Abstract
Objectives
We evaluated the feasibility of performing robot assisted laparoscopic partial adrenalectomy (RALPA) in patients seen at the National Cancer Institute and report the results of our initial experience.
Methods
We reviewed the records of patients with adrenal masses who underwent attempted RALPA from July of 2008 until January of 2010. Demographic, perioperative, and pathologic data were collected. The functional and early oncologic outcomes were examined by the need for steroid replacement and development of recurrent disease, respectively.
Results
Ten patients underwent a total of 13 attempted RALPA for removal of 19 adrenal tumors. There was one open conversion with successful completion of partial adrenalectomy. Of the patients, 80% had a known hereditary syndrome predisposing them to adrenal tumors. One patient had bilateral multifocal adrenal masses with unknown germ line genetic alteration and one patient had a sporadic adrenal mass. Of the 19 tumors removed, 17 were pheochromocytoma and 2 were adrenal-cortical hyperplasia. Two patients underwent partial adrenalectomy on a solitary adrenal gland with one subsequently requiring steroid replacement post-operatively. On postoperative imaging all but one operated adrenal gland demonstrated contrast enhancement. No patient developed local recurrence at a median follow-up of 16.2 months (range 2- 29).
Conclusions
RALPA appears safe and feasible in our early experience. Only one patient in our series required steroid replacement. Local recurrence rates are low but will require longer follow up.
Keywords: Robotic, partial adrenalectomy, adrenal sparing surgery, pheochromocytoma, hereditary syndromes
Objectives
Patients with hereditary syndromes such as von Hippel-Lindau (VHL), multiple endocrine neoplasia type 2 (MEN2), and neurofibromatosis 1 (NF1), are at significant risk of developing adrenal pheochromocytomas during their lifetime, and up to half of the affected patients will have bilateral disease.1 Historically, bilateral total adrenalectomy was preferred treatment for the management of adrenal masses in the hereditary setting. However, even with total extirpation, local recurrence rates have been reported up to 14- 22%.2, 3 In the past decade laparoscopic adrenalectomy has replaced open adrenalectomy as the current standard of care for the majority of adrenal masses owing to the reduced morbidity and improved convalescence. Several large published reports support the use of laparoscopic adrenal surgery over the open approach.4, 5
The benefits of total adrenalectomy with decreased concerns for recurrent disease must be weighed against the maximal preservation of adrenocortical tissue and avoidance of steroid dependence. The morbidity of medical adrenal replacement therapy, an obvious consequence of bilateral total adrenalectomy, may subject patients to increased risks of osteoporosis, Addisonian crisis, and a decreased quality of life.6 Utilization of adrenal-sparing surgery, especially for the hereditary patient population that is at higher risk for bilateral disease, has been long established at our institution utilizing both open and laparoscopic techniques.5, 7
In addition to adrenal masses, many of our hereditary patients present with renal tumors where the same paradigm of organ-sparing surgery is practiced when feasible. Recently, our institution has undergone a shift towards robotics over traditional laparoscopy for the majority of cases when a minimally invasive approach is utilized. Because robotic assistance may have facilitated our ability to perform more complex partial nephrectomies, including resection of large, deep, hilar lesions as well as multifocal renal masses, we have applied these technical considerations to patients with adrenal masses.8, 9 In this study we present our initial experience with RALPA.
Materials and Methods
We retrospectively reviewed the records of patients at the National Institutes of Health (NIH) undergoing RALPA. Demographic, pathologic, perioperative, oncologic, and functional outcome data were collected by chart review. Local recurrence was defined as radiographic evidence of recurrent tumor on the ipsilateral side of partial adrenalectomy. Functional outcome data was determined by the need for steroid replacement after surgery. Patients were considered steroid-dependent if they required steroids at hospital discharge. All patients suspicious for diagnosis of pheochromocytoma received a two week blockade with metyrosine and phenoxybenzamine prior to surgery, as previously reported.2
Surgical technique
Patients were placed in a modified flank position with the robot docked over the ipsilateral shoulder. A standard 3-arm da Vinci® Surgical System and a transperitoneal approach were used in all cases. Pneumoperitoneum at 15mmHg was achieved using a Veress needle for access. Four ports were used for left sided surgery and 5 were placed for right sided surgery including a 5mm liver retractor instrument. Early in our experience a second 5mm assistant port was placed for additional retraction. Figure 1 shows port placement for left (1A) and right sided (1B) partial adrenalectomy. The 30-degree down camera lens was used throughout the operation. The adrenal vein was controlled with Hem-o-Lok® clips in all instances prior to tumor removal. The intraoperative ultrasound was utilized in all cases. While in many cases the tumors were appreciated by inspection of the adrenal gland, ultrasound was used to delineate tumor borders of the dissection and to identify potential additional lesions not initially appreciated on pre-operative cross-sectional imaging. The use of ultrasound is especially important in the hereditary pheochromocytoma patients where multifocality can be common.1
Figure 1.
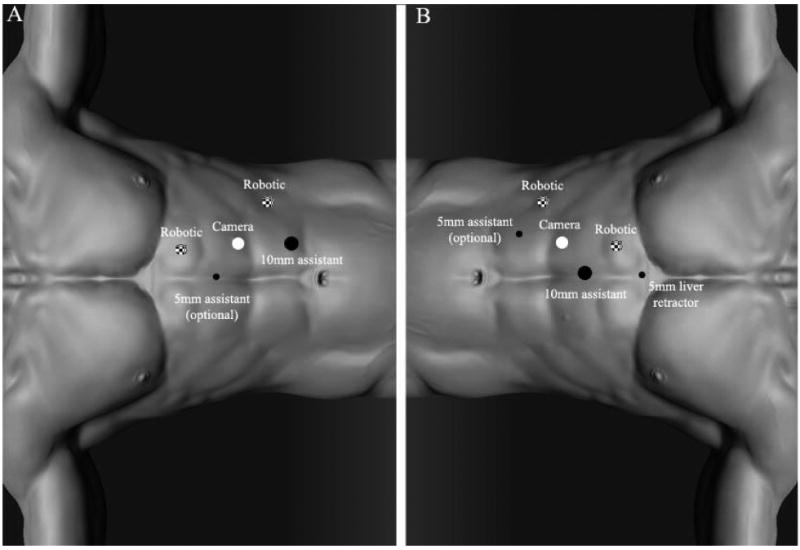
Port placement. A, left side. B, right side.
The strategy for tumor extirpation was based on tumor number and/or genetic alteration status. For patients with known hereditary syndromes (i.e. VHL, NF-1) or multifocal adrenal masses, tumors were resected by a technique previously described at the NIH for both open and laparoscopic approaches.2, 5 Because the majority of patients in our cohort had a pheochromocytoma, we performed a partial or total “demedullation” of the adrenal gland, leaving the majority of cortex intact whenever possible. Briefly, the tumor pseudocapsule was identified and a plane was developed between the tumor and the normal adrenal gland. This plane could be followed circumferentially to a point where the tumor became completely embedded in normal gland. The attached section of normal tissue and tumor was divided using a series of Hem-o-Lok® clips. This provided hemostasis of the resection site and completed tumor extirpation. For sporadic patients with solitary lesions, Hem-o-Lok® clips were deployed at a point further from the lesion to provide a surgical margin between the tumor and normal tissue.
Initial follow up for all patients in this series was at 3 months. It included cross-sectional imaging and catecholamine studies to assess for recurrent disease, enhancement of the residual adrenal remnant, and evidence of biochemical improvement. The subsequent follow up was determined by the treating team.
Results
Ten patients underwent a total of 13 attempted RALPA between July 2008 and January 2010 at the NIH for removal of 19 adrenal tumors. Three patients required bilateral staged RALPA. Among resected tumors 17 were pheochromocytoma and 2 were cortical hyperplasia. All but one of 13 cases was completed with robotic assistance. One open conversion occurred in a patient with prior ipsilateral open partial adrenalectomy resulting in a severe desmoplastic reaction obliterating the plain between the liver, right adrenal gland, and the inferior vena cava. Eight of 10 (80%) patients had known hereditary syndromes including 7 with VHL and one with NF-1. One patient had bilateral multifocal pheochromocytomas with unknown germline mutation and one patient had a sporadic adrenal mass. Partial adrenalectomy was completed in all instances. Details of patient characteristics are displayed in Table 1.
Table 1. Patient characteristics, perioperative and functional outcomes and tumor characteristics.
| Total Patients | 10 |
| Total Procedures | 13 |
| Mean pt age (range) | 32.5 (15.9 to 61.9) |
| Median Follow up months (range) | 5.25 (0 to 28.7) |
| Mean BMI (range) | 28.7 (20 to 48) |
| % Caucasian | 90 |
| % Male | 60 |
| % Right side | 54 |
| Diagnosis (%) | |
| VHL | 7 (70) |
| Neurofibromatosis | 1 (10) |
| Bilateral Multifocal | 1 (10) |
| Sporadic | 1 (10) |
| Median operative time (range)* | 200 (110 to 480) |
| Median EBL (range)* | 150 (25 to 1000) |
| Patients requiring transfusion (%) | 1 (7.5) |
| Median largest tumor size (range) | 2.7 (1.3 to 5.5) |
| Adrenal histology (%) | |
| Pheochromocytoma | 11 (85) |
| Cortical hyperplasia | 2 (15) |
| Recurrence | 0 |
| Steroid replacement post-op (%) | 1 (7.5) |
Does not include 2 patients who had simultaneous partial nephrectomy
Perioperative and functional outcomes along with tumor characteristics are shown in Table 1. The median follow-up was 5.25 months (0-28 months). Four of ten patients (40%) had prior abdominal surgery with two of those patients (20%) having undergone prior open ipsilateral partial adrenalectomy. Two patients had multifocal adrenal masses and two patients had a solitary adrenal gland prior to surgery. We chose to exclude two patients who underwent concomitant partial nephrectomy in our assessment of perioperative outcomes. These cases had longer operative times and estimated blood loss than those with adrenal surgery alone. No patient in the overall cohort sustained perioperative complications related to catecholamine surge such as hypertensive crisis, prolonged hypotension, myocardial infarction, or cerebral vascular accident. In cases completed successfully without conversion, there were no surgical complications related to the partial adrenalectomy portion of the surgery.
Two surgical complications recorded included a bile leak in the aforementioned patient that required open conversion and a ureteral stricture in a second patient undergoing repeat partial nephrectomy at the time of partial adrenalectomy. The bile leak was managed expectantly with intraoperative drain placement and its resolution on postoperative day #4 without sequella, while the ureteral stricture is currently being managed with an indwelling ureteral stent. No patient has developed radiographic or biochemical evidence of ipsilateral adrenal recurrence during follow-up. All but one operated unit revealed evidence of an enhancing residual adrenal remnant. One patient with a history of a prior contralateral partial adrenalectomy underwent a partial adrenalectomy for 6 lesions for multifocal pheochromocytoma and required steroid replacement after surgery.
Comment
Over the past few decades the rate of adrenal surgical procedures in the US has increased by one third.10 While many of these masses are incidentally discovered on cross-sectional imaging and are small and non-functional, up to 15 percent of these lesions may be biochemically active.11 The historical management of these masses has been total adrenalectomy, regardless of tumor size or location or the status of the contralateral adrenal gland. In recent years, however, support has emerged for partial adrenalectomy to avoid potential future side effects of adrenal insufficiency, especially in patients with hereditary adrenal-producing syndromes, bilateral or multifocal lesions, or solitary adrenal glands. It has been reported that after bilateral adrenalectomy up to 35% of patients may experience Addisonian crisis and a 3% mortality risk.12 In addition, steroid replacement has been associated with a decreased quality of life, uncontrolled weight gain, and increased risk of osteoporosis.6
The argument for performing partial adrenalectomy in the sporadic patient with a small unilateral adrenal mass continues to gain credence. A recent literature review of partial adrenalectomy by Kaye et al. found that partial adrenalectomy is increasingly utilized worldwide and does not carry additional morbidity when compared to total adrenalectomy.13 Several studies have even reported less blood loss and shorter operative times during adrenal-sparing surgery procedures when compared to whole-organ removal.14, 15 Additionally, low ipsilateral recurrence rates after partial adrenalectomy have been documented by several authors, further strengthening the argument for an adrenal sparing approach when feasible.15, 16 Some studies have demonstrated that patients with a solitary adrenal gland do not respond equally well to stressful situations when compared to normal controls, underscoring the possibility that partial adrenalectomy may be indicated regardless of the status of the contralateral adrenal gland.16 Although the vast majority of the patients in our cohort have a known history of hereditary syndromes, consideration of adrenal-sparing surgery for all patients regardless of genetic status may play a larger role in the future.
Although laparoscopic adrenalectomy has become the standard of care for the treatment of the majority of adrenal tumors since its emergence in 1992, laparoscopic partial adrenalectomy has received considerably less recognition over a similar period of time.17-19 Safety and feasibility of the procedure have been described mostly for patients with pheochromocytoma or cortisol producing adenomas.5, 14, 20, 21 Walther et al. in 2000 published on 3 VHL patients undergoing laparoscopic partial adrenalectomy for pheochromocytoma via a transperitoneal approach.5 Their median operative time was 324 minutes with an estimated blood loss of 100 cc. No patient required steroid-replacement or had a local recurrence during limited follow-up of 14 months. In the largest series of laparoscopic adrenal-sparing surgery published, Walz et al. compared 100 partial adrenalectomies to 225 total adrenalectomies, all via a retroperitoneal approach.15 The median operative time was 79 minutes and the estimated blood loss was 29 cc for the partial adrenalectomy group. Outcomes showed equivalence of total and partial procedures regarding operative times, blood loss, complications, and cure rates. No local recurrence was documented during a follow-up for up to 120 months. The comprehensive partial adrenalectomy review by Kaye et al consisted of 417 patients from 22 studies including 319 laparoscopic partial adrenal surgeries and 98 open adrenal sparing procedures. In their review, Conn's syndrome (42%) and pheochromocytoma (37%) were the two most common diagnoses. Mean operative time was 130 minutes and the mean EBL was 72 ml. Complication rates were 7.3% in the lap group and 13.6% in the open group. Recurrence rates and steroid dependence rates were 3% and 4.5%, respectively.13 By comparison, our cohort's slightly higher blood loss (150 cc) and longer operative times (200 minutes) can be explained by our patient's higher rates of prior surgical procedures, the resection of multifocal adrenal lesions, as well as some added nuances of robotic surgery (i.e. docking times). In addition, the performance of partial nephrectomy with partial adrenalectomy in two instances further increased the hazard risk of our initial cohort. Despite these factors our complication rates and recurrence risks were similar to prior published reports.5, 15
Robot-assisted total adrenalectomy has emerged as a safe and feasible alternative to laparoscopic adrenalectomy.22-24 Two single case reports have described the use of robotic assistance for partial adenalectomy: one for the removal of a 9mm adrenal nodule, and another one for the resection of a 2 cm metastatic lesion to the adrenal.25, 26 Nevertheless, even with the results of our present series it is difficult to state whether robotic assistance has provided advantages over partial adrenalectomy performed using conventional laparoscopy. One potential advantage of RALPA may be the ability of the surgeon to operate with “two hands” utilizing the assistant to provide adequate exposure compared to using “one hand” for exposure and the other for dissection as with traditional laparoscopy. Additionally, robotic wristed articulation may also facilitate tumor removal with minimal mobilization and manipulation of the remaining adrenal gland, preserving the delicate blood supply and potentially maximizing its survival. Although we have performed partial adrenalectomy using conventional laparoscopy in the past we chose to not proceed with comparative analysis of RALPA with laparoscopic partial adrenalectomy because these analyses would be flawed with numerous unmatched variables including patient selection, tumor number and location, as well as the experience of the operating teams. We therefore report only on the safety and feasibility of RALPA in this early cohort of patients with adrenal masses at the NIH.
Performing partial adrenalectomy for pheochromocytoma in patients with bilateral, large, central tumors were the most challenging in our cohort both because of technical considerations related to tumor size and location as well as the critical importance of organ-sparing surgery in this high risk population. By incorporating knowledge of adrenal anatomy as well as our previous experience with partial adrenalectomy, the majority of these patients remained amenable to adrenal-sparing surgery through a minimally invasive approach. Figure 2 shows pre and post operative CT imaging of a VHL patient who underwent successful staged robot-assisted partial adrenalectomy for bilateral pheochromocytoma. Extirpation of these tumors originating from the adrenal medulla often required a “demedullation” technique, which is demonstrated in intraoperative Figure 3A. In each instance, robotic assistance was utilized to delicately dissect the tumor out from within its surrounding cortex, dividing small vessels with clips or bipolar in the process, minimizing excessive cautery, traction, or manipulation of the resection site. After tumor removal the remnant adrenal gland often appeared as small islands of tissue, each receiving blood supply from separate sources (Figure 3B). Although pheochromocytoma is classically central in its location, by maintaining the plane of dissection between the medulla and cortex the blood supply to the functionally normal adrenal cortical tissue can be preserved. In contrast, cortical hyperplasia, the other lesion treated in this cohort was located in either the medial or lateral limb of the gland. These were removed with limb amputation, leaving the majority of the remnant adrenal intact. Whether or not the early success of our robot-assisted partial adrenalectomy experience was impacted by these surgical strategies is unknown. Clearly, however, the appreciation of adrenal anatomy and the relationship of histology and location remain important as they have influenced our surgical technique and approach.
Figure 2.
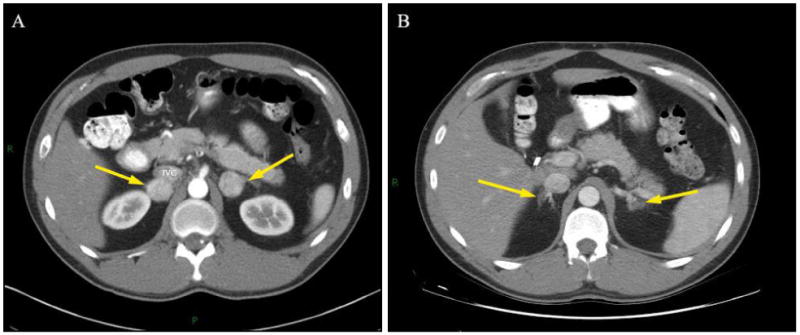
Computerized tomography of a patient with VHL undergoing staged bilateral RALPA. A, pre-operative image shows bilateral pheochromocytomas (arrows). B, post-operative image demonstrating enhancing normal remnant adrenal glands after demedullation (arrows).
Figure 3.
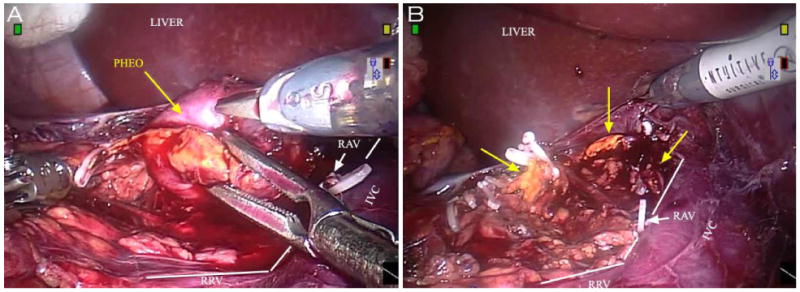
Robotic console view during right RALPA for pheochromocytoma. A, tumor extirpation B, islands of normal remnant adrenal tissue after extirpation (arrows)
Since the initial report of partial adrenalectomy from the NCI in 1999, we have been successfully performing this operation utilizing open, laparoscopic, and currently robot assisted techniques.7 Although the safety and feasibility of partial adrenalectomy in VHL patients have been established and reported in the past, long-term outcomes have not been examined. We recently reported our functional and oncologic outcomes of partial nephrectomy for pheochromocytoma in VHL patients after at least 5 years of follow up.27 Thirty-six partial adrenalectomies were performed in 26 patients with a median follow-up of 9.25 years. Partial adrenalectomy was successfully completed in all instances. A total of 3 patients (11%) developed 5 local recurrences. Long-term steroid replacement therapy was required in three patients (11%). All three of these patients had undergone contralateral adrenal surgery in the past. Six of eight patients (75%) undergoing partial adrenalectomy on a solitary adrenal gland remained steroid free at last follow-up. Based on these findings in the VHL population adrenal-sparing surgery should be the treatment of choice when technically feasible. As a subset analysis of that comprehensive review, robot-assisted techniques can be safely applied to this patient population to successfully perform tumor extirpation and gland preservation in a minimally invasive setting. Early results appear similar to our prior open and laparoscopic series.
The current study has inherent limitations. It is retrospective in nature and our sample size is small. Most patients in this study had an adrenal pheochromocytoma. This is a reflection of our practice patterns rather than a selection bias towards these specific lesions. Functional and oncologic outcomes are not mature and follow-up is limited. Additionally, a number of these patients underwent prior ipsilateral retroperitoneal surgery, concomitant renal surgery, or had multifocal adrenal masses potentially impacting our initial results. Also, a considerable experience of our team with performing these cases via the open or laparoscopic approach may have contributed to the early success of our series. Currently, we cannot definitively state that the robotic approach holds definitive advantages over traditional laparoscopic partial adrenalectomy. However, we have observed that the robotic approach is well suited for resection of adrenal tumors (especially pheochromocytoma) with decreased manipulation of the surrounding adrenal cortex, potentially improving preservation of blood supply to the adrenal remnant. Possible indications for RALPA may include central medullary tumors, partial adrenalectomy in a solitary adrenal gland, and repeat adrenal intervention. Longer follow-up will be necessary to confirm these early observations. Despite the limitations, this is the first series of robot assisted partial adrenalectomy in the literature to document feasibility as well as early functional and oncologic outcomes.
Conclusions
Robot-assisted partial adrenalectomy is safe and feasible in our early experience. Local recurrence and steroid replacement therapy appears to be minimal with limited follow up. Larger studies will need to be conducted to evaluate for potential differences in the outcomes between conventional laparoscopy and robotic assistance for adrenal sparing procedures.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walther MM, Reiter R, Keiser HR, et al. Clinical and genetic characterization of pheochromocytoma in von Hippel-Lindau families: comparison with sporadic pheochromocytoma gives insight into natural history of pheochromocytoma. J Urol. 1999;162:659. doi: 10.1097/00005392-199909010-00004. [DOI] [PubMed] [Google Scholar]
- 2.Diner EK, Franks ME, Behari A, et al. Partial adrenalectomy: the National Cancer Institute experience. Urology. 2005;66:19. doi: 10.1016/j.urology.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Neumann HP, Bender BU, Reincke M, et al. Adrenal-sparing surgery for phaeochromocytoma. Br J Surg. 1999;86:94. doi: 10.1046/j.1365-2168.1999.00974.x. [DOI] [PubMed] [Google Scholar]
- 4.Janetschek G, Finkenstedt G, Gasser R, et al. Laparoscopic surgery for pheochromocytoma: adrenalectomy, partial resection, excision of paragangliomas. J Urol. 1998;160:330. doi: 10.1016/s0022-5347(01)62886-6. [DOI] [PubMed] [Google Scholar]
- 5.Walther MM, Herring J, Choyke PL, et al. Laparoscopic partial adrenalectomy in patients with hereditary forms of pheochromocytoma. J Urol. 2000;164:14. [PubMed] [Google Scholar]
- 6.Oelkers W. Adrenal insufficiency. N Engl J Med. 1996;335:1206. doi: 10.1056/NEJM199610173351607. [DOI] [PubMed] [Google Scholar]
- 7.Walther MM, Keiser HR, Choyke PL, et al. Management of hereditary pheochromocytoma in von Hippel-Lindau kindreds with partial adrenalectomy. J Urol. 1999;161:395. [PubMed] [Google Scholar]
- 8.Boris R, Proano M, Linehan WM, et al. Initial experience with robot assisted partial nephrectomy for multiple renal masses. J Urol. 2009;182:1280. doi: 10.1016/j.juro.2009.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogers CG, Metwalli A, Blatt AM, et al. Robotic partial nephrectomy for renal hilar tumors: a multi-institutional analysis. J Urol. 2008;180:2353. doi: 10.1016/j.juro.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lupo J. Trend in adrenal surgery and adrenal cancer incidence and survival in the era of laparoscopic surgery. Journal of the American College of Surgeons. 2008;207 [Google Scholar]
- 11.Mantero F, Terzolo M, Arnaldi G, et al. A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J Clin Endocrinol Metab. 2000;85:637. doi: 10.1210/jcem.85.2.6372. [DOI] [PubMed] [Google Scholar]
- 12.Asari R, Scheuba C, Kaczirek K, et al. Estimated risk of pheochromocytoma recurrence after adrenal-sparing surgery in patients with multiple endocrine neoplasia type 2A. Arch Surg. 2006;141:1199. doi: 10.1001/archsurg.141.12.1199. [DOI] [PubMed] [Google Scholar]
- 13.Kaye DR, Storey BB, Pacak K, et al. Partial Adrenalectomy: Underused First Line Therapy for Small Adrenal Tumors. J Urol. 2010;184:18. doi: 10.1016/j.juro.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishidoya S, Ito A, Sakai K, et al. Laparoscopic partial versus total adrenalectomy for aldosterone producing adenoma. J Urol. 2005;174:40. doi: 10.1097/01.ju.0000162045.68387.c3. [DOI] [PubMed] [Google Scholar]
- 15.Walz MK, Peitgen K, Diesing D, et al. Partial versus total adrenalectomy by the posterior retroperitoneoscopic approach: early and long-term results of 325 consecutive procedures in primary adrenal neoplasias. World J Surg. 2004;28:1323. doi: 10.1007/s00268-004-7667-y. [DOI] [PubMed] [Google Scholar]
- 16.Nakada T, Kubota Y, Sasagawa I, et al. Therapeutic outcome of primary aldosteronism: adrenalectomy versus enucleation of aldosterone-producing adenoma. J Urol. 1995;153:1775. doi: 10.1016/s0022-5347(01)67303-8. [DOI] [PubMed] [Google Scholar]
- 17.Gagner M, Lacroix A, Bolte E. Laparoscopic adrenalectomy in Cushing's syndrome and pheochromocytoma. N Engl J Med. 1992;327:1033. doi: 10.1056/NEJM199210013271417. [DOI] [PubMed] [Google Scholar]
- 18.Shen ZJ, Chen SW, Wang S, et al. Predictive factors for open conversion of laparoscopic adrenalectomy: a 13-year review of 456 cases. J Endourol. 2007;21:1333. doi: 10.1089/end.2006.450. [DOI] [PubMed] [Google Scholar]
- 19.Vargas HI, Kavoussi LR, Bartlett DL, et al. Laparoscopic adrenalectomy: a new standard of care. Urology. 1997;49:673. doi: 10.1016/s0090-4295(97)00083-6. [DOI] [PubMed] [Google Scholar]
- 20.Jeschke K, Janetschek G, Peschel R, et al. Laparoscopic partial adrenalectomy in patients with aldosterone-producing adenomas: indications, technique, and results. Urology. 2003;61:69. doi: 10.1016/s0090-4295(02)02240-9. [DOI] [PubMed] [Google Scholar]
- 21.Liao CH, Chueh SC, Wu KD, et al. Laparoscopic partial adrenalectomy for aldosterone-producing adenomas with needlescopic instruments. Urology. 2006;68:663. doi: 10.1016/j.urology.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 22.Desai MM, Gill IS, Kaouk JH, et al. Robotic-assisted laparoscopic adrenalectomy. Urology. 2002;60:1104. doi: 10.1016/s0090-4295(02)02011-3. [DOI] [PubMed] [Google Scholar]
- 23.Krane LS, Shrivastava A, Eun D, et al. A four-step technique of robotic right adrenalectomy: initial experience. BJU Int. 2008;101:1289. doi: 10.1111/j.1464-410X.2008.07433.x. [DOI] [PubMed] [Google Scholar]
- 24.Brunaud L, Bresler L, Ayav A, et al. Robotic-assisted adrenalectomy: what advantages compared to lateral transperitoneal laparoscopic adrenalectomy? Am J Surg. 2008;195:433. doi: 10.1016/j.amjsurg.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 25.Rogers CG, Blatt AM, Miles GE, et al. Concurrent robotic partial adrenalectomy and extra-adrenal pheochromocytoma resection in a pediatric patient with von Hippel-Lindau disease. J Endourol. 2008;22:1501. doi: 10.1089/end.2007.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar A, Hyams ES, Stifelman MD. Robot-assisted partial adrenalectomy for isolated adrenal metastasis. J Endourol. 2009;23:651. doi: 10.1089/end.2008.0440. [DOI] [PubMed] [Google Scholar]
- 27.Benhammou JH, Boris RS, Pacek K, Pinto PA, Linehan WM, Bratslavsky G. Functional and oncologic outcomes of partial adrenalectomy for pheochromocytoma in VHL patients after at least 5 years of follow up. Journal of Urology. 2010 doi: 10.1016/j.juro.2010.06.102. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]


