Abstract
Physical and psychological stress can alter the immune system in both humans and animals. Stress is a known risk factor for numerous human diseases, such as infectious and autoimmune diseases, and cancer. Toll-like receptors (TLRs) play a pivotal role in the induction of innate and adaptive immune response. Our previous studies have shown that TLR4 deficiency prevents stress-induced splenocyte reduction. However, the role of TLR2 in stress-mediated lymphocyte reduction is unknown. In this study, we investigated the effects of TLR2 ligands on stress-induced lymphocyte reduction. We also defined whether the phosphoinositide 3-kinases (PI3Ks)/Akt pathway contributes to TLR2-mediated lymphocyte numbers altered by stress. Our data have shown that stimulation of TLR2 by TLR2 ligands peptidoglycan (PGN) or Pam3Csk4 (Pam3) attenuates stress-induced reduction in lymphocyte numbers. However, TLR2 ligand-induced protection from stress-induced lymphocyte reduction is lost in TLR2 deficiency in mice. Furthermore, stimulation of TLR2 by PGN induces protection from stress-induced reduction in the number of splenocytes through PI3K. Moreover, PGN dramatically increases the level of phosphorylation of Akt through a PI3K-dependent manner. Moreover, we found that stimulation of TLR2 by PGN induced protection from stress-induced reduction in splenocyte numbers is abolished in β-arrestin 2 deficient mice. In addition, PGN-induced immune protection in stress-induced changes of cytokine levels appears to require β-arrestin 2, a multifunctional adaptor and signal transducer. Collectively, our study thus demonstrates that stimulation of TLR2-mediated PI3K signaling attenuates splenocyte reduction induced by stress, and that β-arrestin 2 modulates TLR2-mediated immune response following stress.
Keywords: Stress, Lymphocytes, TLR2, PI3K, Akt, β-arrestin 2
1. Introduction
Physical or psychological stress can have a dramatic impact on the immune system in both humans and animals (Yin et al., 2000; Frieri, 2003; Yang and Glaser, 2002; Yin et al., 2006b). Stress is a known risk factor for numerous human diseases, such as infectious and autoimmune diseases (Reiche et al., 2004; Shi et al., 2003; Cao et al., 2007). It has been established that moderate stress such as routine exercise could increase immune responsiveness. Acute stress has been shown to enhance antibody production (Dhabhar and McEwen, 1999). However, chronic stress such as long-term emotional stress can attenuate immune function (Dhabhar and McEwen, 1997; Shi et al., 2003; Frieri, 2003; Zhang et al., 2008a). This effect is at least in part due to the reduction of lymphocytes (Zorrilla et al., 2001; Yin et al., 2000; Shi et al., 2003; Beaulieu et al., 2008). Chronic stress or physiologically exhausting stress has significant suppressive effects on the immune system that includes innate and adaptive immunity, cell-mediated immunity and effector cell function (Reiche et al., 2004; Quan et al., 2001; Hawkley and Cacioppo, 2004). The cellular mechanisms underlying the suppressive effects of stress on the immune system have begun to be further investigated. In order to study the mechanisms of stress-induced immune responses and to design strategies for therapeutic intervention, we established an animal model for restraint stress to study the changes in immune responses during stress. Using this model we have revealed that restraint stress of mice modulates the immune system through a cell apoptotic mechanism (Yin et al., 2000; Shi et al., 2003; Zhang et al., 2008c).
Toll receptors are an ancient and evolutionarily conserved receptor family that is a critical determinant of the innate immune and inflammatory responses (Aderem and Ulevitch, 2000; Gan and Li, 2006; Doyle and O'Neill, 2006). TLRs recognize conserved pathogen-associated molecular patterns (PAMPs) shared by large groups of microorganisms and play an instructive role in induction of innate immune responses and also activation of adaptive immunity (Aderem and Ulevitch, 2000; Medzhitov et al., 1997; Gan and Li, 2006). TLRs (TLR1, TLR2, TLR4, TLR5, and TLR6), which are expressed on the cell surface, are involved in the recognition of structures unique to bacteria or fungi, while TLRs (TLR3, TLR7, TLR8, and TLR9) that are localized in intracellular compartments will recognize viral or bacterial nucleic acids. TLRs, such as TLR2, are abundantly expressed on immune cells, including CD4+ and CD8+ T cells (Zanin-Zhorov et al., 2007; Caramalho et al., 2003; Xu et al., 2005) and dendritic cells (Kaisho and Akira, 2003). TLR-mediated signaling mainly modulates intracellular signaling pathways, such as NF-қB, which play a pivotal role in modulating in innate immunity and inflammatory responses as well as cell survival and cell apoptosis (Zhang and Ghosh, 2001; Aderem and Ulevitch, 2000; O'Neill and Bowie, 2007; Karin and Lin, 2002). Our previous studies have reported that TLR4 contributes to the reduction in splenocyte numbers induced by stress (Zhang et al., 2008c; Zhang et al., 2008b). However, the role of TLR2 in stress-induced reduction in the numbers of splenocytes is not yet known.
The phosphoinositide 3-kinases (PI3Ks) are a conserved family of signal transduction enzymes which play a fundamental role in regulating cell survival (Fruman and Cantley, 2002; Cantley, 2002). The PI3Ks and the downstream serine/threonine kinase Akt (also known as protein kinase B, PKB) modulate cellular activation, inflammatory response, and apoptosis (Cantley, 2002). Recent studies including our own have identified cross-talk between TLR and the PI3K/Akt pathways (Guha and Mackman, 2002; Fukao and Koyasu, 2003; Fukao et al., 2002; Ojaniemi et al., 2003; Zhang et al., 2008c; Li et al., 2010). It has been shown that activation of TLRs leads to activation of the PI3K/Akt signaling pathway (Sarkar et al., 2004; Fukao and Koyasu, 2003; Li et al., 2010). PI3K may be a negative feedback regulator which is crucial to the maintenance and integrity of the immune responses (Fukao and Koyasu, 2003; Ruse and Knaus, 2006). Recent studies have reported that TLR2 ligands inhibit cardiac dysfunction in polymicrobial sepsis through a PI3K/Akt dependent mechanism (Ha et al., 2010). Our previous studies have shown that chronic stress modulates splenocyte numbers through PI3K (Zhang et al., 2008a). However, the role of TLR2 mediated PI3K signaling in stress-induced splenocyte reduction is unknown. Increasing evidence suggests that β-arrestin 2, a multifunctional adaptor and signal transducer, plays a fundamental role in modulating immune responses, such as regulation of chemotactic responses due to its scaffold and adaptor functions (Fong et al., 2002; Beaulieu et al., 2005). β-arrestin 2 induces the activation of Akt through interaction with Akt negative regulator protein phosphatase 2a (PP2A) (Beaulieu et al., 2005). β-arrestin 2 serves as an in vivo negative regulator of TLR-mediated signaling pathways and a stimulator of PI3K/Akt signaling (Wang et al., 2006; Beaulieu et al., 2005; Li et al., 2010). In this study, we investigated the involvement of TLR2 and TLR2-mediated PI3K/Akt signaling. Our data revealed that stimulation of TLR2-mediated PI3K signaling attenuates stress-induced splenocyte reduction and that β-arrestin 2 modulates TLR2-mediated immune response following stress.
2. Materials and Methods
2.1. Mice
Toll-like receptor 2 knockout (TLR2 KO) mice on a C57BL/6 background and wild type C57BL/6 mice were obtained from the Jackson Laboratory. β-arrestin 2 KO mice on a C57BL/6 background was kindly provided by Dr. Robert Lefkowitz, Duke University Medical School. All mice were maintained in the Division of Laboratory Animal Resources at East Tennessee State University (ETSU), a facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAC). All aspects of the animal care and experimental protocols were approved by the ETSU Committee on Animal Care.
2.2. Experimental model of restraint stress
Six- to eight-week-old male mice were subjected to an established chronic physical restraint protocol used in our laboratory as well as others (Yin et al., 2000; Yin et al., 2006b; Zhang et al., 2008a). Briefly, mice were placed in a 50-ml conical centrifuge tube with multiple punctures to allow ventilation. Mice were held horizontally in the tubes for 12 h followed by a 12-h rest. During the rest period food and water were provided ad libitum. Control littermates were kept in their original cage and food and water were provided only during the 12 h rest. At 2 days after physical restraint, mice were sacrificed by CO2 asphyxiation, and the spleens were harvested.
2.3. Experimental protocols
To determine the role of TLR2 signaling in chronic stress-induced reduction in lymphocyte numbers 1 hour before each stress cycle, TLR2 KO mice, β-arrestin 2 KO mice, and their wild type C57BL/6 mice were administrated TLR2 ligands, peptidoglycan (PGN, 50 µg/25 g body weight, i.p. Sigma, St. Louis, MO) (Zhang and Ghosh, 2001; Abrahams et al., 2008; Ha et al., 2010) and Pam3Csk4 (Pam3, 50 µg/25 g body weight, i.p. InvivoGen, San Diego, CA) (Zhang and Ghosh, 2001; Ha et al., 2010). To examine the effect of PI3K/Akt signaling on chronic stress-induced reduction in lymphocyte numbers, we used wortmannin and LY294002 to inhibit PI3K activity, which have been widely used, including in our laboratory and others, to study the role of PI3K in immune responses both in vitro and in vivo (Yin et al., 2006a; Zhang et al., 2008c; Zhang et al., 2008a; Adi et al., 2001). Dose-ranging experiments were performed with wortmannin and LY294002 to identify doses that inhibit the activity of PI3K in vivo without causing morbidity or mortality. TLR2 deficient mice and age-matched wild type C57BL/6 mice were subjected to restraint stress. Parallel groups of mice received an i.p. injection 1 hour before each stress cycle with the PI3K inhibitors, wortmannin (25 µg/25 g body weight, i.p. Sigma) or LY294002 (1 mg/25 g body weight, i.p. Sigma).
2.4. Western blot analysis
Cell lysis was prepared from splenic tissues and immunoblots were performed as described previously (Yin et al., 1999; Yin et al., 2006a; Zhang et al., 2008c). Briefly, the cellular proteins were separated by SDS–polyacrylamide gel electrophoresis and transferred onto Hybond ECL membranes (Amersham Pharmacia, NJ). The ECL membranes were incubated with the appropriate primary antibody, i.e., anti-TLR2, anti-Akt and anti-phospho-Akt (serine 473) (Cell Signal Technology, Beverly, MA), respectively. The blot was exposed to the SuperSignal West Dura Extented Duration substrate (Pierce Biotechnology, Rockford, IL). The signals were quantified by scanning densitometry using a Bio-Image Analysis System (Bio-Rad). The results from each experiment were expressed as relative integrated intensity compared with that of control lymphocytes measured with the same batch.
2.5. Enzyme linked immunosorbent assay (ELISA) for cytokines
Splenic lymphocytes from β-arrestin 2 KO mice and wild type mice were adjusted to a final concentration of 5×105 cells/ ml in 96-well plates. Lymphocytes were treated with concanavalin A (Con A, 10 µg/ ml). The supernatants were harvested after 24 h (IL-2) or 48 h (IL-4) of cultivation. The presence of cytokines in the supernatants was determined using cytokine-specific sandwich ELISA kits (R&D Systems, Minneapolis, MN) as described in our published studies (Zhang et al., 2008c; Zhang et al., 2008b).
2.6. Statistical analysis
The results were presented as Means and SEs. The data were analyzed using one-way analysis of variance (ANOVA) followed by Bonferroni tests to determine where differences among groups existed. A value of p < 0.05 was considered statistically significant.
3. Results
3.1. Stimulation of TLR2 by peptidoglycan (PGN) protects from stress-induced reduction in the number of splenocytes
TLR2 plays a fundamental role in the induction of innate and adaptive immunity (Medzhitov et al., 1997). To examine the role of TLR2 in stress-induced reduction in lymphocyte numbers, we first determined whether stress alters the expression of TLR2. As shown in Fig. 1A, restraint stress of wild type mice significantly decreased the expression of TLR2. Peptidoglycan (PGN) is a TLR2 ligand which activates cellular signaling specifically through TLR2 (Ha et al., 2010; Abrahams et al., 2008). To investigate the effect of PGN on TLR2 level, we administered wild type mice with PGN (50 µg/25 g body weight, i.p.) (Abrahams et al., 2008; Ha et al., 2010) one hour before the initiation of stress. We found that administration of PGN in stressed mice blocked stress-decreased the expression of TLR2 compared to stressed control mice (Fig. 1A). We next examined the role of PGN in stress-induced splenocyte reduction. Wild type mice were treated with or without PGN (50 µg/25 g body weight, i.p.) (Abrahams et al., 2008; Ha et al., 2010) one hour before the initiation of stress. Administration of PGN significantly attenuated stress-induced splenocyte reduction compared to untreated mice (Fig. 1B). Similar results were obtained when we employed Pam3Csk4 (Pam3, 50 µg/25 g body weight, i.p.) as a TLR2 ligand instead of PGN (data not shown). Stimulation of TLR2 by PGN or Pam3 administration did not significantly alter the number of splenocytes in the absence of chronic stress.
Figure 1.
Stimulation of TLR2 by peptidoglycan (PGN) inhibits stress-induced splenocyte reduction. (A) Effect of PGN on TLR2 expression following stress. Wild type (WT) (C57BL/6) mice aged 6–7 weeks were subjected to 12-h of physical stress daily for 2 days. PGN (50 µg/25 g body weight) was injected (i.p.) at 1 hr before the initiation of stress. The expression of TLR2 in the spleen was determined by Western blot. Data are representative of three independent experiments. (B) PGN attenuated stress-induced reduction in the number of splenocytes. C57BL/6 mice aged 6–7 weeks were subjected to 12-h of physical stress daily. PGN (50 µg/25 g body weight) was injected (i.p.) at 1 hr before the initiation of stress. After 2 days stress, total splenocytes were enumerated with a hemocytometer. Means and SEs were calculated from 7 mice per group. * p < 0.01 compared with indicated groups.
3.2. TLR2 is required for Pam3CSK4 (Pam3)-induced protection from stress-induced splenocyte reduction
To determine whether TLR2 contributes to Pam3CSK4 (Pam3)-induced protection from stress-induced splenocyte reduction, we treated TLR2 KO mice and wild type mice one hour before the initiation of stress in the presence or absence of Pam3 administration (50 µg/25 g body weight, i.p) (Abrahams et al., 2008; Doyle and O'Neill, 2006). We found that Pam3-induced protection in the number of splenocytes is abolished in TLR2 KO mice (Fig. 2). Similar results were obtained when we subjected PGN as a TLR2 ligand (data not shown). These data suggest that TLR2 is a critical receptor for PGN-induced immune protection.
Figure 2.
TLR2 deficiency abolishes Pam3CSK4 (Pam3)-induced protection from stress-induced reduction in number of splenocytes. TLR2 KO and WT (C57BL/6) mice aged 6–7 weeks were subjected to 12-h of physical stress daily. Pam3 (50 µg/25 g body weight) was injected (i.p.) at 1 hr before the initiation of stress. After 2 days stress, total splenocytes were enumerated with a hemocytometer. Means and SEs were calculated from 7 mice per group. * p < 0.01 compared with indicated groups. ** p < 0.01 compared with stressed WT group.
3.3. Stimulation of TLR2 by PGN induces protection from stress-induced splenocyte reduction through PI3K
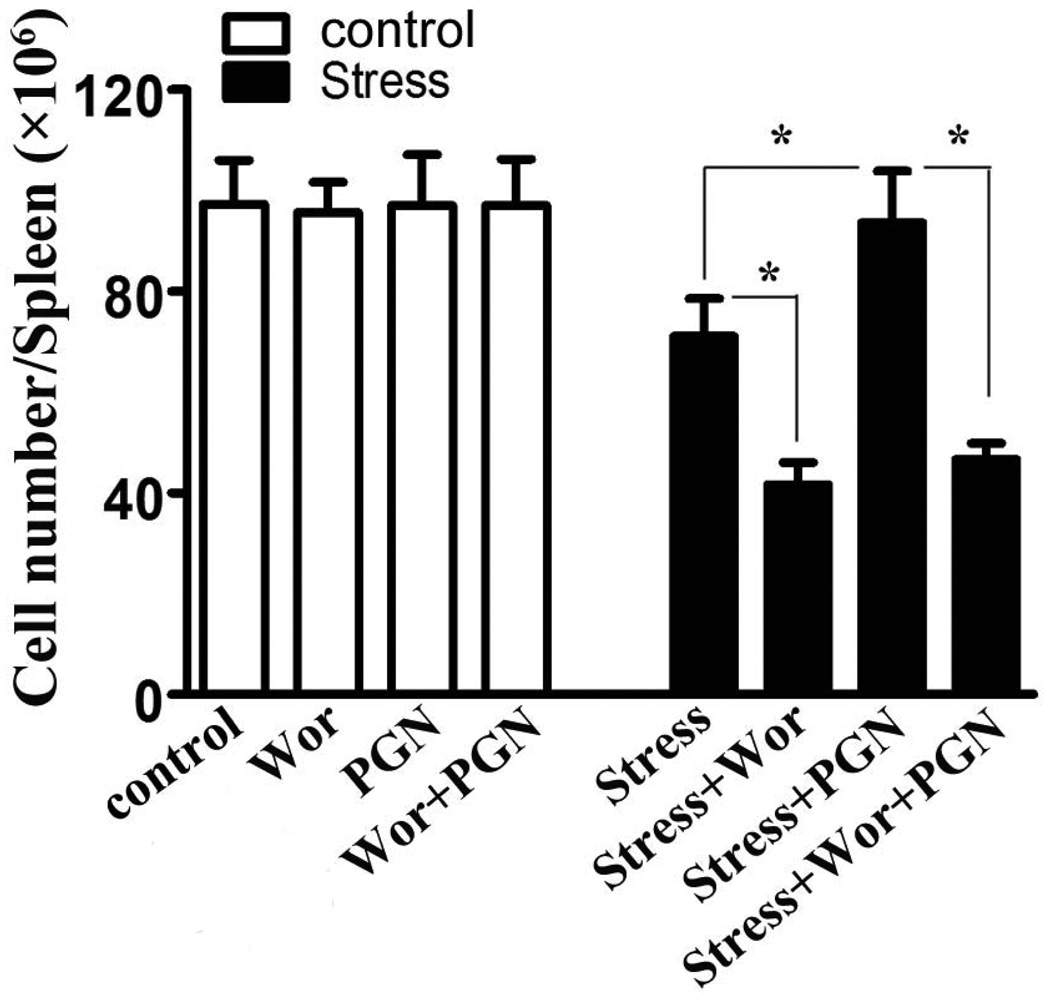
Our previous studies reported that PI3K plays an important role in stress-induced reduction in splenocyte numbers (Zhang et al., 2008c). Recent evidence indicates that stimulation of TLR2 leads to activation of the PI3K signaling (Li et al., 2004; Arbibe et al., 2000). To evaluate the effect of PI3K on lymphocyte number in TLR2-mediated immune response, wild type mice were administered PI3K inhibitor wortmannin or LY294002 one hour before the initiation of each stress cycle with or without PGN administration (Abrahams et al., 2008; Doyle and O'Neill, 2006). PI3K inhibitor, wortmannin or LY294002 has been widely utilized to investigate the role of PI3K in immune response both in vitro and in vivo (Yin et al., 2006a; Zhang et al., 2008a; Zhang et al., 2008c). As shown in Fig. 3, inhibition of PI3K by wortmannin administration did not dramatically alter the number of splenocytes in the absence of stress. However, in the presence of chronic stress, PI3K inhibition significantly decreased the number of splenocytes compared with their stressed control mice (without wortmannin administration). Wortmannin administration exerted an additive effect on chronic stress-induced splenocyte reduction. These data are consistent with our findings (Zhang et al., 2008a). Interestingly, administration of PGN significantly inhibited stress-induced splenocyte reduction compared to stressed control mice (without PGN administration), but this effect was significantly attenuated when PI3K was inhibited by wortmannin administration (Fig. 3). Similar results were obtained when we employed LY294002 as a PI3K inhibitor instead of wortmannin (data not shown). These data demonstrated that stimulation of TLR2 induces protection in lymphocyte numbers which is mediated through a PI3K dependent mechanism.
Figure 3.
Modulation of TLR2 by PGN inhibits stress-induced splenocyte reduction through PI3K. WT (C57BL/6) mice aged 6–7 weeks were subjected to 12-h of physical stress daily. Wortmannin (Wor) (25 µg/25 g body weight, i.p.) was administered 1 hr before the initiation of stress with or without PGN (50 µg/25 g body weight, i.p.). At 2 days after stress, total splenocytes were enumerated with a hemocytometer. Means and SEs were calculated from 5 to 7 mice per group. * p < 0.01 compared with indicated groups.
3.4. TLR2 stimulation enhances the levels of phosphorylation of Akt in stressed mice through a PI3K-dependent manner
Recent evidence suggests that there is cross talk between the TLR and PI3K/Akt signaling pathway (Guha and Mackman, 2002; Fukao and Koyasu, 2003; Ojaniemi et al., 2003). Activation of TLR2 will stimulate PI3K/Akt signaling (Santos-Sierra et al., 2009). We, therefore, determined the effect of PGN on the activation of the PI3K/Akt signaling following stress. As shown in Fig. 4, stimulation of TLR2 by PGN significantly enhanced the levels of phosphorylated Akt (p-Akt) in the spleen compared with those in the absence of PGN administration. Intriguingly, inhibition of PI3K by wortmannin blocked PGN-enhanced levels of p-Akt in the spleen in stressed mice. Although we could not observe wortmannin having a significant effect on the level of p-Akt at 12 hours after stress, in our other experiments, we found that wortmannin administration in mice attenuated p-Akt level at 2 days after stress (data not shown). Similar results were observed in PGN treated mice and in mice treated with LY294002 with or without restraint stress (data not shown). Taken together, stimulation of TLR2 by PGN increases the p-Akt level through a PI3K-dependent mechanism.
Figure 4.
Stimulation of TLR2 increases levels of phosphorylation of Akt in stressed mice via a PI3K-dependent manner. WT (C57BL/6) mice aged 6–7 weeks were subjected to 12-h of physical stress daily. Wortmannin (Wor) (25 µg/25 g body weight, i.p.) was administered 1 hr before the initiation of stress with or without PGN (50 µg/25 g body weight, i.p.). At 12 hours after stress, the spleens were harvested and the levels of phospho-Akt and total Akt were determined by Western blot with specific antibodies. There were five mice per group. Representative results of phospho-Akt and total Akt immunobloting are shown at the top of each pane. * p < 0.01 compared with indicated groups.
3.5. Stimulation of TLR2 by PGN induced protection in the number of splenocytes is abolished in β-arrestin 2 KO mice
We and others have shown that there is a link between TLRs and the PI3K/Akt signaling pathway (Li et al., 2004; Li et al., 2010). β-arrestin 2 is the predominant arrestin protein in T and B lymphocytes (Fong et al., 2002). β-arrestin 2 promotes the activation of Akt through interaction with Akt negative regulator protein phosphatase 2a (PP2A) (Beaulieu et al., 2005). To define whether β-arrestin 2 plays a role in PGN-induced protection in the number of splenocytes, we administered PGN to β-arrestin 2 KO mice. Age and weight matched wild type C57BL/6 mice served as control. One hour after administration of PGN, the mice were subjected to restraint daily for 2 days. PGN-induced protection in the number of splenocytes was abolished in β-arrestin 2 KO mice (Fig. 5). Our data suggest that β-arrestin 2 contributes to PGN-induced protection in stress-induced reduction in lymphocyte numbers.
Figure 5.
β-arrestin 2 deficiency abolishes PGN-induced protection in the number of splenocytes. β-arrestin 2 KO and WT (C57BL/6) mice aged 6–7 weeks were subjected to 12-h of physical stress daily. PGN (50 µg/25 g body weight) was injected (i.p.) at 1 hr before the initiation of stress. After 2 days stress, total splenocytes were enumerated with a hemocytometer. Means and SEs were calculated from 5 to 7 mice per group. * p < 0.01 compared with indicated groups. ** p < 0.01 compared with stressed WT group.
3.6. PGN induced protection in stress-induced changes of cytokine levels is abolished in β-arrestin 2 KO mice
Growing evidence demonstrated that β-arrestin 2 mediates some important immune responses, such as regulation of chemotactic responses and granule release due to its scaffold and adaptor functions (Fong et al., 2002). Recently, we have shown that restraint stress caused dramatic decrease in T help 1 (Th1) cytokine IL-2 level but an increase in the Th2 cytokine IL-4 in wild type mice (Zhang et al., 2008c). Therefore, we next determined the effects of β-arrestin 2 on Th1 and Th2 cytokine production following stimulation of TLR2 by PGN during restraint stress. Two days after restraint stress of β-arrestin 2-deficient mice and wild type mice, culture supernatants from Con A-stimulated splenocytes were analyzed for the levels of Th1 cytokine IL-2 and the Th2 cytokine IL-4 by ELISA. We observed that splenocytes from stressed wild type mice produced dramatically less IL-2 production (Fig. 6A) and significantly more IL-4 production (Fig. 6B) than splenocytes from control wild type mice, which is consistent with the findings from our previous studies (Zhang et al., 2008b). Interestingly, these changes of cytokine production were greatly reduced in PGN administered mice compared with the wild type mice without PGN treatment (Fig. 6). Surprisingly, β-arrestin 2 deficiency in mice abolished PGN-induced immune protection in stress-induced alterations of IL-2 and IL-4 production (Fig. 6). Therefore, PGN-induced immune protection in stress-induced changes of cytokine levels appears to require β-arrestin 2.
Figure 6.
Effect of PGN on splenic lymphocyte IL-2 and IL-4 production in WT mice and β-arrestin 2 deficient mice following stress. β-arrestin 2 KO and WT mice aged 6–8 weeks were injected (i.p.) with PGN (50 µg/25 g body weight) at 1 h before the initiation of each 12-h physical stress daily. At 2 days after stress, splenic lymphocytes were isolated and incubated with Con A (10 µg/ml) for 24 hours (IL-2) or 48 hours (IL-4). Cytokines IL-2 production (A) and IL-4 production (B) were analyzed by ELISA analysis. Means and SEs were calculated from 3 mice per group. * p < 0.01 compared with indicated groups.
4. Discussion
Physical and psychological stress is a known risk factor for numerous human diseases, such as autoimmune diseases and cancer (Reiche et al., 2004; Shi et al., 2003; Cao et al., 2007; Frieri, 2003; Yang and Glaser, 2002). The immunological consequences of stress and the mechanisms by which stress compromises the immune system are important areas of study. However, the cellular and molecular mechanisms associated with deleterious innate immune responses in stress remain to be elucidated. Our previous studies have shown that inhibition of TLR4 attenuates stress-induced reduction in splenocyte numbers, thus suggesting an important role of TLR4 in stress-induced immune responses (Zhang et al., 2008b). Furthermore, we found that activation of PI3K/Akt signaling pathway prevents stress-induced splenocyte reduction (Zhang et al., 2008a). In the current study, we extended these findings to demonstrate that the PI3K/Akt signaling pathway is up-regulated in TLR2 stimulated mice by TLR2 ligands following stress (Figs. 3 and 4). Of great significance, we showed in this study that stimulation of TLR2 induced protection in splenocyte numbers is mediated through a PI3K-dependent manner (Fig. 3). Our studies demonstrated that TLR2 regulates PI3K/Akt in the wild type mice, resulting in protection from stress-induced reduction in splenocyte numbers. In the mice with stimulation of TLR2 by TLR2 ligands, the higher levels of PI3K and Akt prevent mice from the stress-induced splenocyte reduction. Consequently, inhibition of PI3K restores the stress response. Collectively, these results suggest that up-regulation of the PI3K/Akt signaling pathway with stimulation of TLR2 plays a critical role in the protection observed in stimulation of TLR2 following stress-induced reduction in the number of splenocytes.
Recent evidence revealed that there is a cross-talk between TLR signaling and the PI3K/Akt signaling pathway (Arbibe et al., 2000; Fukao and Koyasu, 2003). As an example, stimulation of TLR2 or TLR4 leads to activation of the PI3K/Akt signaling (Arbibe et al., 2000; Marmiroli et al., 1998). Moreover, the PI3K/Akt signaling pathway may be an endogenous negative feedback regulator of TLR4-mediated immune responses (Fukao and Koyasu, 2003). For example, PI3K subunit p85 deficient mice show increased TLR responses to ligand stimulation (Fukao et al., 2002). Our previous studies have reported that activation of PI3K signaling pathway attenuates stress-induced reduction in the number of lymphocytes (Zhang et al., 2008a). Our data showed that the levels of phosphorylated Akt in the spleen of TLR2-stimulated mice are higher than in wild type control mice (Fig. 4). Thus, we speculated that higher levels of splenic PI3K/Akt in the mice with stimulation of TLR2 play an important role for the immune protection that is observed in stressed mice with TLR2 stimulation. In order to test this hypothesis, we administered two structurally different PI3K inhibitors LY294002 or wortmannin to TLR2 stimulated mice before stress. We found that inhibition of PI3K/Akt-dependent signaling abrogated immune protection in the mice with TLR2 stimulation following chronic stress (Fig. 3). LY294002 or wortmannin alone did not alter the number of splenocytes in control mice (Fig. 3) and in our previous studies (Zhang et al., 2008a; Zhang et al., 2008c). Collectively, these results reveal that the PI3K/Akt signaling pathway plays a fundamental role in protection from stress-induced reduction in lymphocyte numbers in the activation of TLR2-mediated signaling. Therefore, these results suggest that activating the PI3K/Akt signaling pathway may be a possible strategy for reducing immune dysfunction associated with chronic stress.
β-arrestin 2 is a cytosolic protein that was initially described as negative regulators of G-protein coupled receptors (GPCRs) functions (Lefkowitz and Shenoy, 2005; Moore et al., 2007). β-arrestin 2 serves as adaptors, scaffolds, and/or signal transducers (Lefkowitz and Shenoy, 2005; Wang et al., 2006). Recent studies have revealed that β-arrestin 2 belongs to a growing list of important molecules that can negatively regulate TLR-mediated signaling pathways. Specifically, it has been shown that a β-arrestin 2 deficient mice are more susceptible to endotoxic shock (Wang et al., 2006; Luan et al., 2005). The interaction of β-arrestin 2 with TRAF6 (tumor necrosis factor receptor-associated factor 6) could be important for β-arrestin 2 negative regulation of the TLR-mediated pathways and activation of PI3K/Akt (Luan et al., 2005; Wang et al., 2006; Witherow et al., 2004). However, relatively little is known about the importance of β-arrestin 2 in T and B lymphocytes, where it is abundantly expressed (Fong et al., 2002). Growing evidence suggests that β-arrestin 2 plays a fundamental role in modulating immune responses, such as regulation of chemotactic responses due to its scaffold functions (Fong et al., 2002; Beaulieu et al., 2005). We and others have revealed that β-arrestin 2 plays anti-apoptotic effects (Lefkowitz and Shenoy, 2005; Revankar et al., 2004; Li et al., 2009). Our data showed that lack of β-arrestin 2 in mice increases the sensitivity of stress-induced lymphocyte reduction. Importantly, we observed that β-arrestin 2 deficiency abolished TLR2 stimulation-induced protection from reduction in splenocyte numbers caused by stress (Fig. 5). These results suggest that β-arrestin 2 contributes to modulate TLR2-mediated protection from splenocyte reduction induced by stress. The splenocyte reduction caused by stress could be mediated by two possible mechanisms: cell death or emigration. Our previous studies have revealed that a significant number of cells in the spleen of stressed mice were undergoing apoptosis, whereas only a few apoptotic cells were detected in the spleen of control mice (Yin et al., 2000). Therefore, stress–induced splenocyte reduction is likely due to the induction of apoptosis rather than the mobilization of splenocytes. We will determine the role of β-arrestin 2 in stress-induced apoptosis in future studies.
We have shown that chronic stress decreases Th1 cytokine levels but increases Th2 cytokine levels in wild type mice (Zhang et al., 2008b). We have also shown that chronic stress in TLR4 deficient mice significantly inhibits changes of Th1 and Th2 cytokines compared with control wild type mice (Zhang et al., 2008c). In the current study, we extended these observations to demonstrate that a deficiency of β-arrestin 2 augments stress-induced changes of cytokines IL-2 and IL-4 levels (Fig. 6). In addition, stimulation of TLR2 by PGN inhibits stress-induced changes of cytokines IL-2 and IL-4 production. Surprisingly, PGN-induced protection in changes of cytokine levels of IL-2 and IL-4 induced by stress was abolished in β-arrestin 2 deficient mice (Fig. 6). Together, these data indicate that PGN-induced immune protection is mediated, at least in part, through a β-arrestin 2 dependent manner. Our previous studies have revealed that adrenalectomy did not significantly affect the stress-induced reduction in the number of lymphocytes (Yin et al., 2000). Thus, the hypothalamo-pituitary-adrenal (HPA) axis is unlikely to participate in modulating the lymphocyte reduction in our established stress mouse model (Yin et al., 2000; Zhang et al., 2008a; Zhang et al., 2008c). This finding is consistent with the proposition by McEwen et al. (McEwen et al., 1997) that the spleen is a relatively privileged site and is inaccessible to endogenously produced corticosteroids.
In summary, to the best of our knowledge, this study is the first report to reveal that stimulation of TLR2 mediated PI3K/Akt signaling diminishes splenocyte reduction induced by stress, and that β-arrestin 2 modulates TLR2 mediated immune response following stress (Fig. 7). These findings further elucidate the actions of TLR2 and β-arrestin 2 in stress responses, and provide new targets for the development of novel anti-immune dysfunction medications.
Figure 7.
Schematic diagram illustrating that stimulation of TLR2 induces protection from stress-induced splenocyte reduction through the PI3K/Akt signaling. Moreover, β-arrestin 2 modulates immune response induced by stress via TLR2-mediated signaling. A fundamental role of PI3K/Akt is to promote cell survival and cell growth.
Acknowledgements
This work was supported in part by NIH grant DA020120-03A1 and ETSU RDC 82061 grant to D. Yin. The authors wish to express their appreciation to Dr. Robert Lefkowitz, Duke University Medical School, for providing β-arrestin 2 knockout mice.
Abbreviations
- TLR2
Toll-like receptor 2
- PI3K
phosphoinositide 3-kinase.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahams VM, Aldo PB, Murphy SP, Visintin I, Koga K, Wilson G, Romero R, Sharma S, Mor G. TLR6 modulates first trimester trophoblast responses to peptidoglycan. J. Immunol. 2008;180:6035–6043. doi: 10.4049/jimmunol.180.9.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- Adi S, Wu NY, Rosenthal SM. Growth factor-stimulated phosphorylation of Akt and p70(S6K) is differentially inhibited by LY294002 and Wortmannin. Endocrinology. 2001;142:498–501. doi: 10.1210/endo.142.1.8051. [DOI] [PubMed] [Google Scholar]
- Arbibe L, Mira JP, Teusch N, Kline L, Guha M, Mackman N, Godowski PJ, Ulevitch RJ, Knaus UG. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat. Immunol. 2000;1:533–540. doi: 10.1038/82797. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Marion S, Rodriguiz RM, Medvedev IO, Sotnikova TD, Ghisi V, Wetsel WC, Lefkowitz RJ, Gainetdinov RR, Caron MG. A beta-arrestin 2 Signaling Complex Mediates Lithium Action on Behavior. Cell. 2008;132:125–136. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Cao L, Hudson CA, Moynihan JA. Chronic foot shock induces hyperactive behaviors and accompanying pro- and anti-inflammatory responses in mice. J. Neuroimmunol. 2007;186:63–74. doi: 10.1016/j.jneuroim.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J. Exp. Med. 2003;197:403–411. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav. Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc. Natl. Acad. Sci. U. S. A. 1999;96:1059–1064. doi: 10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle SL, O'Neill LA. Toll-like receptors: from the discovery of NFkappaB to new insights into transcriptional regulations in innate immunity. Biochem. Pharmacol. 2006;72:1102–1113. doi: 10.1016/j.bcp.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Fong AM, Premont RT, Richardson RM, Yu YR, Lefkowitz RJ, Patel DD. Defective lymphocyte chemotaxis in beta-arrestin2- and GRK6-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 2002;99:7478–7483. doi: 10.1073/pnas.112198299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieri M. Neuroimmunology and inflammation: implications for therapy of allergic and autoimmune diseases. Ann. Allergy Asthma Immunol. 2003;90:34–40. doi: 10.1016/s1081-1206(10)61658-4. [DOI] [PubMed] [Google Scholar]
- Fruman DA, Cantley LC. Phosphoinositide 3-kinase in immunological systems. Semin. Immunol. 2002;14:7–18. doi: 10.1006/smim.2001.0337. [DOI] [PubMed] [Google Scholar]
- Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24:358–363. doi: 10.1016/s1471-4906(03)00139-x. [DOI] [PubMed] [Google Scholar]
- Fukao T, Tanabe M, Terauchi Y, Ota T, Matsuda S, Asano T, Kadowaki T, Takeuchi T, Koyasu S. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat. Immunol. 2002;3:875–881. doi: 10.1038/ni825. [DOI] [PubMed] [Google Scholar]
- Gan L, Li L. Regulations and roles of the interleukin-1 receptor associated kinases (IRAKs) in innate and adaptive immunity. Immunol. Res. 2006;35:295–302. doi: 10.1385/IR:35:3:295. [DOI] [PubMed] [Google Scholar]
- Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J. Biol. Chem. 2002;277:32124–32132. doi: 10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- Ha T, Lu C, Liu L, Hua F, Hu Y, Kelley J, Singh K, Kao RL, Kalbfleisch J, Williams DL, Gao X, Li C. TLR2 ligands attenuate cardiac dysfunction in polymicrobial sepsis via a phosphoinositide 3-kinase-dependent mechanism. Am. J. Physiol Heart Circ. Physiol. 2010;298:H984–H991. doi: 10.1152/ajpheart.01109.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkley LC, Cacioppo JT. Stress and the aging immune system. Brain Behav. Immun. 2004;18:114–119. doi: 10.1016/j.bbi.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Kaisho T, Akira S. Regulation of dendritic cell function through Toll-like receptors. Curr. Mol. Med. 2003;3:373–385. doi: 10.2174/1566524033479726. [DOI] [PubMed] [Google Scholar]
- Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat. Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Li C, Ha T, Kelley J, Gao X, Qiu Y, Kao RL, Browder W, Williams DL. Modulating Toll-like receptor mediated signaling by (1-->3)-beta-D-glucan rapidly induces cardioprotection. Cardiovasc. Res. 2004;61:538–547. doi: 10.1016/j.cardiores.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Li H, Sun X, LeSage G, Zhang Y, Liang Z, Chen J, Hanley G, He L, Sun S, Yin D. Beta-Arrestin 2 regulates toll-like receptor 4-mediated apoptotic signalling through glycogen synthase kinase-3beta. Immunology. 2010;130:556–563. doi: 10.1111/j.1365-2567.2010.03256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Sun X, Zhang Y, Huang J, Hanley G, Ferslew KE, Peng Y, Yin D. Morphine promotes apoptosis via TLR2, and this is negatively regulated by beta-arrestin 2. Biochem. Biophys. Res. Commun. 2009;378:857–861. doi: 10.1016/j.bbrc.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Luan B, Zhang Z, Wu Y, Kang J, Pei G. Beta-arrestin2 functions as a phosphorylation-regulated suppressor of UV-induced NF-kappaB activation. EMBO J. 2005;24:4237–4246. doi: 10.1038/sj.emboj.7600882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmiroli S, Bavelloni A, Faenza I, Sirri A, Ognibene A, Cenni V, Tsukada J, Koyama Y, Ruzzene M, Ferri A, Auron PE, Toker A, Maraldi NM. Phosphatidylinositol 3-kinase is recruited to a specific site in the activated IL-1 receptor I. FEBS Lett. 1998;438:49–54. doi: 10.1016/s0014-5793(98)01270-8. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Biron CA, Brunson KW, Bulloch K, Chambers WH, Dhabhar FS, Goldfarb RH, Kitson RP, Miller AH, Spencer RL, Weiss JM. The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions. Brain Res. Brain Res. Rev. 1997;23:79–133. doi: 10.1016/s0165-0173(96)00012-4. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu. Rev. Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- Ojaniemi M, Glumoff V, Harju K, Liljeroos M, Vuori K, Hallman M. Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur. J. Immunol. 2003;33:597–605. doi: 10.1002/eji.200323376. [DOI] [PubMed] [Google Scholar]
- Quan N, Avitsur R, Stark JL, He L, Shah M, Caligiuri M, Padgett DA, Marucha PT, Sheridan JF. Social stress increases the susceptibility to endotoxic shock. J. Neuroimmunol. 2001;115:36–45. doi: 10.1016/s0165-5728(01)00273-9. [DOI] [PubMed] [Google Scholar]
- Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5:617–625. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Vines CM, Cimino DF, Prossnitz ER. Arrestins block G protein-coupled receptor-mediated apoptosis. J. Biol. Chem. 2004;279:24578–24584. doi: 10.1074/jbc.M402121200. [DOI] [PubMed] [Google Scholar]
- Ruse M, Knaus UG. New players in TLR-mediated innate immunity: PI3K and small Rho GTPases. Immunol. Res. 2006;34:33–48. doi: 10.1385/IR:34:1:33. [DOI] [PubMed] [Google Scholar]
- Santos-Sierra S, Deshmukh SD, Kalnitski J, Kuenzi P, Wymann MP, Golenbock DT, Henneke P. Mal connects TLR2 to PI3Kinase activation and phagocyte polarization. EMBO J. 2009;28:2018–2027. doi: 10.1038/emboj.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar SN, Peters KL, Elco CP, Sakamoto S, Pal S, Sen GC. Novel roles of TLR3 tyrosine phosphorylation and PI3 kinase in double-stranded RNA signaling. Nat. Struct. Mol. Biol. 2004;11:1060–1067. doi: 10.1038/nsmb847. [DOI] [PubMed] [Google Scholar]
- Shi Y, Devadas S, Greeneltch KM, Yin D, Allan MR, Zhou JN. Stressed to death: implication of lymphocyte apoptosis for psychoneuroimmunology. Brain Behav. Immun. 2003;17 Suppl 1:S18–S26. doi: 10.1016/s0889-1591(02)00062-4. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tang Y, Teng L, Wu Y, Zhao X, Pei G. Association of beta-arrestin and TRAF6 negatively regulates Toll-like receptor-interleukin 1 receptor signaling. Nat. Immunol. 2006;7:139–147. doi: 10.1038/ni1294. [DOI] [PubMed] [Google Scholar]
- Witherow DS, Garrison TR, Miller WE, Lefkowitz RJ. beta-Arrestin inhibits NF-kappaB activity by means of its interaction with the NF-kappaB inhibitor IkappaBalpha. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8603–8607. doi: 10.1073/pnas.0402851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Komai-Koma M, Liew FY. Expression and function of Toll-like receptor on T cells. Cell Immunol. 2005;233:85–89. doi: 10.1016/j.cellimm.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Yang EV, Glaser R. Stress-associated immunomodulation and its implications for responses to vaccination. Expert. Rev. Vaccines. 2002;1:453–459. doi: 10.1586/14760584.1.4.453. [DOI] [PubMed] [Google Scholar]
- Yin D, Tuthill D, Mufson RA, Shi Y. Chronic restraint stress promotes lymphocyte apoptosis by modulating CD95 expression. J. Exp. Med. 2000;191:1423–1428. doi: 10.1084/jem.191.8.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin D, Woodruff M, Zhang Y, Whaley S, Miao J, Ferslew K, Zhao J, Stuart C. Morphine promotes Jurkat cell apoptosis through pro-apoptotic FADD/P53 and anti-apoptotic PI3K/Akt/NF-kappaB pathways. J. Neuroimmunol. 2006a;174:101–107. doi: 10.1016/j.jneuroim.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Yin D, Zhang L, Wang R, Radvanyi L, Haudenschild C, Fang Q, Kehry MR, Shi Y. Ligation of CD28 in vivo induces CD40 ligand expression and promotes B cell survival. J. Immunol. 1999;163:4328–4334. [PubMed] [Google Scholar]
- Yin D, Zhang Y, Stuart C, Miao J, Zhang Y, Li C, Zeng X, Hanley G, Moorman J, Yao Z, Woodruff M. Chronic restraint stress modulates expression of genes in murine spleen. J. Neuroimmunol. 2006b;177:11–17. doi: 10.1016/j.jneuroim.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Zanin-Zhorov A, Tal-Lapidot G, Cahalon L, Cohen-Sfady M, Pevsner-Fischer M, Lider O, Cohen IR. Cutting edge: T cells respond to lipopolysaccharide innately via TLR4 signaling. J. Immunol. 2007;179:41–44. doi: 10.4049/jimmunol.179.1.41. [DOI] [PubMed] [Google Scholar]
- Zhang G, Ghosh S. Toll-like receptor-mediated NF-kappaB activation: a phylogenetically conserved paradigm in innate immunity. J. Clin. Invest. 2001;107:13–19. doi: 10.1172/JCI11837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Foster R, Sun X, Yin Q, Li Y, Hanley G, Stuart C, Gan Y, Li C, Zhang Z, Yin D. Restraint stress induces lymphocyte reduction through p53 and PI3K/NF-kappaB pathways. J. Neuroimmunol. 2008a;200:71–76. doi: 10.1016/j.jneuroim.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Woodruff M, Zhang Y, Miao J, Hanley G, Stuart C, Zeng X, Prabhakar S, Moorman J, Zhao B, Yin D. Toll-like receptor 4 mediates chronic restraint stress-induced immune suppression. J. Neuroimmunol. 2008b;194:115–122. doi: 10.1016/j.jneuroim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang Y, Miao J, Hanley G, Stuart C, Sun X, Chen T, Yin D. Chronic restraint stress promotes immune suppression through toll-like receptor 4-mediated phosphoinositide 3-kinase signaling. J. Neuroimmunol. 2008c;204:13–19. doi: 10.1016/j.jneuroim.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, McCorkle R, Seligman DA, Schmidt K. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav. Immun. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]