Abstract
Aim
Impaired heart rate (HR) response to exercise is associated with increased cardiovascular morbidity and mortality. We analyzed whether common variants (rs5443/C825T and rs5442/G814A) in the G-protein β3 subunit (GNB3) gene modulate interindividual variation in β-blocker responses with respect to HR.
Materials & methods
Among 1614 subjects (347 current β-blocker users) of a population-based study, HR during symptom-limited exercise testing was analyzed by multilevel linear regression models adjusted for potential confounders.
Results
In β-blocker users, but not in nonusers, HR was attenuated in rs5443 T allele carriers (TC/TT vs CC) with lower adjusted HR over the entire exercise period from rest to peak workload (3.5 bpm; 95% CI: 1.1–5.8; p < 0.01), and during recovery (4.2 bpm; 95% CI: 0.6–7.8; p = 0.02). The genotype-related HR reducing effect at peak exercise varied by up to 7.5 bpm (CC vs TT), more than a third (35.9%) of the total β-blocker effect (20.9 bpm). By contrast, rs5442 had no impact on any HR-related parameter.
Conclusion
In this population-based sample, a common GNB3 polymorphism (C825T) was significantly related with response to β-blocker therapy with respect to HR during exercise and HR recovery, respectively. Further prospective studies are needed to confirm these associations and to examine their potential clinical relevance.
Keywords: β-blocker, epidemiology, exercise testing, genetic susceptibility, GNB3, heart rate, polymorphism
β-blockers are among the most frequently prescribed cardiovascular drugs and are a pillar in the treatment of chronic heart failure and post-myocardial infarction. In accordance with current guidelines more than 90% of all patients with myocardial infarction in the USA are discharged from hospital with β-blockers [1]. While some trials suggest administering the highest tolerated β-blocker dose in these patients [2], former and current concepts still highlight heart rate (HR) reduction as main predictor for the beneficial effects of chronic β-blocker therapy irrespective of dosage, and suggest individualized dose-titration regimens [3–5].
The use of β-blockers as first-line therapy for uncomplicated hypertension, however, is discussed controversially [6,7], since an association with a greater risk of cardiovascular events has been reported [6]. Furthermore, hypotension and bradycardia – among other adverse effects – contribute to the declining adherences to β-blocker therapy and to dosage reductions below recommendations [5,8]. In this context, perioperative β-blockade in noncardiac surgery has recently been challenged by the Postischemic Evaluation Study (POISE) trial, where the advantage of lower incidence rates of nonfatal myocardial infarctions was diminished by an increase of adverse events during follow-up (higher incidences of stroke, hypotension, bradycardia and increased all-cause mortality) [9].
Heart rate control, particularly under exercise conditions, is a highly complex physiological process. Genetic influences and environmental factors affect interindividual variability [10]. Identification of genetic factors contributing to resting HR, HR regulation during exercise and HR recovery in the presence of β-blocker therapy may have an impact on dose finding in general practice, which is commonly based on an empirical titration process, and could facilitate the differentiation of subjects that will benefit from individuals at risk for adverse events.
In this context, a common variant (rs5443; C825T) in the G-protein β3 subunit (GNB3) gene is a promising candidate gene for modulation of HR control under β-blocker therapy. Heterotrimeric G-proteins are key signal transducers for a large family of membrane receptors, such as adrenoceptors, the muscarinic acetylcholine receptors, and those for angiotensin II, endothelin, bradykinin and vasopressin [11]. GNB3 variants have been associated with different cardiovascular and metabolic disorders and have been shown to affect pharmacologic response to different drugs [12–15]. Several reports have related GNB3 C825T to varying α- and β-adrenergic responses [14,16–18], antihypertensive effects of β-blockers [19] and altered autonomous functions [18,20].
Using a large population-based sample of Caucasian adults, the purpose of this study was to investigate whether common GNB3 variants modulate HR control in the presence of β-blocker therapy during exercise testing.
Materials & methods
Participants
The design of the population-based Study of Health in Pomerania (SHIP) has been published previously [21]. Briefly, SHIP is a population-based study representative of the population in the northeast area of Germany. A total of 4310 baseline subjects were examined between 1997 and 2001. The study was approved by the ethics committee of the University of Greifswald, and all participants gave informed written consent. The first follow-up examination (SHIP-1) was conducted 5 years (median: 5.04 ± 0.62 years) after baseline and comprised of 3300 subjects (83.6% which are still living subjects).
During March 2003 and October 2006, 1707 subjects volunteered once for exercise testing. A total of 41 subjects (2.4%) were excluded because of missing GNB3 genotypes owing to lack of DNA, objections to DNA analyses or genotyping failures. Furthermore, we excluded individuals with pacemakers (n = 7), atrial fibrillation (n = 14), uncertainty regarding β-blocker medication (n = 1), and moderately or severely limited left ventricular function (n = 30) defined by an echocardiographic fractional shortening less than 22% in women and less than 20% in men [22]. There were no heart transplanted patients among the final study sample that included data of 1614 participants (94.6% of those undergoing exercise testing).
Clinical data
Sociodemographic characteristics and medical histories were assessed by computer-aided personal interviews. Hypertension was defined as a resting systolic blood pressure (BP) of 140 mmHg or more, a diastolic BP of 90 mmHg or more or use of antihypertensive medication. As for smoking status, participants were classified as never smoker, exsmoker or current smoker. They were considered physically active if they participated in physical training for at least one hour a week [23]. Height and weight were measured for the calculation of BMI (weight [kg]/height² [m²]). Medication, including doses was recorded by a computer-aided method using the Anatomical Therapeutic Chemical Classification System® (ATC) [101]. Daily β-blocker equal doses were calculated based on the daily-defined-dose concept of the ATC using the most common β-blocker agent among our sample, bisoprolol, as reference [101]. All affected subjects were on the current β-blocker doses for at least 7 days, defined as chronic β-blocker therapy in our study.
Genotyping
DNA was extracted from whole blood, and the GNB3 C825T (rs5443) polymorphism was analyzed as recently described [24]. The rs5442 polymorphism was genotyped by the SNPlex® assay [25]. The genotyping laboratories were unaware of any clinical data of the participants.
Genetic variance at the GNB3 locus
The GNB3 gene is short, encompassing approximately 7210 nucleotides. It is represented by one genetic block, and the entire genetic variance is limited. Most association studies on GNB3 focused on the functional C825T polymorphism (rs5443) [12,26]. In Caucasians, the C825T polymorphism is in almost complete linkage disequilibrium with five other polymorphisms (rs589231113, rs11064426, rs2301339, rs13306405 and rs5446) (Supplementary Figure 1; www.futuremedicine.com/doi/suppl/10.2217/pgs.10.88) [26]. Together, they constitute two principal haplotypes commonly termed C and T, following the nomenclature of the C825T key polymorphisms [26]. rs5442 (G814A), located adjacent to rs5443 is another common GNB3 variant occurring at a minor allele frequency of 5–10% in Caucasians (6.16% in our study population). The A allele of rs5442 occurs virtually in exclusive combination with the rs5443 C allele, while the frequent rs5442 G allele manifests with both rs5443 alleles. This allows the subdivision into T (rs5442G–rs5443T), C1 (rs5442G–rs5443C), and C2 (rs5442A–rs5443G) haplotypes (Supplementary Figure 1). The functional significance of the rs5442 variant has not been addressed so far, although it contains an amino acid exchange in a highly conserved motif present in the Gβ1–Gβ4 family. However, when we subdivided into C1 and C2 haplotypes, findings of this study did not vary. Thus, we present solely the results for the rs5443 C825T polymorphism in this article. The Supplementary material lists further detailed data (see www.futuremedicine.com/doi/suppl/10.2217/pgs.10.88).
Exercise testing
Maximal symptom-limited exercise testing was performed using electromagnetically braked cycle (Ergoselect 100, Ergoline, Bitz, Germany) according to the Jones protocol (stepwise increases in workload of 16 Watt/min, starting with unloaded cycling plus the ergometer-related permanent load) [27]. Electrocardiographic monitoring was performed continuously. HR and BP were obtained at rest, at the end of each stage of exercise and during the first 3 min of recovery.
Chronotropic response
The primary aim of the study was to investigate HR during exercise. For that purpose, each subject's exercise test was divided into five consequent periods of equal duration (rest, 20, 40, 60, 80 and 100% of maximal workload) [28]. Maximal workload was defined as the workload during the last completely finished stage of exercise and age-predicted maximal workload was calculated by the routine algorithm of the ergometer based on standard equations [29]. Peak HR was defined as the maximal HR achieved. Age-predicted maximum HR was estimated by the equation 220 – age [30], which is frequently used in exercise studies [28,31,32]. This formula is also the only one that has been applied in context of β-blocker therapy in a large epidemiological study [31]. Since its validity in different populations has been controversially discussed [33], we additionally applied the equation 205.8 − 0.685 × age [34], which has been proposed as the most accurate general formula [33]. The percentage of maximum age-predicted HR was calculated as the ratio between peak HR and predicted maximum HR for both equations separately. HR reserve was calculated as the difference between maximal age-predicted HR and resting HR. The chronotropic index was calculated as the percentage of HR reserve used at peak exercise (peak HR – resting HR)/(HR reserve) [31,32].
Statistical analyses
Descriptive comparisons between genotypes were made using χ²-test (nominal data) and one-way analysis of variance (ANOVA) (continuous data) with Bonferroni's post hoc test. Values are given as means ± standard deviation (SD) or absolute numbers (percentages). Two-level multilevel linear regression models, using the xtreg command of the statistical software package STATA, with timepoints at level one and individuals at level two, were fitted to estimate the contribution of the genotypes on HR response during exercise (rest, 20, 40, 60, 80 and 100% of maximal workload), and on HR recovery (maximal workload, 1, 2 and 3 min of recovery). This approach is advantageous as it accounts for the correlated data structure across timepoints. Analyses were stratified by β-blocker use. A first model controlled for age and gender. Subsequently, the influence of a large set of confounders was tested: physical activity, BMI, hypertension, systolic and diastolic BP, smoking status, crucial medication (e.g., calcium-channel blockers, angiotensin-converting enzyme [ACE] inhibitors, angiotensin receptor blockers, digitalis and amiodarone), and a summative index of comorbidities (including stroke, diabetes mellitus, chronic obstructive pulmonary disease [COPD], myocardial infarction, asthma, peripheral vascular disease, liver diseases, osteoporosis, gout, rheumatic disorders, thyroid disorders and depression). For β-blocker users, models were additionally controlled for β-blocker doses. Control variables other than gender and age were removed in a backward stepwise strategy when the p-value for single parameters was more than 0.20. The following variables were retained in the model during exercise: age, sex, systolic and diastolic BP, hypertension, smoking status, use of ACE inhibitors and digitalis, and β-blocker doses among β-blocker users. In addition to these variables amiodarone use was retained in the model during recovery.
Because estimates for GNB3 genotype differed very little between the age- and gender-controlled model and the full covariate model, only the latter is presented. Furthermore, adding comorbidities separately into the regression models instead of the global comorbidity index did not affect our results. Wald tests were applied to test multiparameter effects concerning the dummy-coded GNB3 variable (CC genotype as reference category) and its interaction with time. An autoregressive covariance structure was applied to model dependencies between measurements.
To assess the sensitivity of our results to systematic nonresponse in exercise testing and recovery we additionally applied statistical weights. The weights accounted for systematic differences in participants and nonparticipants of exercise testing regarding the variables presented in Table 1.
Table 1.
Baseline characteristics of subjects without and with exercise testing.
| Characteristic | No exercise testing (n = 1415) |
Exercise testing (study population) | ||||
|---|---|---|---|---|---|---|
|
All subjects (n = 1614) |
p-value† |
No β-blocker (n = 1267) |
β-blocker (n = 347) |
p-value‡ | ||
| Age, mean (SD) | 56.1 (16.6) | 51.9 (13.5) | <0.001 | 49.6 (13.2) | 60.1 (11.2) | <0.001 |
| Women, n (%) | 663 (46.9) | 828 (51.3) | 0.325 | 648 (51.1) | 180 (51.9) | 0.810 |
| Diabetes mellitus, n (%) | 190 (13.4) | 116 (7.2) | <0.001 | 50 (3.9) | 54 (15.6) | <0.001 |
| Hypertension, n (%) | 809 (57.2) | 759 (47.1) | <0.001 | 438 (34.6) | 290 (83.9) | <0.001 |
| Myocardial infarction, n (%) | 70 (4.9) | 42 (2.6) | 0.001 | 11 (0.9) | 31 (8.9) | <0.001 |
| Stroke, n (%) | 49 (3.5) | 24 (1.5) | 0.902 | 12 (0.9) | 12 (3.5) | 0.001 |
| Asthma bronchiale, n (%) | 55 (3.9) | 37 (2.3) | 0.009 | 30 (2.4) | 7 (2.0) | 0.705 |
| COPD, n (%) | 93 (6.6) | 67 (4.1) | 0.002 | 49 (3.9) | 18 (5.2) | 0.280 |
| Smoking status | 0.022 | <0.001 | ||||
| – Never smoker, n (%) | 558 (39.7) | 703 (43.6) | 536 (42.3) | 168 (48.6) | ||
| – Exsmoker, n (%) | 447 (31.8) | 513 (31.8) | 377 (29.8) | 136 (39.3) | ||
| – Current smoker, n (%) | 399 (28.4) | 396 (24.6) | 354 (27.9) | 42 (12.1) | ||
| Physically active, n (%) | 157 (11.1) | 274 (17.0) | <0.001 | 589 (46.5) | 139 (40.2) | 0.036 |
| BMI (kg/m2), mean (SD) | 28.1 (5.1) | 27.7 (4.7) | 0.018 | 28.0 (4.3) | 28.1 (4.8) | 0.836 |
| Blood pressure (mmHg) | ||||||
| – Systolic, mean (SD) | 135 (22) | 130 (18) | <0.001 | 129 (17) | 137 (19) | 0.001 |
| – Diastolic, mean (SD) | 81 (11) | 82 (10) | 0.596 | 81 (11) | 82 (11) | 0.854 |
| Resting heart rate, mean (SD) | 69.7 (11.0) | 78.4 (13.3) | <0.001 | 69.6 (9.8) | 62.7 (9.6) | <0.001 |
| Fractional shortening (%), mean (SD) | 37.5 (7.4) | 37.7 (7.6) | 0.231 | 37.6 (7.6) | 38.4 (8.0) | 0.110 |
| GNB3 C825T genotype | 0.605 | 0.873 | ||||
| – CC | 674 (47.6) | 757 (46.9) | 600 (47.3) | 157 (45.3) | ||
| – TC | 593 (41.9) | 694 (43.0) | 543 (42.9) | 151 (43.5) | ||
| – TT | 148 (10.5) | 163 (10.1) | 124 (9.8) | 39 (11.2) | ||
| Medication | ||||||
| β-blocker, n (%) | 390 (27.6) | 347 (21.5) | <0.001 | 0 (0.0) | 347 (100.0) | <0.001 |
| Calcium-channel blocker | <0.001 | <0.001 | ||||
| – Diltiazem or verapamil, n (%) | 35 (2.5) | 11 (0.7) | 10 (0.8) | 1 (0.3) | ||
| – Dihydropyridine, n (%) | 130 (9.2) | 95 (5.9) | 50 (3.9) | 45 (13.0) | ||
| Digitalis, n (%) | 52 (3.7) | 17 (1.1) | 0.995 | 7 (0.6) | 10 (2.9) | <0.001 |
| Amiodarone, n (%) | 5 (0.4) | 1 (0.1) | <0.001 | 0 (0.0) | 1 (0.3) | 0.056 |
| ACE inhibitor, n (%) | 314 (22.2) | 252 (15.6) | <0.001 | 119 (9.4) | 133 (38.3) | <0.001 |
| Angiotensin receptor blocker, n (%) | 91 (6.4) | 88 (5.59) | 0.306 | 40 (3.2) | 49 (14.1) | <0.001 |
| Any antiobstructive drug | 86 (6.1) | 69 (4.3) | 0.035 | 52 (4.1) | 17 (4.9) | 0.510 |
| – β-adrenoceptor agonist, n (%) | 31 (2.2) | 29 (1.8) | 0.164 | 25 (2.0) | 4 (1.2) | 0.311 |
| – β2-adrenoceptor agonist, n (%) | 2 (0.1) | 0 (0.0) | 0.889 | 0 (0.0) | 0 (0.0) | 1.000 |
| – α-adrenoceptor antagonist, n (%) | 21 (1.5) | 4 (0.2) | <0.001 | 0 (0.0) | 4 (1.2) | <0.001 |
Given values are unadjusted. Only subjects without exclusion criteria for the analyses are considered.
Subjects without versus with exercise data.
No β-blocker versus β-blocker among subjects with exercise data (χ2-test or one-way ANOVA test with Bonferroni's post-hoc test, as appropriate).
ACE: Angiotensin-converting enzyme; ANOVA: Analysis of variance; COPD: Chronic obstructive pulmonary disease; SD: Standard deviation.
Estimated HR values are given as adjusted means and 95% CI. A p-value of less than 0.05 was considered statistically significant. Analyses were performed with STATA 10 (StataCorp LP, College Station, TX, USA) and SPSS software version 15.0.1 (SPSS Inc., IL, USA).
Results
Baseline characteristics
Table 1 presents baseline characteristics of subjects without (nonparticipants) and with exercise testing (study population). Nonparticipants were older, had a higher frequency of comorbidities (diabetes mellitus, hypertension, myocardial infarction, asthma bronchiale and COPD) and a higher frequency of taking medication (including β-blockers). Moreover, they were more frequently current smokers, more often physically inactive, had a higher BMI and a lower resting HR (Table 1).
Among the study population of 1614 participants (828 women) with exercise data, 347 (180 women) had a current β-blocker medication, and 1267 had not. There were no statistically significant differences between subjects with and without β-blocker therapy regarding the genotype frequencies of GNB3 rs5443 (Table 1) and GNB3 rs5542 (AA: 0.0 and 0.7%; AG: 11.2 and 11.0%; GG: 87.7 and 80.7%; p = 0.788), respectively. Genotype distributions were in accordance with a Hardy–Weinberg equilibrium (p = 0.84 and p = 0.87, respectively) as well as in line with previous data for German populations [26,35].
The distribution of β-blocker agents and daily equal doses did not differ between the GNB3 C825T genotypes (Table 2). Stratified analyses with respect to β-blocker usage did not reveal any significant associations between the GNB3 C825T genotype and baseline characteristics with the exception of a slightly lower systolic BP at rest in TT allele carriers among β-blocker users (Table 3). As already indicated, GNB3 G814A genotypes were not associated with any of the baseline characteristics in both groups (Supplementary Material).
Table 2.
β-blocker agents, daily doses and GNB3 C825T genotype.
| β-blocker agent | CC (n = 157) | TC (n = 151) | TT (n = 39) |
|---|---|---|---|
| Atenolol, n (%) | 4 (2.5) | 8 (5.3) | 3 (7.7) |
| Metoprolol, n (%) | 3 (1.9) | 1 (0.7) | 1 (2.6) |
| Metoprolol succinate or tartrate, n (%) | 46 (29.3) | 40 (26.5) | 8 (20.5) |
| Bisoprolol, n (%) | 71 (45.2) | 68 (45.0) | 21 (53.8) |
| Carvedilol, n (%) | 3 (1.9) | 8 (5.3) | 0 (0.0) |
| Celiprolol, n (%) | 1 (0.6) | 3 (2.0) | 3 (7.7) |
| Talinolol, n (%) | 7 (4.5) | 5 (3.3) | 1 (2.6) |
| Propanolol, n (%) | 5 (3.2) | 2 (1.3) | 0 (0.0) |
| Betaxolol, n (%) | 1 (0.6) | 1 (0.7) | 0 (0.0) |
| Nebivolol, n (%) | 12 (7.6) | 15 (9.9) | 0 (0.0) |
| Sotalol, n (%) | 4 (2.5) | 0 (0.0) | 2 (5.1) |
| Daily β-blocker equal dose (mg)†, mean (SD) | 5.3 (3.6) | 5.3 (3.7) | 5.8 (3.3) |
Calculations of daily β-blocker equal dose for bisoprolol based on the daily defined-dose concept using the ATC code [101]. No statistical differences between genotype groups (χ2-test or one-way ANOVA test, as appropriate)
ANOVA: Analysis of variance; SD: Standard deviation.
Table 3.
Baseline characteristics of subjects with exercise data (study population) according to β-blocker use and GNB3 C825T genotype.
| Characteristic | No β-blocker | β-blocker | ||||||
|---|---|---|---|---|---|---|---|---|
|
CC (n = 600) |
TC (n = 543) |
TT (n = 124) |
p-value for trend† |
CC (n = 157) |
TC (n = 151) |
TT (n = 39) |
p-value for trend | |
| Age, mean (SD) | 49.2 (13.1) | 50.6 (13.1) | 49.2 (13.4) | 0.165 | 60.6 (11.8) | 59.5 (10.5) | 61.0 (11.2) | 0.612 |
| Women, n (%) | 302 (50.3) | 284 (52.3) | 62 (50.0) | 0.773 | 79 (50.3) | 80 (53.0) | 21 (53.8) | 0.866 |
| Diabetes mellitus, n (%) | 21 (3.5) | 27 (5.0) | 2 (1.6) | 0.165 | 22 (14.0) | 22 (14.6) | 10 (26.3) | 0.155 |
| Hypertension, n (%) | 208 (34.7) | 188 (34.6) | 42 (33.9) | 0.985 | 128 (81.5) | 130 (86.1) | 32 (82.1) | 0.537 |
| Myocardial infarction, n (%) | 4 (0.7) | 6 (1.1) | 1 (0.8) | 0.725 | 15 (9.6) | 11 (7.3) | 5 (12.8) | 0.521 |
| Stroke, n (%) | 5 (0.8) | 4 (0.7) | 3 (2.4) | 0.201 | 4 (2.5) | 5 (3.3) | 3 (7.7) | 0.287 |
| Asthma bronchiale, n (%) | 12 (2.2) | 18 (3.3) | 0 (0.0) | 0.065 | 2 (1.3) | 4 (2.6) | 0 (0.0) | 0.461 |
| COPD, n (%) | 20 (3.3) | 27 (5.0) | 3 (2.4) | 0.238 | 9 (5.7) | 8 (5.3) | 1 (2.7) | 0.756 |
| Smoking status | 0.503 | 0.562 | ||||||
| – Never smoker, n (%) | 258 (43.0) | 231 (42.5) | 47 (37.9) | 72 (45.9) | 77 (51.0) | 19 (50.0) | ||
| – Exsmoker, n (%) | 184 (30.7) | 151 (27.8) | 42 (33.9) | 61 (38.9) | 59 (39.1) | 16 (42.1) | ||
| – Current smoker, n (%) | 158 (26.3) | 161 (29.7) | 35 (28.2) | 24 (15.3) | 15 (9.9) | 3 (7.9) | ||
| Physically active, n (%) | 283 (47.2) | 247 (45.5) | 59 (47.6) | 0.823 | 56 (35.7) | 65 (43.0) | 18 (47.4) | 0.264 |
| BMI (kg/m2), mean (SD) | 28.1 (4.1) | 27.9 (4.5) | 27.9 (4.1) | 0.780 | 28.3 (4.8) | 27.9 (4.8) | 27.7 (4.7) | 0.605 |
| Fractional shortening (%), mean (SD) | 37.7 (7.7) | 37.6 (7.4) | 37.1 (7.3) | 0.780 | 38.3 (7.6) | 38.7 (8.4) | 37.1 (7.4) | 0.616 |
| Medication | ||||||||
| Calcium-channel blocker | 0.412 | 0.618 | ||||||
| – Diltiazem or verapamil, n (%) | 7 (1.2) | 3 (0.6) | 0 (0) | 0 (0) | 1 (0.7) | 0 (0) | ||
| – Dihydropyridine, n (%) | 21 (3.5) | 22 (4.1) | 7 (5.6) | 20 (12.7) | 22 (14.6) | 3 (7.7) | ||
| Digitalis, n (%) | 2 (0.4) | 4 (0.7) | 1 (0.8) | 0.494 | 6 (3.8) | 3 (2.0) | 1 (2.6) | 0.624 |
| Amiodarone, n (%) | 0 (0) | 0 (0) | 0 (0) | 1.000 | 1 (0.6) | 0 (0) | 0 (0) | 0.545 |
| ACE inhibitor, n (%) | 54 (9.0) | 52 (9.6) | 13 (10.5) | 0.859 | 59 (37.6) | 60 (39.7) | 14 (35.9) | 0.878 |
| Angiotensin receptor blocker, n (%) | 14 (2.3) | 24 (4.4) | 2 (1.6) | 0.077 | 22 (14.0) | 19 (12.6) | 8 (20.5) | 0.477 |
| Any antiobstructive drug | 24 (4.0) | 24 (4.4) | 4 (3.2) | 0.820 | 10 (6.4) | 5 (3.3) | 2 (5.3) | 0.460 |
| – β-adrenoceptor agonist | 9 (1.5) | 16 (2.9) | 0 (0.0) | 0.054 | 3 (1.9) | 1 (0.7) | 0 (0.0) | 0.461 |
| – β2-adrenoceptor agonist | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 |
| – α-adrenoceptor antagonist | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 | 3 (1.9) | 1 (0.7) | 0 (0.0) | 0.461 |
| Exercise-related parameters | ||||||||
| Exercise duration (min), mean (SD) | 9.6 (3.1) | 9.2 (3.1) | 9.6 (2.8) | 0.058 | 7.9 (2.6) | 7.9 (2.6) | 7.6 (3.0) | 0.823 |
| Maximal power load (W), mean (SD) | 162 (50) | 155 (51) | 162 (46) | 0.052 | 134 (43) | 134 (43) | 131 (48) | 0.887 |
| Percent of age-predicted workload, mean (SD) | 109 (27) | 110 (30) | 111 (27) | 0.742 | 108 (30) | 104 (31) | 109 (34) | 0.505 |
| Resting HR (bpm), mean (SD) | 81.2 (13.2) | 80.2 (12.8) | 80.2 (11.6) | 0.415 | 71.2 (12.0) | 69.7 (12.7) | 66.3 (8.9) | 0.072 |
| Peak HR (bpm), mean (SD) | 157.3 (19.3) | 155.4 (19.7) | 157.4 (18.8) | 0.238 | 129.4 (23.4) | 126.1 (20.9) | 121.2 (22.0) | 0.100 |
| Percent of age-predicted HR†, mean (SD) | 92 (9) | 92 (9) | 92 (8) | 0.817 | 81 (13) | 79 (12) | 76 (12) | 0.074 |
| Percent of age-predicted HR‡, mean (SD) | 91 (9) | 91 (10) | 91 (9) | 0.553 | 79 (13) | 76 (11) | 74 (12) | 0.058 |
| Chronotropic index, mean (SD)‡ | 0.85 (0.18) | 0.85 (0.19) | 0.85 (0.16) | 0.853 | 0.66 (0.22) | 0.62 (0.19) | 0.59 (0.19) | 0.075 |
| Chronotropic index, mean (SD)§ | 0.84 (0.18) | 0.82 (0.18) | 0.84 (0.16) | 0.580 | 0.63 (0.22) | 0.59 (0.19) | 0.56 (0.19) | 0.108 |
| Systolic resting BP (mmHg), mean (SD) | 120 (16) | 121 (16) | 120 (5) | 0.749 | 123 (18) | 126 (17) | 118 (14) | 0.017 |
| Diastolic resting BP (mmHg), mean (SD) | 85 (11) | 85 (10) | 86 (11) | 0.686 | 85 (12) | 87 (12) | 84 (12) | 0.136 |
| Systolic peak BP (mmHg), mean (SD) | 199 (29) | 196 (27) | 200 (28) | 0.147 | 192 (28) | 191 (29) | 188 (26) | 0.693 |
| Diastolic peak BP (mmHg), mean (SD) | 92 (14) | 91 (13) | 91 (14) | 0.665 | 89 (15) | 90 (15) | 92 (17) | 0.582 |
Given values are unadjusted.
Age-predicted HR calculated by the formula: 205.8−0.685 × age [34]. (Separate analyses for subjects with β-blockers and without β-blockers.)
Comparisons between GNB3 C825T genotypes were made separately for subjects with β-blockers and without β-blockers, respectively, by χ2-test (nominal data) or one-way ANOVA test with Bonferroni's post-hoc test (continuous data).
Age-predicted HR calculated by the formula: 220–age [30].
ACE: Angiotensin-converting enzyme; ANOVA: Analysis of variance; BP: Blood pressure; bpm: Beats per min; COPD: Chronic obstructive pulmonary disease; HR: Heart rate; SD: Standard deviation; W: Watt.
Exercise data
Both exercise duration and maximal workload were lower in subjects with β-blockers compared with those without β-blockers (7.9 ± 2.7 min versus 9.4 ± 3.1 min and 134.0 ± 43.2 Watt (W) versus 158.9 ± 50.2 W; p < 0.001, respectively). These differences were still present after adjustment for age and gender (8.8 min, 95% CI: 8.6–9.0 versus 9.2 min, 95% CI: 9.1–9.3; p = 0.002, and 148.1 W, 95% CI: 144.4–151.7 versus 155.0 W, 95% CI: 153.2–156.8; p = 0.001).
Stratified analyses with respect to the β-blocker status did not reveal any statistically significant differences between the GNB3 genotypes for exercise duration, maximal power load, age predicted workload reached and age-predicted HR reached (Table 3).
Use of β-blockers, GNB3 polymorphisms, HR response & chronotropic incompetence
Compared with participants without β-blockers, those with β-blockers had a lower HR at any timepoint of the exercise regardless of the GNB3 genotype (p < 0.001). After adjustment for all selected confounders the maximum HR at peak exercise was 134.1 bpm (95% CI: 131.8–136.4) in β-blocker users, and 155.0 bpm (95% CI: 154.2–155.8) in non-users (p < 0.001). Thus, the maximum achieved β-blocker effect on HR reduction was 20.9 bpm.
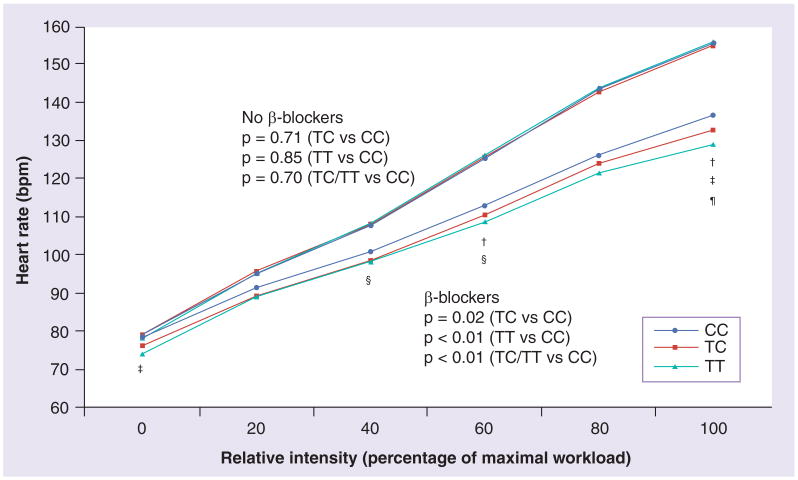
Among subjects without β-blockers, GNB3 genotypes were not associated with HR during any stage of exercise (Figure 1). By contrast, GNB3 genotypes were consistently related to HR during exercise among subjects with β-blockers (Figure 1). Thus, subjects with TC and TT genotype exhibited a reduced HR compared with carriers of the CC genotype. The average HR over the entire exercise period from rest to peak workload was 2.9 bpm (95% CI: 0.4–5.4, p = 0.02) lower in subjects with the TC genotype, and 5.8 bpm (95% CI: 1.9–9.7, p < 0.01) lower in subjects with the TT genotype compared with the CC genotype. Testing heterozygote and homozygous T allele carriers together (recessive genetic model), revealed a 3.5 bpm (95% CI: 1.1–5.8, p < 0.01) lower average HR compared with noncarriers with the T allele.
Figure 1. β-blocker use, GNB3 C825T genotype and heart rate response during exercise.
Full model adjusted for age, sex, systolic and diastolic blood pressure, hypertension, smoking status, use of angiotensin-converting enzyme inhibitors and digitalis, and β-blocker doses among β-blocker users.
p-values at specific time points of exercise (multilevel linear regression model).
†p < 0.05; ‡p < 0.05; §p < 0.05; ¶p < 0.01 (TC/TT vs CC).
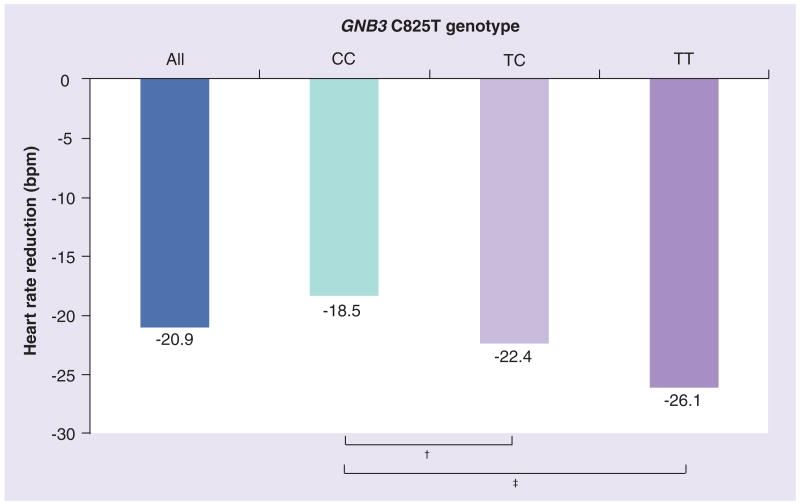
Heart rate differences between GNB3 genotypes were largest at peak exercise. The adjusted HR was 4.7 bpm (95% CI: 0.7–8.7, p = 0.02) lower in subjects with the TC genotype, and 7.5 bpm (95% CI: 1.2–13.9, p = 0.02) lower in subjects with the TT genotype in comparison to the CC genotype. This equaled approximately 35.9% of the maximum β-blocker effect observed (20.9 bpm) between the entire population of β-blocker users and the entire population of the nonusers of β-blocker therapy. Figure 2 illustrates the maximum effects of β-blocker therapy on HR reduction for all β-blocker users as well as stratified for the GNB3 genotype in comparison to the maximum HR in subjects without β-blockers.
Figure 2. Heart rate reduction at peak exercise in.
β-blocker users compared with nonusers. Heart rate reduction at peak exercise in β-blocker users compared with nonusers for all subjects (blue column) and stratified by GNB3 genotype (green column for CC, light purple column for TC and purple column for TT). Values are means adjusted for age, sex, systolic and diastolic blood pressure, hypertension, smoking status, use of angiotensin-converting enzyme inhibitors and digitalis, and β-blocker doses among β-blocker users.
†p < 0.05; ‡p < 0.01 (multilevel linear regression model).
In addition, compared with subjects with the CC genotype (0.66, 95% CI: 0.63–0.70), the adjusted chronotropic index tended to be lower in subjects with the TC (0.62, 95% CI: 0.59–0.65) and the TT (0.59, 95% CI: 0.53–0.66) genotype. This difference was borderline statistically significant after controlling for the resting HR (TT/TC vs CC; p = 0.05).
Again, HR and chronotropic incompetence were not affected by the GNB3 G814A polymorphism, neither if analyzed as single variant, or upon subdifferentiation of the GNB3 C haplotype (Supplementary Material).
HR recovery
Similar to HR during exercise, there was an association between the GNB3 genotype and HR during recovery among participants with β-blockers, but not among those without β-blockers (Figure 3). The average HR during recovery in subjects with the TC and the TT genotype was 4.2 bpm (95% CI: 0.3–8.2, p = 0.03), and 4.3 bpm (95% CI: 1.3–9.8, p = 0.13) lower compared with the CC genotype. Overall, T allele carriers (TT/TC) had a 4.2 bpm (95% CI: 0.6–7.8, p = 0.02) lower HR compared with noncarriers during recovery. These effects were largest at the beginning of the recovery phase, and were independent from peak HR during exercise (Figure 3). Again, GNB3 G814A genotype was not significantly associated with HR during recovery (Supplementary Material).
Figure 3. β-blocker use, GNB3 C825T genotype and heart rate recovery.
Full model: adjusted for age, sex, systolic and diastolic blood pressure, hypertension, smoking status, use of angiotensin-converting enzyme inhibitors and digitalis, and β-blocker doses among β-blocker users. p-values at specific time points of exercise (multilevel linear regression model).
†p < 0.05; ‡p < 0.05; §p < 0.05, ¶p < 0.01 (TC/TT vs CC).
Discussion
Our study is one of the first that addresses issues of pharmacogenomics in a population-based survey. We present evidence that a common GNB3 variant, rs5443 C825T, is associated with HR during rest and any stage of exercise testing in subjects with β-blocker therapy. In particular, the T allele was associated with lower HR during both exercise and recovery. HR differences related to GNB3 were particularly pronounced at peak exercise. The genotype-related variance in HR reduction of more than 7.5 bpm between the TT and the CC genotype at peak exercise equaled to more than a third of the total β-blocker effect of 20.9 bpm that was observed in the entire population of β-blocker users compared with the entire group of nonusers.
The potential impact of GNB3 on hemodynamic regulation has been corroborated with some evidence in previous studies [14,16–18,36–39]. Recently, a study in 79 young healthy subjects reported a greater HR response during a cold pressure test in homozygous carriers of the GNB3 835T allele [18]. Two other studies have suggested that GNB3 variants may modulate β-blockers effects [17, 19]. Schäfers et al. reported an association of the GNB3 T allele with acute hemodynamic effects after administration of propanolol in 71 young healthy males, including lower total peripheral resistances and higher cardiac indices [17]. Another study demonstrated genotype-specific differences of a chronic β-blocker therapy on BP lowering with stronger effects in T allele carriers [19].
Interestingly, in our study HR during rest and exercise was not modulated by GNB3 genotypes in β-blocker-naive subjects, which is in line with a number of previous studies on resting HR [15,40]. By contrast, resting HR was lower in T allele carriers compared with homozygous CC carriers (57.4 ± 1.9 vs 61.1 ± 1.7 bpm) as seen in the study by Schäfers et al. [17]. Similar findings were reported for resting HR in the Hypertension and Ambulatory Recording Venetia (HARVEST) study among 461 untreated hypertensives aged 18–45 years (76.4 ± 0.7 bpm in CC genotypes vs 73.2 ± 1.6 bpm in TT genotypes) [41], although these differences did not reach statistical significance. In addition to our study, the Health, Risk factors, exercise Training and Genetics (HERITAGE) Family Study was the only one to address genotype-dependent HR response during exercise in normotensives without medication [42]. In this study, contrary to our results, the CC genotype was associated with a lower resting HR and a greater training-induced reduction in HR in 255 black subjects. However, there were no differences between the CC, TC and TT genotypes with respect to resting HR (64.0 ± 1.0, 64.4 ± 1.0 and 63.6 ± 1.7 bpm; p = 0.821) or HR during a submaximal cycle ergometer test at 50 W (117.4 ± 1.8, 116.4 ± 2.1 and 113.3 ± 2.4 bpm; p = 0.156) in 473 Caucasians. Thus, these findings are in line with those observed in our study for β-blocker-naive subjects at submaximal exercise stages.
The potential molecular mechanisms underlying our findings remain to be established. Available evidence suggests that the GNB3 825T allele – a silent polymorphism with regard to the amino acid sequence – favors alternative splicing, ultimately resulting in two protein variants termed Gβ3s and Gβ3s225. The pronounced expression of these splice variants in context of the T allele is associated with increased signal transduction [12,14,16,26,38,43]. Because G proteins are involved in the signaling cascades of more than a thousand different receptors, complex phenotypes in association with the Gb3 rs5443 alleles have been described [15]. While for agonists straight forward hypotheses on the actions of the 825T allele may be constructed, chronic effects of an antagonist like chronic β-receptor blockers are difficult to predict. Long-term administration of β-blockers results in enhanced β-adrenoceptor expression [44]. The ratio of β1- and β2-receptor changes and receptor-mediated sensitization processes are influenced by G proteins [11]. It may be speculated that in exercise testing with increasing levels of catecholamines and long-term administration of β-blockers, the degree of G-protein-mediated signaling is most likely to governs the levels of cellular and physiologic effects. Furthermore, long-term β-blockade affects not only the adrenergic system but also central brain and parasympathetic systems. Therefore, a role for effective signal transduction linked to the rs5443 polymorphism appears plausible.
Moreover, it has to be taken into account that classes of β-blockers differ with regard to the degree of β1-selectivity, intrinsic sympathomimetic activity or parallel α1-blocking effects. The vast majority of β-blocker agents used in our study were metoprolol and bisoprolol. Although the distribution of β-blockers between the GNB3 genotypes was not statistically different, we cannot completely exclude that this might have influenced our results given the high variation in receptor affinity of different β-blocker generations. Analyses stratified for different β-blocker classes were not feasible owing to low statistical power. Accumulating evidence suggests that common β-blockers, including metoprolol, bisoprolol and carvedilol are better regarded as inverse agonists than pure antagonists [45]. In this scenario, ligand, receptor and G proteins interact to control their mutual affinities, a process potentially affected by GNB3 variants. While such a mechanism awaits experimental verification for Gβ proteins, elegant studies have actually shown that genetic variants in β-receptors affect the inverse antagonistic behavior of β-blockers [46].
Our study aim was not to identify new polymorphisms that might modulate β-blocker response. But rather we followed a candidate-gene approach and focused on potential effects of a common G-protein variant on HR control of β-blockers. Of course, other gene polymorphisms, for example, genes of the β1-receptor, β2-receptor or CYP2D6 in the case of metoprolol, also play an important role [45]. These questions should be addressed by prospectively designed studies.
The potential clinical relevance of our findings can only be speculated. One the one hand, decreased resting HR has been associated with beneficial effects, including reduced all-cause and cardiovascular mortality in both the general population and in various cardiovascular diseases [4,28]. In the Metoprolol CR/XL Randomised Intervention Trial in-congestive Heart Failure (MERIT-HF) trial, a reduction of total mortality in 1845 heart failure patients was independent from the maximum β-blocker dose reached, meaning that HR reduction was the main predictor of beneficial β-blocker therapy [5]. Conversely, the attenuated HR during exercise and the lower chronotropic index (i.e., chronotropic incompetence), as seen in T allele carriers of our study, might theoretically point toward an adverse prognosis [6,9,31,32]. Thus, chronotropic incompetence was associated with increased total and cardiovascular mortality in various studies in β-blocker-naive subjects [28,31,32,47] as well as with increased all-cause mortality among 3736 patients taking metoprolol tartrate or atenolol in one study [31]. However, it is not feasible to conclude whether the effects observed in our study are advantageous or detrimental, and how it could impact future clinical practice.
Several potential limitations of our investigation merit comment. All subjects volunteered for exercise testing, implying a potential selection bias. Therefore, we additionally applied weights that accounted for systematic differences in participants and nonparticipants of exercise testing. These sensitivity analyses did not suggest a different association between the GNB3 C825T genotype and HR during exercise and recovery. Moreover, it should be noted that no information on the indication for β-blocker therapy was available. With respect to the baseline characteristics, however, it may be assumed that the main indications were hypertension and previous myocardial infarctions (Table 1). Based on the study design we are not able to provide any information on potential contraindications for β-blockers or discontinuation of a previous β-blocker therapy among subjects that were not on this medication during the current examination. Strengths of our study include the availability of extensive data on key risk factors, particularly on β-blocker dosages, comorbidities and on echocardiographically assessed left ventricular function as well as the consistency of the results over multiple statistical models. Despite uncontrollable confounding factors, which are necessarily present in a population-based scenario, associations between the GNB3 C825T genotype and HR control of β-blockers were significant over various statistical models. Repeating our analyses with different combinations of confounders would not have altered our substantial conclusions.
Conclusion
We have demonstrated that a common polymorphism of the GNB3 gene modulates HR control of β-blockers. These findings have to be confirmed and the potential clinical relevance has to be investigated in further studies. Identification and confirmation of such gene–drug interactions may potentially contribute to future pharmacogenetically based dosage regimens for β-blockers or other drugs to facilitate individual dose adjustment, thereby potentially reducing adverse effects.
Supplementary Material
Acknowledgments
The contribution to data collection made by field workers, technicians, interviewers and computer assistants is gratefully acknowledged.
Footnotes
Financial & competing interests disclosure: The work is part of the Community Medicine Research net (CMR) of the University of Greifswald, Germany, which is funded by the Federal Ministry of Education and Research (grant no. ZZ9603), the Ministry of Cultural Affairs, and the Social Ministry of the Federal State of Mecklenburg-West Pomerania. The CMR encompasses several research projects, which share data from the population-based Study of Health in Pomerania (SHIP; http://www.medizin.unigreifswald.de/cm). This work is part supported by the research project Greifswald Approach to Individualized Medicine (GANI_MED). The GANI_MED consortium is funded by the Federal Ministry of Education and Research and the Ministry of Cultural Affairs of the Federal State of Mecklenburg, West Pomerania (03IS2061A). This study was further supported by the future fund of the state government of Mecklenburg-Vorpommern (UG 07 034) and by NIDCR/NIH grant DE0160057. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research: The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
- 1.Lee TH. Eulogy for a quality measure. N Engl J Med. 2007;357(12):1175–1177. doi: 10.1056/NEJMp078102. [DOI] [PubMed] [Google Scholar]
- 2.Gullestad L, Wikstrand J, Deedwania P, et al. What resting heart rate should one aim for when treating patients with heart failure with a β-blocker? experiences from the metoprolol controlled release/extended release randomized intervention trial in chronic heart failure (MERIT-HF) J Am Coll Cardiol. 2005;45(2):252–259. doi: 10.1016/j.jacc.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 3.Kjekshus JK. Importance of heart rate in determining β-blocker efficacy in acute and long-term acute myocardial infarction intervention trials. Am J Cardiol. 1986;57(12):43–49. doi: 10.1016/0002-9149(86)90888-x. [DOI] [PubMed] [Google Scholar]
- 4.Fox K, Borer JS, Camm AJ, et al. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50(9):823–830. doi: 10.1016/j.jacc.2007.04.079. [DOI] [PubMed] [Google Scholar]
- 5.Wikstrand J, Hjalmarson A, Waagstein F, et al. Dose of metoprolol CR/XL and clinical outcomes in patients with heart failure: analysis of the experience in metoprolol CR/XL randomized intervention trial in chronic heart failure (MERIT-HF) J Am Coll Cardiol. 2002;40(3):491–498. doi: 10.1016/s0735-1097(02)01970-8. [DOI] [PubMed] [Google Scholar]
- 6.Messerli FH, Bangalore S, Julius S. Risk/benefit assessment of β-blockers and diuretics precludes their use for first-line therapy in hypertension. Circulation. 2008;117(20):2706–2715. doi: 10.1161/CIRCULATIONAHA.107.695007. [DOI] [PubMed] [Google Scholar]
- 7.Cutler JA, Davis BR. Thiazide-type diuretics and β-adrenergic blockers as first-line drug treatments for hypertension. Circulation. 2008;117(20):2691–2704. doi: 10.1161/CIRCULATIONAHA.107.709931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Persistent use of evidence-based pharmacotherapy in heart failure is associated with improved outcomes. Circulation. 2007;116(7):737–744. doi: 10.1161/CIRCULATIONAHA.106.669101. [DOI] [PubMed] [Google Scholar]
- 9.Devereaux PJ, Yang H, Yusuf S, et al. Effects of extended-release metoprolol succinate in patients undergoing non cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371(9627):1839–1847. doi: 10.1016/S0140-6736(08)60601-7. [DOI] [PubMed] [Google Scholar]
- 10.Ingelsson E, Larson MG, Vasan RS, et al. Heritability, linkage, and genetic associations of exercise treadmill test responses. Circulation. 2007;115(23):2917–2924. doi: 10.1161/CIRCULATIONAHA.106.683821. [DOI] [PubMed] [Google Scholar]
- 11.Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev. 2005;85(4):1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]; ▪ Interesting overview on the main functions of heterotrimeric G proteins in defined cells and tissues.
- 12.Siffert W, Rosskopf D, Siffert G, et al. Association of a human G-protein β3 subunit variant with hypertension. Nat Genet. 1998;18(1):45–48. doi: 10.1038/ng0198-45. [DOI] [PubMed] [Google Scholar]
- 13.Rosskopf D, Schürks M, Rimmbach C, Schafers R. Genetics of arterial hypertension and hypotension. Naunyn Schmiedebergs Arch Pharmacol. 2007;374(5–6):429–469. doi: 10.1007/s00210-007-0133-2. [DOI] [PubMed] [Google Scholar]
- 14.Nürnberger J, Dammer S, Mitchell A, et al. Effect of the C825T polymorphism of the G protein β 3 subunit on the systolic blood pressure-lowering effect of clonidine in young, healthy male subjects. Clin Pharmacol Ther. 2003;74(1):53–60. doi: 10.1016/S0009-9236(03)00087-0. [DOI] [PubMed] [Google Scholar]
- 15.Siffert W. G protein polymorphisms in hypertension, atherosclerosis, and diabetes. Annu Rev Med. 2005;56:17–28. doi: 10.1146/annurev.med.56.082103.104625. [DOI] [PubMed] [Google Scholar]
- 16.Baumgart D, Naber C, Haude M, et al. G protein β3 subunit 825T allele and enhanced coronary vasoconstriction on α(2)-adrenoceptor activation. Circ Res. 1999;85(10):965–969. doi: 10.1161/01.res.85.10.965. [DOI] [PubMed] [Google Scholar]
- 17.Schäfers RF, Nürnberger J, Rutz A, et al. Haemodynamic characterization of young normotensive men carrying the 825T-allele of the G-protein β3 subunit. Pharmacogenetics. 2001;11(6):461–470. doi: 10.1097/00008571-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Kurnik D, Friedman EA, Muszkat M, et al. Genetic variants in the α2C-adrenoceptor and G-protein contribute to ethnic differences in cardiovascular stress responses. Pharmacogenet Genomics. 2008;18:743–750. doi: 10.1097/FPC.0b013e3282fee5a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filigheddu F, Reid JE, Troffa C, et al. Genetic polymorphisms of the β-adrenergic system: association with essential hypertension and response to β-blockade. Pharmacogenomics J. 2004;4(3):154–160. doi: 10.1038/sj.tpj.6500247. [DOI] [PubMed] [Google Scholar]
- 20.Matsunaga T, Nagasumi K, Yamamura T, et al. Association of C825T polymorphism of G protein β3 subunit with the autonomic nervous system in young healthy Japanese individuals. Am J Hypertens. 2005;18(4 Pt 1):523–529. doi: 10.1016/j.amjhyper.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Völzke H, Alte D, Schmidt CO, et al. Cohort profile: the study of health in Pomerania. Int J Epidemiol. 2010 doi: 10.1093/ije/dyp394. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7(2):79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 24.Schürks M, Kurth T, Stude P, et al. G protein β3 polymorphism and triptan response in cluster headache. Clin Pharmacol Ther. 2007;82(4):396–401. doi: 10.1038/sj.clpt.6100159. [DOI] [PubMed] [Google Scholar]
- 25.Tobler AR, Short S, Andersen MR, et al. The SNPlex genotyping system: a flexible and scalable platform for SNP genotyping. J Biomol Tech. 2005;16(4):398–406. [PMC free article] [PubMed] [Google Scholar]
- 26.Rosskopf D, Manthey I, Habich C, et al. Identification and characterization of G β 3s2, a novel splice variant of the G-protein β 3 subunit. Biochem J. 2003;371(Pt 1):223–232. doi: 10.1042/BJ20021208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones N. Clinical Exercise Testing. 3rd. WB Saunders Co.; PA, USA: 1988. pp. 152–155. [Google Scholar]
- 28.Savonen KP, Lakka TA, Laukkanen JA, et al. Heart rate response during exercise test and cardiovascular mortality in middle-aged men. Eur Heart J. 2006;27(5):582–588. doi: 10.1093/eurheartj/ehi708. [DOI] [PubMed] [Google Scholar]
- 29.Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ. Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications. 4th. Lippincott, Williams and Wilkins; PA, USA: 2004. [Google Scholar]
- 30.Astrand I. Aerobic work capacity in men and women with special reference to age. Acta Physiol Scand Suppl. 1960;49(169):1–92. [PubMed] [Google Scholar]
- 31.Khan MN, Pothier CE, Lauer MS. Chronotropic incompetence as a predictor of death among patients with normal electrograms taking β blockers (metoprolol or atenolol) Am J Cardiol. 2005;96(9):1328–1333. doi: 10.1016/j.amjcard.2005.06.082. [DOI] [PubMed] [Google Scholar]; ▪ First study demonstrating that chronotropic incompetence is an independent predictor of death in patients taking β-blockers.
- 32.Lauer MS, Francis GS, Okin PM, Pashkow FJ, Snader CE, Marwick TH. Impaired chronotropic response to exercise stress testing as a predictor of mortality. JAMA. 1999;281(6):524–529. doi: 10.1001/jama.281.6.524. [DOI] [PubMed] [Google Scholar]
- 33.Robergs RA, Landwehr A. The surprising history of the ‘HRmax=220-age’ equation. J Exercise Physiol. 2002;5(2):1–8. [Google Scholar]
- 34.Inbar O, Oren A, Scheinowitz M, Rotstein A, Dlin R, Casaburi R. Normal cardiopulmonary responses during incremental exercise in 20-to 70-yr-old men. Med Sci Sports Exerc. 1994;26(5):538–546. [PubMed] [Google Scholar]
- 35.Rosskopf D, Manthey I, Siffert W. Identification and ethnic distribution of major haplotypes in the gene GNB3 encoding the G-protein β3 subunit. Pharmacogenetics. 2002;12(3):209–220. doi: 10.1097/00008571-200204000-00005. [DOI] [PubMed] [Google Scholar]; ▪ First description of additional SNPs of the GNB3 gene and analyses of their prevalence in Caucasian, black African and Asian populations.
- 36.Wenzel RR, Siffert W, Bruck H, Philipp T, Schafers RF. Enhanced vasoconstriction to endothelin-1, angiotensin II and noradrenaline in carriers of the GNB3 825T allele in the skin microcirculation. Pharmacogenetics. 2002;12(6):489–495. doi: 10.1097/00008571-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell A, Luckebergfeld B, Buhrmann S, et al. Effects of systemic endothelin A receptor antagonism in various vascular beds in men: in vivo interactions of the major blood pressure-regulating systems and associations with the GNB3 C825T polymorphism. Clin Pharmacol Ther. 2004;76(5):396–408. doi: 10.1016/j.clpt.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 38.Meirhaeghe A, Bauters C, Helbecque N, et al. The human G-protein β3 subunit C825T polymorphism is associated with coronary artery vasoconstriction. Eur Heart J. 2001;22(10):845–848. doi: 10.1053/euhj.2000.2400. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell A, Pace M, Nurnberger J, et al. Insulin-mediated venodilation is impaired in young, healthy carriers of the 825T allele of the G-protein β3 subunit gene (GNB3) Clin Pharmacol Ther. 2005;77(6):495–502. doi: 10.1016/j.clpt.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Bagos PG, Elefsinioti AL, Nikolopoulos GK, Hamodrakas SJ. The GNB3 C825T polymorphism and essential hypertension: a meta-analysis of 34 studies including 14,094 cases and 17,760 controls. J Hypertens. 2007;25(3):487–500. doi: 10.1097/HJH.0b013e328011db24. [DOI] [PubMed] [Google Scholar]
- 41.Sartori M, Semplicini A, Siffert W, et al. G-protein β3-subunit gene 825T allele and hypertension: a longitudinal study in young grade I hypertensives. Hypertension. 2003;42(5):909–914. doi: 10.1161/01.HYP.0000097600.58083.EE. [DOI] [PubMed] [Google Scholar]
- 42.Rankinen T, Rice T, Leon AS, et al. G protein β 3 polymorphism and hemodynamic and body composition phenotypes in the HERITAGE Family Study. Physiol Genomics. 2002;8(2):151–157. doi: 10.1152/physiolgenomics.00102.2001. [DOI] [PubMed] [Google Scholar]
- 43.Wilkie MJ, Smith D, Reid IC, et al. A splice site polymorphism in the G-protein β subunit influences antidepressant efficacy in depression. Pharmacogenet Genomics. 2007;17(3):207–215. doi: 10.1097/FPC.0b013e32801a3be6. [DOI] [PubMed] [Google Scholar]
- 44.Wang T, Plumpton C, Brown MJ. Selective β1-adrenoceptor blockade enhances the activity of the stimulatory G-protein in human atrial myocardium. Br J Pharmacol. 1999;128(1):135–141. doi: 10.1038/sj.bjp.0702750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosskopf D, Michel MC. Pharmacogenomics of G protein-coupled receptor ligands in cardiovascular medicine. Pharmacol Rev. 2008;60(4):513–535. doi: 10.1124/pr.108.000612. [DOI] [PubMed] [Google Scholar]; ▪ Overview on the mechanisms and importance of agonists and antagonists of G protein-coupled receptors as drug targets for the treatment of cardiovascular disease.
- 46.Rochais F, Vilardaga JP, Nikolaev VO, Bunemann M, Lohse MJ, Engelhardt S. Real-time optical recording of β1-adrenergic receptor activation reveals supersensitivity of the Arg389 variant to carvedilol. J Clin Invest. 2007;117(1):229–235. doi: 10.1172/JCI30012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lauer MS, Okin PM, Larson MG, Evans JC, Levy D. Impaired heart rate response to graded exercise. Prognostic implications of chronotropic incompetence in the Framingham heart study. Circulation. 1996;93(8):1520–1526. doi: 10.1161/01.cir.93.8.1520. [DOI] [PubMed] [Google Scholar]
Website
- 101.WHO Collaborating Centre for Drug Statistics Methodology; Oslo, Norway: ATC Index with DDDs and the Guidelines for ATC classification and DDD assignment. www.whocc.no/atcddd. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.