Abstract
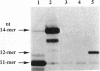
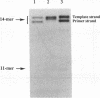
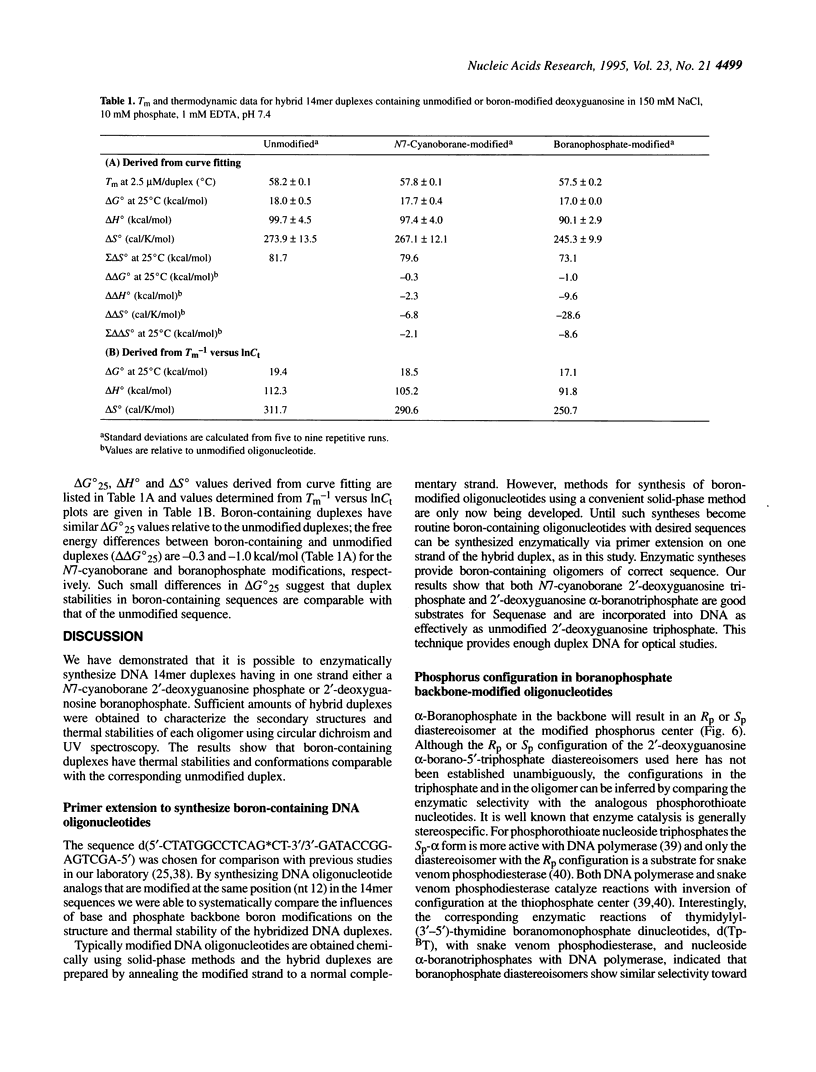
A set of three 14mer oligodeoxyribonucleotides of sequence d(5'-CTATGGCCTCAG*CT-3'/3'-GATACCGGAGTCGA-5') containing G* variants either as 2'-deoxyguanosine phosphate (unmodified), N7-cyanoborane 2'-deoxyguanosine phosphate (base-modified) or 2'-deoxyguanosine boranophosphate (backbone-modified) were synthesized by template-directed primer extension. Both the N7-cyanoborane 2'-deoxyguanosine triphosphate and 2'-deoxyguanosine alpha-boranotriphosphate nucleotides are good substrates for Sequenase. We infer that a single Sp boranophosphate linkage (which has a stereochemistry equivalent to the corresponding Rp thiophosphate analog) is formed in the backbone-modified 14mer. Thermally induced helix-coil transitions were monitored for the hybridized duplexes using UV and circular dichroism (CD) spectroscopy. The CD spectra of the two types of boron-modified hybrids closely resemble the unmodified parent duplex, forming B-type helices in 150 mM NaCl, 1 mM EDTA, 10 mM phosphate, pH 7.4, buffer. UV melting results indicate that both hybrids have stabilities comparable with the parent duplex as measured by Tm or delta G degree 25. These studies indicate that singly modified base- or backbone-boronated DNA are good analogs of normal DNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albergo D. D., Marky L. A., Breslauer K. J., Turner D. H. Thermodynamics of (dG--dC)3 double-helix formation in water and deuterium oxide. Biochemistry. 1981 Mar 17;20(6):1409–1413. doi: 10.1021/bi00509a001. [DOI] [PubMed] [Google Scholar]
- Aurup H., Tuschl T., Benseler F., Ludwig J., Eckstein F. Oligonucleotide duplexes containing 2'-amino-2'-deoxycytidines: thermal stability and chemical reactivity. Nucleic Acids Res. 1994 Jan 11;22(1):20–24. doi: 10.1093/nar/22.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower M., Summers M. F., Powell C., Shinozuka K., Regan J. B., Zon G., Wilson W. D. Oligodeoxyribonucleoside methylphosphonates. NMR and UV spectroscopic studies of Rp-Rp and Sp-Sp methylphosphonate (Me) modified duplexes of (d[GGAATTCC])2. Nucleic Acids Res. 1987 Jun 25;15(12):4915–4930. doi: 10.1093/nar/15.12.4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgers P. M., Eckstein F., Hunneman D. H. Stereochemistry of hydrolysis by snake venom phosphodiesterase. J Biol Chem. 1979 Aug 25;254(16):7476–7478. [PubMed] [Google Scholar]
- Cech C. L., Tinoco I., Jr Circular dichroism calculations for double-stranded polynucleotides of repeating sequence. Biopolymers. 1977 Jan;16(1):43–65. doi: 10.1002/bip.1977.360160105. [DOI] [PubMed] [Google Scholar]
- Eckstein F. Nucleoside phosphorothioates. Annu Rev Biochem. 1985;54:367–402. doi: 10.1146/annurev.bi.54.070185.002055. [DOI] [PubMed] [Google Scholar]
- Freier S. M., Albergo D. D., Turner D. H. Solvent effects on the dynamics of (dG-dC)3. Biopolymers. 1983 Apr;22(4):1107–1131. doi: 10.1002/bip.360220408. [DOI] [PubMed] [Google Scholar]
- Heinemann U., Rudolph L. N., Alings C., Morr M., Heikens W., Frank R., Blöcker H. Effect of a single 3'-methylene phosphonate linkage on the conformation of an A-DNA octamer double helix. Nucleic Acids Res. 1991 Feb 11;19(3):427–433. doi: 10.1093/nar/19.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov V. I., Minchenkova L. E., Schyolkina A. K., Poletayev A. I. Different conformations of double-stranded nucleic acid in solution as revealed by circular dichroism. Biopolymers. 1973;12(1):89–110. doi: 10.1002/bip.1973.360120109. [DOI] [PubMed] [Google Scholar]
- Johnson B. B., Dahl K. S., Tinoco I., Jr, Ivanov V. I., Zhurkin V. B. Correlations between deoxyribonucleic acid structural parameters and calculated circular dichroism spectra. Biochemistry. 1981 Jan 6;20(1):73–78. doi: 10.1021/bi00504a013. [DOI] [PubMed] [Google Scholar]
- Jäger A., Levy M. J., Hecht S. M. Oligonucleotide N-alkylphosphoramidates: synthesis and binding to polynucleotides. Biochemistry. 1988 Sep 20;27(19):7237–7246. doi: 10.1021/bi00419a010. [DOI] [PubMed] [Google Scholar]
- Kibler-Herzog L., Zon G., Uznanski B., Whittier G., Wilson W. D. Duplex stabilities of phosphorothioate, methylphosphonate, and RNA analogs of two DNA 14-mers. Nucleic Acids Res. 1991 Jun 11;19(11):2979–2986. doi: 10.1093/nar/19.11.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. S., Guengerich F. P. Interactions of N7-guanyl methyl- and thioether-substituted d(CATGCCT) derivatives with d(AGGNATG). Chem Res Toxicol. 1993 Nov-Dec;6(6):900–905. doi: 10.1021/tx00036a022. [DOI] [PubMed] [Google Scholar]
- Liaw Y. C., Gao Y. G., Marquez V. E., Wang A. H. Molecular structures of two new anti-HIV nucleoside analogs: 9-(2,3-dideoxy-2-fluoro-beta-D-threo-pentofuranosyl)adenine and 9-(2,3-dideoxy-2-fluoro-beta-D-threo-pentofuranosyl)hypoxanthine. Nucleic Acids Res. 1992 Feb 11;20(3):459–465. doi: 10.1093/nar/20.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longfellow C. E., Kierzek R., Turner D. H. Thermodynamic and spectroscopic study of bulge loops in oligoribonucleotides. Biochemistry. 1990 Jan 9;29(1):278–285. doi: 10.1021/bi00453a038. [DOI] [PubMed] [Google Scholar]
- Maher L. J., 3rd, Dolnick B. J. Comparative hybrid arrest by tandem antisense oligodeoxyribonucleotides or oligodeoxyribonucleoside methylphosphonates in a cell-free system. Nucleic Acids Res. 1988 Apr 25;16(8):3341–3358. doi: 10.1093/nar/16.8.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marky L. A., Canuel L., Jones R. A., Breslauer K. J. Calorimetric and spectroscopic investigation of the helix-to-coil transition of the self-complementary deoxyribonucleotide ATGCAT. Biophys Chem. 1981 Apr;13(2):141–149. doi: 10.1016/0301-4622(81)80013-0. [DOI] [PubMed] [Google Scholar]
- Matsukura M., Shinozuka K., Zon G., Mitsuya H., Reitz M., Cohen J. S., Broder S. Phosphorothioate analogs of oligodeoxynucleotides: inhibitors of replication and cytopathic effects of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7706–7710. doi: 10.1073/pnas.84.21.7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S., McParland K. B., Jayaraman K., Ts'o P. O. Biochemical and biological effects of nonionic nucleic acid methylphosphonates. Biochemistry. 1981 Mar 31;20(7):1874–1880. doi: 10.1021/bi00510a024. [DOI] [PubMed] [Google Scholar]
- Petersheim M., Turner D. H. Base-stacking and base-pairing contributions to helix stability: thermodynamics of double-helix formation with CCGG, CCGGp, CCGGAp, ACCGGp, CCGGUp, and ACCGGUp. Biochemistry. 1983 Jan 18;22(2):256–263. doi: 10.1021/bi00271a004. [DOI] [PubMed] [Google Scholar]
- Reich N. O., Sweetnam K. R. Sequence-dependent effects on DNA stability resulting from guanosine replacements by inosine. Nucleic Acids Res. 1994 Jun 11;22(11):2089–2093. doi: 10.1093/nar/22.11.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo V., Schellman J. A. Matrix-method calculation of linear and circular dichroism spectra of nucleic acids and polynucleotides. Biopolymers. 1984 Mar;23(3):435–470. doi: 10.1002/bip.360230305. [DOI] [PubMed] [Google Scholar]
- Romaniuk P. J., Eckstein F. A study of the mechanism of T4 DNA polymerase with diastereomeric phosphorothioate analogues of deoxyadenosine triphosphate. J Biol Chem. 1982 Jul 10;257(13):7684–7688. [PubMed] [Google Scholar]
- Seela F., Grein T. 7-Deaza-2'-deoxyadenosine and 3-deaza-2'-deoxyadenosine replacing dA within d(A6)-tracts: differential bending at 3'- and 5'-junctions of d(A6).d(T6) and B-DNA. Nucleic Acids Res. 1992 Jul 11;20(13):2297–2306. doi: 10.1093/nar/20.9.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw B. R., Madison J., Sood A., Spielvogel B. F. Oligonucleoside boranophosphate (borane phosphonate). Methods Mol Biol. 1993;20:225–243. doi: 10.1385/0-89603-281-7:225. [DOI] [PubMed] [Google Scholar]
- Sheardy R. D., Levine N., Marotta S., Suh D., Chaires J. B. A thermodynamic investigation of the melting of B-Z junction forming DNA oligomers. Biochemistry. 1994 Feb 15;33(6):1385–1391. doi: 10.1021/bi00172a014. [DOI] [PubMed] [Google Scholar]
- Stein C. A., Subasinghe C., Shinozuka K., Cohen J. S. Physicochemical properties of phosphorothioate oligodeoxynucleotides. Nucleic Acids Res. 1988 Apr 25;16(8):3209–3221. doi: 10.1093/nar/16.8.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovkina E. V., Savchenko E. V., Lokhov S. G., Engels J. W., Wickstrom E., Lebedev A. V. Synthesis of specific diastereomers of a DNA methylphosphonate heptamer, d(CpCpApApApCpA), and stability of base pairing with the normal DNA octamer d(TPGPTPTPTPGPGPC). Nucleic Acids Res. 1994 Jun 25;22(12):2404–2409. doi: 10.1093/nar/22.12.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. P., Longfellow C. E., Freier S. M., Kierzek R., Turner D. H. Laser temperature-jump, spectroscopic, and thermodynamic study of salt effects on duplex formation by dGCATGC. Biochemistry. 1989 May 16;28(10):4283–4291. doi: 10.1021/bi00436a025. [DOI] [PubMed] [Google Scholar]