean lesion area and lesion area:cross-sectional area (CSA) ratio of the aortic sinus. For the en face aortic preparations, area of positive staining for Oil Red O is expressed as a percentage of total aortic area. For immunohistochemical quantitation, positive staining Mac3 is expressed as an area proportional to total lesion area. Cells staining positive for TRAIL, αSMA and apoptosis (TUNEL) were counted as a proportion of the total cells in 0.1 mm2 plaque to create a percentage index. A minimum of 5 areas of plaque were counted per section.
2.4. Plasma analysis
Plasma alanine aminotransferase, aspartate aminotransferase, total cholesterol, low density and high density lipoproteins and random glucose were measured by the biochemistry department at the Northern General Hospital, Sheffield.
2.5. Statistical analysis
Data were analysed by non-parametric testing with the Mann–Whitney U and Kruskal Wallis tests using GraphPad Prism 4 software. Data are shown as median and interquartile range or mean ± SEM unless otherwise stated. Significance is denoted by p < 0.05.
3. Results
3.1. Phenotype and biochemical analysis
Mice were generated from the breeding programme in the expected Mendelian ratio. TRAIL−/−ApoE−/− mice fed on chow for both 8 weeks and 12 weeks weighed more than ApoE−/− controls on chow at the same timepoint (32.81 ± 0.534 vs. 30.31 ± 0.377 g ApoE−/− chow, all n = 8 at 8 weeks, p < 0.01; 33.58 ± 0.375 vs. 29.43 ± 0.702 g ApoE−/− chow, all n = 8 at 12 weeks, p < 0.01).
Plasma cholesterol increased as expected with dietary fat content (Supplementary Tables 1 and 2). There was no significant difference in plasma cholesterol between the two mouse strains studied. Mice fed the Western diet had lower mean random glucose levels compared to the mice fed chow. There was no significant difference in plasma glucose or liver enzymes between the two transgenic strains.
3.2. Expression of TRAIL in murine atherosclerotic plaques
TRAIL was expressed throughout the plaque but predominantly in the smooth muscle cell layer (Fig. 1A and B). A few macrophages in the lesions appeared to express TRAIL (Fig. 1C). TRAIL is a secreted molecule and some positive staining was seen in the extracellular matrix. There was no difference in the % of cells staining positively for TRAIL in lesions after 8 weeks compared to 12 weeks on a Western Diet (data not shown). There were no TRAIL positive cells in ApoE−/−TRAIL−/− mice.
Fig. 1.
TRAIL expression in an atheromatous aortic sinus lesion (8 weeks). (A) TRAIL protein in medial and peri-adventitial cells (arrows denote VSMC). (B) High power of (A) to show individual cell associated expression. (C) Macrophage staining (F480 antigen) in the same lesion as (A) and (B) to show macrophage expression (arrows denote one TRAIL positive and one TRAIL negative macrophage). (D) Negative control (isotype).
3.3. The effect of TRAIL on atheromatous lesion size in the aorta and aortic sinus
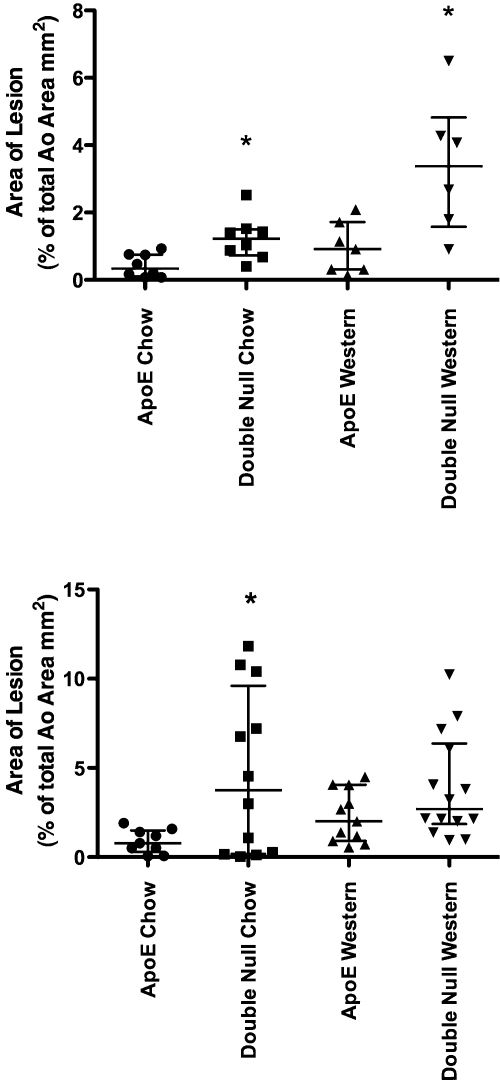
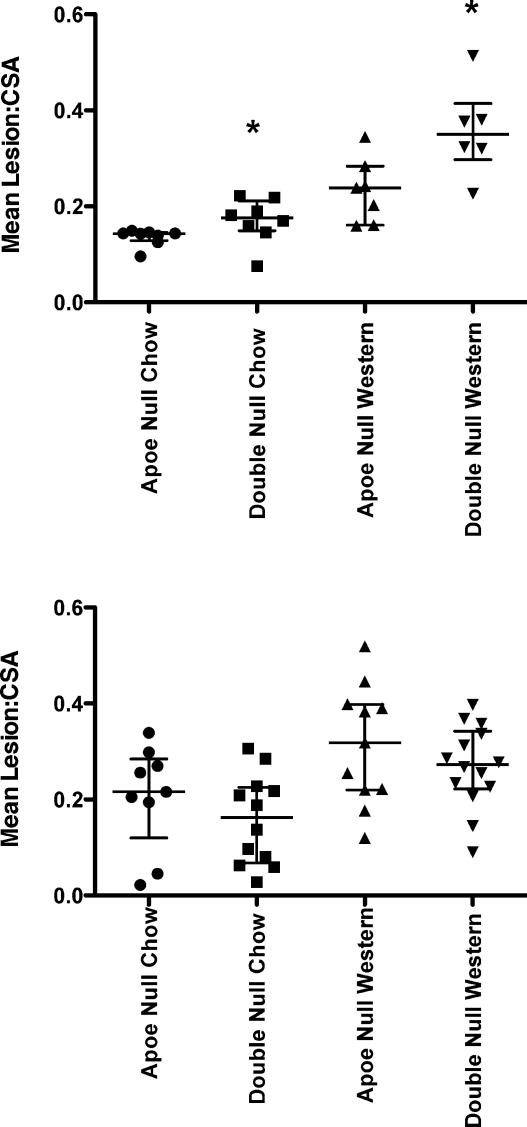
Analysis of lesion area in en face aortae demonstrated that TRAIL−/−ApoE−/− mice fed chow or Western diet for 8 weeks had significantly greater mean lesion area in the aorta compared to ApoE−/− controls (Fig. 2). In the 12-week feeding regime, a difference was only apparent when mice were fed a regular chow diet (Fig. 2B). Analysis of aortic sinus lesion area and lesion:CSA also showed that TRAIL−/−ApoE−/− mice fed chow or Western diets for 8 weeks had a larger lesion area at this site compared to ApoE−/− controls (Fig. 3A). No differences between the groups in lesion area at the aortic sinus were observed at 12 weeks. Representative images of the en face preparation and aortic sinus are shown in Fig. 4.
Fig. 2.
The effect of TRAIL on atheroma development in ApoE−/− mice fed chow or a Western diet. Analysis of atheromatous lesions in en face aortae preparations using Oil red O staining from TRAIL−/−ApoE−/− and ApoE−/− mice fed chow or Western diet for 8 weeks (A) or 12 weeks (B) showed that the absence of TRAIL leads to an increase in % lesion area (expressed as a percentage of total surface area of the aorta). For (A), *p < 0.01 vs. ApoE−/− fed chow and Western, respectively, at 8 weeks, n = 8, Mann–Whitney U Test. For (B), significance only seen on a chow diet after 12 weeks of feeding, *p < 0.001 vs. ApoE−/− chow, n = 9–14, Mann–Whitney U Test.
Fig. 3.
The effect of TRAIL on lesional area at the aortic sinus. ApoE−/− or TRAIL−/−ApoE−/− mice were fed chow or Western diet for 8 weeks (A) or 12 weeks, n = 9–14 (B) and lesion area assessed by Alcian Blue/Elastic Van Gieson staining. ApoE−/− mice on both diets had smaller lesions than TRAIL−/−ApoE−/− mice but only at the 8 week timepoint. *p < 0.05, Mann–Whitney U test.
Fig. 4.
Representative images of atheromatous lesions in ApoE−/− and TRAIL−/−ApoE−/− mice. En face aorta preparations from ApoE−/− mice (A) and TRAIL−/−ApoE−/− mice (B) fed chow for 12 weeks. Note larger lesions at all sites for the TRAIL−/−ApoE−/− mice compared to ApoE−/− mice. Scale bar represents 2 mm. Aortic sinus lesions in ApoE−/− (C) and TRAIL−/−ApoE−/− mice (D) fed Western diet for 8 weeks. Note larger lesions for the TRAIL−/−ApoE−/− mice compared to ApoE−/− mice. Scale bar represents 200 μm.
3.4. The effect of TRAIL on plaque composition
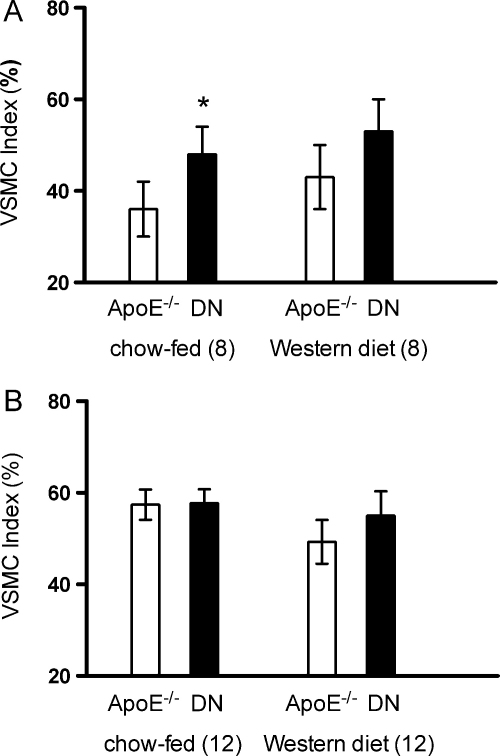
There were more smooth muscle cells in aortic sinus lesions from TRAIL−/−ApoE−/− animals fed both chow and Western diet compared with controls at the early time point of 8 weeks. Significance between the groups (ApoE−/− vs. TRAIL−/−ApoE−/−) was only reached formally at the 8-week time point where mice were fed regular chow (Fig. 5A). Although there was a slight increase, there was no actual significant difference in the VSMC index between TRAIL−/−ApoE−/− and ApoE−/− mice fed chow or Western diet at the 12-week timepoint (Fig. 5B). There was also a suggestion that the increase in smooth muscle cells in the atherosclerotic plaques from TRAIL−/−ApoE−/− animals at 12 weeks coincided with a reduction in apoptosis as analysed by TUNEL but, again, this did not reach statistical significance (Supplementary figure).
Fig. 5.
The effect of TRAIL on atheroma lesion VSMC composition in ApoE−/− mice fed a high fat diet. VSMC indices in aortic sinus lesions from ApoE−/− and TRAIL−/−ApoE−/− mice fed the Western or Paigen diet for 8 weeks. These data show that deletion of TRAIL in ApoE−/− mice fed the Western diet for 8 weeks leads to greater positive staining for α-smooth muscle actin in the lesion (A) compared to mice with functional TRAIL. The data in (B) are from lesions harvested after 12 weeks of feeding. Positive staining in the lesion is expressed as a ratio to total lesion area, data are presented as mean ± SEM, analysis by the Mann–Whitney U test, significant results denoted by *p < 0.05.
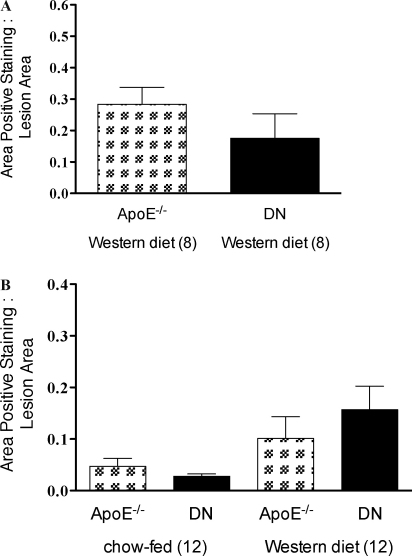
For macrophage staining, immunohistochemistry was not performed on sections from mice fed chow for 8 weeks, as macrophage content of the lesions were too small for analysis. Mice fed a Western diet for 8 or 12 weeks or fed chow for 12 weeks did not reveal any statistically significant difference in macrophage content (Fig. 6A and B).
Fig. 6.
The effect of TRAIL on macrophages in atheromatous lesions. Quantitation of Mac 3 immunohistochemistry of aortic sinus from ApoE−/− and TRAIL−/−ApoE−/− mice fed chow or Western or diet for 8 (A) or 12 (B) weeks. Macrophage staining after 8 weeks on a chow diet was too low to quantify accurately. There was no significant difference in Mac 3 staining between mouse genotypes or with diet. Area of positive staining for Mac 3 is expressed as a ratio to total lesion area, data are presented as mean ± SEM, analysis by the Mann–Whitney U test, significant results denoted by *p < 0.05.
4. Discussion
This is the first report of an atherosclerosis study in double deficient TRAIL and apolipoprotein E mice. In fat fed ApoE−/− mice, TRAIL was expressed predominantly in smooth muscle cells, but also in some endothelial cells and macrophages in aortic sinus lesions. These data on major expression in vascular smooth muscle cells concur with previous studies on human atherosclerosis and mouse aorta from 10 month old ApoE−/− mice [14].
We show here that TRAIL causes an attenuation of atherosclerotic lesion formation in ApoE−/− mice fed a fat-enriched diet. The attenuating effect of TRAIL in the ApoE−/− model of atherosclerosis was only seen in mice fed regular chow or a Western diet for the shortest period of time (8 weeks). These data suggest there may be a temporal component to the attenuating effect of TRAIL on lesion formation. Nevertheless, we cannot exclude the possibility that the effects of TRAIL early in disease formation may not only be lost as the disease develops, but also that, at later stages of lesion development, the effects of TRAIL on lesion progression may change as the developing plaque changes from a lesion characterised by cell proliferation and migration into a more cytopenic lesion with cellular necrosis and apoptosis.
We observed a clear difference in VSMC lesion content in pro-atherosclerotic mice lacking TRAIL where aortic lesions contained more VSMC than in ApoE−/− mice after 8 weeks of Western diet. This effect was only clearly seen at this timepoint and although there was a positive trend for an increase in VSMC lesion content at 12 weeks, this was not significant in either chow or Western diet fed animals. Interestingly, and in support, data on apoptosis at the 12 weeks timepoint also indicated a trend towards a reduction in VSMC apoptosis in Western diet fed TRAIL deficient atherosclerotic animals.
It is notable that our data do not replicate the findings of Secchiero et al. where TRAIL appeared to cause an increase in VSMC lesion content [17]. This may be explained by the different disease models used; Secchiero's mice were rendered diabetic and given recombinant human TRAIL, whereas our mice were not diabetic and had either native TRAIL or no TRAIL.
Secchiero et al. propose that the apparent beneficial effect of TRAIL in atheroma development may be mediated, in part, through promotion of VSMC migration and proliferation, leading to an increase in VSMC content of the fibrous cap, conferring greater plaque stability. However, this does not sit well with in vitro evidence showing that T-cell expression of TRAIL is a potent inducer of VSMC apoptosis [11]. In addition, VSMC proliferation in atheroma development would potentially lead to larger lesions, yet Secchiero's work and the data presented here indicate that TRAIL leads to a reduction in lesion size.
More recent data from Kavurma and Bennett have demonstrated that soluble TRAIL can induce VSMC proliferation in vitro at physiological concentrations (1 ng/ml) and this effect is mediated via activation of NF-κB and induction of insulin-like growth factor-1 receptor [13]. However, the authors also found that at higher concentrations (400 ng/ml), sTRAIL induced VSMC apoptosis. Whilst these levels are far in excess of physiological levels of sTRAIL, TRAIL is also present in the circulation in membrane-bound form. Cross-linkage of the ligand has a more potent cell-killing effect and more closely resembles the membrane-bound form of the ligand found in vivo [18]. It is arguable whether treatment of VSMCs with very high levels of sTRAIL is comparable to treatment with cross-linked or membrane-bound ligand, which is known to induce VSMC apoptosis. Therefore, it is possible that the different experimental models used and the known difference in potency between membrane-bound TRAIL and soluble forms of the ligand may explain these apparently conflicting data. It is not known whether sTRAIL or TRAIL expressed on the surface of T cells has a more dominant effect on cell population control in human physiology.
Recent data from Chan et al. [16] indicate that TRAIL promotes proliferation in a cuff-induced vascular injury. Although this model has some features of atherosclerosis; namely an inflammatory basis and VSMC proliferation, it lacks an atherogenic stimulus which, quite plausibly, might promote apoptotic activity due to TRAIL rather than proliferation.
Since TRAIL is thought to mediate some of its functions through regulation of the immune response and induction of apoptosis of inflammatory cells, it is surprising that our data showed no effect of TRAIL on the macrophage content of the atheromatous plaques. Although a trend was seen for greater macrophage staining in the ApoE−/− mice fed Western diet at 12 weeks these data did not reach significance. Whilst these data do not provide conclusive evidence that deletion of TRAIL affects the macrophage content of atheromatous lesions, this does not exclude TRAIL-induced apoptosis of infiltrating macrophages as a potential mechanism for the reduction in lesion size observed. Evidence from in vitro studies has shown that TRAIL does induce apoptosis of both human and murine macrophages [19], and Secchiero's study demonstrated that administration of rhTRAIL to diabetic ApoE−/− mice led to a reduction in the number of plaque-infiltrating macrophages and an increase in macrophage apoptosis [17]. It is known that infiltration of monocytes and macrophages occurs early in the developing lesion and it is possible that the reduction in lesion size seen here at earlier time-points may be mediated, in part, by control of macrophage numbers by TRAIL.
The observation that deletion of a potentially inflammatory cytokine or mechanism results in enhancement of atherosclerosis is not one in isolation. Though deletion of many inflammatory cytokines and chemokines, when crossed onto an atherosclerotic background in the mouse, produce reduction in plaque volume, deletion of the TNF signalling receptor and deletion of macrophage-specific NF-κB pathways have been associated with enhanced plaque volume [20,21], as seen here with TRAIL deletion. The reasons for this effect are unclear but raise the possibility of beneficial inflammatory reparation mechanisms within the vessel wall. This is quite an attractive hypothesis for the data presented here in relation to TRAIL deletion when the enhanced atherosclerosis was only seen with mild dietary stimuli, an effect overwhelmed by feeding a fat enriched diet for a longer period.
5. Conclusion
The data presented here clearly show that TRAIL can attenuate atheromatous lesion formation. The underlying mechanism appears to be that TRAIL promotes vascular cell apoptosis in response to a mild dietary fat stimulus and that this effect is overcome by extending the length of the fat-feeding period. Whilst the role of TRAIL in vascular biology still remains controversial, these data show the potential for this cytokine system to limit the atherogenic response to moderate atherogenic stimuli in the mouse.
Funding
This work was funded by a British Heart Foundation PhD studentship to VW (FS/04/016). DCR is an NIHR Investigator.
Conflict of interest
None declared.
Acknowledgements
The authors are grateful to Drs. Christina Bursill, Eileen MacNeill and Robin Choudhury from the Department of Cardiovascular Medicine at the University of Oxford for their expertise in histology and immunohistochemistry. MBB students Tanzila Azram and Fiona Moejes performed the TRAIL immunohistochemistry.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.atherosclerosis.2011.01.010.
Appendix A. Supplementary data
References
- 1.Pan G. O.R.K., Chinnaiyan A.M., Gentz R. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 2.Degli-Eposti M.A., Smolak P.J., Walczak H. Cloning and characterization of TRAIL-R3, a novel member of the emerging TRAIL receptor family. J Exp Med. 1997;186:1165–1170. doi: 10.1084/jem.186.7.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emery J.G., McDonnell P., Burke M.B. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 1998;273(23):14363–14367. doi: 10.1074/jbc.273.23.14363. [DOI] [PubMed] [Google Scholar]
- 4.Daniels R.A., Turley H., Kimberley F.C. Expression of TRAIL and TRAIL receptors in normal and malignant tissues. Cell Res. 2005;15:430–438. doi: 10.1038/sj.cr.7290311. [DOI] [PubMed] [Google Scholar]
- 5.Choi J.W., Song J.S., Pai S.H. Associations of serum TRAIL concentrations, anthropometric variables, and serum lipid parameters in healthy adults. Ann Clin Lab Sci. 2004;34(4):400–404. [PubMed] [Google Scholar]
- 6.Lub-de Hooge M.N., de Vries E.G., de Jong S. Soluble TRAIL concentrations are raised in patients with systemic lupus erythematosus. Ann Rheum Dis. 2005;64(6):854–858. doi: 10.1136/ard.2004.029058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimberley F.C., Screaton G.R. Following a TRAIL: update on a ligand and its five receptors. Cell Res. 2004;14(5):359–372. doi: 10.1038/sj.cr.7290236. [DOI] [PubMed] [Google Scholar]
- 8.Secchiero P., Zerbinati C., Rimondi E. TRAIL promotes the survival, migration and proliferation of vascular smooth muscle cells. Cell Mol Life Sci. 2004;61:1965–1974. doi: 10.1007/s00018-004-4197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alladina S.J., Song J.H., Davidge S.T., Hao C., Easton A.S. TRAIL-induced apoptosis in human vascular endothelium is regulated by phosphatidylinositol 3-kinase/Akt through the short form of cellular FLIP and Bcl-2. J Vasc Res. 2005;42:337–347. doi: 10.1159/000086599. [DOI] [PubMed] [Google Scholar]
- 10.Li J.H., Kirkiles-Smith N.C., McNiff J.M. TRAIL induces apoptosis and inflammatory gene expression in human endothelial cells. J Immunol. 2003;171(3):1526–1533. doi: 10.4049/jimmunol.171.3.1526. [DOI] [PubMed] [Google Scholar]
- 11.Sato K., Niessner A., Kopecky S.L. TRAIL-expressing T cells induce apoptosis of vascular smooth muscle cells in the atherosclerotic plaque. J Exp Med. 2006;203(1):239–250. doi: 10.1084/jem.20051062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Secchiero P., Gonelli A., Carnevale E. TRAIL promotes the survival and proliferation of primary human vascular endothelial cells by activating the Akt and ERK pathways. Circulation. 2003;107(17):2250–2256. doi: 10.1161/01.CIR.0000062702.60708.C4. [DOI] [PubMed] [Google Scholar]
- 13.Kavurma M.M., Bennett M.R. Expression, regulation and function of trail in atherosclerosis. Biochem Pharmacol. 2008;75(7):1441–1450. doi: 10.1016/j.bcp.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 14.Michowitz Y., Goldstein E., Roth A. The involvement of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in atherosclerosis. J Am Coll Cardiol. 2005;45(7):1018–1024. doi: 10.1016/j.jacc.2004.12.065. [DOI] [PubMed] [Google Scholar]
- 15.Schoppet M., Al-Fakhri N., Franke F.E. Localization of osteoprotegerin, tumor necrosis factor-related apoptosis-inducing ligand, and receptor activator of nuclear factor-{kappa}B ligand in Monckeberg's sclerosis and atherosclerosis. J Clin Endocrinol Metab. 2004;89(8):4104–4112. doi: 10.1210/jc.2003-031432. [DOI] [PubMed] [Google Scholar]
- 16.Chan J., Prado-Lourenco L., Khachigian L.M. TRAIL promotes VSMC proliferation and neointima formation in a FGF-2-Sp1 phosphorylation-, and NFkappaB-dependent manner. Circ Res. 2010;106(6):1061–1071. doi: 10.1161/CIRCRESAHA.109.206029. [DOI] [PubMed] [Google Scholar]
- 17.Secchiero P., Candido R., Corallini F. Systemic tumor necrosis factor-related apoptosis-inducing ligand delivery shows antiatherosclerotic activity in apolipoprotein e-null diabetic mice. Circulation. 2006;114(14):1522–1530. doi: 10.1161/CIRCULATIONAHA.106.643841. [DOI] [PubMed] [Google Scholar]
- 18.Rus V., Zernetkina V., Puliaev R. Increased expression and release of functional tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) by T cells from lupus patients with active disease. Clin Immunol. 2005;117(1):48–56. doi: 10.1016/j.clim.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan M.J., Ray D., Mo R.R., Yung R.L., Richardson B.C. TRAIL (Apo2 ligand) and TWEAK (Apo3 ligand) mediate CD4+ T cell killing of antigen-presenting macrophages. J Immunol. 2000;164:2897–2904. doi: 10.4049/jimmunol.164.6.2897. [DOI] [PubMed] [Google Scholar]
- 20.Schreyer S.A., Vick C.M., LeBoeuf R.C. Loss of lymphotoxin-alpha but not tumor necrosis factor-alpha reduces atherosclerosis in mice. J Biol Chem. 2002;277(14):12364–12368. doi: 10.1074/jbc.M111727200. [DOI] [PubMed] [Google Scholar]
- 21.Kanters E., Pasparakis M., Gijbels M.J. Inhibition of NF-{kappa}B activation in macrophages increases atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;112(8):1176–1185. doi: 10.1172/JCI18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.