Abstract
Immediate early gene (IEG) expression is coordinated by multiple MAP kinase signaling pathways in a signal specific manner. Stress-activated p38α MAP kinase is implicated in transcriptional regulation of IEGs via MSK-mediated CREB phosphorylation. The protein kinases downstream to p38, MAPKAP kinase (MK) 2 and MK3 have been identified to regulate gene expression at the posttranscriptional levels of mRNA stability and translation. Here, we analyzed stress-induced IEG expression in MK2/3-deficient cells. Ablation of MKs causes a decrease of p38α level and p38-dependent IEG expression. Unexpectedly, restoration of p38α does not rescue the full-range IEG response. Instead, the catalytic activity of MKs is necessary for the major transcriptional activation of IEGs. By transcriptomics, we identified MK2-regulated genes and recognized the serum response element (SRE) as a common promoter element. We show that stress-induced phosphorylation of serum response factor (SRF) at serine residue 103 is significantly reduced and that induction of SRE-dependent reporter activity is impaired and can only be rescued by catalytically active MK2 in MK2/3-deficient cells. Hence, a new function of MKs in transcriptional activation of IEGs via the p38α-MK2/3-SRF-axis is proposed which probably cooperates with MKs’ role in posttranscriptional gene expression in inflammation and stress response.
INTRODUCTION
Growth factors, inflammatory cytokines and various chemical and physical stresses can rapidly induce the transcription of immediate early genes (IEGs) in mammalian cells. The ERK, JNK and p38 MAP kinases regulate the complex intracellular signaling events contributing to IEG expression in a cell-type and stimulus specific manner (1). IEG products are implicated in diverse functions from cellular differentiation (2) to synaptic plasticity (3) and include transcription factors (2), RNA binding proteins (4), apoptosis regulators (5) and enzymes (6).
MK2 and MK3 are two structurally related protein kinases which share activators, substrates and physiological functions (7,8). Since MK2 is expressed to a much higher level than MK3, the phenotype of the MK2 knockout mouse could not be compensated by MK3 and already indicates an essential role of this protein kinase in posttranscriptional regulation of biosynthesis of cytokines, such as TNF and IL-6, at the level of mRNA stability and translation via AU-rich elements (ARE) in the 3′-UTRs (9,10). The role of MK3 in cytokine biosynthesis becomes significant only in a MK2-free background where it significantly contributes to posttranscriptional regulation of TNF biosynthesis (11). As targets for the p38/MK2/3 pathway several ARE-binding proteins involved in regulation of mRNA decay or translation, such as tristetraprolin (TTP), KSRP and hnRNP A0 (12–14), have been identified. So far, it is believed that the inflammatory gene expression is coordinated transcriptionally mainly by the NFkB-pathway together with p38α, while MK2/3 are exclusively involved in posttranscriptional regulation (15).
MK2 knockout and, even more pronounced MK2/3 double knockout (DKO) cells display a significantly reduced p38α level (11). The MK2 level in p38α-deficient cells is also decreased (16), indicating mutual stabilization of both proteins. No functional consequence of reduced p38 levels in MK2/3 deficient cells has been reported so far. In the present study we analyzed stress induced gene expression and p38 signaling in MK2/3-deficient cells. In order to distinguish between the roles of p38α and MKs we employed a catalytic-dead mutant of MK2 which recovers the p38α level in MK2/3-deficient cells without restoration of MKs’ catalytic activity. Defects which cannot be rescued by the catalytic-dead mutant of MK2 could be attributed to the direct MK2/3 catalytic function. Using this strategy, we analyzed stress-dependent activation of IEGs and revealed a coordinated regulation of gene expression by the stress-activated p38/MK2/3 pathway at different levels.
MATERIALS AND METHODS
Cells culture and treatments
All cells were cultured in DMEM containing 10% serum, 2 mM l-glutamine, 100 U of penicillin G/ml and 100 µg of streptomycin/ml. Generation of MK2/3−/− (11) and p38α−/− (17) mouse embryonic fibroblasts (MEFs) were performed as reported earlier. To immortalize primary MEFs, cells were cotransfected with pSV40Tag encoding simian virus 40 large T-antigen and pREP8 plasmid (Invitrogen) in a 10:1 mixture; colonies were selected with 3 mM histidinol (Sigma). For western blots, cells were counted and seeded in six-well plates, starved for 16 h and stimulated as indicated.
Reagents and antibodies
PD184352 was from Upstate Biotechnology. SB-203580, SB-202190, Phorbol 12-myristate 13-acetate (PMA), Anisomycin, Hygromycin-B and MG132 were from Calbiochem. Cycloheximide (CHX), Actinomycin-D and Phalloidin-TRITC were from SIGMA. Antibodies that recognize p38 MAPK, phospho-p38 MAPK, MAPKAPK-2, phospho-MAPKAPK-2 (Thr222), phospho-SRF (Ser103), phospho-MSK1 (Thr581), Elk1 and CREB were from Cell Signaling Technology, Inc. Antibody against phospho-Hsp25 (Ser86) was from Biosource, anti-phospho-CREB (Ser133) was from Upstate, anti-GAPDH (glyceraldehyde-3-phosphate dehydrogenase) antibody was from Chemicon. ERK1/2 antibodies were from BD Transduction Laboratories. Anti-GFP anti-MSK1, anti-SRF, anti-phospho Elk1 (S383) and Horseradish peroxidase-conjugated secondary antibodies were from Santa Cruz.
Vector constructs and cloning
pMMP-IRES retroviral vectors expressing MK2 (11) and pMSCV-p38α (17) were described previously. pGL3-egr1 promoter reporter constructs were provided by Dr J.L. Jameson (18). pcDNA3-Flag-MKK6-EE (19) pcDNA3-myc-MK2, MK2-EE and MK2-K79R plasmids (20) were reported earlier. The plasmids pcDNA3-SRF-WT and -SRF-S103A were gifts from Dr Michael Greenberg (Harvard Medical School, Cambridge) and from Dr Linda Sealy (Vanderbild University, Nashville), respectively.
Retroviral gene transfer
MK2/MK3 double-deficient immortalized mouse embryonic fibroblasts were transduced with the bicistronic pMMP-IRES MK2 or MK2-K79R vectors following the previously reported method (11). The different cell lines were sorted for comparable expression of GFP. For pMSCV or pMSCV-p38 transduction of p38−/− MEFs, retroviral supernatants were generated by transient transfection of the BD EcoPack 2-293 packaging cell line. Virus treated cells were selected on 100–200 µg/ml hygromycin and used for experiments.
Western blotting
Soluble protein extract was run on sodium dodecyl sulfate polyacrylamide gel electrophoresis (10% or 7.5–16% gradient PAGE) gels and transferred to Hybond ECL membranes (Amersham Pharmacia Biotech). Blots were incubated for 1 h in PBS–0.1% Tween-20 containing 5% powdered skim milk. After three washes with PBS–0.1% Tween-20, membranes were incubated for 16 h with the primary antibody at 4°C and for 1 h with horseradish peroxidase-conjugated secondary antibodies (diluted 2000-fold) at room temperature. Blots were developed with an ECL detection kit (Santa Cruz Biotechnology) and the digital chemiluminescence images were taken by a Luminescent Image Analyzer LAS-3000 (Fujifilm).
Real-time PCR
MEFs were stimulated as indicated and RNA was extracted using the NucleoSpin RNA purification method (Machery-Nagel). RNA was reverse-transcribed (Fermentas), and real-time PCR (Q-PCR) was carried out using SYBR Green chemistry for egr1, c-fos and 18S transcripts. Taqman assays (Applied Biosystems) were used for quantifying TTP and actin expression. PCR was performed on a Rotorgene 2000 real-time PCR instrument. The threshold cycle (CT) for each individual PCR product was calculated by the instrument software, and CT values obtained were normalized against 18S rRNA or actin mRNA. Primer sequences to the target genes are available on request.
Immunoprecipitation and kinase assay for MSK
Antipeptide antibody that recognize MSK1 (residues 384–402) was used to immunoprecipitate endogenous MSK1 from cell lysates as described previously (21). MSK1 kinase activity against peptide (GRPRTSSFAEG) was assayed after immunoprecipitation from 0.5 mg of cell lysate as described (22). One unit of activity was that amount of enzyme that incorporates 1 nmol of phosphate into the peptide substrate in 1 min.
Immunocytochemistry
MEFs were seeded on poly-l-lysine-coated coverslips, serum starved over night and then left unstimulated or were stimulated with 10 µg/ml of anisomycin for 40 min. After washing with PBS, cells were fixed with 4% paraformaldehyde and were permeabilised with 0.2% Triton. After 1 h of incubation with 1% BSA/PBS cells were incubated for 1.5 h with primary antibodies: anti-p38 (Mouse IgG1; Cat. 612168; BD) or anti-phospho-p38 (Mouse IgG1; Cat. 612288; BD) followed by Cy3 labeled secondary antibodies (Cat. 115-165-146; Dianova).
DNA microarray experiment
Around 5 × 105 MK2/3 DKO MEFs transduced with MK2 or empty vector were starved over night and then were left unstimulated or were stimulated with 10 µg/ml anisomycin for 90 min. Three independent experiments were done under same conditions. RNA was extracted using the NucleoSpin RNA purification method (Machery-Nagel). Labeled targets for oligonucleotide arrays were prepared using 15 µg of total RNA according to the protocol provided by Affymetrix (Santa Clara, CA, USA). Fragmented biotinylated cRNA (10 µg) in hybridization buffer containing 10% DMSO was hybridized to the Affymetrix Mouse Genome 430 2.0 GeneChip Array® which interrogates over 39 000 transcripts. Raw intensity values were processed using Affymetrix MAS 5.0 software.
Luciferase reporter assays
For co-transfection experiments HeLa cells were seeded in 24 well plates and transfected using polyethylenimine reagent (23). Twenty four hours posttransfection cells were lysed and β-galactosidase and luciferase assays performed as described earlier (24). In case of anisomycin stimulation, transiently transfected HeLa cells were serum starved over night and stimulated for 5 h with 25 ng/ml anisomyin in 1%FCS supplemented medium. Luciferase activity was measured and normalized to that of β-galactosidase. MK2−/− and WT-SRE reporter cell lines were reported previously (25). To generate egr1-promoter reporter cell lines, rescued and control DKO cells were transfected with mouse egr1-promoter reporter constructs—pGL3-mEgr 1-370 or pGL3-mEgr1 1-370mut (all SREs and Ets box mutated) (18) and pBABE-puro plasmid in 7:1 ratio. Cell lines were selected using 2 µg/ml puromycin and clones were pooled and used. For experiments with reporter cell lines, cells were seeded in 12 well plates, serum starved for 20 h and stimulated with 25 ng/ml anisomyin or 100 ng/ml PMA in 1% FCS supplemented medium. Luciferase activity was measured and normalised to total protein content.
RESULTS
Anisomycin, but not PMA-induced IEG expression is affected in MK2/3 double deficient cells
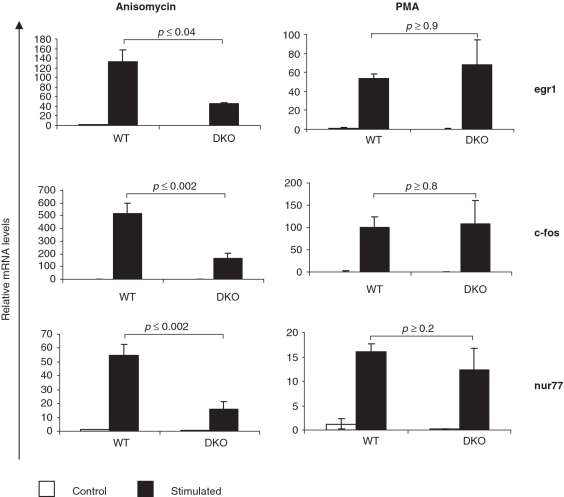
MAP kinases are known to regulate IEG expression in a stimulus-dependent manner (1,26). Previous studies have shown a role for p38 MAPK in the regulation of stress induced IEG expression (27–29). To analyze the role of MK2/3 in stress induced gene expression, we monitored transcript levels of a set of well characterized IEGs including egr1, c-fos and nur77 in MK2/3 DKO fibroblasts. As seen in Figure 1, gene expression induced by anisomycin (10 µg/ml), a stress stimulus and a strong activator of the p38 pathway, is severely affected in MK2/3 DKO fibroblasts. In contrast to anisomycin-stimulated changes in gene expression, gene expression induced by PMA is not significantly altered in MK2/3 DKO fibroblasts. IEG expression induced by H2O2 and UV, two other stress stimuli also gave results similar to that for anisomycin (data not shown).
Figure 1.
Stress-induced IEG expression is compromised in MK2/3 double-deficient cells. Anisomycin- (left panel) and PMA-induced (right panel) expression of egr1, c-fos and nur77 transcripts was analyzed in WT and MK2/3 double-deficient immortalized MEFs. MEFs were starved over night and stimulated for 60 min with PMA or anisomycin (10 µg/ml). IEG transcript levels were determined by RT–PCR and normalized using S18 mRNA. The experiments were performed in triplicate. Anisomycin-dependent stimulation was analyzed in three independent experiments.
Stress induced nuclear signalling via the p38-MSK1-CREB axis is affected in MK2/3 DKO MEFs
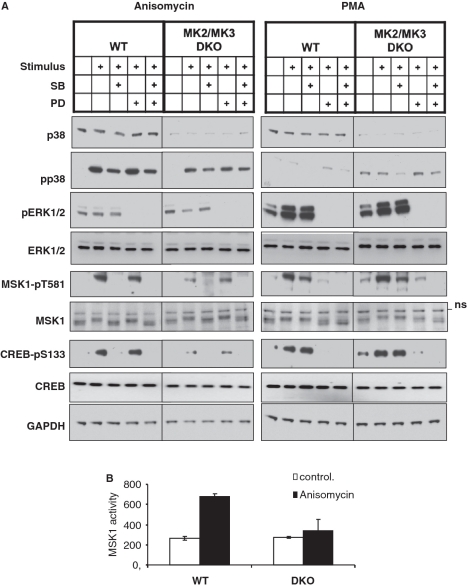
A reduced p38α level was detected in MK2-deficient and, even more pronounced, in MK2/3 double-deficient cells (11,30), making these cells a ‘knockdown’ for p38α as well. p38 was previously shown to regulate stress-induced IEG expression via phosphorylation and activation of MSK1 (22). This led us to hypothesize that the IEG expression defects in MK2/3 DKO cells result from reduced p38 levels. To verify this hypothesis we analyzed MSK1 signaling in MK2/3 DKO fibroblasts. MSK1 is a protein kinase known to be activated via p38 upon stress conditions and via ERKs upon mitogenic stimulation (21). As seen in Figure 2A, anisomycin-induced MSK1-phosphorylation at T581, a regulatory phosphorylation site which correlates with activity of MSK1 (31), is reduced in MK2/3-deficient cells. In contrast, PMA-induced ERK-dependent activation of MSK1 is not impaired. Small molecule inhibitors (SB203580 for p38α/β and PD184352 for activation of MEK1/2) were used to demonstrate the specific activation of the p38 and ERK pathway by anisomycin and PMA, respectively. To compare MSK1 activity between MK2/3 DKO and WT MEFs, we also determined phosphorylation of its substrate cAMP response element-binding protein (CREB). CREB phosphorylation at S133 is also reduced in MK2/3 DKO cells upon anisomycin, but not PMA stimulation (Figure 2A). MSK1 activity was then measured in the lysate of WT and MK2/3 DKO MEFs using the specific substrate peptide crosstide GRPRTSSFAEG (22). As seen from Figure 2B, there is significantly lower MSK1 activity in lysate from anisomycin-stimulated MK2/3 DKO MEFs compared to WT MEFs. Similar results were also obtained for immortalized MEFs of the same genotypes (data not shown).
Figure 2.
MSK1 signaling in primary MK2/3 DKO MEFs. (A) Western blot analysis of p38 expression and anisomycin- and PMA-induced phosphorylation of p38, ERK1/2, MSK and CREB in the absence and presence of the p38α/β-inhibitor SB203580 (SB) and the MEK1/2 inhibitor PD184352 (PD). WT and MK2/3 DKO primary MEFs were starved over night and then were left unstimulated or were stimulated with 10 µg/ml anisomycin for 30 min or with 400 ng/ml PMA for 20 min. Where indicated 5 µM SB203580 or 2 µM PD184352 or both inhibitors were added to the cells 1 h prior to stimulation. As loading control expression of GAPDH was monitored. (B) MSK1 activity measured in non-stimulated and anisomycin-stimulated WT and MK2/3 DKO primary MEFs as described in ‘Materials and Methods’ section. MSK1 activity is represented as ATP incorporation per milligram of protein.
Predominant nuclear localization of p38α and phospho-p38α is unaffected in MK2/3 DKO cells
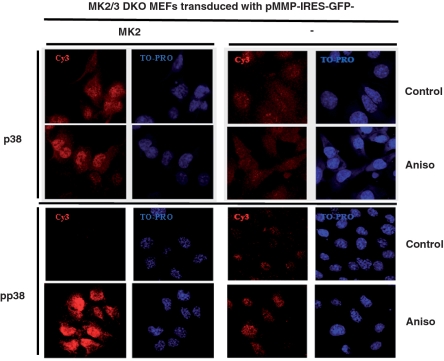
p38 is known to shuttle between nucleus and cytoplasm and MK2 was previously shown to be a major regulator of subcellular localization of p38α (32). Since MSK1 is a nuclear kinase (21) we then asked whether altered subcellular localization of p38α in the absence of MK2/3 could account for defective MSK1 signaling. Intracellular staining of endogenous p38 with pan p38 antibodies as well as with phospho-specific p38 antibodies revealed preferential nuclear localization of p38 in non-stimulated and anisomycin-treated MK2-restored cells (Figure 3). The level of phospho-p38 (pp38) is strongly increased after anisomycin-treatment, but pp38 still localises preferentially in the nucleus. In MK2/3-deficient cells transduced with empty vector alone, the signals for p38 and pp38 are much weaker but still show preferential nuclear localization. A slightly increased basal phosphorylation of the remaining p38 detected in the MK2/3-deficient cells (Figure 3) could also be seen in western blot (Figure 2A PMA-panel and Figure 4A). The specificity of p38 and pp38 staining was confirmed by use of p38α-deficient cells (Supplementary Figure S1). This analysis indicates that reduced MSK1 activation in MK2/3-deficient cells is not due to altered subcellular localization of p38.
Figure 3.
Neither MK2 nor stress stimuli alter preferential nuclear localization of p38α. Subcellular localization of endogenous p38α (upper panel) and pp38α (lower panel) in MK2/3 DKO MEFs transduced with MK2 or empty vector was detected by immunocytochemistry and confocal microscopy. Cells were serum starved over night and then were left non-stimulated or were stimulated with anisomycin (10 µg/ml) for 40 min. The red (Cy3) signal denotes specific p38 or pp38 staining. Nuclei are stained by TO-PRO shown in blue.
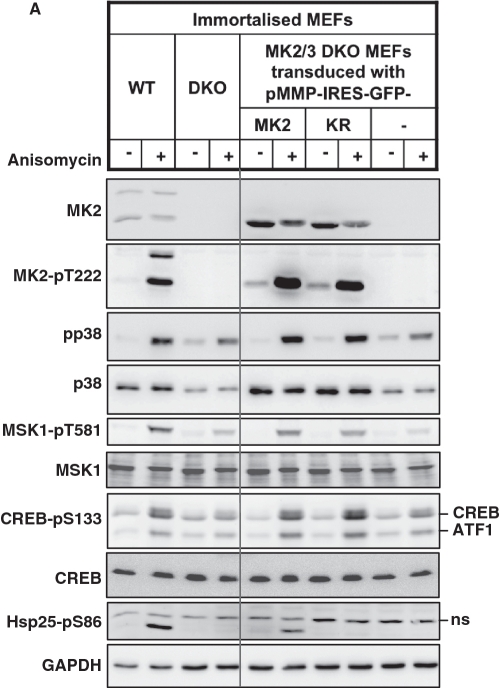
Figure 4.
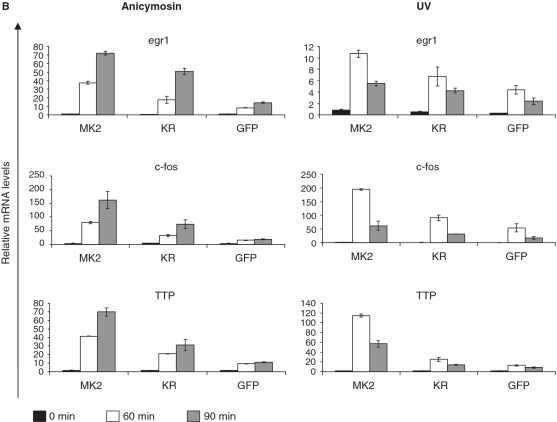
Catalytic activity of MK2 is not necessary for rescue of p38α-MSK1 signaling but indispensable for rescue of the full IEG response. Immortalized MK2/3 DKO MEFs stably transduced with MK2, MK2-K79R or GFP alone were starved over night and then were left non-stimulated or were stimulated with anisomycin (10 µg/ml) or UV (200 J/m2). (A) Rescue of anisomycin-stimulated p38/MSK1/CREB signaling by both, MK2 and catalytic inactive MK2-K79R in MK2/3 DKO MEFs after 30 min of treatment. Phosphorylation of Hsp25 cannot be rescued by MK2-K79R. (B) Rescue of anisomycin- and UV-induced IEG expression (egr1, c-fos, TTP) in MK2/3 DKO MEFs by MK2 and MK2-K79R. Transcript levels after 60 and 90 min were determined by RT-PCR, normalized to the actin signal and displayed. Representative data from six independent experiments performed in duplicates are shown. The inhibitory concentration of anisomycin leads to a more sustained IEG expression while UV-treatment and subinhibitory anisomycin concentration (10–50 ng/ml, not shown) yield a more transient IEG response (cf. Supplementary Figure S3).
Restoration of p38 levels and MSK signaling does not completely rescue stress induced IEG expression
Even though MK2/3 was shown to be necessary for stabilizing p38α, no functional effects due to reduced p38 levels in MK2/3-deficient cells have been reported. Reduced anisomycin-induced MSK1 activity in MK2/3-deficient cells clearly showed defective p38 signaling in these cells. To distinguish between effects of reduction of p38α level and the effects of lack of MK2/3 catalytic activity, a catalytic-dead, p38α-stabilizing mutant of MK2 [MK2-K79R (20,30)] was introduced into the immortalized MK2/3 DKO MEFs and compared with the effects of introduction of the catalytically active wild-type MK2 or empty vector (GFP) alone (Figure 4). The ectopically expressed WT and mutant proteins show similar levels of expression and similar p38-dependent phosphorylation at the regulatory site T222 in the activation loop of MK2. As seen from Figure 4A, MK2-K79R is able to completely rescue the level of p38α and direct p38α signaling as monitored by anisomycin-stimulated phosphorylation of p38α, MSK1 and CREB. In contrast, it could not rescue phosphorylation of Hsp25, a known direct substrate of MK2/3 (33). Only WT MK2 is able to rescue both p38α signaling and Hsp25 phosphorylation.
We further analyzed anisomycin- and UV-stimulated expression of egr1, c-fos and TTP transcripts in these cells (Figure 4B). In the DKO fibroblasts rescued with MK2 increased levels of IEG transcripts, which are similar to the levels in WT cells (not shown), could be detected after 60 and 90 min of UV treatment or stimulation with anisomycin (10 µg/ml) when compared with the GFP control. Used in this concentration, anisomycin also inhibits protein synthesis by interfering with peptidyl transferase of S80 ribosomes. However, when anisomycin is used in subinhibitory concentrations (10–50 ng/ml) (34) comparable activation of p38 and stimulation of IEG is reached after 60 min (Supplementary Figures S2 and 3). Interestingly, as a result of the phenomenon of super-induction (probably due to a decrease of labile transcriptional repressor proteins or mRNA degrading enzymes) a sustained IEG response is seen after 90 min of treatment with the inhibitory anisomycin concentration making the experimental readout more robust. In all experiments, expression of MK2-K79R, which completely rescues p38α level and activity in MK2/3 DKOs, could only partially rescue IEG transcription (Figure 4B), indicating that both catalytic activity of p38α and MK2/3 are necessary for the full IEG response.
Anisomycin-induced IEG transcripts are stable independent of MK2 catalytic activity
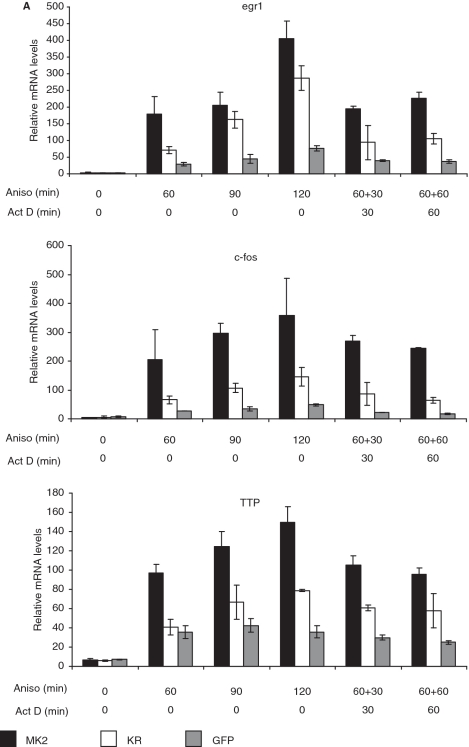
IEG expression is normally transient due to extreme instability of their mRNAs (35). Since it is known that MK2 regulates stability of several mRNAs containing AREs in their 3′-non-translated regions (20), including TTP mRNA (36), we analyzed transcript stability in the given experimental setting by comparing transcript levels after anisomycin stimulation in the absence and presence of the transcriptional inhibitor actinomycin D. In the absence of actinomycin D, all transcripts analyzed are relatively stable and their levels continuously increased from 0 to 120 min of anisomycin treatment. The effect of actinomycin D is clearly seen when comparing the anisomycin treatment of 120 min with the same treatment where actinomycin D was added after 60 min (Figure 5A, ‘120 + 0’ versus ‘60 + 60’). However, no significant rescue-dependent changes in transcript stability can be detected between the different variants of transduced MEFs. Instead, all transcript levels after 60 min of anisomycin stimulation are close to the levels detected after additional 30 or 60 min of actinomycin D treatment. This indicates that the transcripts analyzed are relatively stable in MEFs stimulated with inhibitory concentrations of anisomycin and that their stability is not under stringent control of MK2 or p38α in these cells. This does not exclude lower IEG mRNA stability in other cell types under other experimental conditions.
Figure 5.
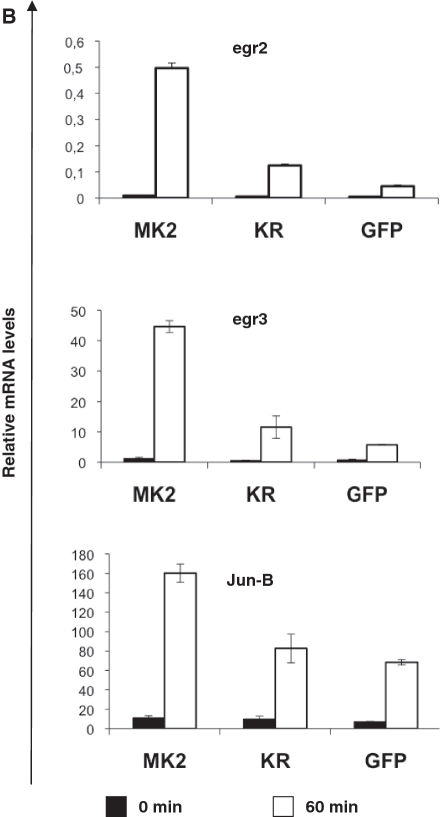
Catalytic activity of MK2 does not change IEG mRNA stability in MEFs but affects IEG transcription. (A) MK2/3 DKO MEFs transduced with MK2, MK2-K79R or empty vector were starved over night and then were left unstimulated or were stimulated with 10 µg/ml anisomycin for 60, 90 and 120 min. Where indicated 5 µg/ml actinomycin D (Act D) was added for a further 30 and 60 min after 60 min of anisomycin treatment leading to total anisomycin treatment of 90 and 120 min, respectively. IEG transcript levels were determined by RT–PCR and normalized to actin mRNA. This experiment is representative of two independently performed experiments each performed in triplicate. (B) For three genes identified in gene array analysis, egr2, egr3, and jun-B (cf. Table 1), transcript levels before (black columns) and 60 min after stimulation with 10 µg/ml anisomycin (white columns) were analyzed by RT–PCR for MK2/3 DKO MEFs transduced with MK2, MK2-K79R or empty vector (GFP).
MK2-dependent anisomycin-stimulated gene expression monitored by microarray analysis
To identify further MK2 target genes and to characterize the common features of their transcriptional regulation we performed a microarray experiment using Affymetrix Mouse Genome 430 2.0 GeneChip Array® which interrogates over 39 000 transcripts, comparing anisomycin-induced gene expression (10 µg/ml anisomycin, 90 min) between MK2/3 double-deficient cells rescued with wild-type MK2 or empty vector (GFP) alone. We filtered genes with at least 3-fold anisomycin-induction in the wild-type-rescued (DKO + MK2) cells and with an absolute signal above a threshold of 500 relative units. The remaining genes were sorted by the difference in their induction by empty vector- or MK2-rescued cells, taking 2-fold difference as threshold. Following these filtering and sorting criteria, 27 genes were identified (Table 1), which show up to 12-fold increased induction in MK2-rescued cells. The genes identified comprise various early growth regulators (egr2, egr3) as well as jun-B and TTP (zinc finger protein 36). For egr2, egr3 and junB, we verified the dependence of their induction on catalytic activity of MK2 by RT–PCR. Indeed, anisomycin-induced expression of these IEG transcripts also depends on catalytic activity of MK2 (Figure 5B).
Table 1.
List of the 27 different anisomycin-induced genes with >2-fold increased MK2-dependent transcript levels in MEFs
| Gene description | Fold induction |
Ratio MK2/ GFP | Position of promoter elements |
||
|---|---|---|---|---|---|
| GFP | MK2 | SRE | CRE | ||
| Zinc finger protein 36 (TTP) | 2.70 | 33.66 | 12.49 | −24 | −2203 |
| Early growth response 2 | 31.9 | 383.66 | 12.04 | −75, −126 | −62 |
| Early growth response 3 | 8.91 | 102.95 | 11.56 | −465, −799 | −583 −634 −2460 −2594 |
| Calponin 3, acidic | 0.37 | 3.49 | 9.32 | −68, −86, −134, −488, −613 | − |
| ADP-ribosylation factor 4 | 0.69 | 5.98 | 8.66 | −285 | −2, −64 |
| Kinesin family member 5B | 0.58 | 3.58 | 6.11 | −562, +33 | − |
| Jun-B oncogene | 3.01 | 16.26 | 5.41 | −61, −318 | −105 |
| DnaJB1 | 0.78 | 3.43 | 4.37 | −82, −244, −704, −796, −826, +1 | − |
| Cell division cycle associated 4 | 0.80 | 3.47 | 4.36 | −10, −485 | − |
| Forkhead box O3 | 1.39 | 5.83 | 4.21 | −414 | −16, −955, −966, −1090 |
| Methionine adenosyltransferase II, α | 0.84 | 3.46 | 4.10 | −103, −861 | − |
| Suppressor of cytokine signaling 3 | 1.14 | 3.94 | 3.45 | −737 | − |
| Nuclear pore membrane protein 121 | 1.51 | 4.91 | 3.26 | −565, −576 | −360, −378 |
| Sirtuin 1 | 1.82 | 5.03 | 2.77 | −495, −721 | − |
| PMA- induced protein 1 | 2.97 | 8.09 | 2.72 | −397 | −59, −66 |
| FBJ osteosarcoma oncogene (c-fos) | 43.7 | 116.2 | 2.66 | −295 | 75, −175, −301, −349 |
| Sprouty homolog 2 (Drosophila) | 2.43 | 6.33 | 2.61 | −249, −460, −626, −769 | −593 |
| Transducer of ErbB-2.1 | 1.45 | 3.53 | 2.44 | −930 | −2280 |
| Insulin induced gene 1 | 1.46 | 3.25 | 2.22 | −122 | −193 |
| Immediate early response 5-like | 3.27 | 7.25 | 2.22 | −63, −127, −985 | − |
| Transformed mouse 3T3 cell double minute 2 | 1.54 | 3.41 | 2.21 | −806 | − |
| Metallothionein 1 | 2.27 | 5.00 | 2.20 | −870, −961 | − |
| Sorting nexin 5 | 1.50 | 3.24 | 2.17 | − | 327, −367 |
| Zinc finger and BTB domain containing 11 | 1.88 | 4.08 | 2.17 | −48, −60, −266, −618, −775, −913, −953 | − |
| Vascular endothelial growth factor A | 2.82 | 5.81 | 2.06 | −230, −265, −303, −694 | −445 |
| Cyclin-dependent kinase inhibitor 1A | 1.67 | 3.42 | 2.05 | −568, −790, +45 | −274 |
| Immediate early response 2 | 16.9 | 34.54 | 2.05 | −29, −511, −534, −725 | −48, −1211 |
Genes identified in gene array were filtered and sorted as described in the text. The fold of induction of the transcript level before and after 90 min of stimulation by 10 µg/ml anisomycin is given for MK2/3 DKO MEFs rescued with MK2 or GFP alone. The ratio between transcript levels of stimulation of MK2 and GFP rescued cells is calculated and used for ranking. SRE and CRE promoter elements identified are given. The numbers represent the relative position of the central nucleotide of the element to the major transcriptional start site (‘−’—no promoter element identified).
Regulatory elements in the promoters of these genes were analyzed: the presence of CREs (CREB-binding elements) was monitored using the ‘CREB Target Gene Database’ (37). In the promoter regions of 16 of the 27 genes one or several CREs could be identified. Interestingly, by literature search combined with prediction using the transcription element search system (TESS) (38), serum response elements (SREs) could be identified in the promoter regions of 26 of the 27 target genes (cf. Table 1). While stimulation of CRE-dependent transcription is known to proceed via p38/MSK1 and CREB (21), the mechanism of SRE-dependent stimulation of these genes might involve serum response factor (SRF) and ternary complex factors (39) but has not been analyzed in regard to the contribution of the p38/MK2/3 pathway so far.
Anisomycin- and UV-induced phosphorylation of SRF at S103 is impaired in MK2/3-double-deficient cells
Since it has been described that SRF is one of the several in vitro substrates of MK2 (7,40), we analyzed SRF phosphorylation in the MK2/3 DKO cells rescued with MK2, MK2-K79R or GFP alone. As seen from Figure 6, catalytically active MK2 is able to rescue anisomycin- (Figure 6A) and UV-stimulated (Figure 6B) SRF phosphorylation at serine residue 103 (pS103). The p38 stabilizing MK2 mutant (MK2-K79R) does not affect stress-induced SRF phosphorylation. A similar effect is observed on stress-dependent phosphorylation of the small heat shock protein Hsp25 at serine 86, a known MK2 site. The p38-dependence of stress-induced SRF phosphorylation was confirmed by the inhibitory effect of p38α/β inhibitor SB202190 (Supplementary Figure S4). Another transcription factor involved in IEG expression is Elk1 (26). We analyzed Elk1 expression and regulatory phosphorylation at serine 383 in parallel and detected a rather strong basal phosphorylation and only weak, if any, anisomycin stimulation of this phosphorylation (Figure 6A). Since stabilization of p38 MAPK by expression of MK2 does not increase Elk1-pS383 upon anisomycin stimulation, a direct phosphorylation of Elk1 at this site by p38 MAPK can be excluded. Furthermore and in accordance with these data, a GAL4-Elk1 reporter system showed PMA-dependent stimulation but no significant anisomycin-stimulation in HeLa cells (Supplementary Figure S5A). A role of the p38 MAPK/MK2 pathway in Elk1 activation in these cells can be further excluded, since transfections of constitutive active (ca) MKK6 and constitutive active MK2 mutant independent of p38 (MK2-EE) (41), which stimulate a SRF reporter, do not stimulate GAL4-Elk1 reporter activation, which, in turn, can only be induced by transfection of caMEKK1 (Supplementary Figure S5B–D).
Figure 6.
Rescue of stress-dependent SRF-S103-phosphorylation in MK2/3 DKO MEFs by MK2. MK2/3 DKO immortalized stably transduced MEFs were starved over night and then were left unstimulated or were stimulated for 30 min with 10 µg/ml of anisomycin (A) or 200 J/m2 UV (B). Phosphorylation of SRF at S103, of Elk1 at S383 (A) and of Hsp25 at S86 were analyzed by western blot. SRF, Elk1 (A), MK2, p38, pp38, Hsp25 and GAPDH levels were analyzed as controls.
Reporter gene assays in HeLa cells indicate a MK2-specific SRE-dependent transcriptional activation probably via SRF
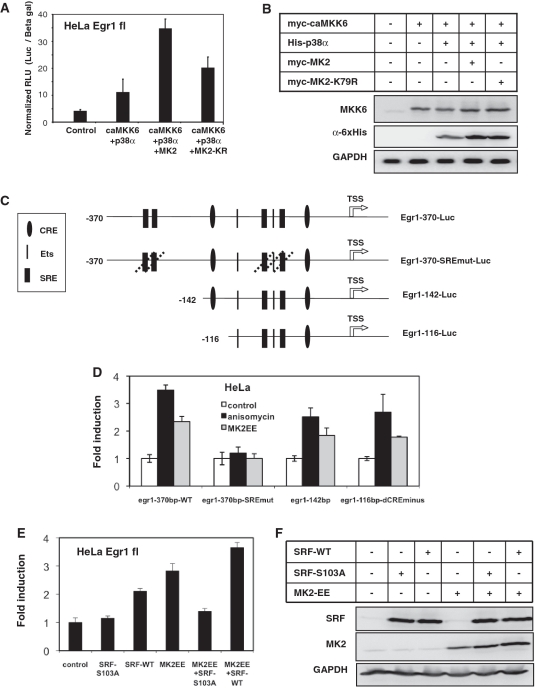
Absence of MK2-dependent IEG mRNA stabilization suggested a role for MK2 in stress induced transcriptional activation. To analyze the role of MK2 in transcriptional activation of gene expression, we first performed an egr1-promoter (full length −1300 to +1) luciferase reporter assay (18) in HeLa cells, because they can be transiently transfected more easily and reproducibly than MEFs. When constitutive active (ca) MKK6 together with p38α was co-transfected into HeLa cells there was a weak stimulation of reporter activity. As expected co-transfection of MK2-K79R mutant enhanced the reporter activity, which could be attributed to the p38-stabilization effect of the MK2 mutant. However, a significant (P < 0.01) higher reporter activity is reached when wild-type MK2 was co-transfected with p38α and caMKK6 (Figure 7A), although both proteins stabilize ectopically expressed His-tagged p38α to a comparable level (Figure 7B). To analyze the role of SRE for MK2-dependent transcriptional activation of the egr1-promoter we used different fragments and mutants of the egr1 promoter (18) schematically presented in Figure 7C. As seen from Figure 7D, anisomycin-dependent and MK2-dependent reporter activation requires the SREs, since their deletion makes the promoter completely unresponsive to anisomycin and MK2-EE, respectively. Furthermore, a minimal promoter of 116 base pair containing two SREs is still responsive to anisomycin and MK2. Interestingly, transfection of caMK2 (MK2-EE) was sufficient for strong induction of the egr1 reporter (Figure 7E). This indicates that catalytic activity of MK2 alone is sufficient to stimulate transcription from the egr1-promoter. To analyze the involvement of SRF in this stimulation, we co-expressed the phosphorylation site mutant SRF-S103A, which should not be able to receive the MK2 signal and, hence, should act negatively interfering. Indeed, co-expression of SFR-S103A significantly inhibits the effect of MK2-EE on egr1 promoter activation (Figure 7E). In contrast, co-expression of wild-type SRF (SRF-WT) to levels comparable to expression of SRF-S103A (Figure 7F) does not inhibit MK2-EE-driven egr1 promoter activation. The effect of SRF-S103A is MK2-specific, since its expression alone does not change promoter activity (Figure 7E). It is described that SRF phosphorylation at S103 enhances its binding to SREs and thus activates transcription (42,43). Although we could not detect a MK2-dependent increase in interaction of phospho-SRF with the egr1 promoter by ChIP (not shown), the above findings strongly suggest that in HeLa cells the transcriptional activation of an IEG promoter by MK2 involves SRE and SRF.
Figure 7.
Egr1 promoter-reporter assays in HeLa cells. (A) Transcriptional activation of the full-length (−1300 to +1) egr1-promoter by MK2 and MK2-K79R in the presence of constitutively active MKK6 and p38α. HeLa cells were transfected with egr1 promoter-luciferase construct alone or together with MKK6, p38α, MK2 or MK2-K79R expression vectors as indicated. Normalized luciferase activity 24 h posttransfection was determined and representative data from three independent experiments performed in triplicate are presented. (B) Parallel western blot analysis showing the expression levels of caMKK6 and p38 in the luciferase assay lysates. (C) Schematic representation of the Egr1 promoter-reporter constructs used for transient transfections of HeLa cells and generation of stably transfected MK2/3 double-deficient (DKO) MEFs. cAMP-responsive elements (CRE), Ets-binding sites (Ets) and SRE are indicated. In Egr1-370-SREmut-Luc all four SREs and SRE flanked Ets box are mutated (18). TSS—transcription start site. (D) Anisomycin-stimulation (25 ng/ml) and stimulation by co-transfection of constitutively active MK2 (MK2-EE) of the different egr1 reporter constructs shown in C in Hela cells after 5 h. (E) Stimulation of the full length egr1 promoter activity by co-expression of constitutively active MK2 (MK2-EE) and inhibition of this stimulation by parallel expression of the phosphorylation site mutant SRF-S103A, but not by wild-type SRF (SRF-WT). (F) Parallel western blot analysis showing the expression levels of MK2 and SRF in the lysates used for luciferase assay in E.
MK2-specific SRE-dependent transcriptional activation in MEFs
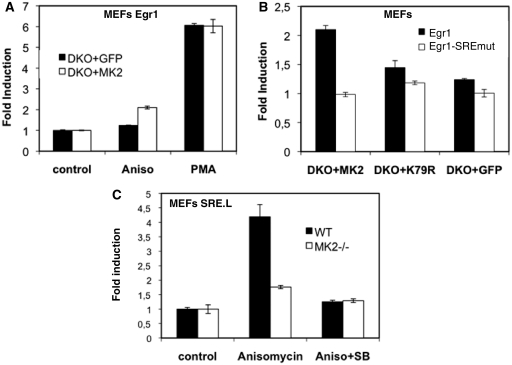
To detect SRE-dependent MK2-specific transcriptional consequences in MEFs, we established six different stably transfected MEF reporter cell lines. In addition to the stably transduced retroviral vectors expressing MK2, MK2K79R or control (GFP), these cell lines carry either a −370 to transcriptional start site (TSS) +1 wild-type Egr1 promoter reporter (Egr1-370-Luc) or the same promoter reporter construct with mutations in all four SREs (Egr1-370-SREmut-Luc, cf. Figure 7C). In contrast to transiently transfected MEF, where reporter genes could not be stimulated by stress (not shown), anisomycin-induced expression of the egr1-reporter construct in stably transfected MK2-rescued MEFs shows a reproducible p38-dependent increase which peaks after ∼4 h (Supplementary Figure S6). Upon PMA treatment, the Egr1-Luc reporter is stimulated in MK2-rescued (DKO + MK2) and control cells (DKO + GFP) to a similar extent (Figure 8A). However, anisomycin-stimulation (25 ng/ml) is only detected for the MK2-rescued (DKO + MK2) but not for the control cells (DKO + GFP). We then compared anisomycin-stimulation of the Egr1-luc and the Egr1-SRFmut-luc reporter constructs (Figure 8B). Only for MK2-rescued cells a clear SRE-dependent stimulation of reporter activity could be detected, indicating that MK2 catalytic activity requires the SREs in the Egr1 promoter for transcriptional activation.
Figure 8.
Egr1 promoter-reporter assays in MK2/3- (A and B) and MK2-deficient (C) MEFs. (A) Egr1-370-Luc (cf. Figure 7C) reporter activity in MK2/3 DKO MEFs rescued with MK2 (white columns) or GFP (black columns) 4 h after stimulation with anisomycin (25 ng/ml) or PMA. (B) Anisomycin-stimulated Egr1-370-Luc reporter (black columns) and Egr1-370-SRFmut-Luc (white columns) activity in MK2/3 DKO MEFs rescued with MK2, MK2-K79RF or GFP. (C) Stably SRE.l-transfected MK2-deficient (white bars) and WT control (black bars) MEFs were serum starved over night and were left unstimulated or were stimulated with anisomycin (aniso) for 5 h. For inhibition of p38, 5 µM SB202190 (SB) was added 1 h prior to stimulation. Reporter luciferase activity was determined, normalized to total protein and induction was calculated. Representative data from three independent experiments are shown.
To further demonstrate SRF-driven transcription we applied a luciferase reporter assay using a derivative of c-fos serum response element, SRE.L, which contains an intact binding site for SRF but lacks the ternary complex factor binding site (ETS-Box) (44,45). Thus, stimulus-induced expression of this reporter could be attributed to direct SRF phosphorylation and activation independent of ternary complex factors. MK2-deficient and WT MEFs stably transfected with the SRE.l-luciferase construct (25) were starved 20 h by serum deprivation and subsequently stimulated with 25 ng/ml anisomycin (Figure 8C). In wild-type MEFs, anisomycin-stimulation leads to 4-fold increase of the SRE-reporter. In contrast, in MK2-deficient MEFs, stimulation of transcription is strongly decreased. Pretreatment of MEFs with a p38α/β MAPK inhibitor completely suppresses anisomycin-stimulated transcriptional activation of the SRF-reporter in wild-type and MK2-deficient MEFs (Figure 8C). Taken together with the data that SRE.L is activated by MK2-EE and caMKK6 in HeLa cells under conditions where GAL4-Elk1 reporter is not activated (Supplementary Figure S5C and D), these data indicate that catalytic activity of MK2 significantly contributes to p38 MAPK-dependent activation of SRE-driven IEGs probably via direct phosphorylation of SRF.
DISCUSSION
In the current study we analyzed stress-induced gene expression defects in MK2/3-deficient cells and could show a novel role for MKs in transcriptional activation of IEGs. IEG activation is highly complex and involves various promoter elements, transcription factors and at least three mammalian MAPK cascades (26). In the c-fos and egr1 promoter the SRE binds SRF together with ternary complex factors, such as Elk-1 or SAP-1, and the cAMP-responsive element (CRE) recruits CREB/ATF-1 (Figure 9). ERKs, JNKs and p38 MAPKs are able to directly phosphorylate and activate Elk-1 (46). The ERK- and p38 MAPK-activated protein kinase MSK1 phosphorylates CREB and ATF1 at S133 and S63, respectively, and further contributes to mitogen- and stress-dependent transcription of IEGs (21,22). Phosphorylation of SRF by the ERK-activated ribosomal S6 kinase (RSK) at S103 enhances its affinity to the SRE (42). SRF has also been identified as target for MK2, but functional consequences of SRF phosphorylation in the context of IEG activation were still open (40).
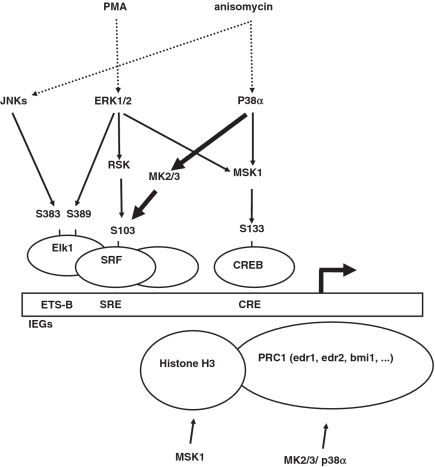
Figure 9.
A model of stress-dependent transcriptional activation of IEGs including the proposed p38 MAPK/MK2/3/SRF/SRE pathway. PMA and anisomycin are indicated as stimuli which are specific for ERK and p38MAPK/JNK activation, respectively. A typical IEG promoter contains SREs and CREs. SRF together with ternary complex factors, such as ELk1, bind to SRE. Elk1 may also bind directly to the ETS-Box (58). CRE-binding protein, CREB, and activating transcription factor (ATF) 1 bind to CRE. Phosphorylations of the different factors at the amino acids indicated contribute to transactivation. MSK1 is activated by both ERKs and p38a and increases CREB/ATF1 activity. ERKs and JNKs directly phosphorylate and activate the TCF Elk1. RSK and MK2/3 are activated by ERKs and p38a, respectively, and stimulate SRF activity. Chromatin remodelling via phosphorylation of histone H3 by MSK1 and components of the PRC 1 by MK2/3 or p38 might further contribute to IEG transcription.
MK2/3-deficient cells show significantly reduced levels of p38α and, hence, could be regarded as a p38α knockdown (11). Here we present evidence that not only p38 level, but p38 function is also affected in MK2/3 double-deficient cells. In MK2/3 DKO cells, the decreased p38α level is partially compensated by an increase in p38α phosphorylation at the critical tyrosine and threonine residue in the activation loop (cf. Figure 4A), probably indicating a decreased feedback control in the pathway via TAB1-phosphorylation by p38α (47). However, this mechanism is not able to fully compensate p38α-signaling. Stress-dependent activation of MSK1, a known substrate of p38 and ERKs, is clearly impaired in MK2/3 DKO cells, while PMA-dependent activation, which proceeds via ERKs, remains normal. Interestingly, stress-dependent signaling to MSK1 can be fully rescued by the catalytic-dead mutant of MK2, indicating that MSK1 is a direct substrate of p38α and that MK2/3 are not involved in its activation (Figure 4A).
Even though previous studies in human cell lines have shown MK2-dependent changes in subcellular localization of p38α, we could not see any significant effect of MK2/3 deficiency on the subcellular localization of endogenous p38 or pp38 in MEF cells. Considering the fact that the intracellular concentration of MK2 determined previously as 6 nmol/g of total protein is ∼3-fold lower than the concentration of p38 MAPK determined in parallel (30), it could be speculated that only a small fraction of total p38α is subjected to nuclear export by MK2 at any time.
In the absence of MK2/3, stress-induced transcriptional activation of IEGs is significantly impaired. In contrast to MSK1 activation, the defect in transcription could neither be rescued through stabilization of p38 by the catalytic-dead mutant of MK2 nor by over expression of exogenous p38 in MK2/3 DKO cells (data not shown). This observation clearly demonstrates that catalytic activity of MK2 is indispensable for full IEG induction upon stress. Since there is also no MK2-dependent stabilization of transcripts detectable, these findings suggest a direct effect of MK2/3 on IEG transcriptional activation. This idea was supported by the identification of various genes which show a more than 2-fold MK2-dependent increase in anisomycin-stimulated transcript induction. The data available suggest the presence of different but overlapping sets of genes regulated by MSKs and MKs. For example, Anisomycin-induced egr1 expression was unaffected in MSK1/2 double knock out fibroblasts (22,48) but is affected in MK2/3 DKO MEFs (Figure 4B). Finally, the direct effect of MK2 is substantiated by demonstration of a MK2-dependent stress-stimulated SRF phosphorylation, by the MK2-dependence of expression of an SRE-reporter and the negative-interfering action of the SRF phosphorylation site mutant. In addition, involvement of a MK2-dependent activation of Elk1, which is also not a substrate of MK2 in an in-gel assay (40), could be further excluded, since no GAL4-Elk1 activation can be obtained by co-expression of caMKK6 or MK2-EE. Hence, we demonstrate for the first time possible functional effects of MK2/3-dependent phosphorylation of SRF at S103 in transcriptional activation of IEGs. MSK1 and MK2/3 are also known to modulate chromatin structure by histone H3 and HMG-14 phosphorylation and interaction with the polycomb repressive complex (PRC), respectively (49–51). Hence, we cannot completely rule out that MK2/3-dependent alterations in chromatin may also contribute to IEG expression.
Via members of the family of myocardin transcriptional co-activators, such as MAL, SRF is able to sense changes in cellular levels of actin monomers (52). G actin sequesters MAL from nuclear SRF. Activation of actin-polymerization by Rho-GTPases releases MAL for nuclear entry and SRF interaction leading to further transcriptional stimulation of IEGs and cytoskeletal genes [recently reviewed in (53)]. It is interesting to note that MK2/3 are involved in both SRF regulation (as demonstrated here) and regulation of actin polymerization via phosphorylation of the small heat shock protein Hsp25/27, which displays phosphorylation-dependent release from the barbed ends of actin filaments (54). Taking into account that MK2/3 shows an activation-dependent redistribution between nucleus and cytoplasm (55), it might further contribute to the complex cross-talk between actin and gene expression. However, under non-inhibitory and inhibitory anisomycin-stimulation of MEFs, there is no MK2-dependent change in actin filaments detectable (Supplementary Figure S7). This observation does not rule out the possibility that MK2 plays a role in regulation the actin cytoskeleton in other cells or in response to other stimulations.
Due to its role in posttranscriptional regulation of cytokine mRNAs including TNF, MK2 is a sought-after candidate target for anti-inflammatory therapy. It is interesting to note that the IEG-targets of MK2 identified by microarray analysis include TTP, SOCS3 and VEGF-A, which are known modulators of inflammatory response. TTP is also a substrate of MK2 and facilitates TNF-mRNA translation only in its phosphorylated form (56,57). The dual action of MK2/3 on TTP phosphorylation and TTP transcription may represent a default mechanism to limit inflammation.
So far, posttranscriptional regulation of cytokine biosynthesis via phosphorylation of ARE-binding proteins by MKs has been documented. Here, the contribution of MKs to transcriptional activation of SRE-dependent genes is demonstrated. The coordinated action of both mechanisms could be essential for efficient regulation of inflammatory gene expression.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Deutsche Forschungsgemeinschaft; PhD-Program (Molecular Medicine) of the Hannover Medical School. Funding for open access charge: Deutsche Forschungsgemeinschaft; Hannover Medical School LOM programm.
Conflict of interest statement. None declared.
ACKNOWLEDGEMENTS
We thank Dr J. Larry Jameson (Chicago) for the reporter plasmids, Dr Margot O’Toole (Cambridge, USA) for help with the gene chip experimental design and discussion of the results, Dr Linda Sealy (Nashville) and Dr Michael Greenberg (Harvard) for the SRF expression constructs and Dr Philip Cohen (Dundee) for discussion of the data.
REFERENCES
- 1.Hazzalin CA, Mahadevan LC. MAPK-regulated transcription: a continuously variable gene switch? Nat. Rev. Mol. Cell. Biol. 2002;3:30–40. doi: 10.1038/nrm715. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen HQ, Hoffman-Liebermann B, Liebermann DA. The zinc finger transcription factor Egr-1 is essential for and restricts differentiation along the macrophage lineage. Cell. 1993;72:197–209. doi: 10.1016/0092-8674(93)90660-i. [DOI] [PubMed] [Google Scholar]
- 3.Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 4.Cao H, Tuttle JS, Blackshear PJ. Immunological characterization of tristetraprolin as a low abundance, inducible, stable cytosolic protein. J. Biol. Chem. 2004;279:21489–21499. doi: 10.1074/jbc.M400900200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schilling D, Pittelkow MR, Kumar R. IEX-1, an immediate early gene, increases the rate of apoptosis in keratinocytes. Oncogene. 2001;20:7992–7997. doi: 10.1038/sj.onc.1204965. [DOI] [PubMed] [Google Scholar]
- 6.Sun H, Charles CH, Lau LF, Tonks NK. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell. 1993;75:487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- 7.Gaestel M. MAPKAP kinases - MKs - two's company, three's a crowd. Nat. Rev. Mol. Cell. Biol. 2006;7:120–130. doi: 10.1038/nrm1834. [DOI] [PubMed] [Google Scholar]
- 8.Ronkina N, Kotlyarov A, Gaestel M. MK2 and MK3: a pair of isoenzymes? Front. Biosci. 2008;13:5511–5521. doi: 10.2741/3095. [DOI] [PubMed] [Google Scholar]
- 9.Kotlyarov A, Neininger A, Schubert C, Eckert R, Birchmeier C, Volk HD, Gaestel M. MAPKAP kinase 2 is essential for LPS-induced TNF-alpha biosynthesis. Nat. Cell. Biol. 1999;1:94–97. doi: 10.1038/10061. [DOI] [PubMed] [Google Scholar]
- 10.Neininger A, Kontoyiannis D, Kotlyarov A, Winzen R, Eckert R, Volk HD, Holtmann H, Kollias G, Gaestel M. MK2 targets AU-rich elements and regulates biosynthesis of tumor necrosis factor and interleukin-6 independently at different post-transcriptional levels. J. Biol. Chem. 2002;277:3065–3068. doi: 10.1074/jbc.C100685200. [DOI] [PubMed] [Google Scholar]
- 11.Ronkina N, Kotlyarov A, Dittrich-Breiholz O, Kracht M, Hitti E, Milarski K, Askew R, Marusic S, Lin LL, Gaestel M, et al. The mitogen-activated protein kinase (MAPK)-activated protein kinases MK2 and MK3 cooperate in stimulation of tumor necrosis factor biosynthesis and stabilization of p38 MAPK. Mol. Cell. Biol. 2007;27:170–181. doi: 10.1128/MCB.01456-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Briata P, Forcales SV, Ponassi M, Corte G, Chen CY, Karin M, Puri PL, Gherzi R. p38-dependent phosphorylation of the mRNA decay-promoting factor KSRP controls the stability of select myogenic transcripts. Mol. Cell. 2005;20:891–903. doi: 10.1016/j.molcel.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Carballo E, Lai WS, Blackshear PJ. Feedback inhibition of macrophage tumor necrosis factor-alpha production by tristetraprolin. Science. 1998;281:1001–1005. doi: 10.1126/science.281.5379.1001. [DOI] [PubMed] [Google Scholar]
- 14.Rousseau S, Morrice N, Peggie M, Campbell DG, Gaestel M, Cohen P. Inhibition of SAPK2a/p38 prevents hnRNP A0 phosphorylation by MAPKAP-K2 and its interaction with cytokine mRNAs. EMBO J. 2002;21:6505–6514. doi: 10.1093/emboj/cdf639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han J, Ulevitch RJ. Emerging targets for anti-inflammatory therapy. Nat. Cell. Biol. 1999;1:E39–40. doi: 10.1038/10032. [DOI] [PubMed] [Google Scholar]
- 16.Sudo T, Kawai K, Matsuzaki H, Osada H. p38 mitogen-activated protein kinase plays a key role in regulating MAPKAPK2 expression. Biochem. Biophys. Res. Commun. 2005;337:415–421. doi: 10.1016/j.bbrc.2005.09.063. [DOI] [PubMed] [Google Scholar]
- 17.Ambrosino C, Mace G, Galban S, Fritsch C, Vintersten K, Black E, Gorospe M, Nebreda AR. Negative feedback regulation of MKK6 mRNA stability by p38alpha mitogen-activated protein kinase. Mol. Cell. Biol. 2003;23:370–381. doi: 10.1128/MCB.23.1.370-381.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duan WR, Ito M, Park Y, Maizels ET, Hunzicker-Dunn M, Jameson JL. GnRH regulates early growth response protein 1 transcription through multiple promoter elements. Mol. Endocrinol. 2002;16:221–233. doi: 10.1210/mend.16.2.0779. [DOI] [PubMed] [Google Scholar]
- 19.Rolli M, Kotlyarov A, Sakamoto KM, Gaestel M, Neininger A. Stress-induced stimulation of early growth response gene-1 by p38/stress-activated protein kinase 2 is mediated by a cAMP-responsive promoter element in a MAPKAP kinase 2-independent manner. J. Biol. Chem. 1999;274:19559–19564. doi: 10.1074/jbc.274.28.19559. [DOI] [PubMed] [Google Scholar]
- 20.Winzen R, Kracht M, Ritter B, Wilhelm A, Chen CY, Shyu AB, Muller M, Gaestel M, Resch K, Holtmann H. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 1999;18:4969–4980. doi: 10.1093/emboj/18.18.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deak M, Clifton AD, Lucocq LM, Alessi DR. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 1998;17:4426–4441. doi: 10.1093/emboj/17.15.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiggin GR, Soloaga A, Foster JM, Murray-Tait V, Cohen P, Arthur JS. MSK1 and MSK2 are required for the mitogen- and stress-induced phosphorylation of CREB and ATF1 in fibroblasts. Mol. Cell. Biol. 2002;22:2871–2881. doi: 10.1128/MCB.22.8.2871-2881.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ehrhardt C, Schmolke M, Matzke A, Knoblauch A, Will C, Wixler V, Ludwig S. Polyethylenimine, a cost-effective transfection reagent. Signal Transduct. 2006;6:179–184. [Google Scholar]
- 24.Proia DA, Nannenga BW, Donehower LA, Weigel NL. Dual roles for the phosphatase PPM1D in regulating progesterone receptor function. J. Biol. Chem. 2006;281:7089–7101. doi: 10.1074/jbc.M511839200. [DOI] [PubMed] [Google Scholar]
- 25.Sousa AM, Liu T, Guevara O, Stevens J, Fanburg BL, Gaestel M, Toksoz D, Kayyali US. Smooth muscle alpha-actin expression and myofibroblast differentiation by TGFbeta are dependent upon MK2. J. Cell. Biochem. 2007;100:1581–1592. doi: 10.1002/jcb.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bebien M, Salinas S, Becamel C, Richard V, Linares L, Hipskind RA. Immediate-early gene induction by the stresses anisomycin and arsenite in human osteosarcoma cells involves MAPK cascade signaling to Elk-1, CREB and SRF. Oncogene. 2003;22:1836–1847. doi: 10.1038/sj.onc.1206334. [DOI] [PubMed] [Google Scholar]
- 27.Lim CP, Jain N, Cao X. Stress-induced immediate-early gene, egr-1, involves activation of p38/JNK1. Oncogene. 1998;16:2915–2926. doi: 10.1038/sj.onc.1201834. [DOI] [PubMed] [Google Scholar]
- 28.Hazzalin CA, Cano E, Cuenda A, Barratt MJ, Cohen P, Mahadevan LC. p38/RK is essential for stress-induced nuclear responses: JNK/SAPKs and c-Jun/ATF-2 phosphorylation are insufficient. Curr. Biol. 1996;6:1028–1031. doi: 10.1016/s0960-9822(02)00649-8. [DOI] [PubMed] [Google Scholar]
- 29.Hazzalin CA, Cuenda A, Cano E, Cohen P, Mahadevan LC. Effects of the inhibition of p38/RK MAP kinase on induction of five fos and jun genes by diverse stimuli. Oncogene. 1997;15:2321–2331. doi: 10.1038/sj.onc.1201403. [DOI] [PubMed] [Google Scholar]
- 30.Kotlyarov A, Yannoni Y, Fritz S, Laass K, Telliez JB, Pitman D, Lin LL, Gaestel M. Distinct cellular functions of MK2. Mol. Cell. Biol. 2002;22:4827–4835. doi: 10.1128/MCB.22.13.4827-4835.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCoy CE, Campbell DG, Deak M, Bloomberg GB, Arthur JS. MSK1 activity is controlled by multiple phosphorylation sites. Biochem. J. 2005;387:507–517. doi: 10.1042/BJ20041501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ben-Levy R, Hooper S, Wilson R, Paterson HF, Marshall CJ. Nuclear export of the stress-activated protein kinase p38 mediated by its substrate MAPKAP kinase-2. Curr. Biol. 1998;8:1049–1057. doi: 10.1016/s0960-9822(98)70442-7. [DOI] [PubMed] [Google Scholar]
- 33.Stokoe D, Engel K, Campbell DG, Cohen P, Gaestel M. Identification of MAPKAP kinase 2 as a major enzyme responsible for the phosphorylation of the small mammalian heat shock proteins. FEBS Lett. 1992;313:307–313. doi: 10.1016/0014-5793(92)81216-9. [DOI] [PubMed] [Google Scholar]
- 34.Mahadevan LC, Edwards DR. Signalling and superinduction. Nature. 1991;349:747–748. doi: 10.1038/349747c0. [DOI] [PubMed] [Google Scholar]
- 35.Greenberg ME, Ziff EB. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984;311:433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- 36.Tchen CR, Brook M, Saklatvala J, Clark AR. The stability of tristetraprolin mRNA is regulated by mitogen-activated protein kinase p38 and by tristetraprolin itself. J. Biol. Chem. 2004;279:32393–32400. doi: 10.1074/jbc.M402059200. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, Odom DT, Koo SH, Conkright MD, Canettieri G, Best J, Chen H, Jenner R, Herbolsheimer E, Jacobsen E, et al. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc. Natl Acad. Sci. USA. 2005;102:4459–4464. doi: 10.1073/pnas.0501076102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schug J. Using TESS to predict transcription factor binding sites in DNA sequence. Curr. Protoc. Bioinformatics. 2008;Chapter 2:Unit 2 6. doi: 10.1002/0471250953.bi0206s21. [DOI] [PubMed] [Google Scholar]
- 39.Treisman R. Journey to the surface of the cell: Fos regulation and the SRE. EMBO J. 1995;14:4905–4913. doi: 10.1002/j.1460-2075.1995.tb00173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heidenreich O, Neininger A, Schratt G, Zinck R, Cahill MA, Engel K, Kotlyarov A, Kraft R, Kostka S, Gaestel M, et al. MAPKAP kinase 2 phosphorylates serum response factor in vitro and in vivo. J. Biol. Chem. 1999;274:14434–14443. doi: 10.1074/jbc.274.20.14434. [DOI] [PubMed] [Google Scholar]
- 41.Engel K, Schultz H, Martin F, Kotlyarov A, Plath K, Hahn M, Heinemann U, Gaestel M. Constitutive activation of mitogen-activated protein kinase-activated protein kinase 2 by mutation of phosphorylation sites and an A-helix motif. J. Biol. Chem. 1995;270:27213–27221. doi: 10.1074/jbc.270.45.27213. [DOI] [PubMed] [Google Scholar]
- 42.Rivera VM, Miranti CK, Misra RP, Ginty DD, Chen RH, Blenis J, Greenberg ME. A growth factor-induced kinase phosphorylates the serum response factor at a site that regulates its DNA-binding activity. Mol. Cell. Biol. 1993;13:6260–6273. doi: 10.1128/mcb.13.10.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miranti CK, Ginty DD, Huang G, Chatila T, Greenberg ME. Calcium activates serum response factor-dependent transcription by a Ras- and Elk-1-independent mechanism that involves a Ca2+/calmodulin-dependent kinase. Mol. Cell. Biol. 1995;15:3672–3684. doi: 10.1128/mcb.15.7.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chihara K, Amano M, Nakamura N, Yano T, Shibata M, Tokui T, Ichikawa H, Ikebe R, Ikebe M, Kaibuchi K. Cytoskeletal rearrangements and transcriptional activation of c-fos serum response element by Rho-kinase. J. Biol. Chem. 1997;272:25121–25127. doi: 10.1074/jbc.272.40.25121. [DOI] [PubMed] [Google Scholar]
- 45.Hill CS, Treisman R. Transcriptional regulation by extracellular signals: mechanisms and specificity. Cell. 1995;80:199–211. doi: 10.1016/0092-8674(95)90403-4. [DOI] [PubMed] [Google Scholar]
- 46.Whitmarsh AJ, Yang SH, Su MS, Sharrocks AD, Davis RJ. Role of p38 and JNK mitogen-activated protein kinases in the activation of ternary complex factors. Mol. Cell. Biol. 1997;17:2360–2371. doi: 10.1128/mcb.17.5.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheung PC, Campbell DG, Nebreda AR, Cohen P. Feedback control of the protein kinase TAK1 by SAPK2a/p38alpha. EMBO J. 2003;22:5793–5805. doi: 10.1093/emboj/cdg552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Darragh J, Soloaga A, Beardmore VA, Wingate AD, Wiggin GR, Peggie M, Arthur JS. MSKs are required for the transcription of the nuclear orphan receptors Nur77, Nurr1 and Nor1 downstream of MAPK signalling. Biochem. J. 2005;390:749–759. doi: 10.1042/BJ20050196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soloaga A, Thomson S, Wiggin GR, Rampersaud N, Dyson MH, Hazzalin CA, Mahadevan LC, Arthur JS. MSK2 and MSK1 mediate the mitogen- and stress-induced phosphorylation of histone H3 and HMG-14. EMBO J. 2003;22:2788–2797. doi: 10.1093/emboj/cdg273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Voncken JW, Niessen H, Neufeld B, Rennefahrt U, Dahlmans V, Kubben N, Holzer B, Ludwig S, Rapp UR. MAPKAP kinase 3pK phosphorylates and regulates chromatin association of the polycomb group protein Bmi1. J. Biol. Chem. 2005;280:5178–5187. doi: 10.1074/jbc.M407155200. [DOI] [PubMed] [Google Scholar]
- 51.Schwermann J, Rathinam C, Schubert M, Schumacher S, Noyan F, Koseki H, Kotlyarov A, Klein C, Gaestel M. MAPKAP kinase MK2 maintains self-renewal capacity of haematopoietic stem cells. EMBO J. 2009;28:1392–1406. doi: 10.1038/emboj.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329–342. doi: 10.1016/s0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- 53.Knoll B, Nordheim A. Functional versatility of transcription factors in the nervous system: the SRF paradigm. Trends Neurosci. 2009;32:432–442. doi: 10.1016/j.tins.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 54.Benndorf R, Hayess K, Ryazantsev S, Wieske M, Behlke J, Lutsch G. Phosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerization-inhibiting activity. J. Biol. Chem. 1994;269:20780–20784. [PubMed] [Google Scholar]
- 55.Engel K, Kotlyarov A, Gaestel M. Leptomycin B-sensitive nuclear export of MAPKAP kinase 2 is regulated by phosphorylation. EMBO J. 1998;17:3363–3371. doi: 10.1093/emboj/17.12.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hitti E, Iakovleva T, Brook M, Deppenmeier S, Gruber AD, Radzioch D, Clark AR, Blackshear PJ, Kotlyarov A, Gaestel M. Mitogen-activated protein kinase-activated protein kinase 2 regulates tumor necrosis factor mRNA stability and translation mainly by altering tristetraprolin expression, stability, and binding to adenine/uridine-rich element. Mol. Cell. Biol. 2006;26:2399–2407. doi: 10.1128/MCB.26.6.2399-2407.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stoecklin G, Stubbs T, Kedersha N, Wax S, Rigby WF, Blackwell TK, Anderson P. MK2-induced tristetraprolin:14-3-3 complexes prevent stress granule association and ARE-mRNA decay. EMBO J. 2004;23:1313–1324. doi: 10.1038/sj.emboj.7600163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zinck R, Cahill MA, Kracht M, Sachsenmaier C, Hipskind RA, Nordheim A. Protein synthesis inhibitors reveal differential regulation of mitogen-activated protein kinase and stress-activated protein kinase pathways that converge on Elk-1. Mol. Cell. Biol. 1995;15:4930–4938. doi: 10.1128/mcb.15.9.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.