Abstract
With the advent of DNA sequencing technologies, more and more reference genome sequences are available for many organisms. Analyzing sequence variation and understanding its biological importance are becoming a major research aim. However, how to store and process the huge amount of eukaryotic genome data, such as those of the human, mouse and rice, has become a challenge to biologists. Currently available bioinformatics tools used to compress genome sequence data have some limitations, such as the requirement of the reference single nucleotide polymorphisms (SNPs) map and information on deletions and insertions. Here, we present a novel compression tool for storing and analyzing Genome ReSequencing data, named GRS. GRS is able to process the genome sequence data without the use of the reference SNPs and other sequence variation information and automatically rebuild the individual genome sequence data using the reference genome sequence. When its performance was tested on the first Korean personal genome sequence data set, GRS was able to achieve ∼159-fold compression, reducing the size of the data from 2986.8 to 18.8 MB. While being tested against the sequencing data from rice and Arabidopsis thaliana, GRS compressed the 361.0 MB rice genome data to 4.4 MB, and the A. thaliana genome data from 115.1 MB to 6.5 KB. This de novo compression tool is available at http://gmdd.shgmo.org/Computational-Biology/GRS.
INTRODUCTION
The development of new DNA sequencing technologies, such as next-generation sequencing (NGS) and single-molecule sequencing, has enabled the research of genomics and functional genomics to advance to new levels (1,2). Due to the dramatic reduction of sequencing cost and increase of sequencing efficiency, these new high-throughput sequencing technologies have become effective and routine applications in the ‘resequencing’ of individual genomes for detecting sequence variation between the individual and the reference genome (3). Resequencing individual genomes can facilitate the investigation of the relationship between sequence and phenotypic variations. To date, several personal human genome sequencing data have been released (2,4–6). Sequencing of individual human genomes is believed to provide molecular basis for personalized medicine. Furthermore, more resequencing data are being generated from various organisms. Individual genome resequencing in animals such as mouse and pig, and in plants such as rice, maize and soybean, has proven to be extremely powerful in investigating genome variations, such as single nucleotide polymorphisms (SNPs), deletions, insertions and rearrangements.
However, how to store and manage the huge amount of sequencing data has become a challenge to biologists. For example, the storage of one 2009 human reference genome (i.e. UCSC hg19 assembly) requires up to 905 MB with the tar.gz compression format (7). Thus, a total of 90 500 MB (nearly 88.38 GB) hard disk storage space with the tar.gz compression format would be required to store data for 100 individuals in genetic disease studies. It is noteworthy that different individuals within one species share higher consensus nucleotide sequence; for example, human has ∼99.9% common genome sequence with DNA sequencing errors of 0.01% (8,9). Moreover, the electronic transfer of sequencing data is a bottleneck, even though some tools have been developed to compress the files and increase the network bandwidth.
Currently, several methods for compressing genomic sequence data have been reported (10–13). However, these compression tools can not process the genome sequence data without the reference SNPs map or information about sequence variations, such as insertions or deletions.
Here, we present a general Genome ReSequencing (GRS) tool for storing and managing the individual genome resequencing data without having to rely on known reference SNPs maps or other information on sequence variation. We demonstrate the power of this GRS tool in processing genome resequencing data, using whole genome sequencing data sets from human and the model plants Arabidopsis and rice.
MATERIALS AND METHODS
Data set
Data sets used to test GRS include KOREF_20090131 and KOREF_20090224, two of the first Korean personal genome sequences (4), and two different versions of the reference genome sequence from Arabidopsis thaliana (TAIR8 and TAIR9) and rice (TIGR5 and TIGR6) (14–16), respectively.
Software availability
GRS is implemented in C and Shell. It will be freely available for non-profit use. The source code and its executable file are available at http://gmdd.shgmo.org/Computational-Biology/GRS.
Architecture of the GRS tool
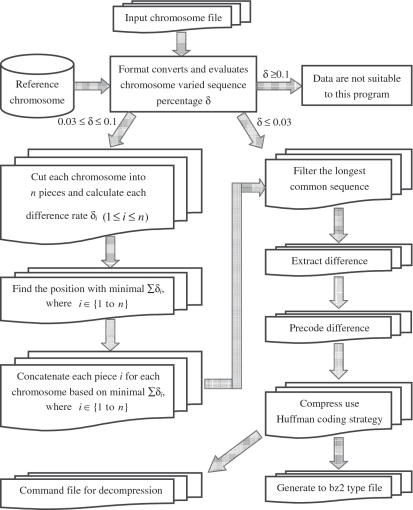
The main modules in GRS connect the input chromosome file, the intermediate data and the final compressed file. The architecture of GRS is shown in Figure 1. When an individual genome sequence data needs to be compressed, GRS first evaluates each chromosome varied sequence percentage (δ) based on the reference chromosome. Then it filters the longest identical nucleotide sequence and extracts the different sequence (δ ≤ 0.03), and precodes the different sequence file that has been generated to reduce the file size. Then, GRS uses the Huffman coding strategy to compress the reduced different sequence file to the bz2 type file and generates the command file to decompress the compressed file. If 0.03 ≤ δ ≤ 0.1, GRS will cut chromosome into n pieces and calculate each different rate δi  to find the position with minimal ∑δi, then compress each piece with the same strategy as the one used for δ ≤ 0.03. Individual genome sequence data that has been compressed using GRS can be later decoded easily with the GRS decoding tool.
to find the position with minimal ∑δi, then compress each piece with the same strategy as the one used for δ ≤ 0.03. Individual genome sequence data that has been compressed using GRS can be later decoded easily with the GRS decoding tool.
Figure 1.
Architecture of the GRS Tool. The main modules in GRS connect the input chromosome file, the intermediate data and the final compressed file. Details of the processing procedure are described in the text.
Evaluation of individual genome sequence variation
Higher percentage of nucleotide sequence variation from the reference genome leads to longer time to run GRS and results in larger compressed file for an individual genome. When the individual genome sequence data needs to be compressed, GRS checks whether the users use the correct reference chromosome data, quantifying the percentage of varied nucleotide sequence in an individual genome is thus required. Here we used the following method to calculate δ. We used the formula  where
where  means the number of different nucleotide sequence between the individual and the reference; i means the type of nucleotide; including A, T, C, G, N, a, t, c, g and n; t means the total DNA base number in the reference genome sequence data.
means the number of different nucleotide sequence between the individual and the reference; i means the type of nucleotide; including A, T, C, G, N, a, t, c, g and n; t means the total DNA base number in the reference genome sequence data.
Recording the longest common local nucleotide sequence and the changed sequence
It is reported that finding and recording the varied nucleotide sequence of two sequences equals to finding their longest common local sequence (17). Using a matrix graph, the longest common sequence can be extracted effectively. Taking two sequences ‘gaNGCTA’ and ‘gNGTNA’ as an example, their longest common sequence is ‘gNGTA’. That is to say that each nucleotide of ‘gaNGCTA’ in x-axis is used to compare with the whole sequence of ‘gNGTNA’ in y-axis, and each common nucleotide in y-axis direction will be marked with a red circle, after the base by base comparison the longest common sequence will be marked in the whole matrix (Supplementary Figure S1). GRS can find the minimal changes between two genome sequences using the modified UNIX diff program (18).
RESULTS
Huffman encoding for varied nucleotide sequence information and individual genome sequence rebuilding
Figure 2a shows the module of presenting the raw information on sequence difference between the individual and the reference sequences generated based on the modified UNIX diff program. When processing the varied sequence information, the ‘>’, ‘<’ and the ‘\n’ between adjacent nucleotides is removed by GRS (Figure 2b). Then ‘a’ (add) is converted to ‘i’ and ‘c’ (change) to ‘h’. In addition, the base information below the ‘d’ (deletion) can be removed since the deleted sequence information can be extracted based on the sequence position such as N5 and N6 (Figure 2a and b). Also, the ‘—’ and the highlighted bases can be removed because these sequences can be recovered by using the sequence at N9 and N10 (Figure 2a and b).
Figure 2.
Processing changes file of DNA base, genome position and recover language. (a) Raw changes between two sequences generated based on the modified UNIX diff program. N1 to N12 indicate the nucleotide sequence position ranging from N1 to N12; ‘a’ is the insertion of nucleotide(s); ‘d’ is the deletion of nucleotide(s) and ‘c’ is the changed nucleotide(s). In addition, symbol ‘,’ between N1 and N2 means positions start from N1 to N2. Symbol ‘>’, ‘<’ and ‘—’ are the keywords when the whole individual genome sequence is rebuilt on basis of the reference genome sequence. (b) Changes file with redundant information deleted. (c) Changes file generated based on the subtracted number, which is more readable to the computer.
Next, ‘,’ is changed to ‘\t’ and ‘\t’ is added to each side of ‘i’, ‘d’ and ‘h’ by GRS to make the rebuilding language more readable by the computer. If there are two numbers at the side of ‘i’, ‘d’ or ‘h’, the second nucleotide position will be recorded using the subtracted number of the first nucleotide position to the second one. Therefore, at each side of ‘i’, ‘d’ and ‘h’, the number N5, N7, N9 and N11 is replaced by the subtracted number of their corresponding nucleotide position N1, N3, N5 and N7 to reduce the file size (Figure 2b and c). Eventually, the individual genome sequence information can be recorded as the format shown in Figure 2c using Huffman coding (19).
To encode the processed individual sequence data more effectively, each nucleotide sequence and its relevant number are recorded with the same binary value since it can be decoded uniquely with the help of ‘i’, ‘d’ and ‘h’. Table 1 shows an example of the encoding strategy using the varied sequence information of A. thaliana chromosome 1 with TAIR8 as the reference and TAIR9 as the individual genome, showing that the larger counts of the symbol are reduced to the shorter encoding value. Then the bit file is able to be generated to the char code, for instance, the taken bits ‘01000001’ presents the corresponding ASCII code ‘A’.
Table 1.
Huffman encoding for DNA base, genome position and recover language
| DNA base | Relevant number | Counts | Encoding value |
|---|---|---|---|
| A | 0 | 95 | 0110 |
| T | 1 | 155 | 000 |
| C | 2 | 132 | 1110 |
| G | 3 | 105 | 1100 |
| N | 4 | 101 | 1010 |
| a | 5 | 110 | 1111 |
| t | 6 | 98 | 0111 |
| c | 7 | 80 | 0010 |
| g | 8 | 106 | 1101 |
| n | 9 | 83 | 0011 |
| d | 31 | 101 110 | |
| h | 15 | 101 111 | |
| i | 54 | 10 110 | |
| \t | 210 | 100 | |
| \n | 168 | 010 |
Each DNA base and its relevant number are encoded with the same binary value based on the Huffman encoding strategy. Shown here is the encoding table for changes file generated for chromosome 1 of the A. thaliana genome using TAIR8 as reference and TAIR9 as the individual genome. Character d means delete sequence, h means change sequence and i means insert sequence.
Performance of GRS
Performance of the GRS tool was tested in three cases. When two Korean genome sequence data (KOREF_20090131 and KOREF_20090224) were used (4), the raw file with 2986.8 MB in size (KOREF_20090224) was reduced to a 18.8-MB compressed file, achieving a ∼159-fold compression rate (Table 2). In addition, we also compressed the raw file of rice genome from 361.0 MB to 4.4 MB with the compression rate ∼82 fold (Table 3), and 115.1 MB of A. thaliana genome to 6.5 KB with nearly 18 133 fold of compression (Table 4). Furthermore, the good performance of GRS was revealed by the calculated compression and decompression time of these three genomes (Supplementary Table S1).
Table 2.
Performance of GRS in compressing the KOREF_20090224 human genome using KOREF_20090131 as the reference
| Chromosome number | Varied sequence percentage (%) | Raw file size | Compressed file size | Compression rate |
|---|---|---|---|---|
| 1 | 0.656 929 | 239.7 MB | 1.3 MB | 184.4 |
| 2 | 0.716 863 | 235.6 MB | 1.3 MB | 181.2 |
| 3 | 0.630 572 | 193.4 MB | 987.4 KB | 200.6 |
| 4 | 0.762 314 | 185.5 MB | 1.1 MB | 168.6 |
| 5 | 0.711 956 | 175.4 MB | 964.9 KB | 186.1 |
| 6 | 0.649 071 | 165.7 MB | 884.9 KB | 191.7 |
| 7 | 0.912 855 | 154.0 MB | 1.0 MB | 154.0 |
| 8 | 0.639 359 | 141.8 MB | 746.4 KB | 194.5 |
| 9 | 0.774 539 | 136.0 MB | 844.0 KB | 165.0 |
| 10 | 0.705 819 | 131.3 MB | 750.4 KB | 179.2 |
| 11 | 0.720 238 | 130.4 MB | 738.0 KB | 180.9 |
| 12 | 0.638 779 | 128.3 MB | 685.6 KB | 191.6 |
| 13 | 0.550 377 | 110.7 MB | 508.4 KB | 223.0 |
| 14 | 0.529 220 | 103.1 MB | 473.4 KB | 223.0 |
| 15 | 0.589 095 | 97.3 MB | 484.6 KB | 205.6 |
| 16 | 0.808 032 | 86.1 MB | 554.7 KB | 158.9 |
| 17 | 0.818 430 | 76.4 MB | 494.1 KB | 158.3 |
| 18 | 0.666 472 | 73.8 MB | 399.0 KB | 189.4 |
| 19 | 0.744 553 | 61.9 MB | 390.4 KB | 162.4 |
| 20 | 0.493 781 | 60.5 MB | 276.0 KB | 224.5 |
| 21 | 0.579 505 | 45.5 MB | 221.2 KB | 210.6 |
| 22 | 0.632 448 | 48.2 MB | 256.3 KB | 192.6 |
| M | 0.108 715 | 16.5 KB | 183.0 B | 94 543.8 |
| X | 3.299 049 | 150.2 MB | 3.1 MB | 48.5 |
| Y | 1.768 076 | 56.0 MB | 578.9 KB | 99.1 |
| The whole genome | 0.804 282 | 2986.8 MB | 18.8 MB | 158.9 |
The verified sequence percentage of each chromosome, the size of raw sequence file and compressed file, as well as the compression rate are shown.
Table 3.
Performance of GRS in compressing rice genome of TIGR6 using TIGR5 as the reference
| Chromosome number | Varied sequence percentage (%) | Raw file size (MB) | Compressed file size | Compression rate |
|---|---|---|---|---|
| 1 | 0.757 801 | 42.0 | 1.4 MB | 30.0 |
| 2 | 0.013 898 | 34.8 | 1.4 KB | 25 453.7 |
| 3 | 0.168 381 | 35.3 | 46.6 KB | 775.7 |
| 4 | 0.096 345 | 34.2 | 35.3 KB | 992.1 |
| 5 | 0.069 046 | 29.0 | 6.0 KB | 4949.3 |
| 6 | 0.000 000 | 30.3 | 0 | |
| 7 | 0.027 041 | 28.8 | 4.0 KB | 7372.8 |
| 8 | 0.479 452 | 27.6 | 115.5 KB | 244.7 |
| 9 | 0.000 000 | 22.3 | 0 | |
| 10 | 1.128 503 | 22.4 | 770.1 KB | 29.8 |
| 11 | 0.188 992 | 27.6 | 2.3 MB | 12.0 |
| 12 | 0.000 000 | 26.7 | 0 | |
| The whole genome | 0.244 122 | 361.0 | 4.4 MB | 82.0 |
The verified sequence percentage of each chromosome, the size of raw sequence file and compressed file, as well as the compression rate are shown.
Table 4.
Performance of GRS in compressing A. thaliana genome of TAIR9 using TAIR8 as the reference
| Chromosome number | Varied sequence percentage (%) | Raw file size (MB) | Compressed file size | Compression rate |
|---|---|---|---|---|
| 1 | 0.016 314 | 29.4 | 715.0 B | 43 116.3 |
| 2 | 0.036 145 | 19.0 | 385.0 B | 51 747.9 |
| 3 | 0.046 910 | 22.7 | 2.9 KB | 6709.0 |
| 4 | 0.000 301 | 17.9 | 1.9 KB | 9647.2 |
| 5 | 0.063 888 | 26.1 | 604.0 B | 45 311.0 |
| The whole genome | 0.032 712 | 115.1 | 6.5 KB | 18 132.7 |
The verified sequence percentage of each chromosome, the size of raw sequence file and compressed file, as well as the compression rate are shown.
DISCUSSION
With the advance of DNA sequencing technologies, more and more genome resequencing projects, such as the International HapMap Project and the 1000 Genomes Project, have been initiated (20,21). As a result, compression of the huge amount of genome sequencing data has become an important issue (10,11). Currently available tools have limitations in effectively processing the large amount of genome reseqencing data. For example, tools developed by Brandon et al. (10) and Christley et al. (11) are limited by not only the known reference SNPs map, but also the possible loss of sequence information, such as large structural variations (SVs) including sequence rearrangements and segment duplications. Even though the advent of sequencing technologies facilitates the processing of individual genome sequence such as reassembling genome sequences using the sequencing reads on basis of the reference genome (3,4), the current sequence compression tools are not very suitable for this purpose. Moreover, comprehensive reference SNPs maps are unavailable for many organisms such as rice, A. thaliana and other species, making it hard to compress these genome resequencing data using the available tools. In this study, we show that GRS is a de novo genome compression approach for compressing resequencing data, which is applicable to the genome data management of many species.
Varied sequence percentage plays a critical role in compressing the genome resequencing data. GRS employs a novel approach to deal with the resequensing data, especially for those data sets with higher variation between the reference genome and the individual genome. The key point of GRS is to splice the reference chromosome and the input chromosome into the same intervals, and then calculate each corresponding pair of the varied sequence percentage δi based on each nucleotide frequency. Subsequently, concatenating piece i to make the modified reference chromosome and modified input chromosome creates the minimum varied sequence percentage based on the δi value (Figure 3). Then the minimum change file can be compressed using GRS and the chromosome piece with a higher value of varied sequence percentage can be compressed using the general and routine file compression method such as 7-Zip. When the chromosome size is too big or the computer memory capability is limited, it is useful to splice the reference chromosome and the input chromosome data. In this study, GRS grouped each chromosome sequencing data of the Korean genome (4), into 50, 25 or 10 million per piece, respectively. Similar compression capabilities were obtained (i.e. file size is ∼19 MB), demonstrating the flexibility and reliability of GRS.
Figure 3.
Method to resolve the minimum varied sequence percentage between the reference and input chromosomes and assemble the pieces together. Here is an example showing that two chromosomes are spliced into nine parts with relevant δi. Part 1, 3, 4, 5, 6, 7 and 8 are put together because δ2 and δ9 with a higher value than others based on the threshold of varied sequence percentage.
CONCLUSIONS
In this article, we designed and implemented a generic tool, GRS, for de novo compression of genome resequencing data. GRS is simple to use and does not need the reference SNPs map, thus can be widely used for many genomes, especially those without reference SNPs. Case studies using the sequencing data of human, rice and A. thaliana genomes have demonstrated the good performance of GRS in sequencing data compression.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
National Key Basic Research Developments Program, Ministry of Science and Technology, P. R. China (2009CB941500, 2006CB101700); National ‘863′ High-Tech Project (2006AA10A102); National Natural Science Foundation of China (30725022 and 30600347); Shanghai Leading Academic Discipline Project (B205). Funding for open access charge: Special Funding for Transgenic Organisms (2008ZX08012-002).
Conflict of interest statement. None declared.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Jianping Hu for editing this manuscript and Xiaobin Wu and Xiaoyi Guo for helpful discussions.
REFERENCES
- 1.Horner DS, Pavesi G, Castrignano T, Meo PDD, Liuni S, Sammeth M, Picardi E, Pesole G. Bioinformatics approaches for genomics and post genomics applications of next-generation sequencing. Brief. Bioinform. 2009;11:181–197. doi: 10.1093/bib/bbp046. [DOI] [PubMed] [Google Scholar]
- 2.Pushkarev D, Neff NF, Quake SR. Single-molecule sequencing of an individual human genome. Nat. Biotechnol. 2009;27:847–852. doi: 10.1038/nbt.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Service RF. The race for the $1000 genome. Science. 2006;311:1544–1546. doi: 10.1126/science.311.5767.1544. [DOI] [PubMed] [Google Scholar]
- 4.Ahn SM, Kim TH, Lee S, Kim D, Ghang H, Kim DS, Kim BC, Kim SY, Kim WY, Kim C, et al. The first Korean genome sequence and analysis: Full genome sequencing for asocio-ethnic group. Genome Res. 2009;19:1622–1629. doi: 10.1101/gr.092197.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wheeler DA, Srinivasan M, Egholm M, Shen Y, Chen L, McGuire A, He W, Chen Y, Makhijani V, Roth GT, et al. The complete genome of an individual by massively parallel DNA sequencing. Nature. 2008;452:872–876. doi: 10.1038/nature06884. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Wang W, Li R, Li Y, Tian G, Goodman L, Fan W, Zhang J, Li J, Zhang J, et al. The diploid genome sequence of an Asian individual. Nature. 2008;456:60–66. doi: 10.1038/nature07484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhead B, Karolchik D, Kuhn RM, Hinrichs AS, Zweig AS, Fujita P, Diekhans M, Smith KE, Rosenbloom KR, Raney BJ, et al. The UCSC Genome Browser database: update. Nucleic Acids Res. 2010;38:D613–D619. doi: 10.1093/nar/gkp939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 9.Snyder M, Du J, Gerstein M. Personal genome sequencing: current approaches and challenges. Genes Dev. 2010;24:423–431. doi: 10.1101/gad.1864110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandon MC, Wallace DC, Baldi P. Data structures and compression algorithms for genomic sequence data. Bioinformatics. 2009;25:1731–1738. doi: 10.1093/bioinformatics/btp319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christley S, Lu Y, Li C, Xie X. Human genomes as email attachments. Bioinformatics. 2009;25:274–275. doi: 10.1093/bioinformatics/btn582. [DOI] [PubMed] [Google Scholar]
- 12.Tembe W, Lowey J, Suh E. G-SQZ: compact encoding of genomic sequence and quality data. Bioinformatics. 2010;26:2192–2194. doi: 10.1093/bioinformatics/btq346. [DOI] [PubMed] [Google Scholar]
- 13.Soliman TH, Gharib TF, Abo-Alian A, Sharkawy ME. A Lossless Compression Algorithm for DNA sequences. Int. J. Bioinform. Res. Appl. 2009;5:593–602. doi: 10.1504/IJBRA.2009.02904. [DOI] [PubMed] [Google Scholar]
- 14.Huala E, Dickerman A, Garcia-Hernandez M, Weems D, Reiser L, LaFond F, Hanley D, Kiphart D, Zhuang J, Huang W, et al. The Arabidopsis Information Resource (TAIR): A comprehensive database and web-based information retrieval, analysis, and visualization system for a model plant. Nucleic Acids Res. 2001;29:102–105. doi: 10.1093/nar/29.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouyang S, Zhu W, Hamilton J, Lin H, Campbell M, Childs K, Thibaud-Nissen F, Malek RL, Lee Y, Zheng L, et al. The TIGR Rice Genome Annotation Resource: improvements and new features. Nucleic Acids Res. 2007;35:D883–D887. doi: 10.1093/nar/gkl976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhee SY, Beavis W, Berardini TZ, Chen G, Dixon D, Doyle A, Garcia-Hernandez M, Huala E, Lander G, Montoya M, et al. The Arabidopsis Information Resource (TAIR): a model organism database providing a centralized, curated gateway to Arabidopsis biology, research materials and community. Nucleic Acids Res. 2003;31:224–228. doi: 10.1093/nar/gkg076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myers EW. An O(ND) Difference Algorithm and Its Variations. Algorithmica. 1986;1:251–266. [Google Scholar]
- 18.Miller W, Myers EW. A File Comparison Program. Software-Pract. Exper. 1985;15:1025–1040. [Google Scholar]
- 19.Huffman D. A method for the construction of minimum redundancy codes. Proc. IRE. 1952;40:1098–1101. [Google Scholar]
- 20.Frazer KA, Ballinger DG, Cox DR, Hinds DA, Stuve LL, Gibbs RA, Belmont JW, Boudreau A, Hardenbol P, Leal SM, et al. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449:851–862. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaiser J. A plan to capture human diversity in 1000 genomes. Science. 2008;319:395. doi: 10.1126/science.319.5862.395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.