Abstract
The influenza viruses are characterized by segmented, negative-strand RNA genomes requiring an RNA-dependent RNA polymerase of viral origin for replication. The particular structure of the influenza virus genome and function of its viral proteins enable antigenic drift and antigenic shift. These processes result in viruses able to evade the long-term adaptive immune responses in many hosts.
1. THE INFLUENZA VIRUSES
The influenza A, B, and C viruses, representing three of the five genera of the family Orthomyxoviridae, are characterized by segmented, negative-strand RNA genomes. Sequencing has confirmed that these viruses share a common genetic ancestry; however, they have genetically diverged, such that reassortment – the exchange of viral RNA segments between viruses – has been reported to occur within each genus, or type, but not across types. Influenza A viruses are further characterized by the subtype of their surface glycoproteins, the hemagglutinin (HA) and the neuraminidase (NA). Influenza viruses have a standard nomenclature that includes virus type; species from which it was isolated (if non-human); location at which it was isolated; isolate number; isolate year; and, for influenza A viruses only, HA and NA subtype. Thus, A/Panama/2007/1999(H3N2) was isolate number 2007 of a human influenza A virus taken in the country of Panama in 1999, and it has an HA subtype 3 and an NA subtype 2. While many genetically distinct subtypes – 16 for HA and 9 for NA – have been found in circulating influenza A viruses, only three HA (H1, H2, and H3) and two NA (N1 and N2) subtypes have caused human epidemics, as defined by sustained, widespread, person-to-person transmission [1].
2. VIRION STRUCTURE AND ORGANIZATION
By electron microscopy, influenza A and B viruses are virtually indistinguishable. They are spherical or filamentous in shape, with the spherical forms on the order of 100 nm in diameter and the filamentous forms often in excess of 300 nm in length. The influenza A virion is studded with glycoprotein spikes of HA and NA, in a ratio of approximately four to one, projecting from a host cell–derived lipid membrane [1]. A smaller number of matrix (M2) ion channels traverse the lipid envelope, with an M2:HA ratio on the order of one M2 channel per 101-102 HA molecules [2]. The envelope and its three integral membrane proteins HA, NA, and M2 overlay a matrix of M1 protein, which encloses the virion core. Internal to the M1 matrix are found the nuclear export protein (NEP; also called nonstructural protein 2, NS2) and the ribonucleoprotein (RNP) complex, which consists of the viral RNA segments coated with nucleoprotein (NP) and the heterotrimeric RNA-dependent RNA polymerase, composed of two “polymerase basic” and one “polymerase acidic” subunits (PB1, PB2, and PA). The organization of the influenza B virion is similar, with four envelope proteins: HA, NA, and, instead of M2, NB and BM2. Influenza C virions are structurally distinct from those of the A and B viruses; on infected cell surfaces, they can form long cordlike structures on the order of 500 μm. However, influenza C virions are compositionally similar, with a glycoprotein-studded lipid envelope overlying a protein matrix and the RNP complex. The influenza C viruses have only one major surface glycoprotein, the hemagglutinin-esterase-fusion (HEF) protein, which corresponds functionally to the HA and NA of influenza A and B viruses, and one minor envelope protein, CM2 [1].
3. GENOME STRUCTURE
The influenza A and B virus genomes each comprise eight negative-sense, single-stranded viral RNA (vRNA) segments, while influenza C virus has a seven-segment genome. (See Table 1.) The eight segments of influenza A and B viruses (and the seven segments of influenza C virus) are numbered in order of decreasing length. In influenza A and B viruses, segments 1, 3, 4, and 5 encode just one protein per segment: the PB2, PA, HA and NP proteins. All influenza viruses encode the polymerase subunit PB1 on segment 2; in some strains of influenza A virus, this segment also codes for the accessory protein PB1-F2, a small, 87-amino acid protein with pro-apoptotic activity, in a +1 alternate reading frame [3]. No analogue to PB1-F2 has been identified in influenza B or C viruses. Conversely, segment 6 of the influenza A virus encodes only the NA protein, while that of influenza B virus encodes both the NA protein and, in a −1 alternate reading frame, the NB matrix protein, which is an integral membrane protein corresponding to the influenza A virus M2 protein [4]. Segment 7 of both influenza A and B viruses code for the M1 matrix protein. In the influenza A genome, the M2 ion channel is also expressed from segment 7 by RNA splicing [5], while influenza B virus encodes its BM2 membrane protein in a +2 alternate reading frame [6, 7]. Finally, both influenza A and B viruses possess a single RNA segment, segment 8, from which they express the interferon-antagonist NS1 protein [8–10] and, by mRNA splicing, the NEP/NS2 [11, 12], which is involved in viral RNP export from the host cell nucleus. The genomic organization of influenza C viruses is generally similar to that of influenza A and B viruses; however, the HEF protein of influenza C replaces the HA and NA proteins, and thus the influenza C virus genome has one fewer segment than that of influenza A or B viruses.
TABLE 1. The genomic segments of influenza A/Puerto Rico/8/1934 (H1N1) virus and their encoded proteins.
The PB2, PA, HA, NP and NA proteins are each coded for by a separate RNA segment. The M2 and NEP are both expressed from spliced mRNAs, while PB1-F2 is encoded in a +1 alternate reading frame. (Kindly provided by Megan L. Shaw.)
| Segment | Segment length in nucleotides | Encoded Protein(s) | Protein length in amino acids | Protein function |
|---|---|---|---|---|
| 1 | 2341 | PB2 | 759 | Polymerase subunit; mRNA cap recognition |
| 2 | 2341 | PB1 | 757 | Polymerase subunit; RNA elongation, endonuclease activity |
| PB1-F2 | 87 | Pro-apoptotic activity | ||
| 3 | 2233 | PA | 716 | Polymerase subunit; protease activity |
| 4 | 1778 | HA | 550 | Surface glycoprotein; major antigen, receptor binding and fusion activities |
| 5 | 1565 | NP | 498 | RNA binding protein; nuclear import regulation |
| 6 | 1413 | NA | 454 | Surface glycoprotein; sialidase activity, virus release |
| 7 | 1027 | M1 | 252 | Matrix protein; vRNP interaction, RNA nuclear export regulation, viral budding |
| M2 | 97 | Ion channel; virus uncoating and assembly | ||
| 8 | 890 | NS1 | 230 | Interferon antagonist protein; regulation of host gene expression |
| NEP/NS2 | 121 | Nuclear export of RNA |
The ends of each vRNA segment form a helical hairpin, which is bound by the heterotrimeric RNA polymerase complex; the remainder of the segment is coated with arginine-rich NP, the net positive charge of which binds the negatively charged phosphate backbone of the vRNA [13–15]. Each vRNA segment possesses noncoding regions, of varying lengths, at both 3′ and 5′ ends. However, the extreme ends of all segments are highly conserved among all influenza virus segments; these partially complementary termini base-pair to function as the promoter for vRNA replication and transcription by the viral polymerase complex. The noncoding regions also include the mRNA polyadenylation signal and part of the packaging signals for virus assembly.
A segmented genome enables antigenic shift, in which an influenza A virus strain acquires the HA segment, and possibly the NA segment as well, from an influenza virus of a different subtype. This segment reassortment can happen in cells infected with different human and animal viruses, and the resulting virus may encode completely novel antigenic proteins to which the human population has no preexisting immunity. Pandemic influenza arises when antigenic shift generates a virus to which humans are susceptible but immunologically naïve. Antigenic shift likely produced the influenza A (H1N1) virus that was the causative agent of the 1918–1919 “Spanish flu,” whose lethality was unparalleled in modern times. Characterization of the reconstructed 1918 influenza virus indicated that its unique constellation of genes was responsible for its extreme virulence, with the HA, NA, and PB1 genes all contributing to its high pathogenicity [16, 17]. The global spread of the pandemic was almost certainly enabled by the acquisition of antigenically novel surface proteins, to which much of the world’s population was immunologically naïve [18].
4. THE INFLUENZA VIRUS REPLICATION CYCLE
4.1 Virus Attachment
Influenza viruses recognize N-acetylneuraminic (sialic) acid on the host cell surface. Sialic acids are nine-carbon acidic monosaccharides commonly found at the termini of many glycoconjugates. Thus, they are ubiquitous on many cell types and in many animal species. The carbon-2 of the terminal sialic acid can bind to the carbon-3 or carbon-6 of galactose, forming α-2,3- or α2,6-linkages; these different linkages result in unique steric configurations of the terminal sialic acid. The sialic acid moiety is recognized and bound by the HA spikes on the surface of influenza viruses, which have a preferential specificity for either α-2,3- or α-2,6-linkages. In human tracheal epithelial cells, α-2,6-linkages predominate, while α-2,3-linkages are more common in duck gut epithelium. Sialic acids with terminalα-2,3-linkages are also present in human respiratory epithelium, though in less abundance than those with α-2,6-linkages [19, 20]; consequently, humans and other primates can be infected by avian influenza viruses, albeit with overall less efficiency than by human strains [21–23].
The differential expression of sialic acids in the mammalian respiratory tract may help to explain the low infectivity but high pathogenicity of some avian strains. In humans, α-2,3-linked sialylated proteins, while less abundant overall, are most prevalent in the lower respiratory tract (bronchioles and alveoli). The lungs are not as accessible to airborne virus particles as is the upper respiratory tract (nasopharynx, paranasal sinuses, trachea, and bronchi), so avian virus infection is relatively rare in humans. However, when avian strains do infect the human lung, a severe and rapidly progressive pneumonia may result; in this clinical setting, fatality rates exceed 60% [24].
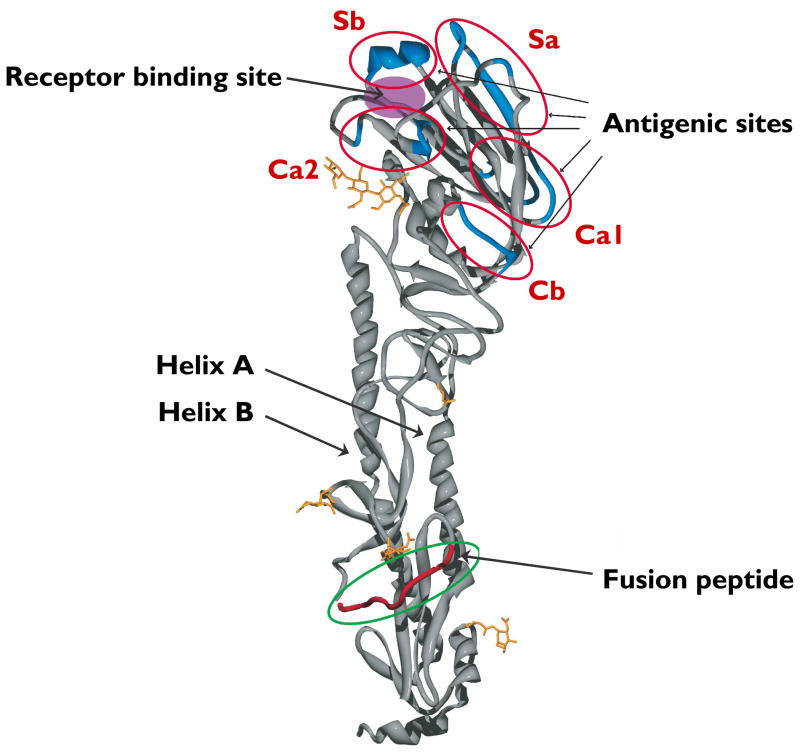
The crystal structure of the HA molecule is a trimer with two structurally distinct regions: a stem, comprising a triple-stranded coiled-coil of alpha-helices, and a globular head of antiparallel beta-sheet, positioned atop the stem [25]. The head contains the sialic acid receptor binding site, which is surrounded by the predicted variable antigenic determinants, designated A, B, C, and D in the H3 subtype [26] and Sa, Sb, Ca1, Ca2, and Cb in the H1 subtype (see Figure 1) [1]. During virus replication, the HA protein is cleaved by serine proteases into HA1 and HA2; this post-translational modification is necessary for virus infectivity. The HA2 portion is thought to mediate the fusion of virus envelope with cell membranes, while the HA1 portion contains the receptor binding and antigenic sites (reviewed in [27]). Antibodies to HA neutralize virus infectivity, so virus strains evolve frequent amino acid changes at the antigenic sites; however, the stem-head configuration of the HA molecule remains conserved among strains and subtypes. These relatively minor changes accumulate in a process called antigenic drift. Eventually, mutations in multiple antigenic sites result in a virus strain that is no longer effectively neutralized by host antibodies to the parental virus, and the host becomes susceptible again to productive infection by the drifted strain.
FIGURE 1. Ribbon diagram of an uncleaved hemagglutinin monomer from the 1918 influenza A virus (H1N1), the causative agent of the “Spanish flu” pandemic.
The head contains the sialic acid receptor-binding site, which is surrounded by the five predicted antigenic sites (Sa, Sb, Ca1, Ca2, and Cb). The stem comprises helices A and B and the fusion peptide, as shown. (Adapted from a figure, kindly provided by James Stevens and Ian Wilson, in [1].)
4.2 Virus Entry
Following attachment of the influenza virus HA protein (or the HEF protein of influenza C virus) to sialic acid, the virus is endocytosed. The acidity of the endosomal compartment is crucial to influenza virus uncoating in two ways. First, low pH triggers a conformational change in the HA, exposing a fusion peptide that mediates the merging of the viral envelope with the endosomal membrane, thus opening a pore through which the viral RNP’s are released into the host cell cytoplasm (reviewed in [28] and [29]). Second, hydrogen ions from the endosome are pumped into the virus particle via the M2 ion channel. The M2 protein, a transmembrane ion channel found only in influenza A virus, has portions external to the viral envelope, along with the HA and NA. The M2 protein is the target of the amantadine class of anti-influenza drugs, which block ion channel activity and prevent virus uncoating [30, 31]; also, because it is a surface protein, it has been proposed as a vaccine component [32, 33]. Internal acidification of the influenza virion via the M2 channel disrupts internal protein-protein interactions, allowing viral RNPs to be released from the viral matrix into the cellular cytoplasm [34].
4.3 Synthesis of Viral RNA
Once liberated from the virion, RNPs are trafficked to the host cell nucleus by means of viral proteins’ nuclear localization signals (NLSs), which direct cellular proteins to import the RNPs and other viral proteins into the host cell nucleus (reviewed in [35]). The nucleus is the location of all influenza virus RNA synthesis – both of the capped, polyadenylated messenger RNA (mRNA) that acts as the template for host-cell translation of viral proteins, and of the vRNA segments that form the genomes of progeny virus. The viral RNA-dependent RNA polymerase – a component of the RNPs imported into the nucleus – uses the negative-sense vRNA as a template to synthesize two positive-sense RNA species: mRNA templates for viral protein synthesis, and complementary RNA (cRNA) intermediates from which the RNA polymerase subsequently transcribes more copies of negative-sense, genomic vRNA.
Unlike host cell mRNA, which is polyadenylated by a specific poly(A) polymerase, the poly(A) tail of influenza virus mRNA is encoded in negative-sense vRNA as a stretch of five to seven uracil residues, which the viral polymerase transcribes into the positive sense as a string of adenosines that form the poly(A) tail [36–38]. Messenger RNA capping occurs in a similarly unique manner, in which the PB1 and PB2 proteins “steal” 5′ capped primers from host pre-mRNA transcripts to initiate viral mRNA synthesis; this process is called “cap snatching” (described further in [39]).
Once polyadenylated and capped, mRNA of viral origin can be exported and translated like host mRNA. Nuclear export of vRNA segments, however, is mediated by the viral proteins M1 and NEP/NS2 [35]. M1 interacts with both vRNA and NP, and is thus thought to bring these two components together within the RNP complex; M1 also associates with the nuclear export protein NEP, which mediates the M1-RNP export via nucleoporins into the cytoplasm.
4.4 Synthesis of Viral Proteins
The envelope proteins HA, NA, and M2 are synthesized, from mRNA of viral origin, on membrane-bound ribosomes into the endoplasmic reticulum, where they are folded and trafficked to the Golgi apparatus for post-translational modification. All three proteins have apical sorting signals that subsequently direct them to the cell membrane for virion assembly. Although comparatively little is known about the translation and sorting of the non-envelope proteins, M1 is thought to play a role in bringing the RNP-NEP complex into contact with the envelope-bound HA, NA, and M2 proteins for packaging at the host cell membrane [1].
4.5 Packaging of RNA and Assembly of Virus
Influenza virus is not fully infectious unless its virions contain a full genome of eight segments (or seven segments, for influenza C virus). Previously, vRNA packaging was thought to be an entirely random process, in which vRNA segments are haphazardly incorporated into budding virus particles, and only those ending up with a complete genome become infectious; however, newer evidence suggests that packaging is a more selective process, in which discrete packaging signals on all vRNA segments insure that a full genome is incorporated into the majority of virus particles [40–43].
4.6 Virus Budding and Release
Influenza virus budding occurs at the cell membrane, probably initiated by an accumulation of M1 matrix protein at the cytoplasmic side of the lipid bilayer. When budding is complete, HA spikes continue to bind the virions to the sialic acid on the cell surface until virus particles are actively released by the sialidase activity of the NA protein. The NA is a mushroom-shaped tetramer, anchored to the viral envelope by a transmembrane domain [44, 45]. It possesses receptor destroying activity, cleaving terminal sialic acid residues from cell-surface glycoproteins and gangliosides to release progeny virus from the host cell. In viruses with inactive or absent NA, or in the presence of neuraminidase inhibitors, virus particles clump at the cell surface and infectivity is consequently reduced. The NA also removes sialic acid residues from the virus envelope itself, which prevents viral particle aggregation to enhance infectivity [46, 47]. The NA is also thought to aid virus infectivity by breaking down the mucins in respiratory tract secretions and allowing the virus to penetrate through to the respiratory epithelium, and it may play a role in virus entry into respiratory epithelial cells [48]. Host antibodies to the NA, as well as neuraminidase inhibitors, prevent virus release from infected cells and thus inhibit viral replication.
5. INFLUENZA VIRUSES AND VACCINE DEVELOPMENT
The influenza viruses are relatively simple, RNA-containing viruses with strongly immunogenic surface proteins, especially the HA. However, their segmented genomes and their error-prone RNA-dependent RNA polymerases enable these viruses to undergo antigenic shift and drift, which in turn results in an evasion of the adaptive immune responses in a range of mammalian and avian species, including humans. Because of their adaptive ability, influenza viruses continue to confound efforts to produce long-lasting vaccines against the disease.
Acknowledgments
Work performed in the laboratory of the authors was partially supported by the NIH Center for Investigating Viral Immunity and Antagonism (1 UC19 AI062623-023), the NIH Center for Research on Influenza Pathogenesis (HHSN266200700010C), and NIH grants UO1 AI070469 and UO1 AI1074539. NMB is supported by a Ruth L. Kirschstein National Research Service Award for Physician-Scientist Research Training in the Pathogenesis of Viral Diseases (NIH grant T32 AI007623; P.I. Mary E. Klotman, M.D.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Palese P, Shaw ML. Orthomyxoviridae: The Viruses and their Replication. In: Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 2.Zebedee SL, Lamb RA. Influenza A virus M2 protein: monoclonal antibody restriction of virus growth and detection of M2 in virions. J Virol. 1988 Aug;62(8):2762–72. doi: 10.1128/jvi.62.8.2762-2772.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W, Calvo PA, Malide D, Gibbs J, Schubert U, Bacik I, et al. A novel influenza A virus mitochondrial protein that induces cell death. Nat Med. 2001 Dec;7(12):1306–12. doi: 10.1038/nm1201-1306. [DOI] [PubMed] [Google Scholar]
- 4.Hatta M, Kawaoka Y. The NB protein of influenza B virus is not necessary for virus replication in vitro. J Virol. 2003 May;77(10):6050–4. doi: 10.1128/JVI.77.10.6050-6054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamb RA, Lai CJ, Choppin PW. Sequences of mRNAs derived from genome RNA segment 7 of influenza virus: colinear and interrupted mRNAs code for overlapping proteins. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4170–4. doi: 10.1073/pnas.78.7.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briedis DJ, Lamb RA, Choppin PW. Sequence of RNA segment 7 of the influenza B virus genome: partial amino acid homology between the membrane proteins (M1) of influenza A and B viruses and conservation of a second open reading frame. Virology. 1982 Jan 30;116(2):581–8. doi: 10.1016/0042-6822(82)90150-7. [DOI] [PubMed] [Google Scholar]
- 7.Horvath CM, Williams MA, Lamb RA. Eukaryotic coupled translation of tandem cistrons: identification of the influenza B virus BM2 polypeptide. EMBO J. 1990 Aug;9(8):2639–47. doi: 10.1002/j.1460-2075.1990.tb07446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dauber B, Heins G, Wolff T. The influenza B virus nonstructural NS1 protein is essential for efficient viral growth and antagonizes beta interferon induction. J Virol. 2004 Feb;78(4):1865–72. doi: 10.1128/JVI.78.4.1865-1872.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Sastre A. Inhibition of interferon-mediated antiviral responses by influenza A viruses and other negative-strand RNA viruses. Virology. 2001 Jan 20;279(2):375–84. doi: 10.1006/viro.2000.0756. [DOI] [PubMed] [Google Scholar]
- 10.Kochs G, Garcia-Sastre A, Martinez-Sobrido L. Multiple anti-interferon actions of the influenza A virus NS1 protein. J Virol. 2007 Jul;81(13):7011–21. doi: 10.1128/JVI.02581-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briedis DJ, Lamb RA. Influenza B virus genome: sequences and structural organization of RNA segment 8 and the mRNAs coding for the NS1 and NS2 proteins. J Virol. 1982 Apr;42(1):186–93. doi: 10.1128/jvi.42.1.186-193.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamb RA, Choppin PW, Chanock RM, Lai CJ. Mapping of the two overlapping genes for polypeptides NS1 and NS2 on RNA segment 8 of influenza virus genome. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1857–61. doi: 10.1073/pnas.77.4.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baudin F, Bach C, Cusack S, Ruigrok RW. Structure of influenza virus RNP. I. Influenza virus nucleoprotein melts secondary structure in panhandle RNA and exposes the bases to the solvent. EMBO J. 1994 Jul 1;13(13):3158–65. doi: 10.1002/j.1460-2075.1994.tb06614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Compans RW, Content J, Duesberg PH. Structure of the ribonucleoprotein of influenza virus. J Virol. 1972 Oct;10(4):795–800. doi: 10.1128/jvi.10.4.795-800.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murti KG, Webster RG, Jones IM. Localization of RNA polymerases on influenza viral ribonucleoproteins by immunogold labeling. Virology. 1988 Jun;164(2):562–6. doi: 10.1016/0042-6822(88)90574-0. [DOI] [PubMed] [Google Scholar]
- 16.Pappas C, Aguilar PV, Basler CF, Solorzano A, Zeng H, Perrone LA, et al. Single gene reassortants identify a critical role for PB1, HA, and NA in the high virulence of the 1918 pandemic influenza virus. Proc Natl Acad Sci U S A. 2008 Feb 26;105(8):3064–9. doi: 10.1073/pnas.0711815105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tumpey TM, Basler CF, Aguilar PV, Zeng H, Solorzano A, Swayne DE, et al. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science. 2005 Oct 7;310(5745):77–80. doi: 10.1126/science.1119392. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed R, Oldstone MB, Palese P. Protective immunity and susceptibility to infectious diseases: lessons from the 1918 influenza pandemic. Nat Immunol. 2007 Nov;8(11):1188–93. doi: 10.1038/ni1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Couceiro JN, Paulson JC, Baum LG. Influenza virus strains selectively recognize sialyloligosaccharides on human respiratory epithelium; the role of the host cell in selection of hemagglutinin receptor specificity. Virus Res. 1993 Aug;29(2):155–65. doi: 10.1016/0168-1702(93)90056-s. [DOI] [PubMed] [Google Scholar]
- 20.Matrosovich MN, Matrosovich TY, Gray T, Roberts NA, Klenk HD. Human and avian influenza viruses target different cell types in cultures of human airway epithelium. Proc Natl Acad Sci U S A. 2004 Mar 30;101(13):4620–4. doi: 10.1073/pnas.0308001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beare AS, Webster RG. Replication of avian influenza viruses in humans. Arch Virol. 1991;119(1–2):37–42. doi: 10.1007/BF01314321. [DOI] [PubMed] [Google Scholar]
- 22.Murphy BR, Hinshaw VS, Sly DL, London WT, Hosier NT, Wood FT, et al. Virulence of avian influenza A viruses for squirrel monkeys. Infect Immun. 1982 Sep;37(3):1119–26. doi: 10.1128/iai.37.3.1119-1126.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian SF, Buckler-White AJ, London WT, Reck LJ, Chanock RM, Murphy BR. Nucleoprotein and membrane protein genes are associated with restriction of replication of influenza A/Mallard/NY/78 virus and its reassortants in squirrel monkey respiratory tract. J Virol. 1985 Mar;53(3):771–5. doi: 10.1128/jvi.53.3.771-775.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gambotto A, Barratt-Boyes SM, de Jong MD, Neumann G, Kawaoka Y. Human infection with highly pathogenic H5N1 influenza virus. Lancet. 2008 Apr 26;371(9622):1464–75. doi: 10.1016/S0140-6736(08)60627-3. [DOI] [PubMed] [Google Scholar]
- 25.Wilson IA, Skehel JJ, Wiley DC. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–73. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- 26.Webster RG, Laver WG, Air GM. Antigenic Variation Among Type A Influenza Viruses. In: Palese P, Kingsbury DW, editors. Genetics of Influenza Viruses. Vienna: Springer-Verlag; 1983. pp. 127–68. [Google Scholar]
- 27.Steinhauer DA. Role of hemagglutinin cleavage for the pathogenicity of influenza virus. Virology. 1999 May 25;258(1):1–20. doi: 10.1006/viro.1999.9716. [DOI] [PubMed] [Google Scholar]
- 28.Sieczkarski SB, Whittaker GR. Viral entry. Curr Top Microbiol Immunol. 2005;285:1–23. doi: 10.1007/3-540-26764-6_1. [DOI] [PubMed] [Google Scholar]
- 29.Stegmann T. Membrane fusion mechanisms: the influenza hemagglutinin paradigm and its implications for intracellular fusion. Traffic. 2000 Aug;1(8):598–604. doi: 10.1034/j.1600-0854.2000.010803.x. [DOI] [PubMed] [Google Scholar]
- 30.Pinto LH, Holsinger LJ, Lamb RA. Influenza virus M2 protein has ion channel activity. Cell. 1992 May 1;69(3):517–28. doi: 10.1016/0092-8674(92)90452-i. [DOI] [PubMed] [Google Scholar]
- 31.Wharton SA, Belshe RB, Skehel JJ, Hay AJ. Role of virion M2 protein in influenza virus uncoating: specific reduction in the rate of membrane fusion between virus and liposomes by amantadine. J Gen Virol. 1994 Apr;75(Pt 4):945–8. doi: 10.1099/0022-1317-75-4-945. [DOI] [PubMed] [Google Scholar]
- 32.Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou WM, Fiers W. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat Med. 1999 Oct;5(10):1157–63. doi: 10.1038/13484. [DOI] [PubMed] [Google Scholar]
- 33.Slepushkin VA, Katz JM, Black RA, Gamble WC, Rota PA, Cox NJ. Protection of mice against influenza A virus challenge by vaccination with baculovirus-expressed M2 protein. Vaccine. 1995;13(15):1399–402. doi: 10.1016/0264-410x(95)92777-y. [DOI] [PubMed] [Google Scholar]
- 34.Martin K, Helenius A. Transport of incoming influenza virus nucleocapsids into the nucleus. J Virol. 1991 Jan;65(1):232–44. doi: 10.1128/jvi.65.1.232-244.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cros JF, Palese P. Trafficking of viral genomic RNA into and out of the nucleus: influenza, Thogoto and Borna disease viruses. Virus Res. 2003 Sep;95(1–2):3–12. doi: 10.1016/s0168-1702(03)00159-x. [DOI] [PubMed] [Google Scholar]
- 36.Li X, Palese P. Characterization of the polyadenylation signal of influenza virus RNA. J Virol. 1994 Feb;68(2):1245–9. doi: 10.1128/jvi.68.2.1245-1249.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo GX, Luytjes W, Enami M, Palese P. The polyadenylation signal of influenza virus RNA involves a stretch of uridines followed by the RNA duplex of the panhandle structure. J Virol. 1991 Jun;65(6):2861–7. doi: 10.1128/jvi.65.6.2861-2867.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robertson JS, Schubert M, Lazzarini RA. Polyadenylation sites for influenza virus mRNA. J Virol. 1981 Apr;38(1):157–63. doi: 10.1128/jvi.38.1.157-163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krug RM. Priming of influenza viral RNA transcription by capped heterologous RNAs. Curr Top Microbiol Immunol. 1981;93:125–49. doi: 10.1007/978-3-642-68123-3_6. [DOI] [PubMed] [Google Scholar]
- 40.Bancroft CT, Parslow TG. Evidence for segment-nonspecific packaging of the influenza a virus genome. J Virol. 2002 Jul;76(14):7133–9. doi: 10.1128/JVI.76.14.7133-7139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duhaut SD, Dimmock NJ. Defective segment 1 RNAs that interfere with production of infectious influenza A virus require at least 150 nucleotides of 5′ sequence: evidence from a plasmid-driven system. J Gen Virol. 2002 Feb;83(Pt 2):403–11. doi: 10.1099/0022-1317-83-2-403. [DOI] [PubMed] [Google Scholar]
- 42.Enami M, Sharma G, Benham C, Palese P. An influenza virus containing nine different RNA segments. Virology. 1991 Nov;185(1):291–8. doi: 10.1016/0042-6822(91)90776-8. [DOI] [PubMed] [Google Scholar]
- 43.Fujii Y, Goto H, Watanabe T, Yoshida T, Kawaoka Y. Selective incorporation of influenza virus RNA segments into virions. Proc Natl Acad Sci U S A. 2003 Feb 18;100(4):2002–7. doi: 10.1073/pnas.0437772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colman PM, Varghese JN, Laver WG. Structure of the catalytic and antigenic sites in influenza virus neuraminidase. Nature. 1983 May 5–11;303(5912):41–4. doi: 10.1038/303041a0. [DOI] [PubMed] [Google Scholar]
- 45.Varghese JN, Laver WG, Colman PM. Structure of the influenza virus glycoprotein antigen neuraminidase at 2.9 A resolution. Nature. 1983 May 5–11;303(5912):35–40. doi: 10.1038/303035a0. [DOI] [PubMed] [Google Scholar]
- 46.Palese P, Compans RW. Inhibition of influenza virus replication in tissue culture by 2-deoxy-2,3-dehydro-N-trifluoroacetylneuraminic acid (FANA): mechanism of action. J Gen Virol. 1976 Oct;33(1):159–63. doi: 10.1099/0022-1317-33-1-159. [DOI] [PubMed] [Google Scholar]
- 47.Palese P, Tobita K, Ueda M, Compans RW. Characterization of temperature sensitive influenza virus mutants defective in neuraminidase. Virology. 1974 Oct;61(2):397–410. doi: 10.1016/0042-6822(74)90276-1. [DOI] [PubMed] [Google Scholar]
- 48.Matrosovich MN, Matrosovich TY, Gray T, Roberts NA, Klenk HD. Neuraminidase is important for the initiation of influenza virus infection in human airway epithelium. J Virol. 2004 Nov;78(22):12665–7. doi: 10.1128/JVI.78.22.12665-12667.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]