Abstract
Memory system circuitry may regulate how cues associated with cocaine are extinguished, and understanding neurosubstrates of extinction may lead to the development of improved treatment strategies for cocaine addiction. Sites within the hippocampus and amygdala were investigated for their role in regulating cocaine cue extinction learning. Initially, rats were trained to self-administer cocaine under a second-order reinforcement schedule (cocaine and cocaine cues present) followed by a 2-week abstinence period. Using lidocaine, rats next underwent bilateral inactivation of the dorsal subiculum (dSUB) or rostral basolateral amygdala (rBLA), asymmetric inactivation of the dSUB and rBLA, unilateral inactivation of the dSUB or rBLA, or ipsilateral inactivation of the dSUB and rBLA prior to cocaine cue extinction training sessions (only cocaine cues present) on 2 consecutive days. Relative to vehicle, bilateral and asymmetric lidocaine treatments in the dSUB and rBLA slowed cocaine cue extinction learning. Specifically, vehicle-treated rats exhibited a significantly larger difference in responding from day 1 to 2 of extinction training than lidocaine-treated rats. In comparison unilateral or ipsilateral lidocaine treatments in the dSUB and rBLA did not slow cocaine cue extinction learning. Rats treated with lidocaine and vehicle exhibited a similar difference in responding from day 1 to day 2 of extinction training. These results indicate that sites within the hippocampus and amygdala need to be functionally active simultaneously in at least one brain hemisphere for acquisition of cocaine cue extinction learning. These results further suggest that a serial circuit within each hemisphere mediates acquisition of cocaine cue extinction learning.
Keywords: Cocaine, Extinction learning, Lidocaine, Neurosubstrates, Self-administration
Introduction
Drug addiction consists of several distinct phases that are modeled in animals by studying acquisition, maintenance, extinction and reinstatement of drug-seeking and drug-taking behavior (See et al., 2003; Kosten et al., 1997; Arroyo et al., 1998; Markou et al., 1993; Comer et al., 1995; 1996). In cocaine self-administration studies, cues often are incorporated into instrumental conditioning procedures to investigate how behavior during different phases of addiction is influenced by their presence or absence (Arroyo et al., 1998; Schindler et al., 2002; See, 2005; Deroche-Gamonet et al., 2002; Atkins et al., 2008). Studies have identified various cortical (e.g., the orbitofrontal, anterior cingulate and prelimbic prefrontal cortex) and subcortical (e.g., the basolateral amygdala, dorsal and ventral hippocampus, nucleus accumbens and dorsal striatum) sites as important neurosubstrates for regulating behavior during the acquisition, maintenance and/or reinstatement phases of addiction (Atkins et al., 2008; Ito et al., 2004; Everitt et al., 2007; See, 2005; See et al., 2003; Kantak et al., 2002a, b; Di Pietro et al., 2006; Black et al., 2004; Thomas et al., 2003; Hearing et al., 2008). However, little is known about neurosubstrates that regulate behavior during extinction, which is of particular interest to clinicians using cue exposure therapy for treating substance use disorders (Obrien et al., 1990; Childress et al., 1993). Most information concerning neurosubstrates of extinction learning comes from fear conditioning studies in animals.
Following acquisition of conditioned fear, extinction is accomplished by re-exposing animals to the conditioned stimulus (CS; e.g., light cue) and omitting the unconditioned stimulus (US; e.g., footshock). Neurosubstrates of extinction learning established in the fear conditioning literature include the basolateral amygdala (BLA) and dorsal hippocampus (DH). Pharmacological disruption of the BLA was shown to impair acquisition of fear extinction (Herry et al., 2006; Sotres-Bayon et al., 2007), indicating the importance of the amygdala for fear extinction learning. Likewise, pharmacological disruption of the DH also impairs acquisition of fear extinction (Corcoran et al., 2005).
Neurosubstrates for extinction of an appetitive CS are less well studied, but the BLA has been implicated. For example, after pharmacological inactivation of the caudal BLA, rats displayed a resistance to extinction of a food CS (McLaughlin and Floresco, 2007). Moreover, when the BLA was inactivated following exposure to a cocaine CS, rats exhibited a disruption in extinction memory consolidation (Fuchs et al., 2006). In these studies, however, the BLA was manipulated and the animals tested for cue extinction following a period of response extinction training (CS and US omitted). As training that involves removal of the CS and US renders animals susceptible to cue-induced reinstatement of responding, it is unclear whether extinction or reinstatement processes were being affected by the manipulations. Extinction training consisting of cue exposure exclusively without prior response extinction training is a model of cue exposure therapy in people. Thus, we investigated if sites within the amygdala and hippocampus, individually or together, could influence cocaine cue extinction learning. This knowledge could potentially lead to treatments that augment cue exposure therapy for substance use disorders.
Materials and methods
Subjects
Male Wistar rats [Crl(WI)BR rats; Charles River Laboratories, Portage, MI, USA], weighing approximately 276–300 g upon arrival, were maintained at 90% of a free-feeding body weight while adjusting for growth throughout the duration of the study by providing 16 g of food per day. Between experimental sessions, rats were allowed unlimited access to water in their home cages. Rats were individually housed in clear plastic cages (43 × 22 × 20 cm) in a temperature-controlled (21–23 °C) and light-controlled (08:00 h on, 20:00 h off) vivarium. Policies and procedures set forth in Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Academy of Sciences) were followed, as well as specific national laws. The Boston University Institutional Animal Care and Use Committee approved all protocols.
Apparatus
Experimental chambers (model ENV-008CT; Med Associates, St Albans, VT, USA) were each equipped with two response levers positioned 8 cm to the left and right of a center-mounted food receptacle and 7 cm from the grid floor. Connected to the food receptacle was a pellet dispenser capable of delivering 45-mg food pellets (Dustless Precision Pellets; Bio-Serv, Frenchtown, NJ, USA). A white stimulus light was mounted 7 cm above each lever. Each chamber was outfitted with a single-channel fluid swivel (Instech Solomon, Plymouth Meeting, PA, USA) and a spring leash assembly, which were connected to a counterbalanced arm assembly (Med Associates) that allowed the animal to move freely in the chamber. A sound-attenuating cubicle (model ENV-108 m; Med Associates) equipped with a house light to provide general illumination, a fan to provide ventilation, and an 8-Ω speaker to provide auditory stimuli, enclosed each chamber. Motor-driven syringe pumps (model PHM-100; Med Associates) located inside each cubicle were used for intravenous drug delivery. A standard personal computer programmed in Medstate Notation and connected to an interface (Med Associates) controlled experimental events. An Olympus BX51 microscope (Olympus Optical, Tokyo, Japan), a Nikon DXM 1200 digital camera (Nikon, Tokyo, Japan) and Image Pro Plus software (version 4.5.1; Media Cybernetics, Silver Spring, MD, USA) were used to evaluate the histology.
Drugs and Intracranial Infusion Procedures
The drugs used were cocaine hydrochloride (National Institute on Drug Abuse, Bethesda, MD, USA), and lidocaine hydrochloride (Sigma-Aldrich, St Louis, MO, USA). Cocaine was dissolved in sterile 0.9% saline containing 3 IU heparin/mL to a final concentration of 2.68 mg/mL. For all self-administration sessions, a 1.0 mg/kg unit infusion dose of cocaine was used and delivered intravenously at a rate of 1.8 mL/min. To attain a dose of 1.0 mg/kg, the infusion volume was adjusted for body weight, resulting in drug delivery times of 1.2 s/100 g body weight in individual rats.
Lidocaine was dissolved in sterile 0.9% saline to a concentration of 200mg/ml. The solution was made fresh on testing days, immediately before intracranial infusions took place. The pH of all infusions, including 0.9% saline, was 5.0. For infusions, 0.5 μl containing either 100μg lidocaine or 0.9% saline was delivered at a rate of 0.5 μl/min into selected brain sites 5 min prior to each of two extinction test sessions. The 28-gauge stainless steel infusion cannula extended 1mm beyond the guide cannula tip. The infusion cannula was left in place for 1 min following the infusion.
Surgery and Histology
Rats were anesthetized with an intraperitoneal injection of 90 mg/kg ketamine plus 10 mg/kg xylazine. To enable intravenous delivery of cocaine or saline during self-administration sessions, a catheter made of silicon tubing (inner diameter, 0.51 mm; outer diameter, 0.94 mm) was implanted into the right jugular vein. The catheter ran subcutaneously under the neck, exited through an incision at the top of the head, and was attached to an L-shaped pedestal mount (Plastics One, Roanoke, VA, USA). Subsequent to catheter implantation, 0.1 mL of a solution containing 1.0 mg of methohexital sodium (Brevital; King Pharmaceuticals, Bristol, TN, USA) was infused intravenously as needed to maintain anesthesia for the remainder of the surgery.
After suturing the neck incision, the rat was placed into a stereotaxic frame, and 22-gauge stainless steel guide cannulae (Plastics One) were implanted. Within the hippocampus, the dorsal subiculum (dSUB) was targeted (anteroposterior [AP], −5.7 mm; lateral, ± 2.5 mm; dorsoventral, −2.3 mm) and within the amygdala, the rostral basolateral nucleus (rBLA) was targeted (AP, −2.0 mm; lateral, ± 4.5 mm; dorsoventral, −7.6 mm). These sites were selected because manipulation of sites within an identified discrete neural circuit may maximize the ability to detect an interaction between the hippocampus and amygdala for regulating cocaine cue extinction learning. Studies have shown that output from the DH occurs mainly through the dSUB (Naber and Witter, 1998), which in turn sends direct projections, though sparse, to the rBLA (Kishi et al., 2006). Moreover, there is direct innervation of the dSUB from the rBLA (Petrovich et al., 2001), and a convergence of outputs from the dSUB and rBLA onto neurons in the lateral part of the nucleus accumbens (Groenewegen et al., 1987; 1999). Therefore, cannulae were implanted into the dSUB of both hemispheres (n = 15); the rBLA of both hemispheres (n = 14); the dSUB of one hemisphere and the rBLA of the contralateral hemisphere (n=23); or the dSUB and rBLA of the same hemisphere (n = 8). Asymmetric and ipsilateral cannulae placements were counterbalanced to left and right sides. Guide cannulae were positioned 1 mm above the intended sites, and placements were based on the bregma coordinate system provided by the Swanson (1992) atlas. The guide cannulae, pedestal and three stainless steel anchoring screws were attached to the skull, and permanently imbedded in dental cement. Two 28-gauge stainless steel obturators (Plastics One) were used to occlude guide cannulae between infusions. Wounds were treated daily with topical Polysporin powder (Johnson and Johnson Consumer Products Company, Skillman NJ, USA) until healed, and rats were allowed 1 week of recovery from surgery before initiation of the study. Catheters were maintained by daily flushing (Monday–Friday) with 0.1 mL of a 0.9% saline solution containing 3 IU of heparin (Baxter Healthcare Corporation, Deerfield, IL, USA) and 6.7 mg of Timentin (Glaxo-SmithKline, Research Triangle Park, NC, USA). On Fridays, a locking solution consisting of glycerol and undiluted (1000 IU/mL) heparin (3:1) was used to fill the catheter dead space and minimize blockages. This solution remained in the catheters until Monday, when it was removed and replaced with the heparin/saline solution prior to the start of behavioral sessions. Additionally, catheters were checked for patency on a weekly basis by infusing a 1.0 mg/0.1 mL solution of Brevital intravenously, which produces a rapid temporary loss of muscle tone when catheters are functional. A new catheter was implanted into either the left jugular vein or right femoral vein to replace a leaking or non-functional catheter. Upon completion of the studies, rats were given an overdose of sodium pentobarbital and then intracardially perfused with saline and 4% paraformaldehyde solution. Brains were extracted, post-fixed in 4% paraformaldehyde for 1–4 h, and then stored in 30% sucrose at 4°C for 3 days. Forty-micrometer coronal sections were collected using a cryostat. Sections were then mounted on gelatin-coated slides and stained to verify bilateral, asymmetric and ipsilateral infusion cannulae placements.
Experimental procedures
Self-Administration Training under a Second-Order Schedule
Prior to surgery, rats were trained to press a lever under a fixed-ratio (FR) 1 schedule of food pellet delivery. After rats learned to rapidly press the lever for 50 pellets, right jugular vein catheters and guide cannulae were implanted. After 1 week of recovery from surgery, 1-h cocaine self-administration sessions were started. Rats were trained to self-administer 1.0 mg/kg cocaine, starting from a fixed-ratio 1 (FR 1) schedule of cocaine delivery and cue presentation. The terminal schedule, after incremental training, was a fixed-interval (FI)-based second-order schedule (FI 5-min [FR5:S]). Under this schedule, where S refers to the 2-s brief stimulus, each session began with the FI 5-min during which every fifth press (FR5) on the active lever resulted in a 2-s presentation of the cocaine-conditioned light cue located above the active lever. Intravenous cocaine delivery was contingent upon completion of an FR 5 on the active lever after the FI 5-min had elapsed. Infusions produced a discrete sound cue emitted by the pump motor for the duration of the infusion. In addition, the stimulus light above the active lever remained illuminated for the duration of the infusion as well as for the 20-s time-out period that followed each infusion, whereas the house light was extinguished during the time-out. Following the 20-s post-infusion time-out period, the schedule reverted to the 5-min FI component and continued as above. During the 1 hr sessions, rats could earn a maximum of 11 infusions. In addition, a 70-db contextual sound cue, continuous white noise, was present for the duration of each session. Thus, cocaine cues consisted of a response-contingent discrete light cue, discrete sound cue (pump motor) and a response-independent background contextual sound cue. Responses on the inactive lever were counted separately, but produced no scheduled consequences. Designation of the active and inactive lever was counterbalanced between right and left levers. Baseline training sessions were conducted 5 days a week during the light phase, and were continued until cocaine intake and responding were stable (number of infusions and responding did not deviate by more than 20%) and the number of responses on the inactive lever was no greater than 10% of active lever responses for each session over a 5-day period.
Cocaine Cue Extinction Testing
When cocaine self-administration baseline behavior was stable, rats underwent two weeks of abstinence prior to extinction tests. As two weeks of cocaine and cocaine cue deprivation make rats more cue reactive (Grimm et al., 2001), rats received ten 1 hr sessions in the operant chambers during the abstinence period for which the levers were retracted and cocaine and both types of cocaine cues were omitted. Inclusion of these sessions dampened the association between cocaine and the experimental chamber while leaving the saliency of the cocaine cues intact. Rats then received two extinction sessions on two consecutive days under the FI 5-min [FR5:S] second-order schedule for which cocaine delivery was omitted. For these 1-hr sessions, each 5 responses on the active lever during each FI resulted in presentation of the 2-sec light cue and the completion of 5 responses after each FI terminated resulted in the pump noise and a 20 sec presentation of the light cue with the house light extinguished, as above. In addition, the contextual sound cue (white noise) was present during extinction sessions. Thus, extinction consisted of sessions that evaluated the rate of lever pressing in the absence of cocaine but in the presence of discrete and contextual cues previously associated with cocaine. Five min prior to each extinction session, rats received infusion of vehicle or lidocaine into the rBLA of both hemispheres (bilateral rBLA); infusion of vehicle or lidocaine into the dSUB of both hemispheres (bilateral dSUB); infusion of vehicle or lidocaine into the dSUB of one hemisphere and the rBLA of the contralateral hemisphere (asymmetric dSUB/rBLA); infusion of lidocaine into the dSUB of one hemisphere with infusion of vehicle only into the contralateral rBLA (unilateral dSUB); infusion of lidocaine into the rBLA of one hemisphere with infusion of vehicle only into the contralateral dSUB (unilateral rBLA); or infusion of vehicle or lidocaine into the dSUB and rBLA of the same hemisphere (ipsilateral dSUB/rBLA). The asymmetric dSUB/rBLA vehicle control group was used additionally as the control group for the unilateral dSUB and unilateral rBLA lidocaine groups, as rats from these latter groups had asymmetric cannula placements.
Data analyses
Three dependent measures were calculated: (i) number of active lever responses; (ii) number of inactive lever responses; and (iii) number of infusions earned during self-administration sessions. Data from the last five cocaine self-administration sessions were averaged to establish a baseline. For extinction sessions, responses on the active lever were expressed as the percent of baseline responses on days 1 and 2 of testing, and as the difference in the number of responses from day 1 to day 2 of testing. Dependent measures were analyzed by one-factor ANOVA, two-factor mixed model repeated measures ANOVA, or t-test, as appropriate. The Tukey procedure was used for follow up testing. Data from seven rats whose cannulae placements were outside the targeted sites were not included in the analyses (see below). In addition, extinction responses of three rats were greater than 3 standard deviations from the mean in their respective treatment and cannulae placement groups; hence their data were not included in the analyses. Final group sizes for each condition were: bilateral dSUB vehicle (n=7); bilateral dSUB lidocaine (n=8); bilateral rBLA vehicle (n=7); bilateral rBLA lidocaine (n=7); asymmetric dSUB/rBLA vehicle (n=8); asymmetric dSUB/rBLA lidocaine (n=7); unilateral dSUB lidocaine (n=7); unilateral rBLA lidocaine (n=7); ipsilateral dSUB/rBLA vehicle (n=4); and ipsilateral dSUB/rBLA lidocaine (n=4).
Results
Histology
For rats used in the bilateral inactivation studies, placements were confirmed in 15 of 19 rats with cannulae aimed at the dSUB and in 16 of 19 rats with cannulae aimed at the rBLA. Placements were confirmed in 15 of 15 rats used in the asymmetric dSUB/rBLA study, 7 of 7 rats used in the unilateral dSUB study, 7 of 7 rats used in the unilateral rBLA study, and 8 of 8 rats used in the ipsilateral dSUB/rBLA study. Placements were within 0.5–0.6 mm of the intended position in the AP plane and within the dSUB and rBLA anatomical ranges (Swanson, 1992). Histological reconstruction of all dSUB and rBLA infusion sites with the theoretical spread of lidocaine is depicted in Fig. 1A–B. As a 0.5 μl volume of lidocaine is estimated to spread spherically with a radius of approximately 0.5 mm from the site of infusion (Tehovnik and Sommer, 1997), these results verify that lidocaine infusions predominantly encompassed the region of interest. Microscopic examination failed to reveal mechanical damage in either the dSUB or rBLA, other than that generated by insertion of the guide and infusion cannulae. Representative low-magnification (x2) photographs of cannulae tracks for dSUB and rBLA placements are shown in Fig. 1C. Furthermore, there was no evidence of cell loss following 2 microinjections of lidocaine, as illustrated in representative high-magnification (x20) photographs of the microinjection areas for the dSUB and rBLA (Fig. 1D).
Figure 1.
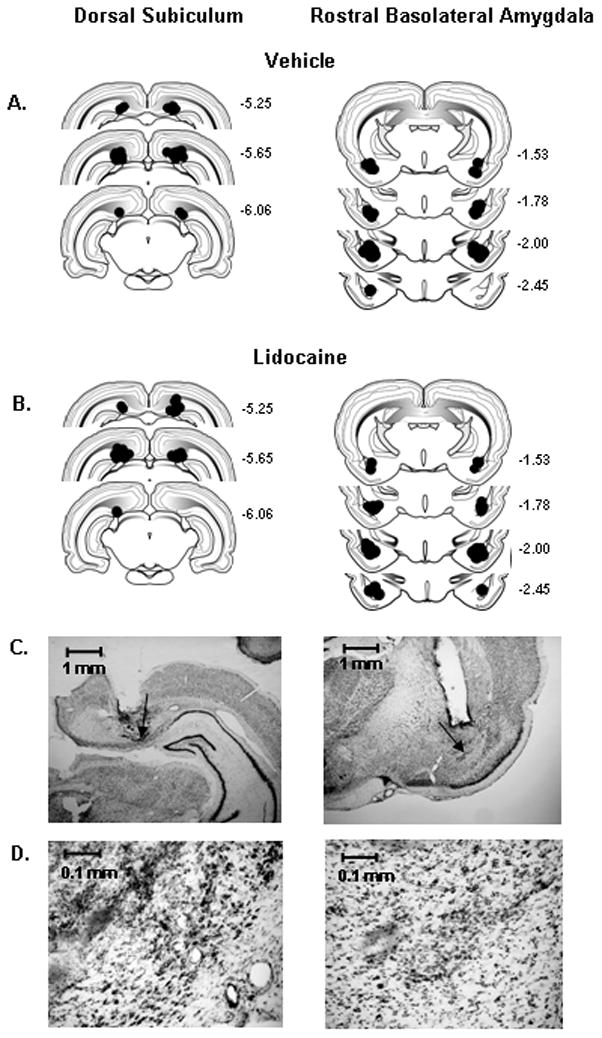
Schematic drawings representing coronal sections of the dorsal subiculum (dSUB) and rostral basolateral amygdala (rBLA) in all placement groups. Circles (0.5 mm radius) indicate the location and theoretical spread of vehicle (A) and lidocaine (B), based on the spherical volume equation for lidocaine (Tehovnik and Sommer, 1997). All drawings are based on the atlas of Swanson (1992), with the anterior-posterior references measured from bregma. Each placement is shown at the midpoint of its anterior-posterior extent. (C) Representative low-magnification (X2) photographs of guide cannulae placements within the dSUB (left) and rBLA (right); arrows indicate infusion site. (D) Representative high magnification (X20) photographs of lidocaine microinjection areas within the dSUB (left) and rBLA (right).
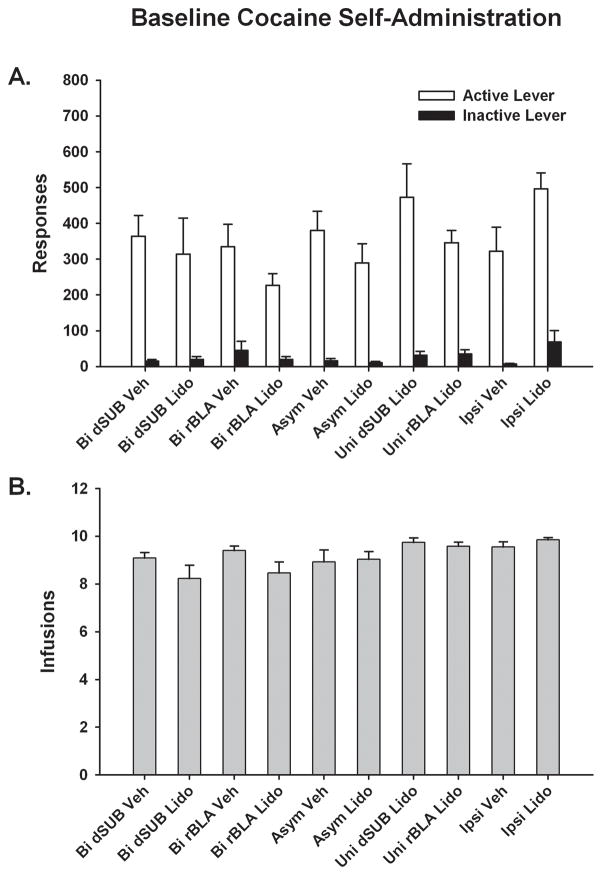
Baseline Cocaine Self-Administration
Prior to cocaine cue extinction training and testing with lidocaine or vehicle, the ten groups of rats had similar baseline cocaine self-administration behavior (Fig. 2A–B). Separate analysis of active lever responses (F[9,56]=1.3, p ≤ 0.27), inactive lever responses (F[9,56]=0.84, p ≤ 0.59) and infusions earned (F[9,56]=2.0, p ≤ 0.06) during baseline sessions showed no significant group differences, as determined by one-factor ANOVA.
Figure 2.
Baseline behavior during cocaine self-administration sessions in each of the ten groups of rats prior to any treatment. Values are the mean ± S.E.M. number of active and inactive lever responses (A) and infusions earned (B). Bi = Bilateral; Asym = Asymmetric; Uni = Unilateral; Ipsi = Ipsilateral; Veh = Vehicle; and Lido = Lidocaine.
Cocaine Cue Extinction Testing
Percent of Baseline Responses
In rats with bilateral dSUB cannulae placements, analysis of active lever responses revealed a significant main effect of extinction day (F[1,13]=11.4, p ≤ 0.01) indicative of fewer responses, overall, on day 2 than on day 1 of extinction training (Fig 3A). The main effect of treatment was not significant (F[1,13]=0.8, p ≤ 0.38), but there was a trend for a treatment X day interaction (F[1,13]=3.2, p≤ 0.09). An examination of Fig 3A suggests that the change in percent of baseline responses from day 1 to day 2 is more apparent in vehicle-treated than lidocaine-treated rats. In bilateral dSUB groups, the overall number of inactive lever responses during extinction averaged 30.9±8.0 for vehicle treatment and 23.2±7.9 for lidocaine treatment, with no significant differences between treatments (F[1,13]=0.5, p ≤ 0.51), over days (F[1,13]=0.3, p ≤ 0.62), or for the treatment X day interaction (F[1,13]=1.5, p ≤ 0.25). In rats with bilateral rBLA cannulae placements, analysis of active lever responses showed a significant main effect of extinction day (F[1,12]=23.8, p<0.001) indicative of fewer responses, overall, on day 2 than on day 1 of extinction training (Fig 3B). Neither the main effect of treatment (F[1,12]=2.3, p ≤ 0.15) nor the treatment X day interaction was significant (F[1,12]=0.63, p ≤ 0.44). In bilateral rBLA groups, analysis of the number of inactive lever responses showed that while the both groups averaged fewer than 30 responses, there was a significant main effect of extinction day (F[1,12]=10.0, p<0.01), but no treatment main effect (F[1,12]=1.4, p ≤ 0.26) or treatment X day interaction (F[1,12]=0.03, p ≤ 0.85). Overall, inactive lever responses were significantly lower on day 2 (13.7±5.0) than on day 1(23.3±5.7).
Figure 3.
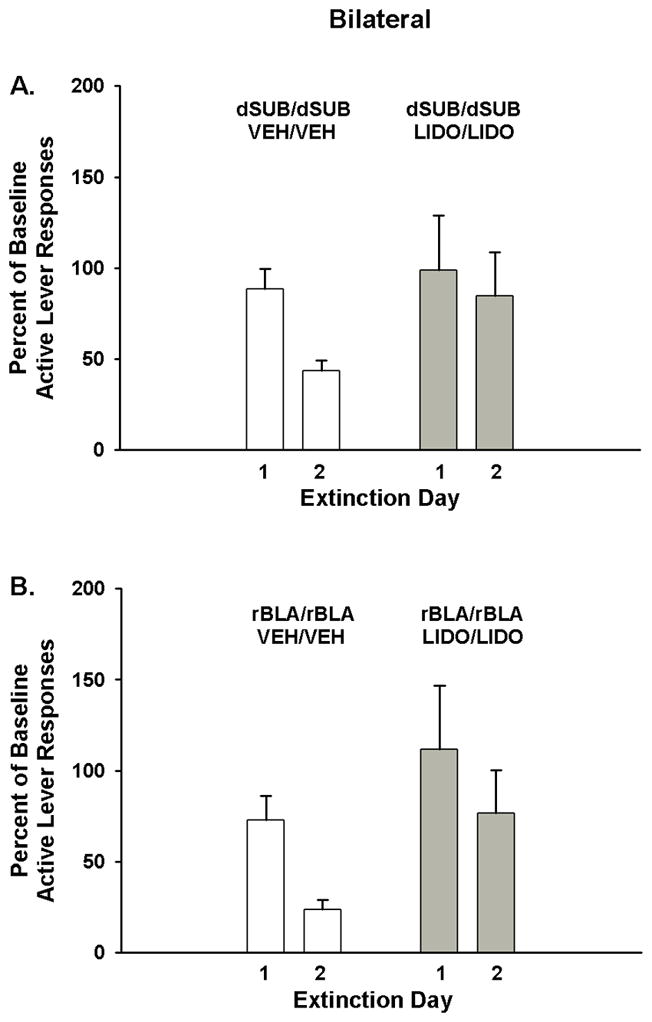
Active lever responding during cocaine cue extinction tests in rats with bilateral dorsal subiculum (dSUB) cannulae placements (A) and bilateral rostral basolateral amygdala (rBLA) cannulae placements (B) and treated with either vehicle (Veh) or lidocaine (Lido). Values are the mean ± S.E.M. active lever responses expressed as the percent of cocaine self-administration baseline responses.
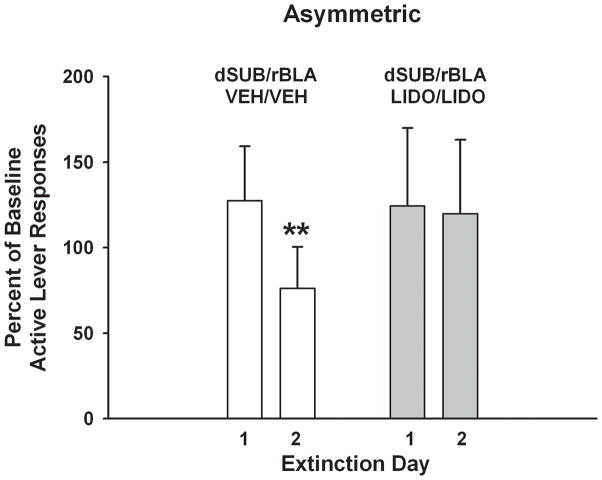
In rats with asymmetric cannulae placements, analysis of active lever responses revealed a significant main effect of extinction day (F[1,13]=8.6, p<0.01) but not of treatment (F[1,13]=0.2, p ≤ 0.69). The treatment X day interaction was significant (F[1,13]=6.0, p<0.03) and Tukey analyses of extinction days in each treatment group revealed that the percent of baseline responses was significantly lower on day 2 than on day 1 in rats that had received vehicle (p<0.002) but not in rats that had received lidocaine (Fig. 4). Analysis of the number of inactive lever responses showed that while the both groups averaged fewer than 25 responses, there was a significant main effect of extinction day (F[1,13]=4.62, p<0.05), but no treatment main effect (F[1,13]=0.5, p ≤ 0.49) or treatment X day interaction (F[1,13]=2.4, p ≤ 0.15). Overall, inactive lever responses were significantly lower on day 2 (10.7±2.2) than on day 1 (19.3±5.8).
Figure 4.
Active lever responses during cocaine cue extinction tests in rats with asymmetric, placements within the dorsal subiculum (dSUB) and rostral basolateral amygdala (rBLA) and treated with either vehicle or lidocaine. Values are the mean ± S.E.M. active lever responses expressed as the percent of cocaine self-administration baseline responses. **p < 0.01 compared to extinction day 1 responses within the same condition.
In a comparison of active lever responses between rats that received lidocaine in the dSUB and vehicle in the contralateral rBLA (unilateral dSUB inactivation) versus rats that received vehicle in both the dSUB and contralateral rBLA (asymmetric control), analysis showed a significant main effect of extinction day (F[1,13]=37.7, p<0.001) indicative of fewer responses, overall, on day 2 than on day 1 of extinction training (Fig 5A). Neither the main effect of treatment (F[1,13]=0.9, p ≤ 0.35) nor the treatment X day interaction was significant (F[1,13]=0.9, p ≤ 0.36). In unilateral dSUB groups, analysis of the number of inactive lever responses showed that while the both groups averaged fewer than 25 responses, there was a significant main effect of extinction day (F[1,13]=6.1, p<0.03), but no treatment main effect (F[1,13]=0.09, p ≤ 0.76) or treatment X day interaction (F[1,13]=1.2, p ≤ 0.29). Overall, inactive lever responses were significantly lower on day 2 (13.5±2.2) than on day 1 (24.1±5.3).
Figure 5.
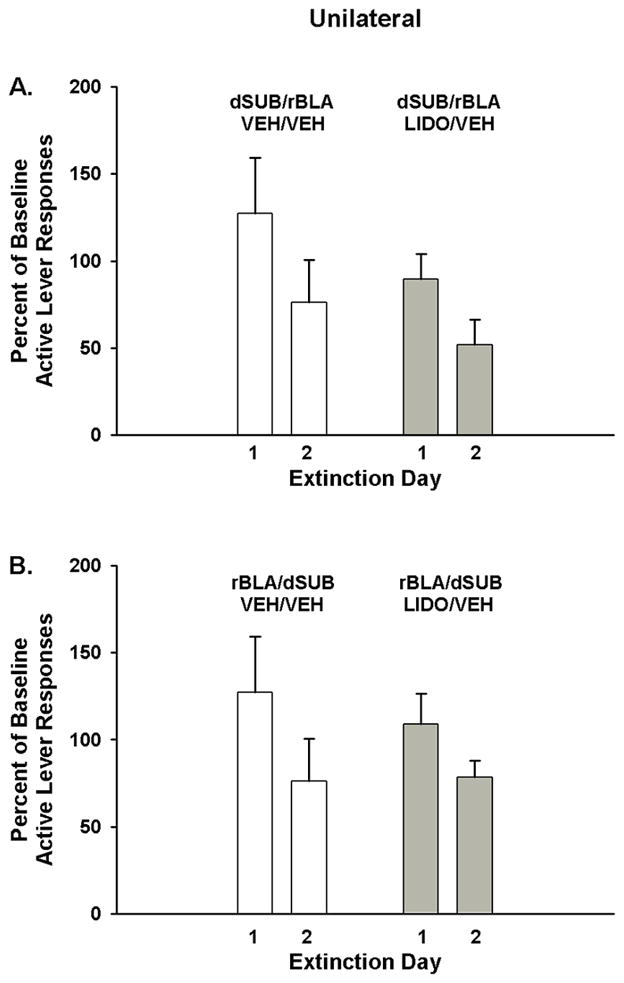
Active lever responding during cocaine cue extinction tests in rats with unilateral manipulation (asymmetric placements) within the (A) dorsal subiculum (dSUB) and (B) rostral basolateral amygdala (rBLA) and treated with either vehicle or vehicle and lidocaine. Values are the mean ± S.E.M. active lever responses expressed as the percent of cocaine self-administration baseline responses.
In a comparison of active lever responses between rats that received lidocaine in the rBLA and vehicle in the contralateral dSUB (unilateral rBLA inactivation) versus rats that received vehicle in both the rBLA and contralateral dSUB (asymmetric control), analysis showed a significant main effect of extinction day (F[1,13]=24.1, p<0.001) indicative of fewer responses, overall, on day 2 than on day 1 of extinction training (Fig 5B). Neither the main effect of treatment (F[1,13]=0.1, p ≤ 0.81) nor the treatment X day interaction was significant (F[1,13]=1.6, p ≤ 0.23). In unilateral rBLA groups, analysis of the number of inactive lever responses showed that while the both groups averaged fewer than 35 responses, there was a significant main effect of extinction day (F[1,13]=6.1, p<0.03), but no treatment main effect (F[1,13]=0.8, p ≤ 0.38) or treatment X day interaction (F[1,13]=0.2, p ≤ 0.63). Overall, inactive lever responses were significantly lower on day 2 (17.1±5.6) than on day 1(29.7±8.3).
In rats with ipsilateral cannulae placements, analysis of active lever responses revealed a significant main effect of extinction day (F[1,6]=32.3, p<0.001) indicative of fewer responses, overall, on day 2 than on day 1 of extinction training (Fig 6). Neither the main effect of treatment (F[1,6]=0.9, p ≤ 0.38) nor the treatment X day interaction was significant (F[1,6]=0.1, p ≤ 0.76). Analysis of the number of inactive lever responses showed that while there was no main effect of extinction day, there was a significant main effect of treatment (F[1,6]=9.3, p<0.02) and a significant treatment X day interaction (F[1,6]=7.0, p<0.04). Tukey analyses of extinction days in each treatment group revealed that inactive lever responses were significantly lower on day 2 than on day 1 (p<0.02) in lidocaine-treated rats (31.3±10.3 vs. 63.8±12.5, respectively), but not in vehicle-treated rats (16.0±7.5 vs. 11.5±5.1, respectively). In addition, lidocaine-treated rats had significantly higher inactive lever responses than vehicle-treated rats on day 1 of extinction testing (p<0.003), but not on day 2.
Figure 6.
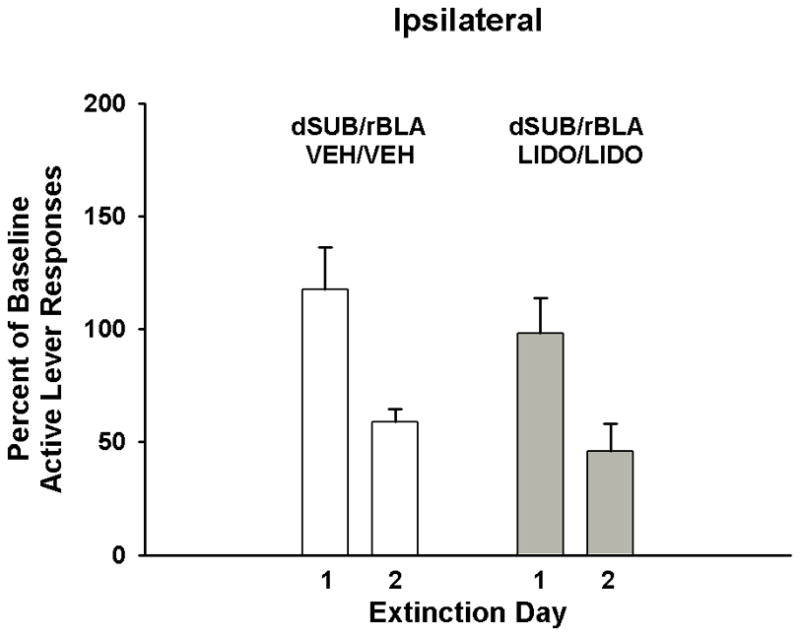
Active lever responding during cocaine cue extinction tests in rats with ipsilateral placements within the dorsal subiculum (dSUB) and rostral basolateral amygdala (rBLA) and treated with either vehicle or lidocaine. Values are the mean ± S.E.M. active lever responses expressed as the percent of cocaine self-administration baseline responses.
Difference in Responses
Expressing data as the difference in active lever responses from day 1 to day 2 of extinction training complement and clarify the above findings to reveal if cocaine cue extinction learning was slowed or if it progressed at a normal rate (Table 1). For rats with bilateral dSUB cannulae placements, the difference in the number of active lever responses from day 1 to day 2 of extinction training was significantly less (t [1,13] = 2.55, p<0.02) in lidocaine-treated rats compared to vehicle-treated rats. Likewise, for rats with bilateral rBLA cannulae placements, the difference in the number of active lever responses from day 1 to day 2 of extinction training was significantly less (t [1,12] = 2.18, p<0.05) in lidocaine-treated rats compared to vehicle-treated rats. In rats with asymmetric cannulae placements, the difference in the number of active lever responses from day 1 to day 2 of extinction training was significantly less (t [1,13] = 2.97, p<0.01) in lidocaine-treated rats compared to vehicle-treated rats as well. Importantly, rats that had received unilateral dSUB or unilateral rBLA lidocaine treatment showed a difference in the number of active lever responses from day 1 to day 2 of extinction training that was similar to rats that had received asymmetric vehicle treatment (t [1,13] = 0.818, p<0.98; t [1,13] = 1.52, p< 0.15, respectively). In addition, ipsilateral lidocaine treatment of the dSUB/rBLA resulted in a difference in the number of active lever responses from day 1 to day 2 of extinction training that also was similar to rats that had received ipsilateral vehicle treatment (t [1,6] = 2.19, p<0.07). Collectively, these findings indicate that cocaine cue extinction learning was slowed by bilateral and asymmetric inactivation of the dSUB and rBLA, but was not impaired by unilateral or ipsilateral inactivation of these sites.
Table 1.
Difference in the Number of Active Lever Responses from Day 1 to Day 2 of Extinction Training
| Placement | Site | Treatment | Difference |
|---|---|---|---|
| Bilateral | dSUB | Vehicle | 155 ± 34 |
| Lidocaine | 27 ± 36 a | ||
| Bilateral | rBLA | Vehicle | 148 ± 24 |
| Lidocaine | 70 ± 25 a | ||
| Asymmetric | dSUB/rBLA | Vehicle | 176 ± 47 |
| Lidocaine | 0.0 ± 33 a | ||
| Unilateral | dSUB | Vehicle | 176 ± 47 |
| Lidocaine | 178 ± 41 | ||
| Unilateral | rBLA | Vehicle | 176 ± 47 |
| Lidocaine | 90 ± 26 | ||
| Ipsilateral | dSUB/rBLA | Vehicle | 157 ± 26 |
| Lidocaine | 263 ± 41 | ||
p < 0.05 compared to corresponding vehicle control group
Discussion
Importance of the Hippocampus and Amygdala in Cocaine Cue Extinction Learning
Neuronal inactivation made prior to extinction training can reveal which brain regions are important neurosubstrates for the learning required to extinguish cocaine-seeking behavior. This approach is similar to the one used to establish the DH and BLA as neurosubstrates of fear extinction in rats (Corcoran et al., 2005; Herry et al., 2006; Falls et al., 1992). Bilateral infusion of lidocaine into either the dSUB or rBLA deterred extinction learning as shown by smaller reductions in cocaine-seeking behavior from day 1 to day 2 of extinction training than in vehicle-treated rats that showed a pronounced reduction in cocaine-seeking behavior from day 1 to day 2 of extinction training. Notably, there was no significant increase from day 1 to day 2 of extinction training in inactive lever responses in any group. All groups either showed no change or decreased inactive lever responses from day 1 to day 2 of extinction training, indicating that rats were not omitting active lever responses to accommodate pressing the inactive lever as an alternative cocaine-seeking strategy for earning response contingent infusions of cocaine. Our findings parallel those reported in the fear extinction literature showing that bilateral inactivation of the DH or BLA prior to extinction training prolongs the expression of fear-conditioned responses (Corcoran et al., 2005; Herry et al., 2006; Falls et al., 1992). However, in the present study, there are two possible interpretations of the effects of bilateral dSUB or rBLA inactivation. One interpretation is that each site is individually important for cocaine cue extinction learning and the other interpretation is that each site is an essential component of a circuit that regulates cocaine cue extinction learning. To discern between these possibilities, the effects of asymmetric inactivation were examined.
Insights from Asymmetric, Unilateral and Ipsilateral Inactivation
Asymmetric inactivation of the dSUB and contralateral rBLA deterred extinction from day 1 to day 2 of extinction training. While such findings suggest that the dSUB and rBLA interact in both hemispheres rather than function independently to regulate cocaine cue extinction learning, it is also possible that the disruption caused by lidocaine was due to an additive influence of each site from its respective hemisphere or to unilateral inactivation of a single site. The results of the unilateral and ipsilateral inactivation groups provide insight into these alternative possibilities.
Since unilateral inactivation was unable to produce a slowing of extinction analogous to the effects of bilateral or asymmetric inactivation, it can be concluded that unilateral inactivation of the dSUB or rBLA is insufficient to disrupt cocaine cue extinction learning. If the effects of asymmetric inactivation of the dSUB/rBLA reflected an additive influence from each site without interaction, we would expect to see a change in responding from day 1 to day 2 of extinction training in the unilateral groups that would fall between the level of reduction observed in rats that received vehicle and rats that received lidocaine in both hemispheres. In addition, if there were an additive influence from individual site inactivation, we would expect that ipsilateral dSUB/rBLA inactivation would deter extinction comparably to the asymmetric or bilateral inactivation groups. Since no deterrence of extinction was observed in the unilateral or ipsilateral groups, the idea that the disruption in extinction learning caused by asymmetric inactivation was due to inactivation of a single site or an additive influence of individual sites is not supported. Moreover, since rats that received unilateral dSUB, unilateral rBLA and ipsilateral dSUB/rBLA inactivation reduced responding from day 1 to day 2 to levels comparable to those observed in the vehicle-treated rats, these findings indicate that intact communication between the dSUB and rBLA in at least one hemisphere is sufficient for normal extinction of cocaine cues. Taken together, the unilateral, ipsilateral, and asymmetric data support the idea that the dSUB and rBLA interact circuit-wise in both hemispheres to regulate cocaine cue extinction learning.
It should be noted that studies have shown that hippocampal and amygdala-dependent learning and memory processes can be lateralized such that the hippocampus and amygdala in the right and left hemispheres may have differential function in rats (Klur et al., 2009; Berlau and McGaugh, 2006; Coleman-Mesches and McGaugh, 1995a, b, c). To control for possible lateralization of function, asymmetric, unilateral and ipsilateral cannulae placements were counterbalanced to right and left sides. If regulation of extinction learning is accomplished predominately from either the right or left hemisphere, we would have observed a disparity in the expression of extinction following inactivation of the right and left sides, as the hemispheres would be disrupted differentially. Additional post-hoc analysis of the difference in active lever responses from day 1 to day 2 of extinction training comparing left hemisphere inactivation with right hemisphere inactivation showed no significant differences in the asymmetric dSUB/rBLA group (56±59 vs. −42±26), unilateral dSUB group (138±42 vs. 208±66), unilateral rBLA group (95±38 vs. 85±42) or ipsilateral dSUB/rBLA group (305±22 vs. 221±79). From these data, it can be inferred that lateralization of brain function was not a factor contributing to whether cocaine cue extinction learning was acquired or deterred after asymmetric, unilateral and ipsilateral brain site inactivation.
Complementary Role of the Hippocampus and Amygdala in Fear and Cocaine Cue Extinction Learning
Both the DH and BLA have been shown to be important in extinction of conditioned fear, and both sites have been shown to interact to regulate learning and memory in fear conditioning (Corcoran et al., 2005; Herry et al., 2006; Falls et al., 1992; Roozendaal and McGaugh, 1997; Roozendaal et al., 1999; McIntyre et al., 2005). However, there is conflicting evidence regarding DH and BLA interaction in regulating extinction of conditioned fear (Berlau and McGaugh, 2006; Vianna et al., 2004). It was shown that post-training inactivation of the DH did not block the enhancement of contextual fear extinction induced by adrenergic activation of the ispilateral BLA (Berlau and McGaugh, 2006). However, post-training infusions would exclusively affect consolidation of extinction rather than acquisition of extinction. A different perspective offered by Vianna and colleagues (2004) highlights a necessary role of the BLA and DH in regulating fear extinction learning. These investigators found that gene expression, protein synthesis, and the extracellular signal-regulated kinase pathway are required in both the BLA and DH for generating extinction of conditioned fear.
The DH and BLA also have been shown to interact in behaviorally arousing associative learning tasks (Izquierdo and Medina, 1997; Lorenzini et al.,1996). Fear conditioning studies suggest that the hippocampus interacts with the amygdala during memory processes by providing contextual information during conditioning (Packard and Teather, 1998). Hence, it is possible for the DH to contribute to contextual encoding of other emotionally evocative learned behaviors, such as drug reinforcement. For example, bilateral inactivation of the DH and BLA with tetrodotoxin and asymmetric inactivation of the DH and contralateral BLA with baclofen/muscimol (but not unilateral or ipsilateral inactivation) selectively attenuated context-induced reinstatement of cocaine-seeking behavior in rats that underwent response extinction training (Fuchs et al., 2005; Fuchs et al., 2007). However, it is notable that these studies employed contextual reinstatement following response extinction. Our data present the first evidence that sites within the hippocampus and amygdala may be involved in the extinction of discrete and contextual cues formerly paired with cocaine.
Together, the bilateral, asymmetric, unilateral and ipsilateral findings provide evidence that the dSUB and rBLA may be components of a serial circuit within each hemisphere that mediates acquisition of cocaine cue extinction learning. This conclusion regarding a serial circuit is based on the fact that when this circuit was disrupted at the same loci in both hemispheres (bilateral dSUB or bilateral rBLA inactivation) or at different loci in opposite hemispheres (asymmetric dSUB/rBLA inactivation), cocaine cue extinction learning was disrupted. If, however, there was intact communication between the dSUB and rBLA on at least one side of the brain (unilateral dSUB inactivation, unilateral rBLA inactivation, ipsilateral dSUB/rBLA inactivation or vehicle-only treatments), cocaine cue extinction learning remained undisturbed. As anatomical studies have shown that there is no established contralateral connectivity between the dSUB and rBLA (Pikkarainen et al., 1999), our findings are consistent with the rationale that the dSUB and rBLA interact serially in each hemisphere to regulate different phases of drug-related learning and memory (Fuchs et al., 2005; Fuchs et al., 2007).
The mechanism by which the dSUB and rBLA interact to regulate cocaine cue extinction learning is unclear. Despite sparse direct connection from the dSUB to the rBLA (Kishi et al., 2006) and from the rBLA to the dSUB (Petrovich et al 2001), it remains possible that these sites interact through direct connection. It also is possible that the communication occurs via projections to downstream structures such as nucleus accumbens core where afferent projections from the dSUB and rBLA converge (Groenewegen et al., 1987;1999; Witter et al., 1990). Given the role of the nucleus accumbens core as a substrate for acquisition of cocaine-seeking behavior (Ito et al., 2004), one possibility is that the dSUB and rBLA combine respective contextual and emotional information at the level of the nucleus accumbens core to extinguish the salience of cocaine cues. Along these lines, past research has demonstrated an important role of the nucleus accumbens core for cocaine cue extinction learning (Torregrossa et al., 2010).
Conclusions
The neurosubstrates of extinction learning identified in the fear conditioning literature (DH and BLA) are complementary to the neurosubstrates of cocaine cue extinction learning identified in the present study (dSUB and rBLA). Cocaine cue extinction learning that is regulated by the dSUB and rBLA is intrahemispheric and is not characterized by laterality in rats. These findings suggest that medications found useful for augmenting cue exposure therapy for anxiety disorders also may be useful for augmenting cue exposure therapy targeting cocaine-related cues. Preclinical findings with the cognitive enhancing drug D-cycloserine support this idea (Nic Dhonnchadha et al., 2010).
Acknowledgments
The authors thank Mr. Jay Gorman for technical assistance. This project was supported by DA024315 from the National Institute on Drug Abuse and in part by SBE-0354378 from the National Science Foundation. The content is solely the responsibility of the authors, and does not necessarily represent the official views of the National Institute on Drug Abuse, National Institutes of Health or National Science Foundation.
Abbreviations
- AP
anteroposterior
- Asym
Asymmetric
- Bi
bilateral
- BLA
basolateral amygdala
- CS
conditioned stimulus
- DH
dorsal hippocampus
- dSUB
dorsal subiculum
- FI
fixed interval
- FR
fixed ratio
- Ipsi
ipsilateral
- Lido
lidocaine
- rBLA
rostral basolateral amygdala
- Uni
unilateral
- US
unconditioned stimulus
- Veh
vehicle
References
- Arroyo M, Markou A, Robbins TW, Everitt BJ. Acquisition, maintenance and reinstatement of intravenous cocaine self-administration under a second-order schedule of reinforcement in rats: Effects of conditioned cues and continuous access to cocaine. Psychopharmacology (Berl) 1998;140:331–344. doi: 10.1007/s002130050774. [DOI] [PubMed] [Google Scholar]
- Atkins AL, Mashhoon Y, Kantak KM. Hippocampal regulation of contextual cue-induced reinstatement of cocaine-seeking behavior. Pharmacol Biochem Behav. 2008;90:481–491. doi: 10.1016/j.pbb.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlau DJ, McGaugh JL. Enhancement of extinction memory consolidation: The role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiol Learn Mem. 2006;86:123–132. doi: 10.1016/j.nlm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Black YD, Green-Jordan K, Eichenbaum HB, Kantak KM. Hippocampal memory system function and the regulation of cocaine self-administration behavior in rats. Behav Brain Res. 2004;151:225–238. doi: 10.1016/j.bbr.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- Coleman-Mesches K, McGaugh JL. Differential involvement of the right and left amygdalae in expression of memory for aversively motivated training. Brain Res. 1995a;670:75–81. doi: 10.1016/0006-8993(94)01272-j. [DOI] [PubMed] [Google Scholar]
- Coleman-Mesches K, McGaugh JL. Muscimol injected into the right or left amygdaloid complex differentially affects retention performance following aversively motivated training. Brain Res. 1995b;676:183–188. doi: 10.1016/0006-8993(95)00108-3. [DOI] [PubMed] [Google Scholar]
- Coleman-Mesches K, McGaugh JL. Differential effects of pretraining inactivation of the right or left amygdala on retention of inhibitory avoidance training. Behav Neurosci. 1995c;109:642–647. doi: 10.1037//0735-7044.109.4.642. [DOI] [PubMed] [Google Scholar]
- Comer SD, Lac ST, Wyvell CL, Carroll ME. Combined effects of buprenorphine and a nondrug alternative reinforcer on i.v cocaine self-administration in rats maintained under FR schedules. Psychopharmacology (Berl) 1996;125:355–360. doi: 10.1007/BF02246018. [DOI] [PubMed] [Google Scholar]
- Comer SD, Lac ST, Wyvell CL, Curtis LK, Carroll ME. Food deprivation affects extinction and reinstatement of responding in rats. Psychopharmacology (Berl) 1995;121:150–157. doi: 10.1007/BF02245624. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci. 2005;25:8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Piat F, Le Moal M, Piazza PV. Influence of cue-conditioning on acquisition, maintenance and relapse of cocaine intravenous self-administration. Eur J Neurosci. 2002;15:1363–1370. doi: 10.1046/j.1460-9568.2002.01974.x. [DOI] [PubMed] [Google Scholar]
- Di Pietro NC, Black YD, Kantak KM. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. Eur J Neurosci. 2006;24:3285–3298. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Hutcheson DM, Ersche KD, Pelloux Y, Dalley JW, Robbins TW. The orbital prefrontal cortex and drug addiction in laboratory animals and humans. Ann N Y Acad Sci. 2007;1121:576–597. doi: 10.1196/annals.1401.022. [DOI] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: Blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Feltenstein MW, See RE. The role of the basolateral amygdala in stimulus-reward memory and extinction memory consolidation and in subsequent conditioned cued reinstatement of cocaine seeking. Eur J Neurosci. 2006;23:2809–2813. doi: 10.1111/j.1460-9568.2006.04806.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Vermeulen-Van der Zee E, te Kortschot A, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23:103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- Hearing MC, See RE, McGinty JF. Relapse to cocaine-seeking increases activity-regulated gene expression differentially in the striatum and cerebral cortex of rats following short or long periods of abstinence. Brain Struct Funct. 2008;213:215–227. doi: 10.1007/s00429-008-0182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Trifilieff P, Micheau J, Luthi A, Mons N. Extinction of auditory fear conditioning requires MAPK/ERK activation in the basolateral amygdala. Eur J Neurosci. 2006;24:261–269. doi: 10.1111/j.1460-9568.2006.04893.x. [DOI] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Medina JH. Memory formation: The sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem. 1997;68:285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2002a;22:1126–1136. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Stimulus-response functions of the lateral dorsal striatum and regulation of behavior studied in a cocaine maintenance/cue reinstatement model in rats. Psychopharmacology (Berl) 2002b;161:278–287. doi: 10.1007/s00213-002-1036-z. [DOI] [PubMed] [Google Scholar]
- Kishi T, Tsumori T, Yokota S, Yasui Y. Topographical projection from the hippocampal formation to the amygdala: A combined anterograde and retrograde tracing study in the rat. J Comp Neurol. 2006;496:349–368. doi: 10.1002/cne.20919. [DOI] [PubMed] [Google Scholar]
- Klur S, Muller C, Pereira de Vasconcelos A, Ballard T, Lopez J, Galani R, Certa U, Cassel JC. Hippocampal-dependent spatial memory functions might be lateralized in rats: An approach combining gene expression profiling and reversible inactivation. Hippocampus. 2009;19:800–816. doi: 10.1002/hipo.20562. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Haile CN, DeCaprio JL, Jatlow PI, Nestler EJ. Acquisition and maintenance of intravenous cocaine self-administration in lewis and fischer inbred rat strains. Brain Res. 1997;778:418–429. doi: 10.1016/s0006-8993(97)01205-5. [DOI] [PubMed] [Google Scholar]
- Lorenzini CA, Baldi E, Bucherelli C, Sacchetti B, Tassoni G. Role of dorsal hippocampus in acquisition, consolidation and retrieval of rat’s passive avoidance response: A tetrodotoxin functional inactivation study. Brain Res. 1996;730:32–39. doi: 10.1016/0006-8993(96)00427-1. [DOI] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology (Berl) 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Miyashita T, Setlow B, Marjon KD, Steward O, Guzowski JF, McGaugh JL. Memory-influencing intra-basolateral amygdala drug infusions modulate expression of arc protein in the hippocampus. Proc Natl Acad Sci U S A. 2005;102:10718–10723. doi: 10.1073/pnas.0504436102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin RJ, Floresco SB. The role of different subregions of the basolateral amygdala in cue-induced reinstatement and extinction of food-seeking behavior. Neuroscience. 2007;146:1484–1494. doi: 10.1016/j.neuroscience.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Naber PA, Witter MP. Subicular efferents are organized mostly as parallel projections: A double-labeling, retrograde-tracing study in the rat. J Comp Neurol. 1998;393:284–297. [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Szalay JJ, Achat-Mendes C, Platt DM, Otto MW, Spealman RD, Kantak KM. D-cycloserine deters reacquisition of cocaine self-administration by augmenting extinction learning. Neuropsychopharmacology. 2010;35:357–367. doi: 10.1038/npp.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan T, Ehrman R. Integrating systemic cue exposure with standard treatment in recovering drug dependent patients. Addict Behav. 1990;15:355–365. doi: 10.1016/0306-4603(90)90045-y. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Amygdala modulation of multiple memory systems: Hippocampus and caudate-putamen. Neurobiol Learn Mem. 1998;69:163–203. doi: 10.1006/nlme.1997.3815. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Pikkarainen M, Ronkko S, Savander V, Insausti R, Pitkanen A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. J Comp Neurol. 1999;403:229–260. [PubMed] [Google Scholar]
- Roozendaal B, Nguyen BT, Power AE, McGaugh JL. Basolateral amygdala noradrenergic influence enables enhancement of memory consolidation induced by hippocampal glucocorticoid receptor activation. Proc Natl Acad Sci U S A. 1999;96:11642–11647. doi: 10.1073/pnas.96.20.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. Basolateral amygdala lesions block the memory-enhancing effect of glucocorticoid administration in the dorsal hippocampus of rats. Eur J Neurosci. 1997;9:76–83. doi: 10.1111/j.1460-9568.1997.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Panlilio LV, Goldberg SR. Second-order schedules of drug self-administration in animals. Psychopharmacology (Berl) 2002;163:327–344. doi: 10.1007/s00213-002-1157-4. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol. 2005;526:140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- See RE, McLaughlin J, Fuchs RA. Muscarinic receptor antagonism in the basolateral amygdala blocks acquisition of cocaine-stimulus association in a model of relapse to cocaine-seeking behavior in rats. Neuroscience. 2003;117:477–483. doi: 10.1016/s0306-4522(02)00665-6. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Acquisition of fear extinction requires activation of NR2B-containing NMDA receptors in the lateral amygdala. Neuropsychopharmacology. 2007;32:1929–1940. doi: 10.1038/sj.npp.1301316. [DOI] [PubMed] [Google Scholar]
- Swanson L. Brain Maps: Structure of the Rat Brain. Elsevier; Amsterdam: 1992. [Google Scholar]
- Tehovnik EJ, Sommer MA. Effective spread and timecourse of neural inactivation caused by lidocaine injection in monkey cerebral cortex. J Neurosci Methods. 1997;74:17–26. doi: 10.1016/s0165-0270(97)02229-2. [DOI] [PubMed] [Google Scholar]
- Thomas KL, Arroyo M, Everitt BJ. Induction of the learning and plasticity-associated gene Zif268 following exposure to a discrete cocaine-associated stimulus. Eur J Neurosci. 2003;17:1964–1972. doi: 10.1046/j.1460-9568.2003.02617.x. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Sanchez H, Taylor JR. D-cycloserine reduces the context specificity of pavlovian extinction of cocaine cues through actions in the nucleus accumbens. J Neurosci. 2010;30:10526–10533. doi: 10.1523/JNEUROSCI.2523-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna MR, Coitinho AS, Izquierdo I. Role of the hippocampus and amygdala in the extinction of fear-motivated learning. Curr Neurovasc Res. 2004;1:55–60. doi: 10.2174/1567202043480170. [DOI] [PubMed] [Google Scholar]
- Witter MP, Ostendorf RH, Groenewegen HJ. Heterogeneity in the dorsal subiculum of the rat. distinct neuronal zones project to different cortical and subcortical targets. Eur J Neurosci. 1990;2:718–725. doi: 10.1111/j.1460-9568.1990.tb00462.x. [DOI] [PubMed] [Google Scholar]