Abstract
The levels of L-arginine, a cationic, semi-essential amino acid, are often controlled within a cell at the level of local availability through biosynthesis. The importance of this temporal and spatial control of cellular L-arginine is highlighted by the tissue specific roles of argininosuccinate synthase (argininosuccinate synthetase) (EC 6.3.4.5), as the rate-limiting step in the conversion of L-citrulline to L-arginine. Since its discovery, the function of argininosuccinate synthase has been linked almost exclusively to hepatic urea production despite the fact that alternative pathways involving argininosuccinate synthase were defined, such as its role in providing arginine for creatine and for polyamine biosynthesis. However, it was the discovery of nitric oxide that meaningfully extended our understanding of the metabolic importance of non-hepatic argininosuccinate synthase. Indeed, our knowledge of the number of tissues that manage distinct pools of arginine under the control of argininosuccinate synthase has expanded significantly.
Keywords: Argininosuccinate synthase, arginine metabolism, nitric oxide, arginase, urea cycle, arginine recycling
Introduction
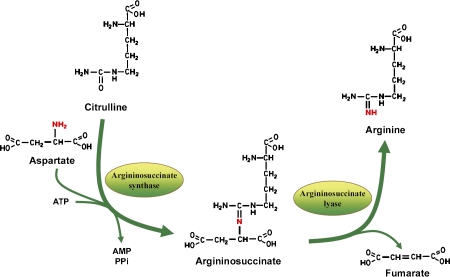
Arginine is required by all tissues in the human body for protein synthesis, and by some tissues for specialized needs. Any de novo biosynthetic pathway for arginine involves the conversion of citrulline to arginine catalyzed by argininosuccinate synthase and argininosuccinate lyase. Specifically, argininosuccinate synthase catalyzes the condensation of citrulline and aspartate to form argininosuccinate, the immediate precursor of arginine. Argininosuccinate lyase then splits argininosuccinate to release fumarate and arginine (Figure 1). First identified in liver, as a rate-limiting enzyme in urea synthesis, argininosuccinate synthase is now recognized as a ubiquitous enzyme in mammalian tissues, and not surprisingly, its expression, localization, and regulation differs significantly depending on the tissue specific needs for arginine.
Figure 1.
De Novo biosynthetic pathway for arginine.
Argininosuccinate synthase plays an important role as the rate-limiting step in providing arginine for an assortment of metabolic processes, both catabolic and anabolic. Thus, the metabolic pathways in which argininosuccinate synthase participates are linked to the varied uses of the amino acid arginine. There are five major pathways in which argininosuccinate synthase plays a key role. These are (a) urea synthesis, (b) nitric oxide synthesis, (c) polyamine synthesis, (d) creatine synthesis, and (e) the de novo synthesis of arginine to maintain serum levels.
In liver, where arginine is hydrolyzed to form urea and ornithine, argininosuccinate synthase is highly expressed, and hormone and nutrients constitute the major regulating factors [1]. In contrast, for nitric oxide-producing cells where arginine is the direct substrate for nitric oxide synthesis, argininosuccinate synthase is expressed at relatively low levels, and in most cases, its regulation has been tied to affecting the phenotypic patterns controlled by nitric oxide signaling [2].
The subcellular localization of argininosuccinate synthase also varies. For example, in endothelial cells argininosuccinate synthase co-localizes with eNOS at caveolae [3] and outside of the Golgi [4]. In kidney, subcellular localization is not well-defined, but seems to be cytosolic in relation to synthesis of arginine for guanidinoacetate production and for the de novo synthesis of arginine for export to maintain serum arginine levels. In liver, argininosuccinate synthase associates with the outside of the mitochondria to complete the urea cycle, which involves both the mitochondrial and cytosolic compartments [5].
The objective of this discussion is to highlight the varied metabolic processes that involve argininosuccinate synthase, its essential role in regulating unique cellular pools of available arginine, and the levels of control that regulate its expression.
Argininosuccinate synthase and the urea cycle
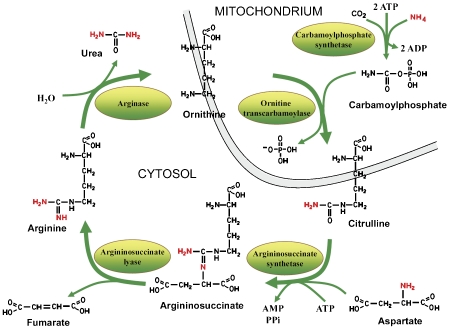
The urea cycle, also known as the Krebs urea cycle or ornithine cycle, was first discovered in 1932 by Hans Krebs [6]. The cycle is comprised of five enzymes and is essentially localized to the liver in mammals, although the kidney does share some capacity to produce urea. The first two enzymes of the urea cycle, carbamoyl phosphate synthetase I and ornithine transcarbamoylase are located within the mitochondrial matrix of periportal hepatocytes [7], while the remaining three enzymes are located in the cytosol. Argininosuccinate synthase is located on the outer mitochondrial membrane [5], utilizing both citrulline and aspartate to produce argininosuccinate. Argininosuccinate lyase catalyzes the cleavage of argininosuccinate to produce arginine and fumarate. Subsequently, arginase splits the arginine to ornithine and urea, which ensures that the arginine generated by the actions of argininosuccinate synthase and argininosuccinate lyase is, for the most part, directed to urea production [8] (Figure 2). Urea is excreted and the ornithine is transported back into the mitochondria to complete the cycle.
Figure 2.
Urea cycle
Immunocytochemical studies by Cohen & Kudal [5] showed that argininosuccinate synthase and argininosuccinate lyase are located in the immediate vicinity of the mitochondria, predominantly next to the cytoplasmic surface of the outer membrane. This finding confirmed previous biochemical studies [9–11] suggesting that these enzymes are organized in situ next to the outer membrane of mitochondria. Perhaps our best understanding as to how argininosuccinate synthase associates with hepatic mitochondria comes from the findings related to adult-onset type II citrullinemia (CTLN2). Unlike classical type I citrullinemia (CTLN1) in children where the deficiency of argininosuccinate synthase results from a mutation in the gene [12], in CTLN2 there is no mutation in the argininosuccinate synthase gene, yet there is still a marked deficiency in liver argininosuccinate synthase protein [13]. To account for the liver specific deficiency of argininosuccinate synthase in CTLN2, Saheki et al. [13] showed association of CTLN2 with a defect in SLC25A13 gene that encodes the mitochondrial Ca2+-dependent as-partate/glutamate transporter known as citrin [14]. Saheki's findings suggested that citrin forms a complex with the three “soluble” cytoplasmic enzymes of the urea cycle, including argininosuccinate synthase. This interaction with citrin may be direct or mediated through some other protein or proteins. Nevertheless, the loss of spatial control attributable to the mutated citrin strongly correlated with reduction of liver argininosuccinate synthase protein in CTLN2, possibly through destabilization and/or degradation [13].
Urea cycle enzymes appear to be closely associated with one another such that substrates and products can be shuttled from one enzyme to another by channeling [11]. Support for channeling comes from studies demonstrating the channeling of the extramitochondrial ornithine to the mitochondrial ornithine transcarbamoylase via its transporter [15], from studies showing the co-localization of carbamoyl phosphate synthetase I and ornithine transcarbamoylase with the inner mitochondrial membrane [16], from labeling studies demonstrating channeling of citrulline from ornithine transcarbamoylase in the mitochondria to argininosuccinate synthase in the cytoplasm, and from studies demonstrating channeling of arginine between argininosuccinate lyase and arginase [10].
Thus the spatial relationship of argininosuccinate synthase relative to ornithine transcarbamoylase in the mitochondria, and argininosuccinate lyase in the cytosol, together with the relatively high expression levels for each of these enzymes, ensures that both substrate and product, and in particular arginine, do not readily exchange with other cellular pools, or even with extracellular sources. Moreover, hepatocytes do not easily exchange arginine with serum, evidenced by their limited capacity to take up arginine [17]. As a result, any other intracellular pool of arginine, as well as serum arginine, is protected [18, 19].
The significance of this arrangement can be illustrated by the grave outcomes observed during liver failure, liver transplants, and other liver diseases, that result in disruption of liver tissue and the release of hepatic arginase. Under these conditions, serum arginine levels can be severely diminished, limiting the available arginine for other tissues [20–23].
Under normal conditions, and in the short term, the urea cycle has sufficient capacity to respond to immediate increases in waste nitrogen load [1, 24, 25], and the rate of urea production essentially depends on the activity of carbamoyl-phosphate synthase I [1]. On the other hand, under conditions where the urea cycle is functioning maximally, argininosuccinate synthase becomes the rate-limiting step [24]. Therefore, it is not surprising that expression of argininosuccinate synthase, relative to urea cycle function, is highly regulated and governed by changes in dietary protein intake, and by hormones such as glucagon, insulin, and glucocorticoids [1]. There are also studies in cultured hepatocytes demonstrating nutrient control over argininosuccinate synthase expression where the addition of arginine was suppressive and the addition of citrulline induced argininosuccinate synthase expression [23, 26, 27].
Both the response to dietary changes and the response to hormones seem to be largely coordinated through transcriptional regulation of the argininosuccinate synthase gene (ASS1). Argininosuccinate synthase mRNA levels are induced by glucocorticoids, cAMP, and glucagon; whereas, insulin and growth hormone seem to act on the developmental regulation of argininosuccinate synthase by modulating the glucocorticoid effect [28, 29]. Interestingly, it has only recently been shown that regulation of argininosuccinate synthase transcription by cAMP maps to a highly conserved cAMP response element 10 kb upstream of the transcription start site in the argininosuccinate synthase promoter [28].
Argininosuccinate synthase involvement in nitric oxide metabolism
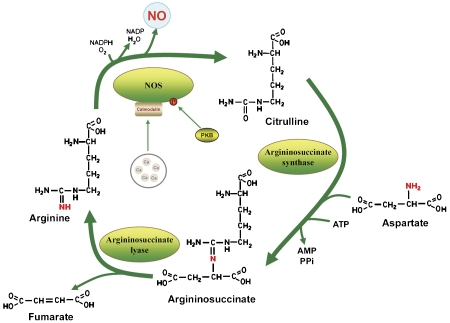
Endothelium derived relaxing factor, first discovered by Furchgott [30] in 1980, was later determined to be a free radical compound called nitric oxide [31]. Nitric oxide is a highly reactive molecule that readily diffuses through cell membranes. As a consequence, nitric oxide cannot be stored inside producing cells like other endogenous messengers; rather, its signaling capacity must be controlled at the levels of synthesis and local availability. The importance of this temporal and spatial control of nitric oxide production is highlighted by vascular endothelial cells where virtually all phenotypic properties are related to nitric oxide bioactivity. Endothelial nitric oxide synthase (eNOS) utilizes L-arginine as the principal substrate, converting it to L-citrulline and nitric oxide. L-citrulline is recycled to L-arginine by two enzymes, argininosuccinate synthase and argininosuccinate lyase, providing the essential arginine for nitric oxide production in endothelial cells [32]. Together, the three enzymes, eNOS, argininosuccinate synthase and argininosuccinate lyase, make up the citrulline-nitric oxide cycle [29] (Figure 3).
Figure 3.
Citrulline-nitric oxide cycle
Although expression of the complete urea cycle is only described in liver, argininosuccinate synthase and argininosuccinate lyase are expressed in an entire range of cell types, often related to their involvement in providing the essential arginine for nitric oxide production. In fact, the capacity to recycle citrulline to arginine appears to be a prerequisite for all nitric oxide-producing cells [33].
For example, cells that constitutively express isoforms of NOS, such as vascular endothelial cells and neurons, all have the capacity to regenerate arginine from citrulline [34–36]. Citrulline formation by pulmonary endothelial cells was shown to increase when nitric oxide production was stimulated, and to diminish when subjected to chronic hypoxia [37]. In neural tissue, histochemical staining for the localization of nitric oxide synthase demonstrated that argininosuccinate synthase coexists with nitric oxide synthase [38]. Furthermore, argininosuccinate synthase and argininosuccinate lyase colocalize in discrete populations of myenteric and submucosal neurons in the canine proximal colon [36].
Likewise, the induction of iNOS, that leads to increased production of nitric oxide in non-hepatic cell types, is always accompanied by the enhancement of argininosuccinate synthase expression [33]. Argininosuccinate synthase and argininosuccinate lyase also co-localize with nNOS throughout the canine gastrointestinal tract [39]. In the guinea-pig trachea, human bronchus and murine proximal colon, the inhibition of electrically induced nitrergic responses by competitive NOS inhibitors was shown to be overcome by both arginine and citrulline treatment. The ability of citrulline treatment to overcome NOS inhibition was attributed to its conversion to arginine by argininosuccinate synthase and argininosuccinate lysase [40, 41].
More recently, Van Geldre et al [42] demonstrated that citrulline recycling was active in rat gastric fundus, and that the recycling of citrulline maintained sufficient arginine during the long-term relaxations of the gastric fundus after food intake. While Swany et al [43] suggested that in the rat cerebellum the citrulline-nitric oxide cycle maintains the essential supply of arginine to support the increased production of nitric oxide.
When Xie et al. [44] transfected argininosuccinate synthase into vascular smooth muscle cells, the resulting overexpression of argininosuccinate synthase dramatically enhanced the capacity of transfected cells to produce nitric oxide over that of untransfected cells despite non-limiting levels of extracellular arginine. As a result, Xie et al. [44, 45] concluded that the capacity to recycle citrulline to arginine is “ratelimiting” to nitric oxide production. Su et al. [37] arrived at a similar conclusion showing that endotoxin inhibited induction of argininosuccinate synthase, and as a consequence, production of nitric oxide in hypoxic pulmonary artery endothelial cells was impaired.
Compelling evidence for the essential role of argininosuccinate synthase in supporting nitric oxide production came from a series of experiments using RNA interference to specifically silence argininosuccinate synthase expression in vascular endothelial cells [32]. Diminished levels of argininosuccinate synthase expression that resulted from siRNA treatment were shown to correlate with impaired nitric oxide production in the presence and absence of effectors. Unanticipated, however, was the significant loss of viable endothelial cells observed after siRNA treatment. The fact that the loss of viability followed reduced expression of Bcl-2, and an increase in caspase activity, strongly suggested a relationship between impaired nitric oxide production and apoptosis. This relationship was supported by the observation that exposure to a nitric oxide donor rescued cell viability despite siRNA treatment [32]. More importantly, these results demonstrated that argininosuccinate synthase was even required for the maintenance of basal production levels of nitric oxide in order to support cell viability.
In endothelial cells, argininosuccinate synthase and argininosuccinate lyase were shown to colocalize with endothelial nitric oxide synthase (eNOS) and specialized structures of the plasma membrane called caveolae [3, 46]. Caveolar domains assemble at the Golgi and traffic to the plasma membrane as stable transport platforms [47]. This may explain the subcellular association of eNOS with Golgi and perinuclear membrane [4, 48, 49]. Evidence also suggests that interaction with other proteins may play a role in eNOS targeting. For example, Schilling et al [50] described two novel eNOS-interacting proteins named NOSIP (for eNOS-interacting protein) and NOSTRIN (for eNOS-trafficking inducer), both of which influence the subcellular localization of eNOS. For NOSIP, targeting of eNOS to the cytoskeleton seems to be one of the protein's major functions [51]. On the other hand, when NOSTRIN is overexpressed, this leads to the translocation of eNOS from the plasma membrane to intracellular vesicular structures [52], possibly involving an endocytic process [53]. In comparison, much less is known about argininosuccinate synthase targeting and how the co-localization of argininosucci-nate synthase and eNOS is maintained to regulate the local availability of substrate for nitric oxide production.
Argininosuccinate synthase in the maintenance of serum arginine levels
In adult human intestines, glutamine/glutamate and proline are converted to citrulline. This citrulline is subsequently released by enterocytes to be taken up mainly by the kidneys for conversion to arginine [4]. Because a significant fraction (~40%) of ingested arginine undergoes catabolism [33], the majority of circulating arginine used for other tissues is provided by the kidneys [54] and from protein turnover [33]. Thus, arginine is often considered to be “semiessential” since in healthy adults endogenous production, essentially by the kidneys, can be sufficient to meet normal metabolic needs [33].
It has been speculated that in the mammalian neonate, enteric arginine synthesis is necessary because mother's milk is relatively deficient in arginine; whereas, the precursors of arginine, proline and glutamine, are quite plentiful [55]. This may also be the reason why the fetal intestine expresses argininosuccinate synthase, providing the metabolic capacity to produce arginine before birth. Nevertheless, at about two to three years of age, the intestine loses the ability to synthesize arginine as a consequence of the loss of argininosuccinate synthase expression. Subsequently, endogenous arginine biosynthesis becomes an inter-organ process, where the net production of citrulline occurs almost exclusively in the enterocytes of the small intestine [56] and absorption of citrulline from circulation takes place essentially in the cortex of the kidney [57] where it is converted to arginine.
In the adult human, elevated expression and co-localization of carbamoyl phosphate synthase I (CPS1) and ornithine transcarbamoylase (OTC) in enterocyte mitochondria favors intestinal synthesis of citrulline from ammonia, HCO3- and ornithine, while the relatively low levels of cytosolic argininosuccinate synthase and argininosuccinate lyase minimizes further conversion of citrulline to arginine. Based on these differences, the intestinal urea cycle enzymes maximize the net synthesis of citrulline from glutamine, and the specific release of citrulline [9].
Similar to the poor uptake of arginine by the liver, citrulline does not undergo significant hepatic uptake and therefore is available for renal arginine synthesis [56]. In the kidney, conversion of citrulline to arginine is catalyzed by argininosuccinate synthase and argininosuccinate lyase. Unlike other pathways involving argininosuccinate synthase, the rate-limiting step in the synthesis of arginine by the kidney seems to be based on citrulline availability [29, 56, 57]. Support for this observation comes from the finding that citrulline supplementation stimulates arginine synthesis in the kidneys, leading to increases in plasma arginine [58].
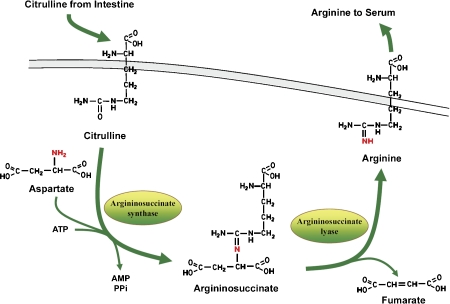
Thus in adults, the majority of endogenous arginine synthesis involves the intestinal/renal axis. Citrulline is released by the intestines followed by uptake in the kidney. In the kidneys, expression of argininosuccinate synthase and argininosuccinate lyase then promotes conversion of citrulline to arginine and its subsequent release into plasma (Figure 4).
Figure 4.
Synthesis of arginine by kidney.
Argininosuccinate synthase provides the essential arginine for creatine biosynthesis
Creatine is distributed throughout the body, with approximately 95% found in skeletal muscle [59] and most of the remaining 5% in brain, liver, kidney and testis. The daily requirement for creatine is achieved either through intestinal absorption of dietary creatine or by de novo creatine biosynthesis [60]. Two enzymes are involved in creatine biosynthesis. AGAT (L-Arginine-glycine amidinotransferase) converts arginine and glycine into ornithine and guanidinoacetate, and GAMT (Guanidinoacetate methyltransferase) catalyzes S-adenosyl-L-methionine-dependent methylation of guanidinoacetate to yield creatine and S-adenosyl-L-homocysteine. The formation of guanidinoacetate is normally the rate-limiting step of creatine biosynthesis, and in this respect, the feedback repression by creatine, the end product of the pathway, acts to conserve the utilization of the essential amino acid, methionine, and semiessential amino acid, arginine.
Again, different body tissues seem to play a role in creatine synthesis. In humans, synthesis of guanidinoacetate occurs mainly in the kidney followed by transport to the liver where guanidinoacetate is subsequently methylated to produce creatine [60]. Thus the kidney-liver axis for creatine synthesis provides creatine to peripheral tissues, and in particular to muscle.
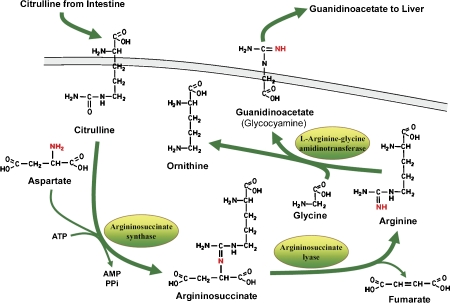
In the human kidney, guanidinoacetate is synthesized by the transamidation of the guanidine group from arginine to glycine. The synthesis of guanidinoacetate is dependent on the endogenous synthesis of arginine from citrulline catalyzed by argininosuccinate synthase and argininosuccinate lyase. However, regulation of argininosuccinate synthase in kidney tissue, as it relates to the synthesis of guanidinoacetate, is not well-defined. Evidence, however, suggests that in this case again, the rate-limiting step may be the availability of citrulline that is provided by the intestines, and the negative feedback regulation of creatine at AGAT [60] (Figure 5).
Figure 5.
Synthesis of guanidinoacetate by the kidney.
Argininosuccinate synthase defines the pool of arginine for arginase and subsequent polyamine synthesis
The arginine derived from argininosuccinate synthase and argininosuccinate lyase may also be diverted by arginase to the production of ornithine for polyamine biosynthesis [9]. Ornithine is a precursor of putrescine, the base molecule of polyamines. In this case, arginine production by argininosuccinate synthase regulation would presumably be tied to cell cycle regulatory events, and perhaps to the induction and regulation of ornithine decarboxylase in response to specific hormones, growth factors, or cell cycle regulatory signals. Importantly, argininosuccinate synthase appears to provide a distinct pool of intracellular arginine for arginase and the subsequent synthesis of polyamines, much like that observed for nitric oxide production [61]. This observation is supported by results demonstrating that polyamine synthesis is dependent on the intracellular localization and synthesis of arginine from citrulline [62, 63].
Argininosuccinate synthase expression in tumor tissue
Some tumor types downregulate argininosuccinate synthase expression producing an intrinsic dependence on extracellular arginine due to the inability to synthesize endogenous arginine for growth [64]. This dependence on extracellular arginine is known as “arginine auxotrophy”, and tumors of this type include heptocellular carcinoma, malignant melanoma, malignant pleural mesothelioma, osteosarcoma, prostate and renal cancer [64]. Although arginine deprivation therapy, as an anticancer strategy, has been investigated for several decades [65–69], it is only recently that it has shown encouraging activity in patients with specific tumor types [70].
Arginine deiminase (ADI), an enzyme isolated from Mycoplasma [71, 72], hydrolyzes arginine to citrulline and ammonia. In its native form, it is strongly antigenic with a half-life of 5 hours [73]. However, conjugation to 20,000 mw polyethylene glycol (ADI-PEG20) decreases antigenicity and dramatically increases serum half-life, allowing weekly administration that reduces plasma arginine to undetectable levels [65]. For example, ADI-PEG 20, with its prolonged halflife and ability to suppress detectable levels of circulating arginine, has been assessed in patients with malignant melanoma and hepatocellular carcinoma. Both are arginine auxotrophic tumors [65]. Izzo et al. [74] reported a 47% (complete and partial) response rate to this drug in a phase I/II study of patients with advanced hepatocellular carcinoma. Whereas, Ascierto et al. [75] documented an overall 25% (complete and partial) response rate for this treatment in patients with advanced metastatic melanoma. Importantly, several patients in both studies were observed to have a sustained response to treatment. Based on reports like these, ADI-PEG 20 has received an orphan drug designation in both the United States and, more recently, in Europe for the treatment of hepatocellular carcinoma.
Just recently, Kobayashi et al [76] suggested that reduced expression of argininosuccinate synthase in patients with osteosarcoma may provide a novel predictive biomarker related to their risk of metastasis. Moreover, the authors also suggested that it may be possible to exploit the arginine auxotrophic phenotype of this osteosarcoma by depleting extracellular arginine [70].
In contrast, there are also tumor types that demonstrate a dependency on elevated argini-nosuccinate synthase expression. For example, Szolsarek et al [77] demonstrated that epithelial ovarian tumor tissue was highly dependent on elevated argininosuccinate synthase expression. Based on this observation, the authors suggested that the dependency of this ovarian tumor on elevated argininosuccinate synthase may be exploited, providing an important diagnostic marker as well as potential therapeutic target.
Regulation of argininosuccinate synthase expression
Since the early publications on argininosuccinate synthase in the 1970s, our understanding of the properties and biological role of this enzyme has changed greatly. Initially, the non-hepatic role of this enzyme was assumed to be a vestige; and as such, the role and regulation of argininosuccinate synthase, outside of that needed for urea production, developed slowly. However, interest in argininosuccinate synthase, and particularly its regulation, has grown due in great part because of its involvement in nitric oxide production [29].
In humans, there are 14 copies of the argininosuccinate synthase gene, including 13 pseudogenes scattered across the genome. The ASS1 gene, located on chromosome 9, appears to be the only functional gene involved in argininosuccinate synthase expression. This is supported by the findings that mutations in the chromosome 9 copy of ASS1 cause citrullinemia [12, 13, 78].
About 800 bp of the promoter region for the human ASS1 gene have been characterized [79] showing a TATA box, six potential Sp1 binding sites (GC boxes) and one potential AP-2 site [80, 81]. The three GC boxes in the immediate promoter were shown to act synergistically and to be required in order to achieve full promoter activation [81]. Surprisingly, no CAAT sequence (C/EBP binding site), nor GRE (glucocorticoid responsive), or CRE (cAMP responsive-) elements were found in the early characterization of the argininosuccinate synthase promoter.
Recently, however, Guei et al [28], through functional analysis of DNase I hypersensitive sites, located a cAMP response element for the human ASS1 gene at ~10 kb upstream of the transcription start site. This discovery helped reconcile earlier findings showing liver-specific enhancement of ASS1 gene expression by TSE1 (tissue-specific extinguisher locus 1) regulation [82, 83], and the fact that transcription of the ASS1 gene in rat liver was stimulated by cAMP in nuclear run-on assays [84]. TSE1 encodes the regulatory subunit Rl alpha of protein kinase A (PKA) [85].
According to Tsai et al [86], the proximal region of the ASS1 promoter also contains an E-box that is recognized by c-Myc and HIF-1alpha, as well as a GC-box that is recognized by Sp4 in melanoma cells. Under non-induced conditions, the E-box element bound HIF-1 alpha, and under induced conditions (arginine depletion) HIF-1 alpha was replaced by c-Myc, at least in two of the melanoma cell lines, but not in another. In all cases, however, Sp4 was shown to constitutively bind the GC-box regardless of arginine availability. Taken together, their results suggested that the transcriptional induction of ASS1 expression, relative to arginine depletion, involved the interplay between the positive transcriptional regulators c-Myc and Sp4, and the negative regulator HIF-1alpha. Moreover, this level of regulation in melanoma cells was taken to confer resistance to arginine deiminase based arginine depletion studies.
Since most all phenotypic properties of endothelial cells relate to nitric oxide signaling, eNOS has been highly studied in response to physiological cues such as shear stress and cytokines [87–90]. On the other hand, relatively little is known about the regulatory mechanisms that control argininosuccinate synthase expression in this context. Nevertheless, what has been elucidated, thus far, has provided a picture that supports the central role of arininosuccinate synthase and its coordinate regulation with eNOS in nitric oxide production.
For example, Mun et al [91] examined the differential expression of genes in human umbilical vein endothelial cells (HUVECs) under static and laminar shear stress conditions. Among the 20 genes whose expression was increased by laminar shear stress conditions, and simultaneously decreased by cellular senescence, was ASS1. Based on their findings, the authors suggested that argininosuccinate synthase expression was critical both for basal and laminar shear stress-induced nitric oxide production. This observation was consistent with Goodwin et al [32] who demonstrated through siRNA knockdown studies, that argininosuccinate synthase expression was required to support both basal and induced nitric oxide production in vascular endothelial cells.
Goodwin et al [92] also showed that both argininosuccinate synthase and eNOS expression were significantly reduced by treatment with physiological levels of TNF-alpha in vascular endothlelial cells. Not surprisingly, the loss argininosuccinate synthase and eNOS expression was accompanied by a dramatic decrease in the capacity of the TNF-alpha-treated cells to produce nitric oxide. Moreover, the down regulation of argininosuccinate synthase expression by TNF-alpha was shown to be mediated through a similar transcriptional mechanism by which TNF-alpha down-regulates eNOS, involving Sp1/Sp3 elements in the proximal promoter [87]. Inhibitor studies suggested that repression of argininosuccinate synthase expression by TNF-alpha was mediated, at least in part, via the NF-kappaB signaling pathway [93]. Critically, these results revealed the extent to which TNF-alpha impairs vascular endothelial function via the coordinate down-regulation of both eNOS and argininosuccinate synthase expression [92].
On the other hand, the peroxisome proliferator-activated receptor gamma (PPAR-gamma) agonist, troglitazone, was shown to support nitric oxide production in vascular endothelial cells through the up-regulation of argininosuccinate synthase expression [94]. Because the induction of argininosuccinate synthase was inhibited by treatment with the transcriptional inhibitor, 1 -D-ribofuranosylbenzimidazole (DRB), this demonstrated that troglitazone mediated its response essentially at the transcriptional level. In keeping with this finding, a distal PPAR-gamma response element (PPRE) at –2471 to –2458 was identified that mediated the PPAR-gamma agonist response. These results extended the repertoire of mechanisms by which PPAR-gamma agonists promote vascular health [95, 96]. In the case of troglitazone, one of its vascular protective properties directly relates to the maintenance of an adequate supply of arginine for nitric oxide production through the upregulation of argininosuccinate synthase [94].
Less is known at a more global level involving co-regulators and co-repressors that interact with specific transcription factors on the ASS1 gene promoter to alter rates of expression. Thus, critical information related to the interplay of promoter elements that allow tissue specific expression of argininosuccinate synthase is quite limited.
Argininosuccinate synthase has been shown to associate with raft-like membrane microdomains, caveolae, at the plasma membrane [3]. The major constituent of caveolae is caveolin-1 [97], which is expressed in many cell types, including endothelial and epithelial cells. Importantly, a major phenotype of caveolin-1 knockout mice relates to nitric oxide signaling. Nitric oxide release from caveolin-1-deficient cells is higher compared to wild-type cells, and secondary effects of nitric oxide are increased, demonstrating activation of eNOS through deinhibition in the absence of caveolin-1 [98]. Conversely, direct binding of eNOS to the scaffolding domain (positions 82-101) of caveolin-1 has been shown to decrease nitric oxide production in vitro and in cell culture [46, 99, 100]. However, little is known as to whether caveolin may play a similar role in affecting either the activity or the subcellular localization of argininosuccinate synthase. Indeed, the only known factor shown to affect localization of arginino-succinate synthase is citrin where mutations in the SLC25A13 gene that encodes citrin were shown to cause adult-onset type II citrullinemia [13]. In this case, the mutated citrin does not allow argininosuccinate synthase mitochondrial association. As a consequence, argininosuccinate synthase appears to be rapidly degraded accounting for the low levels found in hepatocytes of affected individuals [13].
Open reading frames in the 5′-leaders of messenger RNAs are emerging as important mediators of transcript-specific translational controls. In most instances, upstream open-reading frames (uORFs) regulate the expression of a downstream ORF on the same mRNA (cis-regulation). In this case, those mRNAs with uORFs are typically expressed as the only species of that mRNA in a particular cell type [101]. In contrast, the transcription of argininosucci-nate synthase mRNA, at least in vascular endo-thelial cells, is initiated from multiple start sites, generating mRNAs with different lengths and overlapping 5′-untranslated regions (UTRs) [102]. In fact, nearly 10% of argininosuccinate transcripts are longer variants that contain an out-of-frame, upstream open reading frame (uORF) encoding a ~4.4 kDa protein, referred to as Argininosuccinate synthase Regulatory Protein (ARP). ARP downregulates argininosuccinate synthase expression in trans at the translational level [103]. Consistent with the ratelimiting role of argininosuccinate synthase in the citrulline/nitric oxide cycle, the increase in argininosuccinate synthase protein that resulted from the knockdown of ARP was accompanied by an increase in nitric oxide production. Likewise, when ARP was over-expressed in endothelial cells, argininosuccinate synthase expression was suppressed, resulting in reduced nitric oxide production [103].
Since argininosuccinate synthase is responsible for maintaining the available arginine for varied metabolic processes, it seems logical that acute or immediate changes in activity may also be necessary to accommodate the multiple roles that the arginine product plays in cell function. However, such immediate responses require regulation that goes beyond changes in transcription. Nevertheless, there are very few documented reports that discuss posttranslational events that may regulate the function of argininosuccinate synthase. In fact the bulk of published literature suggests that argininosuccinate synthase regulation occurs primarily at the transcriptional level [29].
There are, however, some recent findings that have changed this view. For example, it has been reported that the NADPH sensor protein, HSCARG, interacts with argininosuccinate synthase to downregulate its activity in epithelial cells [104]. In fact, it has been suggested that the protein-protein interaction of HSCARG with argininosuccinate synthase may provide a mechanism by which the epithelial cell maintains an intracellular balance between its redox state and nitric oxide levels.
On the other hand, Hao et al [105] showed that human argininosuccinate synthase can be inactivated by reversible nitrosylation at Cys-132 in response to lipopolysaccharide-treatment in both cultured smooth muscle cells and in mice. Mutagenesis studies confirmed that the nitrosylation of Cys-132 was both necessary and sufficient to inhibit argininosuccinate synthase. Based on their results, the authors suggested that post-translational modification of arginino-succinate synthase by nitrosylation might provide important feedback mechanism whereby nitric oxide can limit arginine availability in nitric oxide producing cells [105].
Possibly the first report demonstrating that argininosuccinate was a phosphoprotein came in 2008 when Imani et al [106], using a phosphoproteomic analysis, reported that argininosuccinate synthase in HeLa cells was a phosphoprotein. However, their study did not consider the biological relevance of argininosuccinate synthase phosphorylation. Subsequently, Corbin et al [107] showed that argininosuccinate synthase was indeed phosphorylated in vascular endothelial cells in response to VEGF treatment by protein kinase A (PKA). Interestingly, PKA was already known to enhance nitric oxide production via phosphorylation and activation of eNOS [48].
Although reports on the post-transcriptional regulation of argininosuccinate synthase are presently meager in number, it is becoming clear that there exists an assortment of new mechanisms that affect argininosuccinate synthase expression and activity. As such, argininosuccinate synthase offers a distinctive paradigm for regulation that has expanded beyond transcriptional regulation to now include localization, translation and post-translational controls. This array of regulatory strategies which a cell uses to define and control arginine levels further endorses the physiological significance of argininosuccinate synthase in mammalian arginine metabolism.
Acknowledgements
This work was supported in part by American Heart Association 0455228B, Mary and Walter Traskiewicz Memorial Fund, USF Foundation Grant 250047, James & Esther King Biomedical Research Program Grant, DOH Florida, NIH RO1 HL083153-01A2
References
- [1].Morris SM., Jr. Regulation of enzymes of the urea cycle and arginine metabolism. Annu Rev Nutr. 2002;22:87–105. doi: 10.1146/annurev.nutr.22.110801.140547. [DOI] [PubMed] [Google Scholar]
- [2].Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- [3].Flam BR, Hartmann PJ, Harrell-Booth M, Solo-monson LP, Eichler DC. Caveolar localization of arginine regeneration enzymes, argininosuccinate synthase, and lyase, with endothelial nitric oxide synthase. Nitric Oxide. 2001;5:187–197. doi: 10.1006/niox.2001.0340. [DOI] [PubMed] [Google Scholar]
- [4].Fulton D, Babbitt R, Zoellner S, Fontana J, Acevedo L, McCabe TJ, Iwakiri Y, Sessa WC. Targeting of endothelial nitric-oxide synthase to the cytoplasmic face of the Golgi complex or plasma membrane regulates Akt- versus calcium-dependent mechanisms for nitric oxide release. J Biol Chem. 2004;279:30349–30357. doi: 10.1074/jbc.M402155200. [DOI] [PubMed] [Google Scholar]
- [5].Cohen NS, Kuda A. Argininosuccinate synthetase and argininosuccinate lyase are localized around mitochondria: an immunocytochemical study. J Cell Biochem. 1996;60:334–340. doi: 10.1002/(SICI)1097-4644(19960301)60:3%3C334::AID-JCB5%3E3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- [6].Stubbs M, Gibbons G. Hans Adolf Krebs (1900-1981)…his life and times. IUBMB Life. 2000;50:163–166. doi: 10.1080/152165400300001462. [DOI] [PubMed] [Google Scholar]
- [7].Meijer AJ, Lamers WH, Chamuleau RA. Nitrogen metabolism and ornithine cycle function. Physiol Rev. 1990;70:701–748. doi: 10.1152/physrev.1990.70.3.701. [DOI] [PubMed] [Google Scholar]
- [8].Ratner S. Enzymes of arginine and urea synthesis. Adv Enzymol Relat Areas Mol Biol. 1973;39:1–90. doi: 10.1002/9780470122846.ch1. [DOI] [PubMed] [Google Scholar]
- [9].Davis PK, Wu G. Compartmentation and kinetics of urea cycle enzymes in porcine enterocytes. Comp Biochem Physiol B Biochem Mol Biol. 1998;119:527–537. doi: 10.1016/s0305-0491(98)00014-5. [DOI] [PubMed] [Google Scholar]
- [10].Cheung CW, Cohen NS, Raijman L. Channeling of urea cycle intermediates in situ in permeabilized hepatocytes. J Biol Chem. 1989;264:4038–4044. [PubMed] [Google Scholar]
- [11].Watford M. Channeling in the urea cycle: a metabolon spanning two compartments. Trends Biochem Sci. 1989;14:313–314. doi: 10.1016/0968-0004(89)90156-4. [DOI] [PubMed] [Google Scholar]
- [12].Gao HZ, Kobayashi K, Tabata A, Tsuge H, Iijima M, Yasuda T, Kalkanoglu HS, Dursun A, Tokatli A, Coskun T, Trefz FK, Skladal D, Mandel H, Seidel J, Kodama S, Shirane S, Ichida T, Makino S, Yoshino M, Kang JH, Mizuguchi M, Barshop BA, Fuchinoue S, Seneca S, Zeesman S, Knerr I, Rodes M, Wasant P, Yoshida I, De Meirleir L, Abdul Jalil M, Begum L, Horiuchi M, Katunuma N, Nakagawa S, Saheki T. Identification of 16 novel mutations in the argininosuccinate synthetase gene and genotype-phenotype correlation in 38 classical citrullinemia patients. Hum Mutat. 2003;22:24–34. doi: 10.1002/humu.10230. [DOI] [PubMed] [Google Scholar]
- [13].Saheki T, Kobayashi K, Iijima M, Horiuchi M, Begum L, Jalil MA, Li MX, Lu YB, Ushikai M, Tabata A, Moriyama M, Hsiao KJ, Yang Y. Adult-onset type II citrullinemia and idiopathic neonatal hepatitis caused by citrin deficiency: involvement of the aspartate glutamate carrier for urea synthesis and maintenance of the urea cycle. Mol Genet Metab. 2004;81(Suppl 1):S20–26. doi: 10.1016/j.ymgme.2004.01.006. [DOI] [PubMed] [Google Scholar]
- [14].Kobayashi K, Sinasac DS, Iijima M, Boright AP, Begum L, Lee JR, Yasuda T, Ikeda S, Hirano R, Terazono H, Crackower MA, Kondo I, Tsui LC, Scherer SW, Saheki T. The gene mutated in adult-onset type II citrullinaemia encodes a putative mitochondrial carrier protein. Nat Genet. 1999;22:159–163. doi: 10.1038/9667. [DOI] [PubMed] [Google Scholar]
- [15].Cohen NS, Cheung CW, Raijman L. Channeling of extramitochondrial ornithine to matrix ornithine transcarbamylase. J Biol Chem. 1987;262:203–208. [PubMed] [Google Scholar]
- [16].Powers-Lee SG, Mastico RA, Bendayan M. The interaction of rat liver carbamoyl phosphate synthetase and ornithine transcarbamoy-lase with inner mitochondrial membranes. J Biol Chem. 1987;262:15683–15688. [PubMed] [Google Scholar]
- [17].White MF, Gazzola GC, Christensen HN. Cationic amino acid transport into cultured animal cells. I. Influx into cultured human fibroblasts. J Biol Chem. 1982;257:4443–4449. [PubMed] [Google Scholar]
- [18].Maher AD, Kuchel PW, Ortega F, de Atauri P, Centelles J, Cascante M. Mathematical modelling of the urea cycle. A numerical investigation into substrate channelling. Eur J Biochem. 2003;270:3953–3961. doi: 10.1046/j.1432-1033.2003.03783.x. [DOI] [PubMed] [Google Scholar]
- [19].Panza JA, Garcia CE, Kilcoyne CM, Quyyumi AA, Cannon RO., 3rd Impaired endothelium-dependent vasodilation in patients with essential hypertension. Evidence that nitric oxide abnormality is not localized to a single signal transduction pathway. Circulation. 1995;91:1732–1738. doi: 10.1161/01.cir.91.6.1732. [DOI] [PubMed] [Google Scholar]
- [20].Prins HA, Houdijk AP, van Lambalgen AA, Teerlink T, Meijer S, Thijs LG, van Leeuwen PA. Paradoxical changes in organ blood flow after arginase infusion in the non-stressed rat. Shock. 1998;9:422–427. doi: 10.1097/00024382-199806000-00006. [DOI] [PubMed] [Google Scholar]
- [21].Morris CR, Poljakovic M, Lavrisha L, Machado L, Kuypers FA, Morris SM., Jr. Decreased arginine bioavailability and increased serum arginase activity in asthma. Am J Respir Crit Care Med. 2004;170:148–153. doi: 10.1164/rccm.200309-1304OC. [DOI] [PubMed] [Google Scholar]
- [22].Langle F, Roth E, Steininger R, Winkler S, Muhlbacher F. Arginase release following liver reperfusion. Evidence of hemodynamic action of arginase infusions. Transplantation. 1995;59:1542–1549. [PubMed] [Google Scholar]
- [23].Morris SM., Jr. Arginine metabolism: boundaries of our knowledge. J Nutr. 2007;137:1602S–1609S. doi: 10.1093/jn/137.6.1602S. [DOI] [PubMed] [Google Scholar]
- [24].Morris SM., Jr. Regulation of enzymes of urea and arginine synthesis. Annu Rev Nutr. 1992;12:81–101. doi: 10.1146/annurev.nu.12.070192.000501. [DOI] [PubMed] [Google Scholar]
- [25].Beliveau Carey G, Cheung CW, Cohen NS, Brusi-low S, Raijman L. Regulation of urea and citrulline synthesis under physiological conditions. Biochem J. 1993;292((Pt 1)):241–247. doi: 10.1042/bj2920241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schimke RT. Repression of enzymes of arginine biosynthesis in mammalian tissue culture. Biochim Biophys Acta. 1962;62:599–601. doi: 10.1016/0006-3002(62)90250-0. [DOI] [PubMed] [Google Scholar]
- [27].Schimke RT. Enzymes of Arginine Metabolism in Mammalian Cell Culture. I. Repression of Argininosuccinate Synthetase and Argininosuccinase. J Biol Chem. 1964;239:136–145. [PubMed] [Google Scholar]
- [28].Guei TR, Liu MC, Yang CP, Su TS. Identification of a liver-specific cAMP response element in the human argininosuccinate synthetase gene. Biochem Biophys Res Commun. 2008;377:257–261. doi: 10.1016/j.bbrc.2008.09.118. [DOI] [PubMed] [Google Scholar]
- [29].Husson A, Brasse-Lagnel C, Fairand A, Renouf S, Lavoinne A. Argininosuccinate synthetase from the urea cycle to the citrulline-NO cycle. Eur J Biochem. 2003;270:1887–1899. doi: 10.1046/j.1432-1033.2003.03559.x. [DOI] [PubMed] [Google Scholar]
- [30].Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- [31].Toda N, Nakanishi-Toda M. Nitric oxide: ocular blood flow, glaucoma, and diabetic retinopathy. Prog Retin Eye Res. 2007;26:205–238. doi: 10.1016/j.preteyeres.2007.01.004. [DOI] [PubMed] [Google Scholar]
- [32].Goodwin BL, Solomonson LP, Eichler DC. Argininosuccinate synthase expression is required to maintain nitric oxide production and cell viability in aortic endothelial cells. J Biol Chem. 2004;279:18353–18360. doi: 10.1074/jbc.M308160200. [DOI] [PubMed] [Google Scholar]
- [33].Wu G, Morris SM., Jr. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336((Pt 1)):1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].McCormick SM, Eskin SG, McIntire LV, Teng CL, Lu CM, Russell CG, Chittur KK. DNA mi-croarray reveals changes in gene expression of shear stressed human umbilical vein endothelial cells. Proc Natl Acad Sci USA. 2001;98:8955–8960. doi: 10.1073/pnas.171259298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hecker M, Sessa WC, Harris HJ, Anggard EE, Vane JR. The metabolism of L-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: cultured endothe-lial cells recycle L-citrulline to L-arginine. Proc Natl Acad Sci USA. 1990;87:8612–8616. doi: 10.1073/pnas.87.21.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Shuttleworth CW, Burns AJ, Ward SM, O'Brien WE, Sanders KM. Recycling of L-citrulline to sustain nitric oxide-dependent enteric neuro-transmission. Neuroscience. 1995;68:1295–1304. doi: 10.1016/0306-4522(95)00193-m. [DOI] [PubMed] [Google Scholar]
- [37].Su Y, Block ER. Hypoxia inhibits L-arginine synthesis from L-citrulline in porcine pulmonary artery endothelial cells. Am J Physiol. 1995;269:L581–587. doi: 10.1152/ajplung.1995.269.5.L581. [DOI] [PubMed] [Google Scholar]
- [38].Arnt-Ramos LR, O'Brien WE, Vincent SR. Immunohistochemical localization of argininosuccinate synthetase in the rat brain in relation to nitric oxide synthase-containing neurons. Neuroscience. 1992;51:773–789. doi: 10.1016/0306-4522(92)90519-8. [DOI] [PubMed] [Google Scholar]
- [39].Daniel EE, Wang YF, Salapatek AM, Mao YK, Mori M. Arginosuccinate synthetase, argi-nosuccinate lyase and NOS in canine gastrointestinal tract: immunocytochemical studies. Neurogastroenterology & Motility. 2000;12:317–334. doi: 10.1046/j.1365-2982.2000.00208.x. [DOI] [PubMed] [Google Scholar]
- [40].Shuttleworth CW, Conlon SB, Sanders KM. Regulation of citrulline recycling in nitric oxide-dependent neurotransmission in the murine proximal colon. Br J Pharmacol. 1997;120:707–713. doi: 10.1038/sj.bjp.0700949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ellis JL, Conanan N. L-citrulline reverses the inhibition of nonadrenergic, noncholinergic relaxations produced by nitric oxide synthase inhibitors in guinea pig trachea and human bronchus. J Pharmacol Exp Ther. 1994;269:1073–1078. [PubMed] [Google Scholar]
- [42].Van Geldre LA, Timmermans JP, Lefebvre RA. L-citrulline recycling by argininosuccinate synthetase and lyase in rat gastric fundus. European Journal of Pharmacology. 2002;455:149–160. doi: 10.1016/s0014-2999(02)02584-0. [DOI] [PubMed] [Google Scholar]
- [43].Swamy M, Salleh MJ, Sirajudeen KN, Yusof WR, Chandran G. Nitric oxide (no), citrulline - no cycle enzymes, glutamine synthetase and oxidative stress in anoxia (hypobaric hypoxia) and reperfusion in rat brain. Int J Med Sci. 2010;7:147–154. doi: 10.7150/ijms.7.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Xie L, Gross SS. Argininosuccinate syn-thetase overexpression in vascular smooth muscle cells potentiates immunostimulant-induced NO production. J Biol Chem. 1997;272:16624–16630. doi: 10.1074/jbc.272.26.16624. [DOI] [PubMed] [Google Scholar]
- [45].Xie L, Hattori Y, Tume N, Gross SS. The preferred source of arginine for high-output nitric oxide synthesis in blood vessels. Semin Perina-tol. 2000;24:42–45. doi: 10.1016/s0146-0005(00)80054-3. [DOI] [PubMed] [Google Scholar]
- [46].Feron O, Belhassen L, Kobzik L, Smith TW, Kelly RA, Michel T. Endothelial nitric oxide syn-thase targeting to caveolae. Specific interactions with caveolin isoforms in cardiac myo-cytes and endothelial cells. J Biol Chem. 1996;271:22810–22814. doi: 10.1074/jbc.271.37.22810. [DOI] [PubMed] [Google Scholar]
- [47].Tagawa A, Mezzacasa A, Hayer A, Longatti A, Pelkmans L, Helenius A. Assembly and trafficking of caveolar domains in the cell: caveolae as stable, cargo-triggered, vesicular transporters. J Cell Biol. 2005;170:769–779. doi: 10.1083/jcb.200506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Fulton D, Fontana J, Sowa G, Gratton JP, Lin M, Li KX, Michell B, Kemp BE, Rodman D, Sessa WC. Localization of endothelial nitric-oxide synthase phosphorylated on serine 1179 and nitric oxide in Golgi and plasma membrane defines the existence of two pools of active enzyme. J Biol Chem. 2002;277:4277–4284. doi: 10.1074/jbc.M106302200. [DOI] [PubMed] [Google Scholar]
- [49].Sessa WC, Garcia-Cardena G, Liu J, Keh A, Pollock JS, Bradley J, Thiru S, Braverman IM, Desai KM. The Golgi association of endothelial nitric oxide synthase is necessary for the efficient synthesis of nitric oxide. J Biol Chem. 1995;270((30)):17641–17644. doi: 10.1074/jbc.270.30.17641. [DOI] [PubMed] [Google Scholar]
- [50].Schilling K, Opitz N, Wiesenthal A, Oess S, Tik-kanen R, Muller-Esterl W, Icking A. Translo-cation of endothelial nitric-oxide synthase involves a ternary complex with caveolin-1 and NOSTRIN. Mol Biol Cell. 2006;17:3870–3880. doi: 10.1091/mbc.E05-08-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Schleicher M, Brundin F, Gross S, Muller-Esterl W, Oess S. Cell cycle-regulated inactivation of endothelial NO synthase through NOSIP-dependent targeting to the cytoskeleton. Mol Cell Biol. 2005;25:8251–8258. doi: 10.1128/MCB.25.18.8251-8258.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zimmermann K, Opitz N, Dedio J, Renne C, Muller-Esterl W, Oess S. NOSTRIN: a protein modulating nitric oxide release and subcellular distribution of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2002;99:17167–17172. doi: 10.1073/pnas.252345399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Icking A, Matt S, Opitz N, Wiesenthal A, Muller-Esterl W, Schilling K. NOSTRIN functions as a homotrimeric adaptor protein facilitating internalization of eNOS. J Cell Sci. 2005;118:5059–5069. doi: 10.1242/jcs.02620. [DOI] [PubMed] [Google Scholar]
- [54].Rogers QR, Freedland RA, Symmons RA. In vivo synthesis and utilization of arginine in the rat. Am J Physiol. 1972;223:236–240. doi: 10.1152/ajplegacy.1972.223.1.236. [DOI] [PubMed] [Google Scholar]
- [55].Davis TA, Nguyen HV, Garcia-Bravo R, Fiorotto ML, Jackson EM, Lewis DS, Lee DR, Reeds PJ. Amino acid composition of human milk is not unique. J Nutr. 1994;124:1126–1132. doi: 10.1093/jn/124.7.1126. [DOI] [PubMed] [Google Scholar]
- [56].Windmueller HG, Spaeth AE. Source and fate of circulating citrulline. Am J Physiol. 1981;241:E473–480. doi: 10.1152/ajpendo.1981.241.6.E473. [DOI] [PubMed] [Google Scholar]
- [57].Brosnan ME, Brosnan JT. Renal arginine metabolism. J Nutr. 2004;134:2791S–2795S. doi: 10.1093/jn/134.10.2791S. discussion 2796S-2797S. [DOI] [PubMed] [Google Scholar]
- [58].Levillain O, Hus-Citharel A, Morel F, Bankir L. Localization of arginine synthesis along rat nephron. Am J Physiol. 1990;259:F916–923. doi: 10.1152/ajprenal.1990.259.6.F916. [DOI] [PubMed] [Google Scholar]
- [59].Walker JB. Creatine: biosynthesis, regulation, and function. Adv Enzymol Relat Areas Mol Biol. 1979;50:177–242. doi: 10.1002/9780470122952.ch4. [DOI] [PubMed] [Google Scholar]
- [60].Nasrallah F, Feki M, Kaabachi N. Creatine and creatine deficiency syndromes: biochemical and clinical aspects. Pediatr Neurol. 2010;42:163–171. doi: 10.1016/j.pediatrneurol.2009.07.015. [DOI] [PubMed] [Google Scholar]
- [61].Flam BR, Eichler DC, Solomonson LP. Endo-thelial nitric oxide production is tightly coupled to the citrulline-nitric oxide cycle. Nitric Oxide. 2007;17:115–121. doi: 10.1016/j.niox.2007.07.001. [DOI] [PubMed] [Google Scholar]
- [62].Shen LJ, Beloussow K, Shen WC. Modulation of arginine metabolic pathways as the potential anti-tumor mechanism of recombinant arginine deiminase. Cancer Lett. 2006;231:30–35. doi: 10.1016/j.canlet.2005.01.007. [DOI] [PubMed] [Google Scholar]
- [63].Topal G, Brunet A, Walch L, Boucher JL, David-Dufilho M. Mitochondrial arginase II modulates nitric-oxide synthesis through non-freely exchangeable L-arginine pools in human endothelial cells. J Pharmacol Exp Ther. 2006;318:1368–1374. doi: 10.1124/jpet.106.103747. [DOI] [PubMed] [Google Scholar]
- [64].Delage B, Fennell DA, Nicholson L, McNeish I, Lemoine NR, Crook T, Szlosarek PW. Argin-ine deprivation and argininosuccinate synthetase expression in the treatment of cancer. Int J Cancer. 2010;126:2762–2772. doi: 10.1002/ijc.25202. [DOI] [PubMed] [Google Scholar]
- [65].Ensor CM, Holtsberg FW, Bomalaski JS, Clark MA. Pegylated arginine deiminase (ADI-SS PEG20,000 mw) inhibits human melanomas and hepatocellular carcinomas in vitro and in vivo. Cancer Res. 2002;62:5443–5450. [PubMed] [Google Scholar]
- [66].Bach SJ, Swaine D. The Effect of Arginase on the Retardation of Tumour Growth. Br J Cancer. 1965;19:379–386. doi: 10.1038/bjc.1965.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Yeatman TJ, Risley GL, Brunson ME. Depletion of dietary arginine inhibits growth of metastatic tumor. Arch Surg. 1991;126:1376–1381. doi: 10.1001/archsurg.1991.01410350066010. discussion 1381-1372. [DOI] [PubMed] [Google Scholar]
- [68].Scott L, Lamb J, Smith S, Wheatley DN. Single amino acid (arginine) deprivation: rapid and selective death of cultured transformed and malignant cells. Br J Cancer. 2000;83:800–810. doi: 10.1054/bjoc.2000.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Philip R, Campbell E, Wheatley DN. Arginine deprivation, growth inhibition and tumour cell death: 2. Enzymatic degradation of arginine in normal and malignant cell cultures. Br J Cancer. 2003;88:613–623. doi: 10.1038/sj.bjc.6600681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wheatley DN. Controlling cancer by restricting arginine availability–arginine-catabolizing enzymes as anticancer agents. Anticancer Drugs. 2004;15:825–833. doi: 10.1097/00001813-200410000-00002. [DOI] [PubMed] [Google Scholar]
- [71].Miyazaki K, Takaku H, Umeda M, Fujita T, Huang WD, Kimura T, Yamashita J, Horio T. Potent growth inhibition of human tumor cells in culture by arginine deiminase purified from a culture medium of a Mycoplasma-infected cell line. Cancer Res. 1990;50:4522–4527. [PubMed] [Google Scholar]
- [72].Takaku H, Matsumoto M, Misawa S, Miya-zaki K. Anti-tumor activity of arginine deiminase from Mycoplasma argini and its growth-inhibitory mechanism. Jpn J Cancer Res. 1995;86:840–846. doi: 10.1111/j.1349-7006.1995.tb03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Takaku H, Takase M, Abe S, Hayashi H, Miyazaki K. In vivo anti-tumor activity of arginine deiminase purified from Mycoplasma arginini. Int J Cancer. 1992;51:244–249. doi: 10.1002/ijc.2910510213. [DOI] [PubMed] [Google Scholar]
- [74].Izzo F, Marra P, Beneduce G, Castello G, Val-lone P, De Rosa V, Cremona F, Ensor CM, Holts-berg FW, Bomalaski JS, Clark MA, Ng C, Curley SA. Pegylated arginine deiminase treatment of patients with unresectable hepatocellular carcinoma: results from phase I/II studies. J Clin Oncol. 2004;22:1815–1822. doi: 10.1200/JCO.2004.11.120. [DOI] [PubMed] [Google Scholar]
- [75].Ascierto PA, Scala S, Castello G, Daponte A, Simeone E, Ottaiano A, Beneduce G, De Rosa V, Izzo F, Melucci MT, Ensor CM, Prestayko AW, Holtsberg FW, Bomalaski JS, Clark MA, Savaraj N, Feun LG, Logan TF. Pegylated arginine deiminase treatment of patients with metastatic melanoma: results from phase I and II studies. J Clin Oncol. 2005;23:7660–7668. doi: 10.1200/JCO.2005.02.0933. [DOI] [PubMed] [Google Scholar]
- [76].Kobayashi E, Masuda M, Nakayama R, Ichi-kawa H, Satow R, Shitashige M, Honda K, Ya-maguchi U, Shoji A, Tochigi N, Morioka H, To-yama Y, Hirohashi S, Kawai A, Yamada T. Reduced argininosuccinate synthetase is a predictive biomarker for the development of pulmonary metastasis in patients with osteosarcoma. Mol Cancer Ther. 2010;9:535–544. doi: 10.1158/1535-7163.MCT-09-0774. [DOI] [PubMed] [Google Scholar]
- [77].Szlosarek PW, Grimshaw MJ, Wilbanks GD, Hagemann T, Wilson JL, Burke F, Stamp G, Balkwill FR. Aberrant regulation of argininosuccinate synthetase by TNF-alpha in human epithelial ovarian cancer. Int J Cancer. 2007;121:6–11. doi: 10.1002/ijc.22666. [DOI] [PubMed] [Google Scholar]
- [78].Kobayashi K, Jackson MJ, Tick DB, O'Brien WE, Beaudet AL. Heterogeneity of mutations in argininosuccinate synthetase causing human citrullinemia. J Biol Chem. 1990;265:11361–11367. [PubMed] [Google Scholar]
- [79].Jinno Y, Matuo S, Nomiyama H, Shimada K, Matsuda I. Novel structure of the 5’ end region of the human argininosuccinate synthetase gene. J Biochem (Tokyo) 1985;98:1395–1403. doi: 10.1093/oxfordjournals.jbchem.a135407. [DOI] [PubMed] [Google Scholar]
- [80].Jinno Y, Nomiyama H, Matuo S, Shimada K, Matsuda I, Saheki T. Structure of the 5′ end region of the human argininosuccinate synthetase gene. J Inherit Metab Dis. 1985;8:157–159. doi: 10.1007/BF01819307. [DOI] [PubMed] [Google Scholar]
- [81].Anderson GM, Freytag SO. Synergistic activation of a human promoter in vivo by transcription factor Sp1. Mol Cell Biol. 1991;11:1935–1943. doi: 10.1128/mcb.11.4.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Chin AC, Fournier RE. A genetic analysis of extinction: trans-regulation of 16 liver-specific genes in hepatoma-fibroblast hybrid cells. Proc Natl Acad Sci U S A. 1987;84:1614–1618. doi: 10.1073/pnas.84.6.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ruppert S, Boshart M, Bosch FX, Schmid W, Fournier RE, Schutz G. Two genetically defined trans-acting loci coordinately regulate overlapping sets of liver-specific genes. Cell. 1990;61:895–904. doi: 10.1016/0092-8674(90)90200-x. [DOI] [PubMed] [Google Scholar]
- [84].Morris SM, Jr., Moncman CL, Rand KD, Dizikes GJ, Cederbaum SD, O’Brien WE. Regulation of mRNA levels for five urea cycle enzymes in rat liver by diet, cyclic AMP, and glucocorticoids. Arch Biochem Biophys. 1987;256:343–353. doi: 10.1016/0003-9861(87)90455-3. [DOI] [PubMed] [Google Scholar]
- [85].Boshart M, Weih F, Nichols M, Schutz G. The tissue-specific extinguisher locus TSE1 encodes a regulatory subunit of cAMP-dependent protein kinase. Cell. 1991;66:849–859. doi: 10.1016/0092-8674(91)90432-x. [DOI] [PubMed] [Google Scholar]
- [86].Tsai WB, Aiba I, Lee SY, Feun L, Savaraj N, Kuo MT. Resistance to arginine deiminase treatment in melanoma cells is associated with induced argininosuccinate synthetase expression involving c-Myc/HIF-1alpha/Sp4. Mol Cancer Ther. 2009;8:3223–3233. doi: 10.1158/1535-7163.MCT-09-0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Anderson HD, Rahmutula D, Gardner DG. Tumor necrosis factor-alpha inhibits endothelial nitric-oxide synthase gene promoter activity in bovine aortic endothelial cells. J Biol Chem. 2004;279:963–969. doi: 10.1074/jbc.M309552200. [DOI] [PubMed] [Google Scholar]
- [88].Lai PF, Mohamed F, Monge JC, Stewart DJ. Downregulation of eNOS mRNA expression by TNFalpha: identification and functional characterization of RNA-protein interactions in the 3’UTR. Cardiovasc Res. 2003;59:160–168. doi: 10.1016/s0008-6363(03)00296-7. [DOI] [PubMed] [Google Scholar]
- [89].Barsacchi R, Perrotta C, Bulotta S, Moncada S, Borgese N, Clementi E. Activation of endothelial nitric-oxide synthase by tumor necrosis factor-alpha: a novel pathway involving sequential activation of neutral sphingomyelinase, phosphatidylinositol-3’ kinase, and Akt. Mol Pharmacol. 2003;63:886–895. doi: 10.1124/mol.63.4.886. [DOI] [PubMed] [Google Scholar]
- [90].Takahashi S, Mendelsohn ME. Synergistic activation of endothelial nitric-oxide synthase (eNOS) by HSP90 and Akt: calcium-independent eNOS activation involves formation of an HSP90-Akt-CaM-bound eNOS complex. J Biol Chem. 2003;278:30821–30827. doi: 10.1074/jbc.M304471200. [DOI] [PubMed] [Google Scholar]
- [91].Kwon H, Pak Y. Prolonged tyrosine kinase activation of insulin receptor by pY27-caveolin-2. Biochem Biophys Res Commun. 2009. [DOI] [PubMed]
- [92].Goodwin B, Pendleton L, Levy M, Solomonson L, Eichler D. Tumor Necrosis Factor-{alpha} Reduces Substrate Availability for Nitric Oxide Production via Down-Regulation of Argininosuccinate Synthase. Am J Physiol Heart Circ Physiol. 2007;293:H1115–1121. doi: 10.1152/ajpheart.01100.2006. [DOI] [PubMed] [Google Scholar]
- [93].Rothwarf DM, Karin M. The NF-kappa B activation pathway: a paradigm in information transfer from membrane to nucleus. Sci STKE. 1999;1999:RE1. doi: 10.1126/stke.1999.5.re1. [DOI] [PubMed] [Google Scholar]
- [94].Goodwin BL, Corbin KD, Pendleton LC, Levy MM, Solomonson LP, Eichler DC. Troglita-zone up-regulates vascular endothelial argininosuccinate synthase. Biochem Biophys Res Commun. 2008;370:254–258. doi: 10.1016/j.bbrc.2008.03.089. [DOI] [PubMed] [Google Scholar]
- [95].Polikandriotis JA, Mazzella LJ, Rupnow HL, Hart CM. Peroxisome proliferator-activated receptor gamma ligands stimulate endothelial nitric oxide production through distinct perox-isome proliferator-activated receptor gamma-dependent mechanisms. Arterioscler Thromb Vasc Biol. 2005;25:1810–1816. doi: 10.1161/01.ATV.0000177805.65864.d4. [DOI] [PubMed] [Google Scholar]
- [96].Cho DH, Choi YJ, Jo SA, Jo I. Nitric oxide production and regulation of endothelial nitric-oxide synthase phosphorylation by prolonged treatment with troglitazone: evidence for involvement of peroxisome proliferator-activated receptor (PPAR) gamma-dependent and PPARgamma-independent signaling pathways. J Biol Chem. 2004;279:2499–2506. doi: 10.1074/jbc.M309451200. [DOI] [PubMed] [Google Scholar]
- [97].Fra AM, Williamson E, Simons K, Parton RG. De novo formation of caveolae in lymphocytes by expression of VIP21-caveolin. Proc Natl Acad Sci U S A. 1995;92:8655–8659. doi: 10.1073/pnas.92.19.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- [99].Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem. 1997;272:18522–18525. doi: 10.1074/jbc.272.30.18522. [DOI] [PubMed] [Google Scholar]
- [100].Ghosh S, Gachhui R, Crooks C, Wu C, Lisanti MP, Stuehr DJ. Interaction between caveolin-1 and the reductase domain of endothelial nitric-oxide synthase. Consequences for catalysis. J Biol Chem. 1998;273:22267–22271. doi: 10.1074/jbc.273.35.22267. [DOI] [PubMed] [Google Scholar]
- [101].Meijer HA, Thomas AA. Control of eukaryotic protein synthesis by upstream open reading frames in the 5’-untranslated region of an mRNA. Biochem J. 2002;367:1–11. doi: 10.1042/BJ20011706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Pendleton LC, Goodwin BL, Flam BR, Solomon-son LP, Eichler DC. Endothelial argininosuc-cinate synthase mRNA 5’-untranslated region diversity. Infrastructure for tissue-specific expression. J Biol Chem. 2002;277:25363–25369. doi: 10.1074/jbc.M111677200. [DOI] [PubMed] [Google Scholar]
- [103].Pendleton LC, Goodwin BL, Solomonson LP, Eichler DC. Regulation of endothelial argininosuccinate synthase expression and NO production by an upstream open reading frame. J Biol Chem. 2005;280:24252–24260. doi: 10.1074/jbc.M500106200. [DOI] [PubMed] [Google Scholar]
- [104].Zhao Y, Zhang J, Li H, Li Y, Ren J, Luo M, Zheng X. An NADPH sensor protein (HSCARG) down-regulates nitric oxide synthesis by association with argininosuccinate synthetase and is essential for epithelial cell viability. J Biol Chem. 2008;283:11004–11013. doi: 10.1074/jbc.M708697200. [DOI] [PubMed] [Google Scholar]
- [105].Hao G, Xie L, Gross SS. Argininosuccinate Synthetase is Reversibly Inactivated by S-Nitrosylation in Vitro and in Vivo. Journal of Biological Chemistry. 2004;279:36192–36200. doi: 10.1074/jbc.M404866200. [DOI] [PubMed] [Google Scholar]
- [106].Imami K, Sugiyama N, Kyono Y, Tomita M, Ishihama Y. Automated phosphoproteome analysis for cultured cancer cells by two-dimensional nanoLC-MS using a calcined tita-nia/C18 biphasic column. Anal Sci. 2008;24:161–166. doi: 10.2116/analsci.24.161. [DOI] [PubMed] [Google Scholar]
- [107].Corbin KD, Pendleton LC, Solomonson LP, Eichler DC. Phosphorylation of argininosucci-nate synthase by protein kinase A. Biochem Biophys Res Commun. 2008;377:1042–1046. doi: 10.1016/j.bbrc.2008.10.056. [DOI] [PubMed] [Google Scholar]