Abstract
Background
Biliary Atresia (BA) is notable for marked ductular reaction and rapid development of fibrosis. Activation of the Hedgehog (Hh) pathway promotes the expansion of populations of immature epithelial cells that co-express mesenchymal markers and may be pro-fibrogenic. We examined the hypothesis that in BA excessive Hh activation impedes ductular morphogenesis and enhances fibrogenesis by promoting accumulation of immature ductular cells with a mesenchymal phenotype.
Methods
Livers and remnant extrahepatic ducts from BA patients were evaluated by QRT-PCR and immunostaining for Hh ligands, target genes, and markers of mesenchymal cells or ductular progenitors. Findings were compared to children with genetic cholestatic disease, age-matched deceased donor controls, and adult controls. Ductular cells isolated from adult rats with and without bile duct ligation were incubated with Hh ligand-enriched medium ± Hh-neutralizing antibody to determine direct effects of Hh ligands on EMT marker expression.
Results
Livers from pediatric controls showed greater innate Hh activation than adult controls. In children with BA, both intra- and extra-hepatic ductular cells demonstrated striking up-regulation of Hh ligand production, and increased expression of Hh target genes. Excessive accumulation of Hh-producing cells and Hh-responsive cells also occurred in other infantile cholestatic diseases. Further analysis of the BA samples demonstrated that immature ductular cells with a mesenchymal phenotype were Hh-responsive. Treating immature ductular cells with Hh ligand-enriched medium induced mesenchymal genes; neutralizing Hh ligands inhibited this.
Conclusions
BA is characterized by excessive Hh pathway activity, which stimulates biliary EMT and may contribute to biliary dysmorphogenesis. Other cholestatic diseases show similar activation, suggesting this is a common response to cholestatic injury in infancy.
Keywords: Pediatric liver disease, immature epithelial cells, biliary morphogenesis, Hedgehog Pathway, Cell differentiation
INTRODUCTION
The etiology, pathogenesis and pathophysiology of biliary atresia (BA) remain uncertain. Most cases appear to be acquired perinatally in a process thought to involve an abnormal immune response to viral infection leading to destruction of the extrahepatic bile ducts (1). The defining feature of BA is fibro-inflammatory destruction of the extrahepatic bile duct. However, the intrahepatic biliary system is also involved in the process. At the time of diagnosis liver biopsy shows expanded, fibrotic portal triads that contain increased numbers of bile duct profiles (ductal proliferation), some containing plugs of bile and prominent is the ductular reaction (increase in duct-like structures) at the limiting plate (2). Many BA patients will already have advanced biliary fibrosis or cirrhosis by 4-8 weeks of age. As there may be an intimate, even mechanistic relationship between the biliary dysmorphogensis and rapidly progressive fibrosis in BA, it is important to understand the pathways involved in order to begin to intervene in the process and to improve outcome.
The Hedgehog (Hh) pathway is critically important in organogenesis and tissue remodeling, and Hh activation appears to be involved in fibrogenesis in hepato-biliary disease (3, 4). Furthermore, Hh signaling regulates epithelial-mesenchymal transitions (EMT; expression of typically mesenchymal markers in epithelial cell types) of cholangiocytes in experimental obstructive cholestasis, and intrahepatic bile ducts in PBC patients show evidence of EMT (5). These findings suggest the importance of Hh activation and related EMT of duct and ductal cells in biliary diseases. Recent work has show that EMT is active in BA (6, 7). We have previously shown that apoptotic stress stimulates liver epithelial cells to produce Hh ligands (8) and that such cells release membranous particles that contain biologically active Hh ligands, which are capable of inducing expression of Hh target genes in Hh-responsive cells (9). Hh pathway activation was recently proven to inhibit TRAIL-mediated apoptosis in biliary cells (10, 11), which provides them a survival advantage at the cost of acquiring/retaining a more mesenchymal (and less epithelial) phenotype. Recent work showed that dsRNA viral infection, a presumed initiator of BA, induces TRAIL-mediated apoptosis in cultured biliary epithelial cells (12, 13). It is plausible, therefore, that immature ductular cells in infants with BA would have experienced a stimulus to increase production of Hh ligands and that surviving cells might provide a self-perpetuating source of Hh ligands to promote ductular EMT and fibrosis. Hence, we evaluated the relationship of Hh activation to EMT in human BA. The results show that hyperactive Hh signaling and ductular EMT are prominent features of BA and may be involved in the biliary dysmorphogenesis and fibrosis that are characteristic of the disease.
MATERIALS AND METHODS
Liver tissues
The tissues studied were drawn from banks of formalin-fixed paraffin embedded as well as liver samples snap-frozen immediately after they were obtained, maintained at Children's Memorial Hospital, Chicago IL, The Johns Hopkins Hospital, Baltimore MD, and Duke University Medical Center, Durham NC. They comprised liver specimens obtained at the time of liver transplantation from 12 BA patients (all acquired type, age range 5-23 months, median 20 months); 18 children with other cholestatic disease (7 progressive familial intra-hepatic cholestasis (PFIC) type 1 (FIC1 disease), 6 PFIC type 2 (BSEP disease), and 5 Allagille's syndrome (AGS)). Extrahepatic biliary remnants from 5 BA patients were obtained at the time of Kasai portoenterostomy. Normal control samples consisted of liver from 6 child-aged deceased donors (age range 8-28 months) and normal resected liver from 5 adults with metastatic colorectal cancer. Tissues were studied in accordance with NIH and institutional guidelines for research involving human subjects.
Primary cholangiocyte isolation and in vitro experiments
To determine whether or not the acquisition of a more mesenchymal phenotype in ductular cells was Hh-dependent, expression of the fibroblast marker Fibroblastic Specific Protein 1, FSP1 (14) was examined in primary culture of small (immature or less differentiated) and large (more differentiated) (16) cholangiocytes acquired from rats with and without bile duct ligation. In vitro manipulation of cholangiocytes was achieved by treatment with Hh ligand enriched conditioned medium collected from 6-day cultures of myofibroblasts. Cholangiocytes were isolated using a monoclonal antibody (from R. Faris, Brown University, Providence, RI) as described (17). Purity of cholangiocytes was confirmed by cytochemistry for γ-glutamyl transpeptidase (γGT). Freshly isolated cholangiocytes were incubated with MF conditioned medium in the presence of Hh neutralizing antibody (5E1, Developmental Studies Hybridoma Bank, Iowa City, IA) or control IgG (R&D)(10μg/ml). Normal freshly isolated cholangiocytes that were treated with unconditioned medium served as controls. Six hours later cell pellets or cytospins were harvested for subsequent mRNA and protein analysis respectively.
Molecular techniques
Real-time reverse-transcription polymerase chain reaction (RT-PCR) was performed using established protocols as previously described (5); details are in Supplemental Materials and Methods. High-quality total RNA was extracted from snap-frozen liver tissue from 9 BA patients, 18 non-BA cholestatic disease, 6 child-aged deceased donors, and 5 adult controls, as well as rat cholangiocytes.
Immunohistochemistry
Paraffin embedded formalin fixed liver specimens were used for immunohistochemistry examination as previously described (18); details are in Supplementary Materials and Methods and Supplemental Table 1.
Immunocytochemistry
Primary cholangiocytes after treatment were cyto-spun onto VWR superfrost® plus micro slides (VWR Int., USA) using the Shandon Cytospin 4 (Thermo Scientific, UK) at 600 rpm for 5min and examined as described in Supplemental Material and Methods.
Statistical Analysis
To evaluate the significance of changes in mRNA expression between groups, levels of target genes were compared and analyzed by using JMP 8.0.1 statistical software (SAS). Because data didn't follow a normal distribution, non-parametric tests were employed. Namely, after normalization to housekeeping gene, data were expressed as ratio to level of the target gene in the 6 child-aged control (CTL) specimens. Wilcoxon rank-sum test was then employed to assess the significance of differences in gene expression. QRT-PCR results were, thus, graphically depicted by using box-and-whisker plots to show the degree of dispersion. Single dots represent target gene level corresponding to each patient. The bottom and top of the black box are the 25th and 75th percentile while the band near the middle of the box correspond to the 50th percentile (the median). To further examine the covariance between selected genes, correlation and linear regression analyses were performed. Data were plotted as a scatter of points on a graph, and the regression line displayed with relevant significance (P value) and strength of correlation (coefficient of determination, r2) between the selected genes shown.
RESULTS
Hh activation and EMT are greater in child-aged liver than adult controls
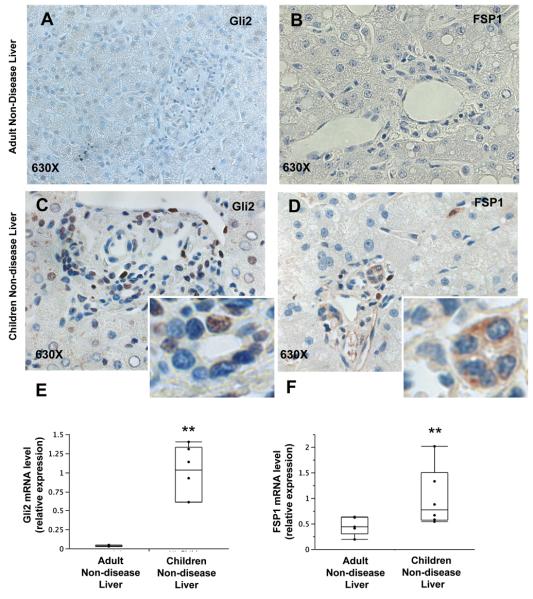
Liver sections from adult and child-aged controls were analyzed for expression of the Hh inducible gene product glioblastoma (Gli)-2 and a marker of fibroblastic cells, FSP1 (also called S100A4). Immunohistochemistry demonstrated greater expression of Gli2 in child-aged control liver than adult controls (Figure 1A, C, E) indicating innately more active Hh signaling, leading to greater numbers of Hh responsive cells in early life. This is to be expected given earlier evidence for Hh pathway activity along the ductal plates of developing livers (19), and the general role of Hh signaling in organogenesis (20). FSP1 expression was also greater in interlobular ductal cells in child-aged control liver than adult control liver (Figure 1B, D, F), demonstrating that ductular epithelial cells in children have a greater tendency to exhibit features of mesenchymal cells and implying that EMT is more active within portal tracts in children. These findings were confirmed by the greater mRNA expression of Gli2 and FSP1 in child-age liver than in liver of adult controls (Figure 1).
Figure 1. Liver from child-aged controls displays higher Hedgehog pathway activation and FSP1 expression compared to adult controls.
Non-disease liver (CTL) from adult and pediatric controls were analyzed at protein and gene levels for Gli2 and FSP1 expression. (A-D) IHC was performed in representative patients (N=2 per group) to localize the cell types expressing the Hedgehog transcription factor Gli2 (A, C) and the EMT marker FSP1 (B, D) at baseline. Final magnification 630X. (E-F) QRT-PCR was performed to quantitative differences in gene expression of Gli2 and FSP1 between child-aged (N=6) and adult (N=5) CTL livers. Data are displayed by box-and-whisker plots. Significance of difference in gene expression was evaluated by Wilcoxon rank-sum test. ** Significant Wilcoxon rank-sum test vs NL control.
Prominent Hh activation in biliary atresia and other neonatal cholestatic diseases
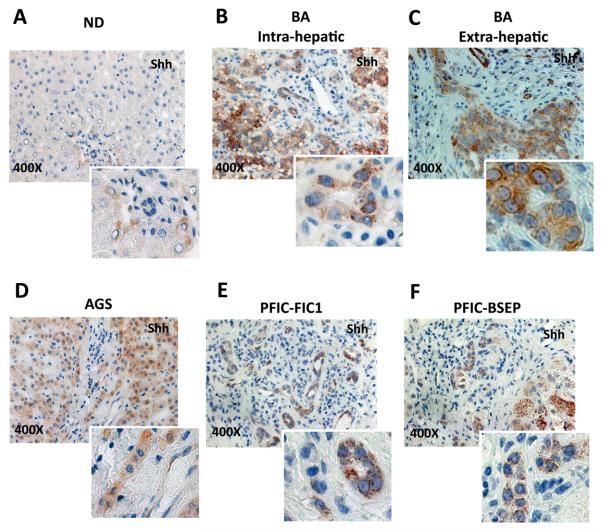
In order to determine if increased production of Hh ligands might provide a trigger for accumulation of Hh-responsive cells in the livers of children with BA and the specificity of this response, expression of Sonic hedgehog (Shh) ligand was examined in liver samples from BA patients and non-diseased (ND) age-matched controls using real time PCR and immunohistochemistry (IHC). Results in the BA group were also compared to Shh expression in the livers of children with FIC1 disease, BSEP disease, and Alagille's syndrome. In addition, paraffin-embedded remnant extrahepatic ducts from 5 BA patients were evaluated for Shh expression using IHC.
Livers from BA patients and non-BA cholestatic disease tended to express more Shh mRNA than livers from ND pediatric controls (Supplemental Figure 1). Disease-related focal induction of Shh production was more apparent when IHC was used to assess ligand expression (Figure 2). Compared to ND control livers, in which Shh-expressing cells were identified very infrequently, livers from patients with BA and all other cholestatic diseases demonstrated striking accumulation of Shh-expressing cells in and around portal tracts. Ductular cells in the extrahepatic biliary remnants from BA patients were also strongly Shh-positive (Figure 2C). Hence, it appears that various cholestatic insults in children up-regulate expression of Shh in residual ductular-type cells.
Figure 2. Up-regulation of Sonic Hedgehog ligand production in children with biliary atresia and other types of cholestatic liver disease.
Livers from patients with BA (N=12), children with various other types of cholestatic liver disease (AGS, FIC1 and BSEP, N=18), and age-matched controls without liver disease (nondiseased, ND, N=6), and all available extrahepatic biliary remnants from BA patients (N=5) were examined for Shh ligand production by IHC. Representative pictures at 400X of magnification, with inserts showing magnified views to facilitate visualization of Shh-producing cells.
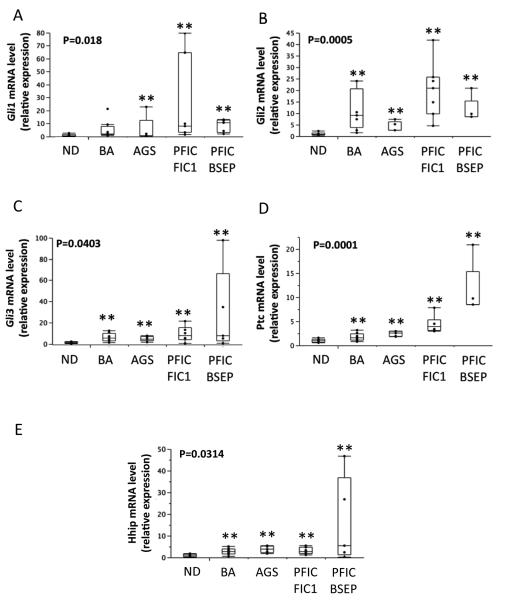
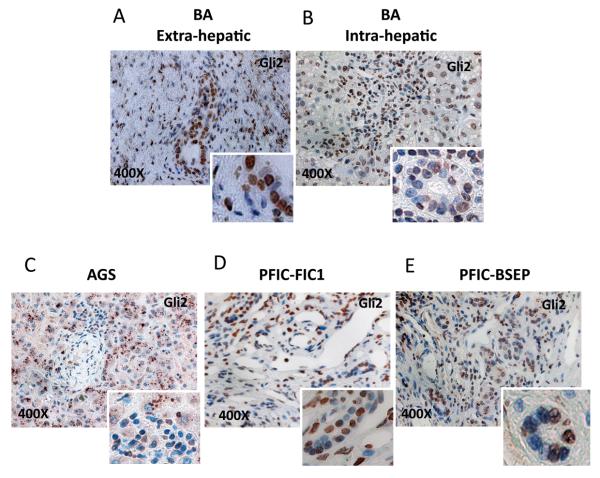
To assess the potential relevance of increased Hh ligand production, the mRNA expressions of several Hh target genes, Gli1, Gli2, Gli3, Ptc, and Hh interacting protein (Hhip), were determined by QRT-PCR in the same cohorts. Hh target gene expression was significantly increased in the livers of patients with BA and non-BA cholestatic disease (Figure 3). To confirm that such transcript increases were accompanied by changes in protein levels, immunohistochemistry was used to assess expression of Gli2, a transcription factor that is a Hh-target gene product. Populations of ductular-appearing cells and stromal cells in and around portal tracts were particularly enriched with Hh-responsive cells (i.e., cells with Gli2-positive nuclei) in all neonatal cholestatic diseases (Figure 4). The extrahepatic biliary remnants of BA patients also harbored large numbers of ductular and stromal cells that expressed Gli2 (Figure 4A). These findings were not observed in child-aged control livers, and together with the data shown in Figure 1, suggest that Hh activation in congenital biliary diseases occurs locally in the areas where diverse causes of cholestatic injury stimulate production of Hh ligands.
Figure 3. Up-regulation of Hedgehog target gene expression in children with biliary atresia and other types of cholestatic liver disease.
Patients with BA, children with various other types of cholestatic liver disease, and age-matched controls without liver disease (non diseased, ND), were examined for expression of the Hh-target genes. QRT-PCR analysis was performed in liver tissue from patients with BA (N=9), Alagille's syndrome (AGS, N=5), progressive familial intrahepatic cholestasis type 1 (FIC1, N=7), progressive intrahepatic cholestasis type 2 (BSEP, N=6), and age-matched CTL (N=6). (A-E) mRNA expression of Gli1, Gli2, Gli3, Ptc, and Hhip. Gene expression data in diseased livers are expressed relative to expression levels in control subjects and graphically depicted as box-and-whisker plots. Significance was evaluated by Wilcoxon rank-sum test. ** Significant Wilcoxon rank-sum test vs NL control.
Figure 4. Nuclear accumulation of Gli2 protein in children with biliary atresia and other types of cholestatic liver disease.
(A-D) Gli2 protein expression was confirmed by IHC analysis in all ND livers (Figure 1) and all available extrahepatic biliary remnants from BA patients (A), BA livers (B), and all livers from patients with AGS (C), FIC1 (D) and BSEP (E). Representative pictures at 400X of magnification, with inserts showing magnified views to facilitate visualization of cells with nuclear accumulation of Gli2 protein.
Evidence for EMT in BA
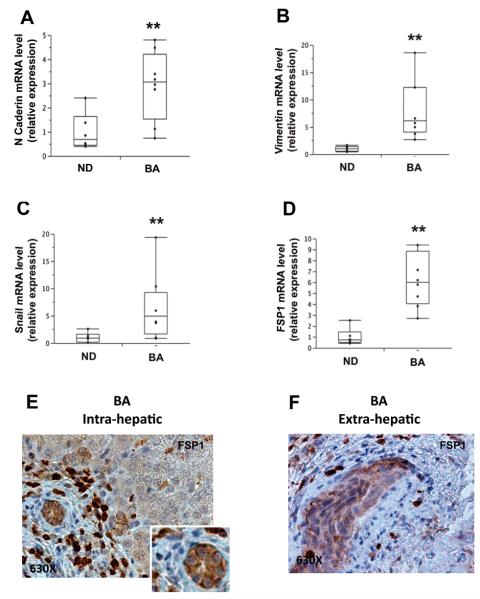
Other groups have already reported evidence for EMT in BA (6, 7). Therefore, to assess how Hh pathway activation might relate to EMT in pediatric biliary disease, we focused further analysis on tissue samples from BA patients and measured liver mRNA expressions of four established EMT markers, N-Cadherin, Vimentin, FSP1, and Snail. All showed significantly increased expression in BA relative to child-aged control liver (Figure 5) and were highly correlated with each other (Supplemental Figure 2A-C), confirming earlier reports of increased hepatic EMT in BA patients. Of substantial interest, FSP1 localized by immunohistochemistry both to cells with fibroblast-like morphology and to epithelial cells in interlobular duct structures (Figure 5E-F, Supplemental Figure 2D-E) and co-localized with the marker for immature or progenitor ductular epithelium, keratin 7 (KRT7), in epithelial cells in interlobular duct structures (Figure 6A, C). The extrahepatic biliary remnants from BA patients showed virtually identical findings (Figure 6B, D). The localization of FSP1 to KRT7-expressing cells within the biliary epithelium indicates the enrichment of ductular structures with immature ductal epithelial cells that have a relatively mesenchymal phenotype. The aggregate data, therefore, suggest that during the remodeling process in response to bile duct injury, epithelial-mesenchymal transitions occur and promote the accumulation of cells with a more mesenchymal (and less epithelial) phenotype.
Figure 5. Hedgehog activation in biliary atresia was accompanied by EMT in ductular cells.
(A-D) QRT-PCR analysis of EMT markers (i.e. N-Cadherin, Vimentin,, Snail, FSP1) in livers from pediatric patients with BA (N=9) and age-matched controls (CTL) (N=6). Gene expression data in disease livers are expressed relative to levels in control subjects and presented as box-and-whisker plots. Significance was evaluated by Wilcoxon rank-sum test. ** Significant Wilcoxon rank-sum test vs NL control. (E-F) Immunohistochemical analysis for FSP1 was performed in both livers (E) and extrahepatic biliary remnants (F) from BA patients. Final magnifications 630X (E-F).
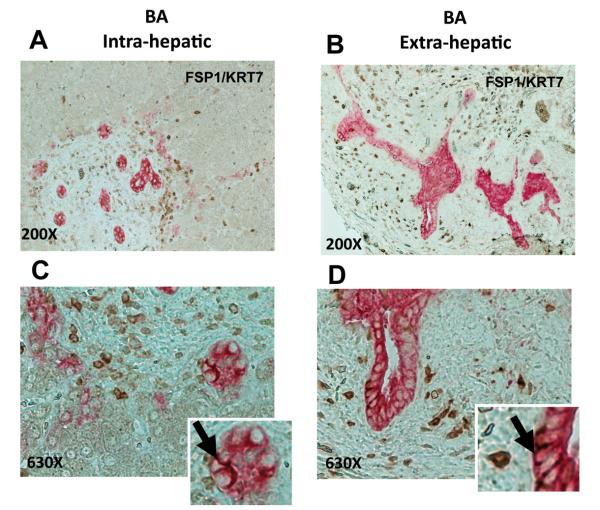
Figure 6. Expressions of mesenchymal marker (FSP1) and marker of immature liver epithelial cells (KRT7) co-localize in ductular epithelial of BA patients.
To further characterize the FSP1 expressing cells, additional double immunocytochemistry (A-D) for FSP1 (stained brown) and KRT7 (stained pink) was performed in the same sections. (A, C) FSP1(+)/KRT7(+) intrahepatic bile ducts of representative patients with BA. (B, D) Similar staining in the extrahepatic biliary remnants from representative BA patients. Final magnification 200X (A-B) and 630X (C-D). Inserts show magnified view of double positive cells in intrahepatic ducts (C) and extrahepatic biliary epithelium (D).
Relationship between Hh activity and EMT in BA
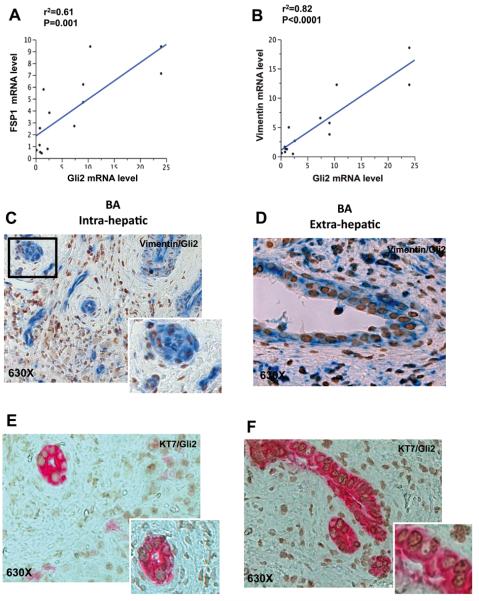
To determine to what extent Hh activity and EMT are related in BA, we examined the correlation/co-localization of the Hh transcription factor Gli2 with the mesenchymal cell markers vimentin and FSP1 in ductular cells within BA liver and extrahepatic bile duct remnants. Gli2 mRNA expression correlated closely with the expressions of both vimentin and FSP1 (Figure 7A, B). If EMT is dependent upon Hh activity as suggested by the close correlations of mRNA expressions, Gli2 should localize to ductular-appearing cells that express mesenchymal genes. Immunohistochemistry showed clear co-localization of Gli2 and vimentin to epithelial cells in interlobular duct structures (Figure 7C, Supplemental Figure 3A) and in extrahepatic biliary remnants (Figure 7D, Supplemental Figure 3B). Further IHC demonstrated nuclear localization of Gli2 in some of the KRT7-positive ductular-appearing cells within BA livers (Figure 7E, Supplemental Figure 3C) and extrahepatic biliary remnants (Figure 7F Supplemental Figure 3D). Taken together with the data in Figures 5 and 6, these findings suggest that Hh activation is a driving force that promotes EMT (thereby inhibiting MET) in BA, and that a major location of EMT in this disease is the Hh-responsive biliary epithelium that is enriched with relatively immature ductular cells.
Figure 7. Cells undergoing EMT in liver samples from BA patients are Hedgehog responsive.
(A-B) Covariance between the Hh target gene Gli2 and the EMT markers FSP1 and Vimentin was analyzed in patients with BA; data were plotted to demonstrate results of the linear regression analysis. Significance (P=value) and strength of correlation (coefficient of determination, r2) are indicated. (C-D) Double immunohistochemistry for Gli2 (brown) and Vimentin (blue) was performed to confirm that ductular cells acquiring mesenchymal markers were Hh responsive. Representative pictures of BA liver are presented at 630X (C), with magnified view in inserts. Similar staining results were observed in the extrahepatic biliary remnants of representative BA patients (D). Double immunohistochemistry for Gli2 (brown) and the progenitor marker, KRT7 (pink), were done in serial sections from the same cases. Gli2/KRT7 staining in intrahepatic bile ducts (E) and extrahepatic biliary remnants (F). Final magnifications 630X (E-F).
Relationships between Hh pathway activation, EMT, and accumulation of immature bile ductules in BA
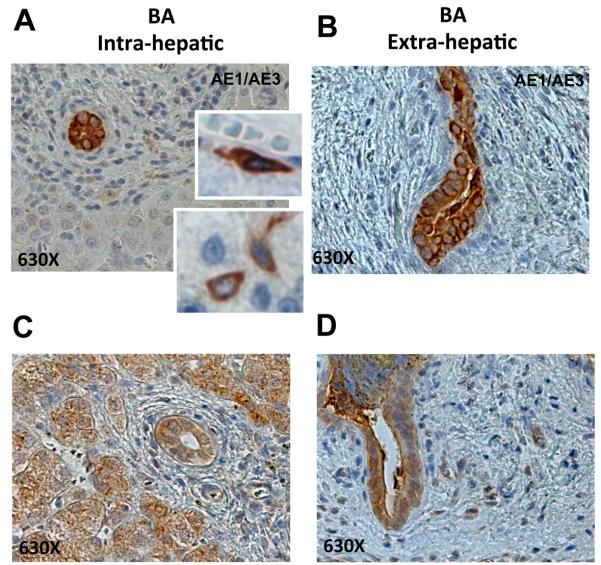
Hh signaling is a characteristic of immature liver cells, and pathway activity is progressively silenced during the differentiation process (19). In addition, cells that are undergoing EMT typically repress expression of epithelial markers, and the resultant inhibition of MET permits them to retain a more mesenchymal/migratory phenotype (21, 22). These data, together with the new evidence of nuclear Gli2 in subpopulations of KRT7 ductular cells in BA (Figure 7E-F), suggested to us that Hh responsive biliary cells with mesenchymal features might be relatively immature. In order to examine this possibility further, expression of AE1/AE3 (Figure 8A-B, Supplemental Figure 4A-B)) and EpCam (Figure 8C-D, Supplemental Figure 4C-D) were assessed by immunohistochemistry of sections from BA livers (Figure 8A, C, Supplemental Figure 4A,C) and extrahepatic biliary remnants (Figure 8B, D, Supplemental Figure 4B, D). Results demonstrated striking accumulation of cells that expressed these progenitor markers in ductular structures of BA livers and extrahepatic biliary remnants. Occasional MF-like AE1/AE3(+) stromal cells also localized immediately adjacent to intrahepatic ductular structures, further suggesting that epithelial progenitors with mesenchymal features accumulate in BA (Figure 8A). In addition to the intra- and extra-hepatic ductular cells that stained strongly for EpCam, hepatocytic-appearing cells in peri-portal areas were also positive for this stem/progenitor cell marker (Figure 8C).
Figure 8. EMT localizes to the progenitor cell compartment in human BA samples.
(A-D) Immunostaining for additional progenitor markers was performed to further assess the accumulation of immature ductular cells in BA livers (A, C) and extrahepatic biliary remnants (B, D). (A-B) AE1/AE3, (C-D) Epcam. Representative pictures are displayed at 630X. Higher magnification views are also provided in inserts to facilitate visualization of marker-positive cells.
Cholangiocytes exposed to Hh ligand undergo EMT
To gain mechanistic insight into the relationship between Hh signaling and EMT of biliary epithelium, normal adult primary cholangiocytes were exposed to Hh ligand enriched medium without and with antibody blockade of Hh signaling, with FSP1 expression as the marker for mesenchymal transition. Pooled samples of small and large cholangiocytes incubated in MF-conditioned medium and non-specific IgG (CTL IgG) expressed FSP1 mRNA approximately 8-fold greater than those exposed to fresh (unconditioned) medium containing CTL IgG (Supplemental Figure 5A). MF are known to be a rich source of Hh ligands (15, 23) and Hh ligand neutralizing antibody (5E1) reduced FSP1 mRNA expression by more than 50% (i.e., to approximately 3-fold control and less than half of cells treated with MF-conditioned medium + CTL IgG), identifying Hh ligand as the MF-derived soluble factor responsible for inducing FSP1 expression in the cholangiocytes. FSP1 protein expression in treated cholangiocytes was also assessed by specific antibody immunofluorescence. Cholangiocytes cultured in fresh (unconditioned) medium containing CTL IgG expressed essentially no FSP1 protein, whereas a subpopulation (~40%) of these cells acquired robust fluorescence for FSP1 when treated with MF-conditioned medium plus CTL IgG (Supplemental Figure 5B, C). Incubation with Hh ligand neutralizing antibody 5E1 reduced FSP fluorescence to near background low levels. Further analysis demonstrated that the subpopulation of small cholangiocytes from rats with ongoing biliary injury (i.e. BDL) was significantly more enriched with FSP1-expressing cells than the subpopulation of large cholangiocytes (Supplemental Figure 5D). These findings taken together indicate that Hh signaling induces small, relatively immature cholangiocytes to undergo EMT (and/or prevents them from completing MET), thereby causing them to acquire (or retain) some characteristics of myofibroblastic cells.
DISCUSSION
This work suggests that activation of Hh signaling is an important mechanism in the evolution of the biliary pathology in BA. In an evolving model of BA pathogenesis, viral infection of cells in the extrahepatic bilary tree is suspected to be the primary trigger for the development of BA (1), though the subsequent downstream events that lead to the full spectrum of BA pathology are not fully understood. In the model we propose, viral infection (or some other, yet-to-be-discovered insult) introduces an apoptotic stress within the extrahepatic biliary system (13, 24). In order for bile ducts to survive this injury, a remodeling process is initiated, which involves locally increased production of Hh ligands. Excessive exposure to these multi-potent morphogenic factors at critical time points during development, however, is counter-productive because it disrupts normal maturation of the extrahepatic biliary tree and promotes fibrogenesis. Hence, in BA, over-activation of the Hh pathway actually obstructs biliary drainage and incites intrahepatic cholestasis, which then triggers (and perpetuates) similar responses in the intrahepatic biliary tree.
Our results provide novel evidence for tremendous up-regulation of Hh ligand production in the damaged extrahepatic biliary remnants and intrahepatic bile ducts of patients with BA. These multipotent morphogenic factors are released locally, particularly when ligand-producing cells undergo apoptosis. The ligands released by stressed or dying cells then interact with Hh receptors on viable Hh-responsive cells, activating Hh signaling in these cells. Nuclear accumulation of the Hh-regulated transcription factor, Gli2, and increased expression of various other Hh-target genes, including Gli1, Ptc, and Hhip, provide evidence for hyperactive Hh signaling. While Hh pathway activation may enhance ductular epithelial cell survival, it may also interfere with epithelial maturation and result in accumulation of dysmorphic ductular structures that co-express various markers of immature duct cells and stromal cells, a characteristic pathologic finding in BA. Whether this occurs by regression of mature cholangiocytes to a mesenchymal phenotype (via stimulation of EMT) and/or decreased movement of mesenchymal progenitors into an epithelial phenotype (via inhibition of MET) remains to be explored. Both responses are known to result when Hh signaling increases. Hh activation may also lead to the accumulation of Hh-responsive stromal cells, which have the capacity to generate fibrous matrix. Hence, activation of the Hh pathway could occur in response to viral biliary infection, and could substantially contribute to biliary dysmorphogenesis and fibrosis. There is much to learn about the interplay of Hh signaling with the innate and adaptive immune systems that clearly have roles in the disease process. However, Hh activation in response to what may have been a minor viral infection may be the critical event that leads to perpetuation of biliary injury and amplification of the disease process through induction of EMT.
Our analysis of livers from children with several other types of neonatal cholestatic disease demonstrates that injury-related induction of Hh ligand production and resultant activation of Hh signaling in residual biliary epithelia are not unique to BA. Rather, these responses appear to be conserved mechanisms that are triggered by various, presumably diverse, causes of cholestatic injury to developing bile ducts. Hh pathway activation, ductular abnormalities, and progressive biliary fibrosis also occur in adults with immune-mediated biliary injury attributed to primary biliary cirrhosis or primary sclerosing cholangitis. (3) Taken together, these observations suggest that similar Hh-regulated mechanisms may be involved in biliary repair, while disease-related differences in cellular targets and duration of injury, plus individual differences in the capacity for Hh ligand production and/or susceptibility of residual cells to Hh pathway activators, dictate the ultimate outcome of the biliary damage.
Our data demonstrate greater Hh activation in child-age liver relative to adult liver, probably relating to ongoing organ development. Hedgehog signaling is thought to contribute to the maintenance of hepatic progenitors throughout life (19), and our data demonstrate that pathway activity is higher during post-natal development than in adulthood. In the process of liver repair and remodeling in BA, Hh signaling seems to be further activated. The Hh-responsive nuclear transcription proteins, Gli1-3, were expressed significantly more in the livers from BA patients than in child-age controls, indicating overall hyperactivity of Hh signaling in BA. Moreover, we showed by immunohistochemical localization that Shh ligand was greatly expressed in interlobular and extra-hepatic bile duct structures, where Gli2 showed nuclear localization. This focal hyper-expression of Shh in Hh responsive immature bile duct epithelial cells suggests the same cells that produce Shh respond to the signal it provides in an autocrine fashion. Gli2 was also expressed in fibroblastic-appearing cells in the periportal stroma. While one cannot say for certain, this suggests that the Shh signal emanating from remodeling interlobular bile ducts may act in paracrine fashion to induce Hh response just outside of the portal plate. We also showed increased expression of Ptc and Hhip in BA liver. This suggests an overall increase in Hh responsive cells, although it could also result from increased expression of these Hh receptors per responsive cell. In any case, the findings leave little doubt that Hh signaling is active in BA, and that it is focused in and around the portal triad containing remodeling bile duct structures. The possibility exists that the exuberant Hh activation seen in BA results in part because the system is primed from its already active state at this stage of human development. This may account for the remarkable bile ductular reaction seen in the disease.
The data acquired from study of the livers from children with BA support an association between Hh activation and EMT. Gli2 expression (marker for Hh activation) and expression of mesenchymal cell markers co-localized to the epithelium of interlobular duct structures, which in BA are prolific. As Hh contributes to remodeling and repair efforts in experimental and human biliary disorders (5, 15, 18, 26), finding it active during biliary tree remodeling in BA is not surprising. Finding Hh activation in association with EMT suggests that in the process of Hh-driven remodeling, the immature biliary epithelium adopts/retains a less mature, and more mesenchymal, phenotype. Mesenchymal cells in adult livers are capable of both responding to and producing Hh ligands (15, 23), and activation of Hh signaling in such cells contributes to fibrogenic repair of adult liver damage.(5, 15, 26-28) Thus, Hh-related EMT may contribute to fibrogenesis in BA both by providing Hh ligands, which function as paracrine signals to activate neighboring fibroblastic cells in the portal mesenchyme, and by stimulating epithelial cells themselves to acquire more mesenchymal characteristics, including matrix elaboration and remodeling.
It has long been recognized that the cells apparently contributing to fibrosis formation in BA are located within and closely around portal areas and have a myofibroblastic phenotype (29, 30). It was thought that these cells invade the area in response to inflammation (31). Other observations, however, suggest alternative mechanisms might also contribute to the expansion of myofibroblastic populations in BA. In an early study, collagen-producing fibroblastic cells accumulated near proliferating ductular structures (32). Subsequently, fibrosis-related cytokines, chemokines and matrix-remodeling proteins such as metaloproteinases were observed in ductular-type epithelial cells, themselves (31, 33, 34). The demonstration of EMT (i.e., co-expression of epithelial and mesenchymal markers) in ductular epithelium (6, 7) extended evidence that the ductular cells promoted fibrosis during BA, and represented an important paradigm shift in regard to potential origins of fibrosis-generating cells in this disease. These studies, however, could not resolve whether the observed enrichment of bile ductules with “transitioning” epithelial cells in BA reflected enhanced EMT of cholangiocytes or inhibited MET of less mature (and inherently more mesenchymal) cholangiocyte progenitors. In the present study, we were able to show that healthy adult liver-derived cholangiocytes treated with Hh ligand in vitro undergo EMT. Further analysis indicated that the sub-population of small cholangiocytes (which are known to harbor cholangiocyte progenitors) was most likely to express mesenchymal markers during cholestatic injury that activated Hh signaling in vivo. The aggregate data, therefore, suggest that activating Hh signaling in immature ductular cells promotes EMT, while repressing MET. Consequently, the cells acquire/retain a more mesenchymal (and less epithelial) phenotype in which they clearly elaborate fibrogenic factors (e.g., Hh ligands) that are capable of promoting the growth of neighboring fibroblastic cells. In addition, the Hh-responsive ductular cells themselves might produce matrix, since Hh ligands are known to up-regulate mesenchymal cell expression of various matrix-encoding genes.(5, 35) While as yet unproven, the latter concept merits consideration given recent work from Kaestner's group, which proved that arresting the epithelial differentiation of immature ductular cells led such cells to remain mesenchymal and elaborate collagen matrix.(36) Hence, our findings suggest that the association of Hh activation markers with mesenchymal markers in biliary epithelial cells in BA is not coincidence; rather it represents an important mechanism of the biliary dysmorphogenesis and progressive biliary fibrosis that characterize BA.
Supplementary Material
Patients with BA, children with various other cholestatic liver diseases, and age-matched controls without liver disease (nondiseased, ND), were examined for Shh ligand expression. QRT-PCR analysis was performed in liver tissue from patients with BA (N=9), Alagille's syndrome (AGS, N=5), progressive familial intrahepatic cholestasis type 1 (FIC1, N=7), progressive intrahepatic cholestasis type 2 (BSEP, N=6), and age-matched CTL (N=6). (A) Shh ligand mRNA expression. Gene expression data in diseased livers are expressed relative to levels in control subjects and graphically depicted using box-and-whisker plots. As evaluated by Wilcoxon rank-sum test, no significant differences in Shh expression were noted among the groups.
(A-C) Covariance between the EMT-driving gene, Snail, and two other typical mesenchymal markers, Vimentin and FSP1, was evaluated in BA patients. Data were plotted to demonstrate the results of linear regression analysis. Significance (P=value) and strength of correlation (coefficient of determination, r2) are shown. (D-E) Pictures demonstrating FSP1 staining in the liver (intrahepatic, D) and extrahepatic biliary remnant (E) of representative BA patients. Magnification 200X.
Immunohistochemical analysis for Gli2/Vimentin (A-B) and Gli2/KRT7 (C-D) was performed in both livers (A, C) and extrahepatic biliary remnants (B, D) from BA patients. Final magnifications 100X
Immunohistochemical analysis for AE1/AE3 (A-B) and Epcam (C-D) was performed in both livers (A, C) and extrahepatic biliary remnants (B, D) from BA patients. Final magnifications 200X
QRT-PCR (A) and immunocytochemistry (B) of pooled small and large primary cholangiocytes from healthy adult rats were used to assess FSP1. (A) FSP1 mRNA expression after treatment with unconditioned medium (white bars) containing control IgG or MF-conditioned with control IgG (black bars) or Hh neutralizing antibody (grey bars). (B) Representative cholangiocyte cytospins. Final Magnification 400X, inserts show magnified field of areas pointed by the arrows. (C) Quantification of FSP1 positive cholangiocytes. FSP1(+) (red bars) and FSP1 (−) (black bars) cholangiocytes were counted under under 200X final magnification. Per each field, FSP1 positive (i.e. red (+) and DAPI(+)) (red bars) and FSP1 negative (i.e. red (−) and DAPI(+)) were counted and the final data were expressed as percentage of total cells/field. (D) QRTPCR analysis of FSP1 expression in small (more immature) and large (mature) subpopulations of primary cholangiocytes isolated from rats with ongoing biliary fibrosis (i.e. BDL). Data are expressed relative to control group (white bars) and displayed as mean − Standard Error Mean (SEM). Comparisons between groups were performed using the two-tailed Student's t-test. Significance was accepted at the 5% level. (*P<0.05, ** P<0.005).
Table 1.
Markers of Hh signaling and EMT examined
| Name | Abbreviation | Function |
|---|---|---|
| Sonic Hedgehog | Shh | Hh ligand |
| Indian Hedgehog | Ihh | Hh ligand |
| Patched | Ptc | Cell surface receptor for Hh ligands promoting Hh response; marker for Hh responsiveness |
| Hedgehog interacting protein | Hhip | Hh-responsive gene product trans-membrane protein; marker for Hh responsiveness |
| Glioblastoma-1 | Gli1 | Hh-responsive gene product; transcription factor-Hh signal activator |
| Glioblastoma-2 | Gli2 | Hh-responsive gene product; transcription factor-Hh signal activator |
| Glioblastoma-3 | Gli3 | Hh-responsive gene product; transcription factor-Hh signal repressor |
| Fibroblast-specific protein 1 |
FSP1 or S100A4 |
Marker of fibroblastic transformation |
| Keratin 7 | KRT7 | Marker if immature biliary epithelium |
| N-Cadherin | N-Cadherin | Mesenchymal marker |
| Vimentin | Vimentin | Intermediate filament marker of EMT |
| Snail | Snail | Nuclear transcription factor regulating EMT |
| Epithelial cell adhesion molecule |
Epcam | Marker of epithelial progenitor status or carcinoma transformation |
| CD133 | CD133 | Transmembrane protein marker of progenitor cells |
| Cytokeratin AE1/AE3 |
AE1/AE3 | Marker of epithelial progenitor status |
ACKNOWLEDGMENTS
We thank F. Meng, Juliet Venter, Ayako Suzuki and Marzena Zdanowicz for their advice and technical support. We are also grateful to WC Stone for his administrative support to this study. The 5E1 antibody developed by Thomas M Jessel was obtained from the Developmental Studies Hybridoma Bank.
Financial Supports:
This work was supported by grants from the National Institutes of Health RO1 DK077794 (to AMD), R01DK080736 and R01DK081417 (to RAA). Portions of the study were supported by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology to G.A. from Scott & White Hospital and a VA Merit Award, and by funds provided by the Liver Foundation for Kids, Lemont IL.
List of Abbreviations
- BA
Biliary Atresia
- Epcam
epithelial cell adhesion molecule
- EMT
Epithelial to mesenchymal transition
- γGT
γ-glutamyl transpeptidase
- Gli
glioblastoma
- Hh
Hedgehog
- Hhip
Hh interacting protein
- KRT7
keratin 7
- KRT19
keratin 19
- MET
mesenchymal to epithelial transition
- MF
myofibroblasts
- PTC
Patched
- PBC
Primary biliary cirrhosis
Footnotes
Disclosure:
The authors have declared that no conflicts of interest exist
REFERENCES
- 1.Mack CL. The pathogenesis of biliary atresia: evidence for a virus-induced autoimmune disease. Semin Liver Dis. 2007;27:233–242. doi: 10.1055/s-2007-985068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brough AJ, Bernstein J. Conjugated hyperbilirubinemia in early infancy. A reassessment of liver biopsy. Hum Pathol. 1974;5:507–516. doi: 10.1016/s0046-8177(74)80003-1. [DOI] [PubMed] [Google Scholar]
- 3.Omenetti A, Diehl AM. The adventures of sonic hedgehog in development and repair. II. Sonic hedgehog and liver development, inflammation, and cancer. Am J Physiol Gastrointest Liver Physiol. 2008;294:G595–598. doi: 10.1152/ajpgi.00543.2007. [DOI] [PubMed] [Google Scholar]
- 4.Ominetti A, Diehl AM. Sonic Hedgehog Pathway. In: Dufour J-F, Clavien P-A, editors. Singaling Pathways in LIver Diseases. Springer-Verlag; Berlin Heidleberg: 2009. [Google Scholar]
- 5.Omenetti A, Porrello A, Jung Y, Yang L, Popov Y, Choi SS, Witek RP, et al. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. J Clin Invest. 2008;118:3331–3342. doi: 10.1172/JCI35875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diaz R, Kim JW, Hui JJ, Li Z, Swain GP, Fong KS, Csiszar K, et al. Evidence for the epithelial to mesenchymal transition in biliary atresia fibrosis. Hum Pathol. 2008;39:102–115. doi: 10.1016/j.humpath.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Harada K, Sato Y, Ikeda H, Isse K, Ozaki S, Enomae M, Ohama K, et al. Epithelial-mesenchymal transition induced by biliary innate immunity contributes to the sclerosing cholangiopathy of biliary atresia. J Pathol. 2009;217:654–664. doi: 10.1002/path.2488. [DOI] [PubMed] [Google Scholar]
- 8.Jung Y, Witek RP, Syn WK, Choi SS, Omenetti A, Premont R, Guy CD, et al. Signals from dying hepatocytes trigger growth of liver progenitors. Gut. 2010;59:655–665. doi: 10.1136/gut.2009.204354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witek RP, Yang L, Liu R, Jung Y, Omenetti A, Syn WK, Choi SS, et al. Liver cell-derived microparticles activate hedgehog signaling and alter gene expression in hepatic endothelial cells. Gastroenterology. 2009;136:320–330. doi: 10.1053/j.gastro.2008.09.066. e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurita S, Mott JL, Almada LL, Bronk SF, Werneburg NW, Sun SY, Roberts LR, et al. GLI3-dependent repression of DR4 mediates hedgehog antagonism of TRAIL-induced apoptosis. Oncogene. 2010 doi: 10.1038/onc.2010.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mott JL, Kobayashi S, Bronk SF, Gores GJ. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene. 2007;26:6133–6140. doi: 10.1038/sj.onc.1210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harada K, Sato Y, Isse K, Ikeda H, Nakanuma Y. Induction of innate immune response and absence of subsequent tolerance to dsRNA in biliary epithelial cells relate to the pathogenesis of biliary atresia. Liver Int. 2008;28:614–621. doi: 10.1111/j.1478-3231.2008.01740.x. [DOI] [PubMed] [Google Scholar]
- 13.Harada K, Sato Y, Itatsu K, Isse K, Ikeda H, Yasoshima M, Zen Y, et al. Innate immune response to double-stranded RNA in biliary epithelial cells is associated with the pathogenesis of biliary atresia. Hepatology. 2007;46:1146–1154. doi: 10.1002/hep.21797. [DOI] [PubMed] [Google Scholar]
- 14.Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol. 1995;130:393–405. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Omenetti A, Yang L, Li YX, McCall SJ, Jung Y, Sicklick JK, Huang J, et al. Hedgehog-mediated mesenchymal-epithelial interactions modulate hepatic response to bile duct ligation. Lab Invest. 2007;87:499–514. doi: 10.1038/labinvest.3700537. [DOI] [PubMed] [Google Scholar]
- 16.Alpini G, McGill JM, Larusso NF. The pathobiology of biliary epithelia. Hepatology. 2002;35:1256–1268. doi: 10.1053/jhep.2002.33541. [DOI] [PubMed] [Google Scholar]
- 17.Omenetti A, Syn WK, Jung Y, Francis H, Porrello A, Witek RP, Choi SS, et al. Repair-related activation of hedgehog signaling promotes cholangiocyte chemokine production. Hepatology. 2009;50:518–527. doi: 10.1002/hep.23019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung Y, McCall SJ, Li YX, Diehl AM. Bile ductules and stromal cells express hedgehog ligands and/or hedgehog target genes in primary biliary cirrhosis. Hepatology. 2007;45:1091–1096. doi: 10.1002/hep.21660. [DOI] [PubMed] [Google Scholar]
- 19.Sicklick JK, Li YX, Melhem A, Schmelzer E, Zdanowicz M, Huang J, Caballero M, et al. Hedgehog signaling maintains resident hepatic progenitors throughout life. Am J Physiol Gastrointest Liver Physiol. 2006;290:G859–870. doi: 10.1152/ajpgi.00456.2005. [DOI] [PubMed] [Google Scholar]
- 20.Ingham PW, Placzek M. Orchestrating ontogenesis: variations on a theme by sonic hedgehog. Nat Rev Genet. 2006;7:841–850. doi: 10.1038/nrg1969. [DOI] [PubMed] [Google Scholar]
- 21.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang L, Wang Y, Mao H, Fleig S, Omenetti A, Brown KD, Sicklick JK, et al. Sonic hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. J Hepatol. 2008;48:98–106. doi: 10.1016/j.jhep.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erickson N, Mohanty SK, Shivakumar P, Sabla G, Chakraborty R, Bezerra JA. Temporal-spatial activation of apoptosis and epithelial injury in murine experimental biliary atresia. Hepatology. 2008;47:1567–1577. doi: 10.1002/hep.22229. [DOI] [PubMed] [Google Scholar]
- 25.Zheng S, Luo Y, Wang W, Xiao X. Analysis of the pathomorphology of the intra- and extrahepatic biliary system in biliary atresia. Eur J Pediatr Surg. 2008;18:98–102. doi: 10.1055/s-2008-1038360. [DOI] [PubMed] [Google Scholar]
- 26.Omenetti A, Popov Y, Jung Y, Choi SS, Witek RP, Yang L, Brown KD, et al. The hedgehog pathway regulates remodelling responses to biliary obstruction in rats. Gut. 2008;57:1275–1282. doi: 10.1136/gut.2008.148619. [DOI] [PubMed] [Google Scholar]
- 27.Choi SS, Syn WK, Karaca GF, Omenetti A, Moylan CA, Witek RP, Agboola KM, et al. Leptin promotes the myofibroblastic phenotype in hepatic stellate cells by activating the hedgehog pathway. J Biol Chem. 2010 doi: 10.1074/jbc.M110.168542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi SS, Witek RP, Yang L, Omenetti A, Syn WK, Moylan CA, Jung Y, et al. Activation of Rac1 promotes hedgehog-mediated acquisition of the myofibroblastic phenotype in rat and human hepatic stellate cells. Hepatology. 2010;52:278–290. doi: 10.1002/hep.23649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shirahase I, Ooshima A, Tanaka K, Yamabe H, Inomata Y, Ozawa K. Immunohistochemical demonstration of collagen types III and IV and myofibroblasts in the liver of patients with biliary atresia. J Pediatr Surg. 1994;29:639–644. doi: 10.1016/0022-3468(94)90730-7. [DOI] [PubMed] [Google Scholar]
- 30.Ramm GA, Nair VG, Bridle KR, Shepherd RW, Crawford DH. Contribution of hepatic parenchymal and nonparenchymal cells to hepatic fibrogenesis in biliary atresia. Am J Pathol. 1998;153:527–535. doi: 10.1016/S0002-9440(10)65595-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petersen M, Drews U, Schweizer P. Induction of bile ducts in embryonic liver by mesenchyme: a new perspective for the treatment of biliary atresia? Eur J Pediatr Surg. 2001;11:382–390. doi: 10.1055/s-2001-19731. [DOI] [PubMed] [Google Scholar]
- 32.de Freitas LA, Chevallier M, Louis D, Grimaud JA. Human extrahepatic biliary atresia: portal connective tissue activation related to ductular proliferation. Liver. 1986;6:253–261. doi: 10.1111/j.1600-0676.1986.tb00288.x. [DOI] [PubMed] [Google Scholar]
- 33.Al-Masri AN, Flemming P, Rodeck B, Melter M, Leonhardt J, Petersen C. Expression of the interferon-induced Mx proteins in biliary atresia. J Pediatr Surg. 2006;41:1139–1143. doi: 10.1016/j.jpedsurg.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 34.Faiz Kabir Uddin Ahmed A, Ohtani H, Nio M, Funaki N, Iwami D, Kumagai S, Sato E, et al. In situ expression of fibrogenic growth factors and their receptors in biliary atresia: comparison between early and late stages. J Pathol. 2000;192:73–80. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH657>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 35.Choi SS, Omenetti A, Witek RP, Moylan CA, Syn WK, Jung Y, Yang L, et al. Hedgehog pathway activation and epithelial-to-mesenchymal transitions during myofibroblastic transformation of rat hepatic cells in culture and cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1093–1106. doi: 10.1152/ajpgi.00292.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Z, White P, Tuteja G, Rubins N, Sackett S, Kaestner KH. Foxa1 and Foxa2 regulate bile duct development in mice. J Clin Invest. 2009;119:1537–1545. doi: 10.1172/JCI38201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patients with BA, children with various other cholestatic liver diseases, and age-matched controls without liver disease (nondiseased, ND), were examined for Shh ligand expression. QRT-PCR analysis was performed in liver tissue from patients with BA (N=9), Alagille's syndrome (AGS, N=5), progressive familial intrahepatic cholestasis type 1 (FIC1, N=7), progressive intrahepatic cholestasis type 2 (BSEP, N=6), and age-matched CTL (N=6). (A) Shh ligand mRNA expression. Gene expression data in diseased livers are expressed relative to levels in control subjects and graphically depicted using box-and-whisker plots. As evaluated by Wilcoxon rank-sum test, no significant differences in Shh expression were noted among the groups.
(A-C) Covariance between the EMT-driving gene, Snail, and two other typical mesenchymal markers, Vimentin and FSP1, was evaluated in BA patients. Data were plotted to demonstrate the results of linear regression analysis. Significance (P=value) and strength of correlation (coefficient of determination, r2) are shown. (D-E) Pictures demonstrating FSP1 staining in the liver (intrahepatic, D) and extrahepatic biliary remnant (E) of representative BA patients. Magnification 200X.
Immunohistochemical analysis for Gli2/Vimentin (A-B) and Gli2/KRT7 (C-D) was performed in both livers (A, C) and extrahepatic biliary remnants (B, D) from BA patients. Final magnifications 100X
Immunohistochemical analysis for AE1/AE3 (A-B) and Epcam (C-D) was performed in both livers (A, C) and extrahepatic biliary remnants (B, D) from BA patients. Final magnifications 200X
QRT-PCR (A) and immunocytochemistry (B) of pooled small and large primary cholangiocytes from healthy adult rats were used to assess FSP1. (A) FSP1 mRNA expression after treatment with unconditioned medium (white bars) containing control IgG or MF-conditioned with control IgG (black bars) or Hh neutralizing antibody (grey bars). (B) Representative cholangiocyte cytospins. Final Magnification 400X, inserts show magnified field of areas pointed by the arrows. (C) Quantification of FSP1 positive cholangiocytes. FSP1(+) (red bars) and FSP1 (−) (black bars) cholangiocytes were counted under under 200X final magnification. Per each field, FSP1 positive (i.e. red (+) and DAPI(+)) (red bars) and FSP1 negative (i.e. red (−) and DAPI(+)) were counted and the final data were expressed as percentage of total cells/field. (D) QRTPCR analysis of FSP1 expression in small (more immature) and large (mature) subpopulations of primary cholangiocytes isolated from rats with ongoing biliary fibrosis (i.e. BDL). Data are expressed relative to control group (white bars) and displayed as mean − Standard Error Mean (SEM). Comparisons between groups were performed using the two-tailed Student's t-test. Significance was accepted at the 5% level. (*P<0.05, ** P<0.005).