Abstract
The activity of β-catenin, commonly dysregulated in human colon cancers, is inhibited by the vitamin D receptor (VDR), and this mechanism is postulated to explain the putative anti-cancer activity of vitamin D metabolites in the colon. We investigated the effect of a common FokI restriction site polymorphism (F/f) in the human VDR gene as well as the effect of anti-tumorigenic 1,25-dihydroxyvitamin D3 (1,25D) and pro-tumorigenic lithocholic acid (LCA) VDR ligands on β-catenin transcriptional activity. Furthermore, the influence of a major regulatory protein of β-catenin, the APC tumor suppressor gene, on VDR-dependent inhibition of β-catenin activity was examined. We report herein that β-catenin-mediated transcription is most effectively suppressed by the VDR FokI variant F/M4 when 1,25D is limiting. Using Caco-2 colorectal cancer cells, it was observed that VDR ligands, 1,25D and LCA, both suppress β-catenin transcriptional activity, though 1,25D exhibited significantly greater inhibition. Moreover, 1,25D, but not LCA, suppressed endogenous expression of the β-catenin target gene DKK-4 independent of VDR DNA-binding activity. These results support β-catenin sequestration away from endogenous gene targets by 1,25D-VDR. This activity is most efficiently mediated by the FokI gene variant F/M4, a VDR allele previously associated with protection against colorectal cancer. Interestingly, we found the inhibition of β-catenin activity by 1,25D-VDR was significantly enhanced by wildtype APC. These results reveal a previously unrecognized role for 1,25D-VDR in APC/β-catenin cross-talk. Collectively, these findings strengthen evidence favoring a direct effect on the Wnt-signaling molecule β-catenin as one anti-cancer target of 1,25D-VDR action in the colorectum.
Keywords: Wnt signaling; lithocholic acid; 1,25-dihydroxyvitamin D3; single nucleotide polymorphisms; colorectal cancer
INTRODUCTION
The vitamin D receptor (VDR) is a ligand-activated transcription factor with a domain structure homologous to other nuclear receptor superfamily members [1]. VDR contains several common gene polymorphisms, including a FokI restriction site polymorphism (F/f) in exon II of the 5′ region of the gene. This polymorphism in human VDR results in two allelic isoforms of differing length (f/M1 and F/M4) [2,3]. Differential carriage of the isoforms is thought to impact VDR transcriptional activity [3] and vitamin D hormone related disease risk [4–8]. When bound to VDR, 1,25-dihydroxyvitamin D3 (1,25D), the active hormonal metabolite of vitamin D, activates target genes via vitamin D responsive element (VDREs). Classic VDR target genes involved in bone mineral homeostasis include CYP24A1, osteopontin and parathyroid hormone [3,9–11]. In addition to its role in bone mineral metabolism, VDR mediates neoclassical actions in the differentiation, proliferation, cell cycle regulation and apoptosis of a number of cell types including the colonic epithelium [9,12–19]. VDR has also been shown to act as a low affinity bile acid sensor for lithocholic acid (LCA) and other secondary bile acids that act as colon carcinogens [20–23]. The general anti-tumor activity (e.g., pro-differentiation, pro-apoptosis) as well as adverse consequences of competitive ligands, such as the secondary bile acids, have been suggested to explain the positive associations between ‘low vitamin D status’ and colon cancer risk, leading to the emerging concept that VDR may act directly as a tumor suppressor gene.
Dysregulated Wnt signaling with hallmark β-catenin nuclear translocation, accumulation, and upregulation of TCF/LEF-controlled genes is a common early event in the majority of human colon cancers [24]. In the nucleus, β-catenin acts as a transcriptional coactivator of TCF/LEF in the promotion of oncogenes such as MYC, CCND1, JUN, CDX1 [24,25]. Cytoplasmic levels of β-catenin are regulated by a degradative complex composed of axin, adenomatous polyposis coli (APC) tumor suppressor, and GSK3-β which phosphorylates β-catenin for ubiquitination and degradation by the proteasome [24,26]. β-catenin also forms complexes with E-cadherin and other proteins in the cytoplasm to form tight junctions between cells [24]. Cells treated with 1,25D demonstrate E-cadherin induction [27] while the corepressor, Snail1, inhibits the expression of E-cadherin and VDR [28–30]. Our group [31], as well as two other laboratories [27,32] have demonstrated a 1,25D-enhanced interaction between VDR and β-catenin that results in increased VDRE dependent transcriptional activity and inhibition of β-catenin dependent TCF/LEF transcriptional activity. The effect of β-catenin on VDR/VDRE activity has been attributed to the activating function domain (AF-2) located in the C terminus of VDR [32], whereas the exact nature of the interaction with the VDR itself is less clear. Shah et al. [32], found high levels of exogenous β-catenin could restore the transcriptional function of a VDR mutant containing a glutamine (Q) for glutamic acid (E) at amino acid 420 located in the AF-2 domain or co-activator ‘platform’ of VDR; mutation of E420 in previous studies has been shown to result in complete loss of transcriptional activity [33,34]. The findings of Shah et al. [32], confirm previous work demonstrating the importance of the glutamic acid residue at position 420 in mediating coactivator binding to VDR and transactivation of a VDRE containing promoter [33,34], but suggest that the transcriptional impact of mutation at this site is in part dependent on the availability of β-catenin [32]. While mutants of E420, specifically E420Q, appear capable of interacting with β-catenin [32], these results need confirmation as they suggest a non-classical compensatory role for β-catenin in VDR-dependent gene transcription for which biologic significance is currently unknown. Taken in total, these results support a molecular interaction between β-catenin and VDR activity that may be modified by 1,25D and provide a mechanistic link between vitamin D hormone exposure and Wnt signaling molecules whose biologic role may explain the consistent association in human studies between low vitamin D hormone levels and colorectal cancer risk.
Although it has been demonstrated that VDR interacts with β-catenin, several important questions remain. For example, while 1,25D-mediated suppression of β-catenin transcriptional activity has been observed in APC mutated cell lines [27], the influence of wildtype APC on the VDR and β-catenin interaction, in the presence and absence of 1,25D, is unknown. This raises important questions as to the relative contribution of VDR in maintenance of cellular homeostasis in the presence or absence of a major regulator of nuclear β-catenin such as APC. Because loss of APC is such a critical early event in colorectal tumorigenesis, understanding the role of VDR within the context of APC status is of prominent clinical relevance particularly in conceptualizing strategies for prevention among high risk individuals where the effects of APC loss may be enhanced or possibly mitigated depending on 1,25D status. In addition, the ability of β-catenin to interact with the VDR E420 mutant protein, and the effect of that interaction on VDRE transcriptional activity in the presence of exogenous β-catenin has not been investigated in colon cancer cells. The action of potentially carcinogenic secondary bile acids such as LCA, on the interaction between VDR and β-catenin, and the functional impact of these low-affinity VDR ligands on β-catenin transcriptional activity is also currently unknown. Furthermore, our own work demonstrates that human VDR containing a common f/M1 single nucleotide polymorphism (SNP), which introduces an ATG start site three amino acids upstream from the F/M4 variant start site, is less effective in mediating VDRE-dependent transcription than the F/M4 variant which lacks the first three N terminal amino acids [3]. However, the influence of the common VDR M1/M4 variant on inhibition of β-catenin activity is unknown. Thus, we sought to further characterize the interaction between VDR and β-catenin as well as elucidate the contribution of the AF-2 domain in VDR to the interaction of the two proteins, along with determining whether the presence of the low affinity VDR ligand LCA, the presence or absence of APC, or M1/M4 genetic variation influence the interaction between VDR and β-catenin either physically and/or functionally.
MATERIALS AND METHODS
GST Pulldown Assay
35S-Methionine labeled β-catenin and either full-length VDR or VDR truncation mutant proteins were generated in an in vitro transcription/translation (IVTT) system. The reaction mixture contained VDR or β-catenin DNA templates, TNT Quick Master Mix (Promega, Madison, WI), HALT protease inhibitor (Thermo Fisher Scientific, Rockford, IL) and 35S-Methionine (Perkin-Elmer, Waltham, MA). Glutathione Sepharose beads (GE Healthcare, Piscataway, NJ) coupled to β-catenin or VDR were pre-blocked using chilled TEZ wash buffer containing 10 mM Tris, pH 7.6, 1 mM EDTA, 0.3 mM zinc chloride, 5 mM DTT, 10% Tween 20, 140 mM KCl, Bovine Serum Albumin, and HALT protease inhibitor. The VDR (either bound to beads or in the IVTT mix) was then exposed for 1 hour to ethanol vehicle, 10−6 M 1,25D, or 10−4 M lithocholic acid (LCA). The beads and IVTT mixture were then incubated with gentle shaking for 30 minutes to allow for protein-protein interaction. Next, the beads were washed with TEZ wash buffer, placed into loading buffer (4% SDS, 10% β-mercaptoethanol, 125 mM Tris-HCl, pH 6.8, 20% glycerol, 0.008% bromophenol blue) and boiled for two minutes. Samples were separated by gradient 5–15% SDS PAGE and visualized using autoradiography.
Mammalian Two Hybrid Assay
Mammalian two hybrid experiments were conducted using HT-29-APC colon cancer cells which contain an inducible (metallothionein promoter), stably expressed plasmid for APC. Cells were plated at 70,000 cells/well in a 24 well plate. The cells were co-transfected utilizing bait (BD) and prey (AD) fusion constructs, pFR-Luc reporter gene and renilla control plasmid via liposome-mediated transfection with Lipofectamine LTX and PLUS reagent (Invitrogen, Carlsbad, CA). The cells were incubated with the transfection mixture overnight and then treated with ethanol vehicle or 10−8 M 1,25D. In order to induce the APC plasmid, 150 μM zinc chloride or water vehicle was added with the 1,25D. After 24 hour incubation, cells were lysed and subjected to a luciferase assay (Promega). Independent experiments were conducted with triplicate samples for each treatment group. In order to rule out an effect of zinc, the APC inducing agent, on the interaction with VDR and β-catenin, HT-29-β galactosidase cells (which contain a zinc-inducible β-galactosidase plasmid) were co-transfected, treated and lysed in the same fashion as the HT-29-APC cells. Caco-2 colon cancer cells were utilized to investigate the effect of LCA on the interaction of VDR and β-catenin. Subconfluent cells were transfected as described above and then treated with ethanol vehicle, 10−8 M 1,25D, or 70 μM lithocholic acid prior to cell lysis and luciferase assay.
Western blot
HT-29-APC cells were plated (2 million cells/10 cm plate) and allowed to grow to 80% confluency prior to treatment with sterile water, 150 μM or 300 μM zinc chloride. After treatment, cells were incubated at 37°C, 5% CO2 overnight and then lysed in a 1.5 M Tris-HCl (pH 8), 137 mM sodium chloride, 10% glycerol, 1% Nonidet P-40, 2 mM EDTA lysis buffer containing HALT protease inhibitor. Loading buffer (4% SDS, 10% β-mercaptoethanol, 125 mM Tris-HCl, pH 6.8, 20% glycerol, 0.008% bromophenol blue) was added to each sample and samples boiled for two minutes. Samples were separated on a 7.5% Next Gel™ (ISC BioExpress, Kaysville, UT). Proteins were then transferred to a Hybond-P membrane (GE Healthcare, Piscataway, NJ). The membrane was blocked in 5% blotto (5% non-fat dry milk in tris-buffered saline) and washed in 0.1% TBST (0.1% Tween-20 in TBS) prior to immunoblotting. The membrane was incubated with primary anti-APC mouse monoclonal IgG1 antibody (Calbiochem, Gibbstown, NJ) diluted 1:250 at room temperature for two hours with rocking. The membrane was then washed in 0.1% TBST at room temperature prior to secondary antibody incubation. Next, the membrane was incubated with goat anti-mouse HRP conjugated secondary antibody (1:5000) for one hour at room temperature with rocking. The membrane was washed again and excess liquid removed prior to exposure of the membrane to ECL Advance (GE Healthcare) and KODAK BioMax XAR film (Carestream Molecular Imaging, Rochester, NY).
IVTT samples were separated on a 5–15% polyacrylamide gel and then were transferred to an Immobilon P membrane (Millipore, Billerica, MA) and blocked with 3% blotto. The membrane was incubated for 48 hours with anti-β-catenin primary antibody conjugated to horseradish peroxidase (BD Biosciences, San Jose, CA) diluted 1:300 in 1% blotto. Membranes were washed with TBST and then developed as described above.
TOPFlash assay
HT-29 colon cancer cells were plated (70,000 cells/well in a 24 well plate) and then transfected with varying amounts of f/M1 or F/M4 VDR expression plasmid, β-catenin vector, renilla control plasmid and TOPFlash or FOPFlash along with Lipofectamine LTX (Invitrogen) and PLUS reagent (Invitrogen). TOPFlash is a reporter gene which contains three TCF/LEF binding sites in the promoter and is used to measure β-catenin transcriptional activity while FOPFlash contains mutated TCF/LEF binding sites and thus serves as a negative control [25]. The cells were incubated with the transfection mixture overnight and then treated with ethanol vehicle or 10−8 M 1,25D. After 24 hour incubation, cells were lysed and subjected to luciferase assay.
Caco-2 colon cancer cells were utilized to determine the effect of LCA on β-catenin transcriptional activity. Subconfluent cells were plated at 70,000 cells/well in a 24 well plate and transfected with F/M4 VDR expression plasmid, β-catenin vector, renilla control plasmid and TOPFlash or FOPFlash as described above treated with ethanol vehicle, 10−8 M 1,25D, or 70 μM lithocholic acid prior to cell lysis and luciferase assay.
In order to assess the effect of APC on β-catenin transcriptional activity in the presence of the 1,25D hormone, HT-29-APC cells were plated (70,000 cells/well in a 24 well plate) and then transfected as described above with f/M1 or F/M4 VDR expression plasmid, β-catenin vector, renilla control plasmid and TOPFlash. The cells were allowed to incubate with the transfection mixture overnight and then treated with vehicle or 150 μM zinc chloride (for APC induction) in addition to ethanol vehicle or 10−8 M 1,25D. After a 24 hour incubation, cells were lysed and subjected to luciferase assay.
VDR-VDRE Transcription Assay
Subconfluent Caco-2 colon cancer cells were plated (70,000 cells/well in a 24 well plate) and then transfected as described above with a β-catenin expression plasmid, renilla control DNA, a VDRE-luciferase reporter vector, and pSG5-hVDR (F/M4) or pSG5-E420A (contains an alanine (A) substitution for glutamic acid (E) at amino acid 420). The VDRE-reporter vector contains a 5500 bp fragment of the promoter region from the human vitamin D3 24-hydroxylase (CYP24A1) gene linked to luciferase and is used to assess VDR transcriptional activity. The cells were incubated with the transfection mixture overnight and then treated with ethanol vehicle, 10−8 M 1,25D, or 70 μM LCA. After 24 hour, cells were lysed and subjected to luciferase assay.
RT-PCR
Subconfluent Caco-2 colon cancer cells were plated (720,000 cells/60 mm dish) and then transfected with 600 ng pSG5 vector, 600 ng pSG5-hVDR vector or 600 ng pSG5-Δ131–141 (a VDR mutant with a deletion that results in loss of VDRE binding, data not shown [35]) along with Lipofectamine LTX and PLUS reagent. The cells were allowed to incubate with the liposome transfection mixture overnight and then treated with ethanol vehicle, 10−7 M 1,25D, or 70 μM LCA. After a 24-hour incubation period, the cells were harvested and RNA extracted using the Aurum Total RNA Mini Kit (Biorad, Hercules, CA). cDNA was generated using the iScript cDNA Synthesis Kit (Biorad) with the following thermocycling conditions: 5 min at 25°C, 30 min at 42°C, 5 min at 85°C and then held at 4°C until removed and placed in −20°C for storage. Both the endogenous control, GAPDH and the target gene DKK-4 were labeled with FAM. PCR was performed using the Applied Biosystems (ABI, Foster City, CA) 7500 Fast with 50 ng cDNA per reaction, Taqman Fast Universal PCR Master Mix (2X), No AmpErase UNG (ABI), and Taqman Gene Expression Assay (ABI). The following thermocycling conditions were utilized: 95°C for 20 sec and then 40 cycles of 95°C for 3 seconds and 60° for 30 sec. Expression was normalized to GAPDH endogenous control and fold-change determined by comparing each ligand treated sample to the ethanol treatment sample for that transfection group.
Statistical Analyses
For statistical analyses of the experimental results, we employed several methods where appropriate. A Student’s t-test was employed in the case of comparisons of two categories; while analysis of variance (ANOVA) was utilized in cases with more than two treatment categories. If the ANOVA yielded a significant F-statistic, Tukey’s Honestly Significant Differences (Tukey’s HSD) test was used to account for multiple comparisons. Finally, regression models were employed to evaluate whether there were statistically significant dose-dependent trends observed with specific treatments.
RESULTS
VDR interaction with β-catenin
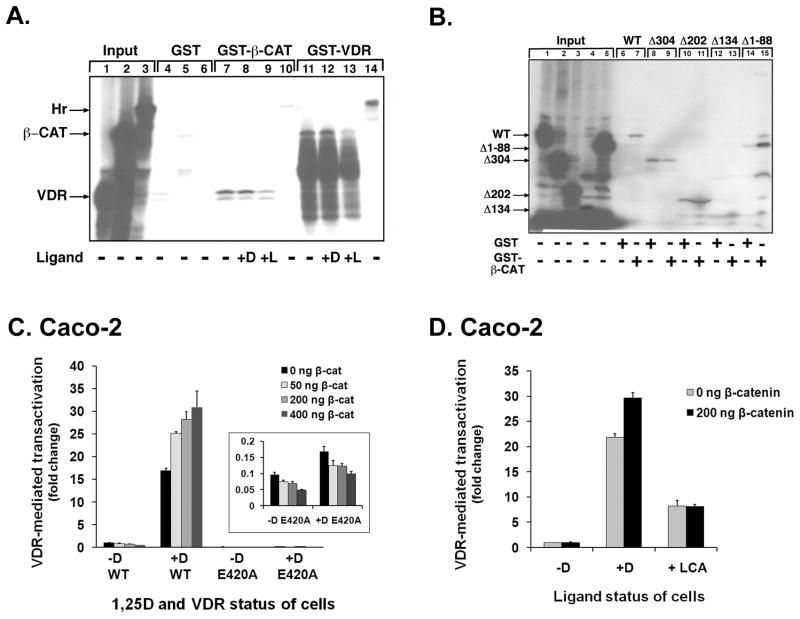
We first sought to demonstrate the ability of VDR to interact with β-catenin, and to clarify the role of the 1,25D ligand in this interaction. The experiments involved an in vitro, GST fusion protein “pulldown” assay to probe the association of VDR and β-catenin reported previously [27,31]. Representative results from a total of 4 independent GST-pulldown experiments are shown in Figure 1A. Lanes 1–3 contain 5% aliquots of radiolabeled protein to indicate the migration pattern and level of synthesis of each protein in the gel. No significant binding occurred between the protein-containing lysates and the GST sepharose control beads (Lanes 4–6). Lanes 7, 8, 11 & 12 illustrate the 1,25D-independent interaction of VDR and β-catenin shown previously by our group [31] and others [27]. The VDR corepressor hairless (Hr) was used as a positive control with GST-VDR beads and as a negative control with the GST-β-catenin beads. Significant interaction of Hr was observed with the GST-VDR beads (lane 14), but not with the GST-β-catenin beads (Lane 10) validating the utility of the in-vitro transcription/translation (IVTT) pull-down assay system. To confirm that the multiple bands in our β-catenin input and pull-down samples (lanes 2, 11–13) were β-catenin or β-catenin fragments, we separated IVTT input samples on a 5–15% gradient gel and either exposed to autoradiography or subjected to western blot probed with β-catenin primary antibody. The banding patterns derived from autoradiography and from the western blot were identical, leading us to conclude that the IVTT system was functional and generated β-catenin protein (data not shown).
Figure 1. Probing of protein-protein interactions between β-catenin and human VDR.
A. GST fusion proteins were used to assess binding of radiolabeled bait and glutathione-immobilized prey proteins. Human VDR, β-catenin (β-CAT) or rat hairless (Hr) expression plasmids (1.0 μg) containing the T7 promoter were used as a template in an in vitro transcription/translation (IVTT) reaction to generate 35S-methionine-labeled bait proteins. VDR-containing lysates were also incubated with 10−6 M 1,25D (+D), 10−4 M lithocholic acid (+L), or ethanol vehicle (−) as indicated below the lanes 7–9. The lysates were then combined with either 20 μL of glutathione-S-transferase (GST) fusion protein alone bound to Sepharose beads (GST; lanes 4–6), GST-WT-VDR (lanes 7–10) or GST-β-catenin-S37A (lanes 11–14) for 30 min. GST-VDR beads were pre-incubated with the indicated ligands at the same concentration employed for the lysates (lanes 11–13). The beads were then washed extensively and the amount of coprecipitated VDR, β-catenin, or hairless was detected by electrophoresis of denatured bead samples followed by autoradiography. The arrows indicate the migration position of VDR, β-catenin and hairless. Aliquots (5%) of all radiolabeled protein inputs are shown at far left (lanes 1–3). The autoradiography gel is representative of 4 independent experiments.
B. VDR truncation mutants define one region of the receptor required for contact with human β-catenin. WT and C-terminally truncated VDR proteins were generated in the IVTT system and incubated with either 20 μL of GST-β-CAT-Sepharose or GST-Sepharose only (as indicated below each lane) for 30 min. The beads were then washed and analyzed as described in the legend to Fig. 1A. Three C-terminal truncated receptor mutants are shown, with a stop codon inserted at the indicated residue position in VDR (Δ304, Δ202, Δ134). An N-terminal truncated receptor that is missing the first 88 amino acids was also employed (Δ1–88). The autoradiography gel is representative of 3 independent experiments.
C. WT and E420A mutated VDR was transfected into Caco-2 cells along with the indicated amount of β-catenin, human CYP24A1 promoter-luciferase reporter and renilla plasmid. Cells were treated with ethanol vehicle or 10−8 M 1,25D prior to luciferase assay. VDR-mediated transactivation was measured as firefly luciferase output. Firefly values were normalized for transfection efficiency (using renilla luciferase) and expressed as the ratio of Firefly/Renilla relative light units (RLUs) prior to calculation of fold effects. The data illustrated are representative of 3 independent experiments with triplicate samples in each group. Error bars indicate standard deviation. When the change in activity from 0 to 400 ng of β-catenin was compared with a t-test for the WT VDR −1,25D and +1,25D groups, the increased activity was statistically significantly greater for the +1,25D group (P = 0.002), and regression modeling revealed a statistically significant dose-dependent increase in activity with increased β-catenin (P-trend = 0.001) for the wildtype VDR in the presence of ligand. A Student’s t-test and ANOVA indicated that the E420A VDR exhibited less than 1% of the activity of WT VDR, both in the presence (P = 0.0001) and absence (P = 0.003) of 1,25D; differences remained statistically significant after employing Tukey’s HSD. No increase in activity for E420A was observed with the addition of β-catenin (P = 0.22 with ANOVA).
D. WT VDR was transfected into Caco-2 cells along with the indicated amount of β-catenin, human CYP24A1 promoter-luciferase reporter and renilla plasmid. Cells were treated with ethanol vehicle, 10−7 M 1,25D, or 70 μM LCA prior to luciferase assay. VDR-mediated transactivation was measured as firefly luciferase output, and then normalized as in (C). The data shown are representative of 3 independent experiments with triplicate samples in each group. Error bars indicate standard deviation. As evaluated with ANOVA, 1,25D and LCA both stimulated transactivation (P = 0.0001 for both ligands); results remained significant after adjustment with Tukey’s HSD. Transfection of β-catenin further augmented transcription by 1,25D-VDR (P = 0.0004), but not by LCA-VDR (P = 0.93); Student’s t-tests.
Next we sought to identify the region of VDR involved in the β-catenin interaction. A mapping experiment utilizing the GST-pull down method with truncated VDR proteins was performed. Representative results from 3 independent experiments are shown in Figure 1B. Based upon densitometric scanning of three independent experiments and statistical analysis, the wild-type (WT) VDR band is significantly (8.3-fold over control, P < 0.05) and reproducibly more intense with GST-β-catenin than with GST-only beads (compare lanes 6 and 7), whereas the truncated Δ304, Δ202, and Δ134 VDR proteins on average display the same band intensity with the GST-only (lanes 8, 10 and 12, respectively) and GST-β-catenin lanes (lanes 9, 11 and 13, respectively). This indicates that these truncated VDRs are unable to specifically associate with β-catenin and only bind non-specifically to the GST beads. In contrast, the Δ1–88 N-terminal VDR truncation shows a strong and statistically significant (7.1-fold over control, P < 0.05) band in the presence of GST-β-catenin beads (lane 15) that is almost absent in the control lane (lane 14). Thus, removal of the C- but not the N-terminus of VDR appears to significantly impact β-catenin interaction, at least in the context of the GST in vitro assay system, and implicates the C-terminal activation function (AF-2) of VDR as one region required for β-catenin interaction.
VDR AF-2 is required for β-catenin binding and potentiation of VDR activity
To address the effect of β-catenin on VDR-mediated transcription and the role of the AF-2 domain of VDR in the interaction, we utilized a VDR transcription assay in transfected Caco-2 cells with a VDRE-reporter vector that contains a large fragment of the promoter region from the human vitamin D3 24-hydroxylase (CYP24A1) gene linked to luciferase. Results in Figure 1C demonstrate minimal VDR-mediated transcription in the presence of WT VDR and the absence of 1,25DIn contrast, 1,25D results in a 16-fold increase in VDR transcriptional activity when compared to the vehicle control. When the change in activity from 0 to 400 ng of β-catenin was compared with a t-test for the WT VDR −1,25D and +1,25D groups, the increased activity was statistically significantly greater for the +1,25D group (P = 0.002). Furthermore, this 16-fold increase in VDR activity is augmented to 25–30 fold stimulation in transcriptional activity by dose-dependent expression of β-catenin, with regression modeling revealing a statistically significant trend (P-trend = 0.001). Thus, β-catenin can act as a bone fide VDR coactivator to facilitate transcription from a VDRE in the context of the CYP24A1 gene promoter, an authentic 1,25D regulated-gene. To address the putative compensatory role for β-catenin in the absence of a functioning AF-2 domain, we utilized an E420A mutant of VDR which contains an alanine (A) substitution for a glutamic acid (E) at amino acid 420 which differs from the glutamine (Q) substitution used by Shah et al. [32]. As evaluated with Student’s t-tests and ANOVA, the E420A VDR displays less than 1% of the activity of WT VDR with the maximal dose of β-catenin, both in the presence (P = 0.0001) and absence (P = 0.003) of 1,25D (Fig. 1C, inset). These pairwise comparisons remained statistically significant at the 99% confidence level after applying the Tukey HSD test. Importantly, when exogenous β-catenin is added, there is no compensatory increase in 1,25D-dependent transactivation exhibited by E420A VDR (Fig. 1C, inset; P = 0.22 using ANOVA). Moreover, mammalian two-hybrid assays in Caco-2 cells with β-catenin bait and WT or E420A VDR prey revealed no interaction between β-catenin and E420A VDR (data not shown). Thus, in contrast to the results of Shah et al. [32] who reported both a significant interaction between β-catenin and E420Q VDR, and an increase in E420Q activity by overexpression of exogenous β-catenin in HEK-293 kidney cells, our results suggest that in Caco-2 colon cancer cells, such a mechanism is unlikely.
VDR ligands modulate interaction with β-catenin
Because of the 1) consistent association in human studies between low vitamin D hormone levels and colorectal cancer risk, and 2) the proposed role of secondary bile acids in colorectal carcinogenesis and their low affinity binding to VDR, we next investigated the effect of lithocholic acid (LCA) on the interaction between VDR and β-catenin in the in vitro GST-pulldown experiment (Fig. 1A). This was followed by an examination of the effect of both 1,25D and LCA ligands on the physical and functional association of VDR and β-catenin in a cellular context of transfected Caco-2 colon cancer cells (Fig. 1D and Fig. 2). First, the interaction of VDR-containing IVTT lysates and β-catenin-GST beads was consistently attenuated (an average of 41% based on densitometric scanning) in the presence of LCA (Fig. 1A, lane 9) when compared to VDR in the absence and presence 1,25D (lanes 7 and 8, respectively). These results are supported by a similar decrease in the pull down signal when β-catenin lysates are incubated with VDR-GST beads in the presence of LCA (Fig. 1A, compare lanes 11–13).
Figure 2. Effect of 1,25D and LCA on the interaction of VDR and β-catenin, transcriptional activity of β-catenin and endogenous DKK-4 expression.
A. Representative graph of mammalian two hybrid assay utilizing Caco-2 cells to assess the effect of 1,25D and LCA on the interaction of VDR and β-catenin. Caco-2 cells were transfected with VDR prey, β-catenin bait, and then treated with vehicle (−1,25D), 10−8 M 1,25D or 70 μM LCA. Cells were then lysed and subjected to luciferase assay. VDR and β-catenin interaction was measured as firefly luciferase output (RLUs). Error bars indicate standard deviation. In the presence of 1,25D, interaction between VDR and β-catenin was increased 12-fold, while LCA increased the association by 8-fold (P-value for ANOVA = 0.0001; after Tukey’s HSD all pairwise comparisons remained statistically significant at a 99% confidence level).
B. TOPFlash assay measuring the effect of VDR on β-catenin transcriptional activity. HT29 cells were transfected the indicated amounts of exogenous F/M4 VDR, as well as human β-catenin and the TOPFlash vector. Cells were treated with vehicle −1,25D) or 10−9 M 1,25D for 24 hours prior to cell lysis and luciferase assay. Transcriptional activity was measured as firefly luciferase light output. Error bars indicate standard deviation. Using regression analyses, a statistically significant dose-response trend for suppression of beta-catenin activity with increasing VDR in the presence of ligand was observed (P-trend = 0.0001).
C. Representative graph of the TOPFlash assay in Caco-2 cells transfected with F/M4 VDR, TOPFlash vector, and β-catenin. Cells were treated with vehicle (−1,25D), 10−8 M 1,25D or 70 μM LCA and β-catenin transcriptional activity was measured as firefly luciferase output. Error bars indicate standard deviation. ANOVA reveals suppression of β-catenin activity at 25 ng VDR by 1,25-D but not LCA (* P = 0.04; though pairwise comparisons are not statistically significant after employing Tukey’s HSD). At 150 ng VDR, LCA was less effective at inhibiting β-catenin transactivation as compared to 1,25D (30%; ** P = 0.019 and 97%; P = 0.001), respectively; pairwise comparisons remained significant after Tukey’s HSD.
D. Quantitative RT-PCR to evaluate the effect of 1,25D and LCA on DKK-4 expression in Caco-2. Cells were transfected with 0 ng, 600 ng wild-type VDR, or 600 ng DNA-binding mutant VDR, and treated with either vehicle or 10−7 M 1,25D (bars left of dashed line), or with vehicle, 10−7 M 1,25D, or 70 μM LCA (bars right of dashed line) 24 hours prior to RNA extraction. PCR was performed using the Applied Biosystems 7500 Fast. Expression was normalized to GAPDH endogenous control. Fold change was determined by comparing ligand treated samples to the vehicle treated sample for that transfection group. The reference group is assigned a value of one. All statistical analyses were student’s t-tests. * P = 0.001 when comparing the −1,25D and +1,25D groups that did not receive exogenous VDR. Cells with mutant and wildtype VDR demonstrated similar DKK-4 expression (P = 0.0004 for both M4 and mutant VDR). Data in all panels are representative of 3 independent experiments with triplicate samples in each group.
Next, we observed that both 1,25D (10−7 M) and LCA (70 μM) were able to stimulate VDR-mediated transactivation (Fig. 1D) of the CYP24A1 reporter vector in transfected Caco-2 cells (22-fold versus 8-fold, respectively; P = 0.0001 using ANOVA). These results remained significant at the 99% confidence level after conducting the Tukey’s HSD procedure. A student’s t-test revealed that transfection of β-catenin (200 ng) resulted in a further augmentation of transcription by 1,25D-VDR (up to 30-fold; P = 0.0004) but not by LCA-VDR (Fig. 1D, bars on right; P = 0.93). Thus, while both 1,25D and LCA can bind to and activate VDR, the level of transactivation elicited by LCA-VDR is attenuated and, unlike 1,25D-VDR, the LCA-VDR complex cannot employ β-catenin as an efficient coactivator for transcriptional stimulation of VDRE-controlled genes such as CYP24A1.
To further address the potential modulatory effects of 1,25D and LCA on the interaction of VDR and β-catenin, we utilized the mammalian two hybrid system. These experiments were conducted in transfected Caco-2 cells treated with ethanol vehicle, 1,25D or 70 μM LCA. Figure 2A demonstrates that in the presence of 1,25D, the interaction of VDR and β-catenin is increased 12-fold, while LCA increases VDR-β-catenin association by 8-fold (compare the 3 bars at far right), indicating that LCA-VDR is 35% less effective than 1,25D-VDR in binding to β-catenin in the context of the mammalian two-hybrid system (P-value for ANOVA = 0.0001; Tukey’s HSD revealed that all pairwise comparison remained significant at the 99% confidence level).
To determine if the 1) observed enhancement of the interaction between VDR and β-catenin in the presence of 1,25D, and 2) the less efficient stimulation of VDR-β-catenin association in the presence of LCA has an effect on β-catenin transcriptional activity, we utilized the TOPFlash assay. The TOPFlash vector contains a TCF/LEF driven luciferase reporter gene to measure β-catenin-dependent transcriptional activation. These experiments were optimized in HT-29 colon cancer cells. Employing regression modeling, a significant dose-dependent suppression (ranging from a 1.5 fold to a 5-fold attenuation) of β-catenin transcription was evident with increasing amounts of VDR in the presence of 10−9 M 1,25D (P-trend = 0.0001), supporting and extending previous reports on the effect of 1,25D in the TOPFlash assay [27,31,32] (Fig. 2B). We next determined the effect of LCA on β-catenin transcriptional activity and compared LCA and 1,25D directly in the same experiment. Caco-2 cells were transfected and treated with vehicle, 1,25D or LCA as described in the legend to Figure 2C. As analyzed using ANOVA, this experiment demonstrates that in Caco-2 cells (Fig. 2C) there is a potent 1,25D- and VDR-dependent suppression of β-catenin transcriptional activity similar to that observed in HT-29 cells (Fig. 2B). When low levels of VDR (25 ng) were transfected into the cells, suppression by 1,25D, but not by LCA, was observed (Fig. 2C, middle set of bars; P-value with ANOVA = 0.04; though pairwise results were not statistically significant after Tukey’s HSD). At higher levels of transfected VDR (150 ng), LCA-VDR is only modestly effective in suppressing β-catenin activity (30% inhibition, P = 0.019), while 1,25D-VDR is very effective (97%, P = 0.001) in the inhibition of β-catenin transactivation. All pairwise comparisons remained statistically significant at the 99% confidence level after conducting the Tukey’s HSD procedure. The results of Fig. 1D and Fig. 2A-C indicate that 1,25D and LCA likely differ in their interaction with VDR potentially via the creation of distinct receptor conformations upon ligand binding, with 1,25D-VDR being more effective than LCA-VDR in binding co-factors and neutralizing β-catenin; results supported by our previous studies with LCA-VDR [36].
1,25D mediated inhibition of β-catenin transcriptional activity results in suppression of β-catenin target genes
In order to determine if the observed suppression of β-catenin transcriptional activity by VDR in the TOPFlash assay is biologically relevant, we next assessed whether VDR acts to suppress a β-catenin target gene, DKK-4 [37]. For these studies we utilized real time polymerase chain reaction (RT-PCR) specific for DKK-4 in Caco-2 cells transiently transfected with VDR and treated with ethanol vehicle, 1,25D or LCA. Figure 2D demonstrates that Caco-2 cells transfected with VDR and treated with 1,25D have 51–55% lower expression levels of DKK-4 than cells which did not receive 1,25D. Furthermore, cells transfected with the DNA-binding mutant VDR demonstrated similar DKK-4 expression as cells transfected with wild-type VDR (P = 0.0004 for both M4 and mutant VDR, using student’s t-tests). Cells treated with LCA did not display significant suppression of DKK-4. Importantly, in the absence of transfected exogenous VDR, we observed a modest, statistically significant (P ≤ 0.001; student’s t-test) 1,25D suppression of DKK-4 expression by endogenous VDR (Fig. 2D, first and fourth set of bars). In conclusion, Figure 2D demonstrates that 1,25D-VDR likely interacts with and suppresses β-catenin transcriptional activity, thus resulting in the inhibition of an endogenous β-catenin target gene in the context of the natural chromatin environment, while LCA-VDR does not participate in β-catenin neutralization to affect expression of DKK-4.
APC enhances the interaction of VDR and β-catenin
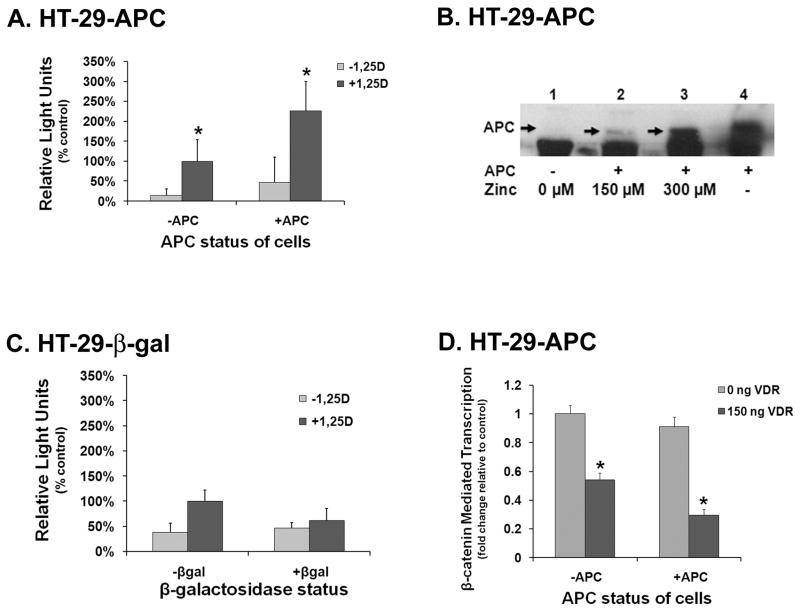
The work of Palmer et al. [27], suggests that the VDR/β-catenin interaction in the presence of 1,25D occurs independently of full length APC protein. Because of the critical function of APC as a tumor suppressor to sequester β-catenin from the nucleus and its common and early loss in colorectal cancers, we sought to better understand the VDR-β-catenin interaction in the context of cellular APC status. For these experiments, we used a mammalian two hybrid system with HT-29-APC cells which contain truncated endogenous APC and a stably expressed, zinc inducible full length APC plasmid under the control of a metallothionein promoter [38]. When compared to the vehicle controls, HT-29-APC cells treated with 1,25D showed a 6.8-fold increase in β-catenin-VDR interaction in the absence (Fig. 3A, compare first and second bars) and a 4.9-fold increase in the presence of induced full length APC (Fig. 3A, compare third and fourth bars). These results are consistent with Fig. 2A which also demonstrated a 1,25D-enhanced interaction between VDR prey and β-catenin bait proteins in a Caco-2 mammalian two hybrid assay, as well as previous studies showing a hormone-dependent interaction between VDR and β-catenin independent of APC [32]. Moreover, treatment with 150 μM zinc (modest induction of full length APC) significantly enhanced the interaction of VDR and β-catenin over those groups that did not express full-length APC, both in the absence (Fig. 3A, compare first and third bars) and presence of 1,25D (Fig. 3A, compare second and fourth bars), with the latter comparison (second and fourth bars) representing the greater increase in VDR binding to β-catenin (+1,25D/+APC, 2.3-fold increase). Thus, both 1,25D and APC serve to enhance the interaction between VDR and β-catenin, with the greatest stimulation observed when both modulators are present.
Figure 3. APC enhances both the interaction of VDR and β-catenin as well as the VDR-mediated suppression of β-catenin transcriptional activity.
A. A mammalian two hybrid assay was utilized in HT-29-APC inducible cells to assess if VDR and β-catenin interact within an intact cell in the presence and absence of full length APC. HT-29-APC cells were transfected with VDR prey, β-catenin bait, and renilla plasmids and then treated with vehicle or 10−8 M 1,25D as well as vehicle or 150 μM zinc chloride (to induce full length APC). Cells were then lysed and subjected to luciferase assay. Interaction was measured as firefly luciferase output. Firefly output numbers were normalized using a firefly/renilla ratio to control for transcription efficiency. Percent change in the firefly/renilla ratio was determined by comparing all groups to the −APC(−Zn)/+1,25D group. This reference group is assigned a value of 100%. Error bars indicate standard deviation. * P < 0.001 when comparing the −APC and +APC groups which received 1,25D.
B. Western blot of HT-29-APC cell lysates treated with zinc. HT-29-APC cells were treated with vehicle, 150 μM zinc chloride or 300 μM zinc chloride (lanes 1, 2 and 3, respectively) and allowed to incubate overnight prior to lysis. Lysates were then subjected to western blotting, using a chemiluminescence detection system and then exposed to film. HCT-116 cell lysates were used as an APC positive control (lane 4).
C. Mammalian two hybrid assay in HT-29-βgalactosidase cells. HT-29-βgalactosidase cells were transfected with VDR prey, β-catenin bait, and renilla plasmids and then treated with vehicle or 10−8 M 1,25D as well as vehicle or 150 μM zinc chloride (to induce β-galactosidase). Cells were then lysed and subjected to luciferase assay. Interaction was measured as firefly light output. Firefly light output numbers were normalized using a firefly/renilla ratio to control for transfection efficiency. Percent change in the firefly/renilla ratio was determined by comparing all groups to the −βgalactosidase (−Zn)/+1,25D reference group which is assigned a value of 100%. Error bars indicate standard deviation.
D. TOPFlash assay in HT-29-APC cells to assess the functional effects of full length APC on β-catenin transcriptional activity. F/M4 VDR was transfected into HT-29-APC cells along with a TOPFlash vector and renilla plasmid. Cells were treated with ethanol vehicle or 10−9 M 1,25D as well as vehicle or 150 μM zinc chloride prior to lysing and luciferase assay. Transcriptional activity was measured as firefly luciferase light output and the firefly values normalized by calculating fold effects via a +1,25D/−1,25D ratio. Fold transcription was determined by comparing the +1,25D/−1,25D ratio of each VDR group to the reference group of 0 ng VDR and no full length APC. The reference group is assigned a value of one. Error bars indicate standard deviation. * P < 0.001 when comparing the APC and +APC groups which received 1,25D. The data in all panels (except B) are the mean of three independent experiments with triplicate samples in each group.
Next, to confirm that full length APC protein was induced by 150 μM zinc in the mammalian two-hybrid experiments (Fig. 3A), we subjected lysates of HT-29-APC cells treated with vehicle, 150 μM and 300 μM zinc to western blotting with anti-APC antibody. As expected, untreated lysates (Fig. 3B, lane 1) had no induction of full length APC protein. Lysates from cells treated with 150 μM (lane 2) showed modest induction of full length APC while lysates from cells treated with 300 μM (lane 3), revealed the strongest signal for full length APC. HCT-116 cell lysates which contain endogenous full length APC were included as a positive control (lane 4).
To rule out non-specific effects of zinc, we used HT-29-βgalactosidase cells containing a stably integrated β-galactosidase plasmid under the control of the metallothionein promoter. For the purposes of comparing between the +1,25D hormone groups, the −βgalactosidase (−Zn)/+1,25D treatment group served as the reference group against which all samples were compared. When the mammalian two hybrid experiments were repeated in the HT-29-βgalactosidase cells, a 2.6-fold increase in VDR-β-catenin interaction was observed in the presence of 1,25D in the cells not treated with zinc (Fig. 3C; first and second bars; similar to that detected in HT-29 APC cells, Fig. 3A), but there was no significant effect of 1,25D on the interaction in the zinc treated cells (Figure 3C, compare third and fourth bars). The mechanism for this attenuation of VDR-β-catenin interaction in the presence of 1,25D and zinc (only in the HT-29-βgalactosidase cells) is unknown, but the important point is that the presence of zinc alone did not increase the interaction of VDR and β-catenin in the cells treated with zinc and 1,25D when compared to the cells treated with zinc, vehicle and 1,25D (compare second and fourth bars). Therefore, we conclude that the increased interaction of VDR and β-catenin observed in the presence of 1,25D and full length APC is due to the presence of full length APC and not due to the zinc inducing agent.
To determine if the observed enhancement of the interaction between VDR and β-catenin in the presence of intact APC has an effect on β-catenin transcriptional activity either in the presence or absence of 1,25D, we then utilized the TOPFlash assay (Fig. 3D). We again measured a significant 50% suppression of β-catenin transcriptional activity in the presence of 1,25D with VDR in the absence of full length APC (compare first and second bar), and a 70% suppression in the presence of VDR and full length APC (compare first and fourth bar) when both groups are compared to the −APC, 0 ng VDR reference group. We observed a statistically significant and reproducible 20% decrease in β-catenin transcriptional activity in the presence of 1,25D-VDR plus full length APC when compared to 1,25D-VDR in the absence of full length APC (Fig. 3D, compare second and fourth bars). A modest induction of APC (150 μM zinc chloride treatment) was utilized to allow detection of the additive effect of 1,25D-VDR mediated suppression of β-catenin transcriptional activity. The FOPFlash control assay displayed no significant β-catenin transcriptional activity (data not shown). As in our previous study, we ruled out independent effects of zinc by repeating these same experiments in HT-29-βgalactosidase cells. Again, there was no demonstrable effect of the zinc treatment on β-catenin transcriptional activity (see Fig. 4C; P = 0.73 for M4 variant with student’s t-test). Therefore, we conclude that the consistently observed 20% additional suppression of β-catenin transcriptional activity in the presence of full length APC is due to the presence of intact APC and not due to the zinc inducing agent.
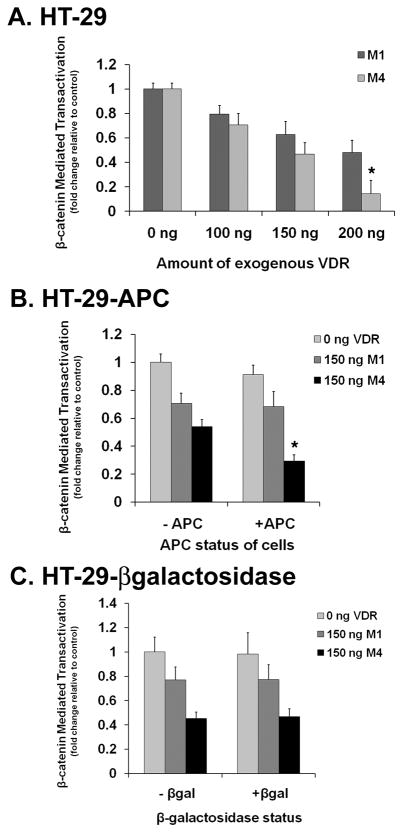
Figure 4. Differential effect of FokI isoforms and APC on β-catenin transcriptional activity.
A. Varying amounts of exogenous f/M1 and F/M4 VDR were transfected into HT-29 parental cells along with a TOPFlash vector and renilla plasmids. Cells were treated with ethanol vehicle or 10−9 M 1,25D prior to lysing and luciferase assay. Transcriptional activity was measured as luciferase light output and normalized by utilizing a +1,25D/−1,25D ratio of the firefly output. Fold transcription was determined by comparing the +1,25D/−1,25D firefly ratio of each VDR concentration to the reference group of 0 ng VDR for each isoform of VDR. The two reference groups are assigned a value of one. Error bars indicate standard deviation. Regression models revealed statistically significant trends (P = 0.0001) for both variants with increasing doses of VDR. A Student’s t-test indicated that the suppression of the TCF/LEF reporter was significantly greater for the M4 variant as compared to the M1 variant (* P = 0.0001).
B. 150 ng of f/M1 and F/M4 VDR were transfected into HT-29-APC cells with a TOPFlash vector and renilla plasmid. Cells were treated with vehicle, 150 μM zinc, and/or 10−9 M 1,25D prior to lysing and luciferase assay. Zinc was used to induce the expression of APC (see Fig. 3). Transcriptional activity was measured as luciferase light output. The firefly output was normalized as in (A) and fold transcription determined by comparing the +1,25D/−1,25D ratio of each VDR group to the reference group of 0 ng VDR and no full length APC. The reference group is assigned a value of one. Error bars indicate standard deviation. P = 0.45 for the comparison of −APC vs. +APC in the M1 group (Student’s t-test). * P < 0.0001 for the comparison of −APC vs. +APC in the M4 group (Student’s t-test). All pairwise comparisons remained significant at the 99% confidence level after Tukey’s HSD.
C. 150 ng exogenous f/M1 and F/M4 VDR were transfected into HT-29-βgalactosidase cells with a TOPFlash vector and renilla plasmid. Treatments and transcriptional activity was determined as in (B). Using a student’s t-test, no changes in β-catenin transcriptional activity was observed with the addition of β-gal in either the M1 (P = 0.97) or M4 (P = 0.73) variant. The data in all panels are the mean of three independent experiments with triplicate samples in each group.
FokI polymorphism in VDR affects interaction with β-catenin
In the studies presented above, we employed the common F/M4 allele form of the VDR gene. Our previous studies indicated that the f/M1 VDR polymorphism, common in the population (~0.4 allele frequency) [39–43], exhibits an 3.2-fold and 2.1-fold reduced transcriptional activity compared to the F/M4 isoform at 10−9 M and 10−8 M 1,25D treatment, respectively [3]. We introduced the f/M1 VDR polymorphic variant into the TOPFlash assay to determine if the f/M1 variant differentially affects β-catenin transcriptional levels. For both the F/M4 and f/M1 forms of the VDR (which are equally expressed in transfected cells [3]) we observed a strong VDR dose-dependent suppression of β-catenin transcriptional activity in the presence of 1,25D (Fig. 4A). Regression models yielded P-trends = 0.0001 for both variants with increasing doses of exogenous VDR. However, the F/M4 isoform demonstrated more efficient suppression of the TCF/LEF reporter (30–90% change with F/M4 versus 20–50% with f/M1) with lower inputs of VDR. A student’s t-test revealed that the change in activity between the F/M4 and f/M1 variants was statistically significant (P = 0.0001). For these experiments, we also utilized a mutated TCF/LEF site containing luciferase reporter gene assay (FOPFlash) as a negative control. No significant β-catenin transcriptional activity was observed with the FOPflash assay (data not shown).
We then repeated the above experiment in the HT-29-APC cells to determine the effect of the FokI variants in the presence of full length APC and 1,25D on the suppression of β-catenin transcriptional activity (Fig. 4B). Consistent with Fig. 4A, 1,25D-VDR(M1) is less effective at suppressing β-catenin compared to 1,25D-VDR(M4) in the absence of exogenous APC (30% versus 50% inhibition, respectively; compare second and third bars; P = 0.0001 using ANOVA; pairwise comparisons all significant at 99% confidence level with Tukey’s HSD). Significantly, in the presence of APC, the suppression by 1,25D-VDR(M1) was not altered (P = 0.45 compared to no APC; student’s t-test); while β-catenin inhibition by 1,25D-VDR(M4) is appreciably more pronounced (70%; P = 0.0001). A modest induction of full length APC (150 μM) was utilized to allow detection of an additive effect of 1,25D-VDR on β-catenin transcriptional activity. We ruled out independent effects of zinc by repeating these experiments in HT-29-βgalactosidase cells with no differential effect of the zinc treatment (compare −βgalactosidase to +βgalactosidase) on β-catenin transcriptional activity (Fig. 4C; P = 0.97 for M1 and P = 0.73 for M4 using a student’s t-test). Therefore, we conclude that the ability of APC to enhance F/M4 VDR-mediated suppression of β-catenin transcriptional activity, but not f/M1 VDR-directed suppression, is due to the presence of intact APC and not due to the zinc inducing agent.
DISCUSSION
Effect of ligands on VDR and β-catenin interaction
Extensive prior work supports 1,25D as a pro-differentiation, anti-growth hormone in colorectal cancer cells [11–13,15,16,19,44], biologic effects that have been suggested to explain the observation that low vitamin D levels increase the risk of colorectal cancer. Our results confirm a potent 1,25D-enhanced physical interaction between VDR and β-catenin [27,32] and demonstrate that this interaction results in functional suppression of β-catenin-mediated transcription of a TCF/LEF reporter gene, as well as inhibition of an endogenous β-catenin target gene, namely DKK-4. These results further strengthen the evidence that VDR, through a direct interaction with β-catenin in the presence of 1,25D, acts to modulate the Wnt signaling pathway in the colonocyte, effectively functioning as a tumor suppressor.
As a mechanism for bile acid carcinogenesis, we hypothesize that the association between the secondary bile acid lithocholic acid (LCA) and colorectal cancer risk may be partly mediated through attenuation of the VDR and β-catenin interaction. Our GST pull down studies, two hybrid experiments, and TOPFlash results support a role for LCA in suppressing the potent 1,25D-stimulated interaction between VDR and β-catenin. LCA clearly disrupts the interaction between VDR and β-catenin in our GST pulldown experiments (Fig. 1A), and is less effective than 1,25D in eliciting the physical interaction between VDR and β-catenin (Fig. 2A). Most significant is the demonstration that under conditions of moderate VDR expression, LCA-VDR is unable to inhibit β-catenin activity while 1,25D-VDR leads to significant suppression (Fig. 2C). Moreover, the interaction of 1,25D-VDR with β-catenin not only neutralizes the ability of β-catenin to serve as a coactivator for TCF/LEF-driven target genes involved in proliferation, but also β-catenin is then able to serve as a VDR coactivator to boost VDRE-mediated transactivation by 1,25D-VDR, but not in the LCA-VDR-β-catenin complex (Fig. 1C–D). Thus, LCA may be considered a partial agonist in that it is able to bind VDR, but with a lower affinity than 1,25D, and it endows the receptor with properties that are distinct from those of 1,25D-VDR. Taken together, these results indicate that 1,25D shifts the balance of β-catenin in favor of VDRE target genes and away from TCF/LEF sites. Consequently, differentiation, cell cycle regulation and apoptosis are favored. In contrast, LCA shifts the balance of β-catenin in favor of target genes with TCF/LEF sites and away from VDRE target genes resulting in proliferation (Fig. 5). It is important to consider the relative occupancy of VDR by 1,25D and LCA in experiments that compare the activity of these two VDR ligands. Based on competition-displacement curves for LCA binding to VDR occupied by radio-labeled 1,25D [21], approximately 30,000 more molecules of LCA than 1,25D are required to achieve receptor occupation by an equal number of molecules of each ligand. We utilized 1,25D concentrations in excess of the Kd for VDR binding in some experiments, but the bioeffects are also achievable with concentrations of 1,25D as low as 1 nM (e.g., Fig. 2B), therefore, the effective concentrations of LCA (30–70 μM) are actually approximately 50,000 times those of the effective 1,25D concentration. This demonstrates that our experiments were carried out at a range of LCA concentrations that would attain up to the same number of molecules of this ligand bound to VDR as is the case for 1,25D.
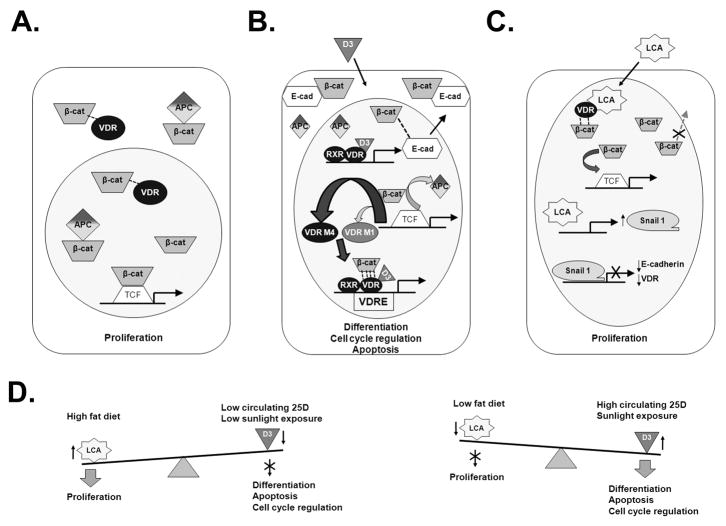
Figure 5. Proposed model of VDR-β-catenin interactions.
A. Absence of 1,25D. There is weak interaction (indicated by single line) of VDR, β-catenin and APC. β-catenin is able to freely translocate to the nucleus and bind to the promoter of target genes resulting in cell proliferation.
B. Presence of 1,25D (D3). There is a robust interaction (indicated by four lines) of 1,25D-VDR and β-catenin (bottom), with stronger binding/sequestration by the F/M4 VDR isoform (center). The β-catenin in this complex is unable to activate TCF/LEF-driven proliferation genes, and instead is diverted towards VDRE-mediated transcription of 1,25D target genes regulating differentiation, cell cycle regulation and apoptosis. The 1,25D-VDR-RXR complex (with or without β-catenin) also binds to the VDRE in the promoter region of the E-cadherin gene to upregulate this protein which then facilitates the movement of β-catenin from the nucleus to the plasma membrane (top).
C. Presence of lithocholic acid. LCA-VDR does not interact as robustly (indicated by two lines) with β-catenin compared to 1,25D-VDR. Thus, in the presence of an increased LCA/1,25D ratio, there is a relative elevation in the free pool of β-catenin resulting in more TCF/LEF-mediated expression of proliferative genes (top). LCA also upregulates the Snail1 transcriptional repressor (center) which has been previously shown to inhibit the expression of E-cadherin and VDR (bottom) and to prevent the nuclear exit of β-catenin, further increasing the pool of nuclear β-catenin and shifting the balance towards upregulation of β-catenin proliferative target genes.
D. The role of dietary and environmental factors. A high fat diet, which results in the production of elevated colonic LCA, and/or low circulating 25D and low sunlight exposure shifts the “equilibrium” towards proliferation over differentiation. Conversely, high circulating 25D and adequate sunlight exposure, coupled with a low-fat diet, will lead to colonocytes that support the processes of differentiation, apoptosis and cell cycle regulation.
Finally, the ability of 1,25D, but not LCA, to suppress DKK-4 (Fig. 2D), a β-catenin target gene [37,45] lends further support to our hypothesis for differential activity of 1,25D-VDR versus LCA-VDR. Importantly, the effect of 1,25D in suppressing DKK-4 is enhanced in the presence of additional exogenous VDR, and a VDR mutant that cannot bind to the VDRE can still suppress TOPFlash activity (data not shown) or DKK-4 (Fig. 2D). Taken together, these observations suggest that 1,25D-VDR inhibits DKK-4 expression, at least in part, via direct interaction with and neutralization of β-catenin. Suppression of DKK-4 by 1,25D-VDR has significant implication for colorectal cancer, as DKK-4 has been reported to be significantly upregulated in a series of 29 human colorectal tumor samples, with matched normal control tissue possessing undetectable expression [45]. In this work, there was also a reported inverse correlation between the expression of DKK-4 and that of VDR [45]. Thus, even though DKK-4 has previously been considered a Wnt “antagonist” similar to the 1,25D-upregulated DKK-1 gene, the fact that DKK-4 is both targeted and upregulated by β-catenin, and is significantly elevated in colonic tumor tissue [45,46] suggests that DKK-4 may in fact possess unique tumorigenic properties. In further support of this hypothesis, while DKK-1 is potently downregulated in human colon cancers [47,48], upregulation of DKK-4 correlates with a more neoplastic phenotype because ectopic DKK-4 expression increases the migration and invasion properties of colon cancer cells, and conditioned media from DKK-4-expressing cells enhanced the capacity of human microvascular endothelial cells to migrate and form capillary-like tubules [45]. Thus, opposing regulation of DKK-1 and DKK-4 in colonic tissue by 1,25D-VDR may play a significant role in the molecular mechanism of chemoprotection by vitamin D.
VDR AF-2 domain is important for VDR-β-catenin interaction
VDR contains a highly conserved ligand binding domain [1,49–51] which includes the activating function (AF-2) region [52]. The AF-2 domain recruits co-modulators [50] and contains two highly conserved amino acids, leucine-417 and glutamate-420, which are required for 1,25D-dependent interaction between VDR and co-activators [33,34]. The present data support previous reports from our [31] and other laboratories [27,32] that VDR, when bound to 1,25D, utilizes β-catenin as a coactivator for VDRE-dependent transcriptional activity, and additionally demonstrates a dose-dependent effect of β-catenin on VDRE transcriptional activity. Furthermore, our data reveal that β-catenin cannot be used as a coactivator of VDRE transcriptional activity when VDR amino acid 420 in the AF-2 domain is mutated from a glutamate to an alanine. Together with our transcription assays (Fig. 1C) and mammalian two-hybrid analysis in Caco-2 cells with β-catenin bait and WT or E420A VDR prey (data not shown), this implicates the VDR AF-2 as an interacting domain that contacts β-catenin for the purpose of transcriptional activity enhancement, similar to that proposed for other nuclear receptors such as the androgen or retinoic acid receptors [53,54]. Therefore, amino acid 420 of the VDR AF-2 domain is necessary for VDR to interact with β-catenin as a co-activator of VDR target genes and, unlike the previous results of Shah et al. [32] where excess β-catenin appeared to compensate for the more likely structurally and functionally conservative E to Q substitution at amino acid 420, the presence of alanine at 420 completely abrogated VDR function even in the presence of excess amounts of β-catenin. Therefore, in the context of Caco-2 cells, overexpression of β-catenin does not compensate for the presence of an E420A mutation in VDR. Finally, unlike wild-type VDR, E420A VDR cannot mediate 1,25D-dependent suppression of TOPFlash activity in Caco-2 cells, further suggesting that E420 is required for β-catenin-VDR interaction (data not shown).
Differential suppression of β-catenin transcriptional activity by FokI variants
In previous work by our group, we reported that the presence of the VDR FokI polymorphism affects the interaction of VDR with TFIIB (3). Here we report for the first time our novel results which reveal that while both VDR isoforms are capable of suppressing β-catenin transcriptional activity, the VDR f/M1 isoform is less efficient than the VDR F/M4 at sequestering β-catenin from TCF/LEF binding sites, with F/M4 suppressing β-catenin transcriptional activity 3.4-fold more effectively than f/M1. This difference was statistically significant and most evident when 1,25D was limiting. This effect is likely physiologically relevant because VDR is mostly unoccupied with ligand under normal physiologic conditions (serum 1,25D = 0.10 nM) and therefore most sensitive to small fluctuations in 1,25D levels when the F/M4 isoform is expressed. However, we do not know the intracellular concentrations of 1,25D, and the colon is capable of 1α-hydroxylation of 25D. Therefore, the local cellular concentration in normal, adenoma and neoplastic colonic epithelium will require measurement to assess the in vivo impact of VDR polymorphisms in colorectal cancer. Nonetheless, these results with F/M4 versus f/M1 are consistent with our earlier work showing that the common f/M1 variant allele exhibits lower transcriptional activity, and the present study further elucidates the diversity of the VDR FokI polymorphism to confer differential functionality with respect to VDR interaction with multiple transcription factors, now including TFIIB and β-catenin.
Numerous epidemiological studies have been conducted to evaluate the role of the common functional FokI polymorphism in VDR as a heritable risk factor for colorectal neoplasia with variable findings across studies [5–8,41,55–60]. Inconsistencies across epidemiologic studies may in part be explained by lack of information on measured 25D levels, an important modifier of individual risk. The results herein suggest that the FokI VDR allelic variants differ in their activity in a manner that is dependent on available ligand. Collectively, past experiments and the present data suggest that vitamin D levels may modify the expression of the colorectal cancer risk differentially among the two functionally distinct allelic variants. This conclusion is supported by the observation that risk of colorectal cancer is increased with the f/M1 allele when environmental factors thought to modify available 25D levels, such as poor diet and high body mass index (BMI), are considered [7,55,60].
APC enhances VDR-β-catenin interaction
An extensive body of work has established APC as a major regulator of β-catenin in the Wnt signaling pathway. More recent efforts, including those herein, demonstrate a potentially important role for VDR as a regulator of β-catenin cellular activity [27,30,32]. Given the common loss of APC in colorectal cancers and our interest in the potential association between tissue availability of 1,25D and colorectal cancer risk, we asked whether or not VDR was equally potent in the absence and presence of APC in redirecting β-catenin to VDRE elements and inhibiting transcription from TCF/LEF promoter elements. While Palmer et al. [27] reported APC independent VDR inhibition of β-catenin-mediated TCF/LEF transcription, we discovered a statistically significant further enhancement of β-catenin-mediated TCF/LEF inhibition by VDR in the presence of overexpressed wildtype APC protein. While the mechanism of APC enhancement on the interaction between VDR and β-catenin is currently unknown, these data warrant additional investigation as they suggest that APC may form a complex with VDR and β-catenin in the presence of 1,25D and/or facilitates the transfer of β-catenin to VDR bound to 1,25D. We have not as yet shown directly that APC influences expression of β-catenin downstream targets such as DKK-4. This tempers our conclusion regarding APC impact, but does not negate the statistically significant effect of APC on β-catenin-VDR interaction/signaling. Furthermore, we demonstrated for the first time that the presence of full length APC protein only enhanced suppression of β-catenin activity in the presence of the VDR F/M4 ‘high activity’ isoform, not the VDR f/M1 isoform. The apparent resistance of the VDR f/M1 isoform to the enhancing effect of APC may be due in part to differences in the amino acid sequence at the N-terminus of the two VDR isoforms, and additional work will be needed to determine if the shorter F/M4 variant, because of its structure, has a greater ability to interact with, or be secondarily enhanced by, the presence of intact APC. It is also important to point out that one potential limitation of the current study is that some of the experimental systems employed reporter-based assays and protein overexpression. Ultimately, future studies will be required to assess the cellular localization of endogenous APC, VDR and β-catenin in the presence and absence of 1,25D. In addition, further evaluation of the functional interaction between endogenous VDR and β-catenin (as demonstrated in Fig. 2D) is warranted to confirm and extend this work. Thus, while additional studies will be essential to identify the underlying mechanism of the observed enhancing effects of APC on the inhibitory action of polymorphic VDR on β-catenin mediated transactivation, the current results implicate a novel cross talk between APC and VDR in the regulation of β-catenin activity, and consequently VDR effects on β-catenin target genes.
Proposed model
We have expanded on the developing models for the molecular mechanism underlying VDR-1,25D crosstalk with the β-catenin signaling pathway in colon cancer cells, and the role of APC, VDR polymorphisms, and bile acid ligands to generate a proposed mechanism (Fig. 5). Based on the data reported herein, as well as those of previous studies [27,31,61], we propose that a complex is formed between VDR, β-catenin and APC in the cytoplasm of the cell. In the absence of 1,25D (Fig. 5A), this interaction is sub-optimal with a significant proportion of β-catenin present in the nucleus where it stimulates TCF-mediated transcription of cell proliferation genes.
In contrast, 1,25D (Fig. 5B) facilitates a strong interaction of VDR, β-catenin, and APC which results in the nuclear sequestration of β-catenin from the TCF/LEF binding sites. Previous work, as well as our own data (Fig. 1C–D), has shown a potentiation in 1,25D-VDR/VDRE-mediated transcription in the presence of β-catenin [27,31,32]. Thus, we propose that a significant fraction of VDR, β-catenin and 1,25D form a complex in the nucleus that is diverted away from TCF-controlled genes and towards VDRE-containing target genes. The F/M4 VDR isoform appears to be more efficacious than f/M1 VDR in binding and suppressing β-catenin activity (Fig. 4 and Fig. 5B, bold arrow). Furthermore, 1,25D-VDR has also been shown to expedite the translocation of β-catenin from the nucleus to the plasma membrane [27,30] likely by inducing the transcription of E-cadherin [27]. Thus, the presence of 1,25D leads to reduced transcriptional activity of β-catenin target genes, and greater VDRE-specific transactivation resulting in cell differentiation, apoptosis and cell cycle regulation.
When bound to lithocholic acid, LCA-VDR does not interact as tightly with β-catenin as does 1,25D-VDR, nor does LCA-VDR elicit suppression of β-catenin activity that is as effective as 1,25D-VDR (Fig. 2 and 5C). Thus, the relative free pool of β-catenin in the nucleus is greater as the ratio of LCA/1,25D increases, allowing more β-catenin to bind to TCF/LEF and to initiate transcription of TCF/LEF target genes. Moreover, LCA upregulates Snail1 [28,29] which antagonizes the anti-proliferative effects of 1,25D, and blocks 1,25D/VDR-mediated export of β-catenin from the nucleus of SW480-ADH cells [30]. Snail1 mediates these effects via downregulation of E-cadherin and VDR expression [28,30]. The suppression of VDR (or low 1,25D levels) can also lead to further increases in colonic LCA concentrations because in colonocytes 1,25D-VDR upregulates CYP3A4, an enzyme that hydroxylates and detoxifies LCA. Thus, we propose that the balance of colonic cellular proliferation, differentiation, apoptosis and cell cycle regulation is influenced by the relative concentrations of LCA and 1,25D (Fig. 5D). A high fat diet resulting in high concentrations of LCA in the colon, and low circulating 25D and/or restricted sunlight exposure, will favor cellular proliferation and consequently, an increased risk of developing colorectal cancer. If the converse is true, then differentiation, apoptosis and cell cycle regulation will be favored resulting in reduced cancer risk. Superimposed on these dietary/environmental factors are the genetic parameters of VDR polymorphisms, as well as the APC status of the individual.
Acknowledgments
Grant support: This work was supported, in whole or in part, by National Institutes of Health Grant DK33351 (to MRH and PWJ) and 1K07CA106269 (to ETJ). This work was also supported by a Technology and Research Initiative Fund (TRIF) ASU-UA Collaborative on Biomedical Research Grant (to PAT and PWJ).
Abbreviations used
- 1,25D
1,25-dihydroxyvitamin D3
- 25D
25(OH)D3
- AF-2
activating function-2 domain
- APC
adenomatous polyposis coli
- CRC
colorectal cancer
- CYP24A1
vitamin D3 24-hydroxylase
- IVTT
in-vitro transcription/translation
- LCA
lithocholic acid
- SNP
single nucleotide polymorphism
- VDR
vitamin D receptor
- VDRE
vitamin D responsive element
References
- 1.Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood RJ, Fleet JC. The genetics of osteoporosis: vitamin D receptor polymorphisms. Annu Rev Nutr. 1998;18:233–258. doi: 10.1146/annurev.nutr.18.1.233. [DOI] [PubMed] [Google Scholar]
- 3.Jurutka PW, Remus LS, Whitfield GK, et al. The polymorphic N terminus in human vitamin D receptor isoforms influences transcriptional activity by modulating interaction with transcription factor IIB. Mol Endocrinol. 2000;14(3):401–420. doi: 10.1210/mend.14.3.0435. [DOI] [PubMed] [Google Scholar]
- 4.Fang Y, van Meurs JB, d’Alesio A, et al. Promoter and 3′-untranslated-region haplotypes in the vitamin d receptor gene predispose to osteoporotic fracture: the rotterdam study. Am J Hum Genet. 2005;77(5):807–823. doi: 10.1086/497438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park K, Woo M, Nam J, Kim JC. Start codon polymorphisms in the vitamin D receptor and colorectal cancer risk. Cancer Lett. 2006;237(2):199–206. doi: 10.1016/j.canlet.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 6.Peters U, McGlynn KA, Chatterjee N, et al. Vitamin D, calcium, and vitamin D receptor polymorphism in colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 2001;10(12):1267–1274. [PubMed] [Google Scholar]
- 7.Slattery ML, Murtaugh M, Caan B, Ma KN, Wolff R, Samowitz W. Associations between BMI, energy intake, energy expenditure, VDR genotype and colon and rectal cancers (United States) Cancer Causes Control. 2004;15(9):863–872. doi: 10.1007/s10552-004-1048-6. [DOI] [PubMed] [Google Scholar]
- 8.Wong HL, Seow A, Arakawa K, Lee HP, Yu MC, Ingles SA. Vitamin D receptor start codon polymorphism and colorectal cancer risk: effect modification by dietary calcium and fat in Singapore Chinese. Carcinogenesis. 2003;24(6):1091–1095. doi: 10.1093/carcin/bgg059. [DOI] [PubMed] [Google Scholar]
- 9.Holt PR, Arber N, Halmos B, et al. Colonic epithelial cell proliferation decreases with increasing levels of serum 25-hydroxy vitamin D. Cancer Epidemiol Biomarkers Prev. 2002;11(1):113–119. [PubMed] [Google Scholar]
- 10.Whitfield GK, Jurutka PW, Haussler CA, et al. Nuclear Vitamin D Receptor: Structure-Function, Molecular Control of Gene Transcription, and Novel Bioactions. In: Feldman D, Pike JW, GLorieux FH, editors. Vitamin D. 2. Vol. 1. London: Elsevier Academic Press; 2005. pp. 219–261. [Google Scholar]
- 11.Zhao X, Feldman D. Regulation of vitamin D receptor abundance and responsiveness during differentiation of HT-29 human colon cancer cells. Endocrinology. 1993;132(4):1808–1814. doi: 10.1210/endo.132.4.8384998. [DOI] [PubMed] [Google Scholar]
- 12.Halline AG, Davidson NO, Skarosi SF, et al. Effects of 1,25-dihydroxyvitamin D3 on proliferation and differentiation of Caco-2 cells. Endocrinology. 1994;134(4):1710–1717. doi: 10.1210/endo.134.4.8137734. [DOI] [PubMed] [Google Scholar]
- 13.Haussler MR, Whitfield GK, Haussler CA, et al. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13(3):325–349. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- 14.Hulla W, Kallay E, Krugluger W, Peterlik M, Cross HS. Growth control of human colon-adenocarcinoma-derived Caco-2 cells by vitamin-D compounds and extracellular calcium in vitro: relation to c-myc-oncogene and vitamin-D-receptor expression. Int J Cancer. 1995;62(6):711–716. doi: 10.1002/ijc.2910620611. [DOI] [PubMed] [Google Scholar]
- 15.Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer. 2003;3(8):601–614. doi: 10.1038/nrc1144. [DOI] [PubMed] [Google Scholar]
- 16.Scaglione-Sewell BA, Bissonnette M, Skarosi S, Abraham C, Brasitus TA. A vitamin D3 analog induces a G1-phase arrest in CaCo-2 cells by inhibiting cdk2 and cdk6: roles of cyclin E, p21Waf1, and p27Kip1. Endocrinology. 2000;141(11):3931–3939. doi: 10.1210/endo.141.11.7782. [DOI] [PubMed] [Google Scholar]
- 17.Shabahang M, Buras RR, Davoodi F, et al. Growth inhibition of HT-29 human colon cancer cells by analogues of 1,25-dihydroxyvitamin D3. Cancer Res. 1994;54(15):4057–4064. [PubMed] [Google Scholar]
- 18.van de Wetering M, Sancho E, Verweij C, et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111(2):241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 19.Ylikomi T, Laaksi I, Lou YR, et al. Antiproliferative action of vitamin D. Vitam Horm. 2002;64:357–406. doi: 10.1016/s0083-6729(02)64010-5. [DOI] [PubMed] [Google Scholar]
- 20.Hamada K, Umemoto A, Kajikawa A, Seraj MJ, Monden Y. In vitro formation of DNA adducts with bile acids. Carcinogenesis. 1994;15(9):1911–1915. doi: 10.1093/carcin/15.9.1911. [DOI] [PubMed] [Google Scholar]
- 21.Makishima M, Lu TT, Xie W, et al. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296(5571):1313–1316. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- 22.Nagengast FM, Grubben MJ, van Munster IP. Role of bile acids in colorectal carcinogenesis. Eur J Cancer. 1995;31A(7–8):1067–1070. doi: 10.1016/0959-8049(95)00216-6. [DOI] [PubMed] [Google Scholar]
- 23.Reddy BS, Watanabe K, Weisburger JH, Wynder EL. Promoting effect of bile acids in colon carcinogenesis in germ-free and conventional F344 rats. Cancer Res. 1977;37(9):3238–3242. [PubMed] [Google Scholar]
- 24.Fodde R, Smits R, Clevers H. APC, signal transduction and genetic instability in colorectal cancer. Nat Rev Cancer. 2001;1(1):55–67. doi: 10.1038/35094067. [DOI] [PubMed] [Google Scholar]
- 25.Korinek V, Barker N, Morin PJ, et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275(5307):1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 26.Lee E, Salic A, Kruger R, Heinrich R, Kirschner MW. The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol. 2003;1(1):E10. doi: 10.1371/journal.pbio.0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer HG, Gonzalez-Sancho JM, Espada J, et al. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol. 2001;154(2):369–387. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fukase K, Ohtsuka H, Onogawa T, et al. Bile acids repress E-cadherin through the induction of Snail and increase cancer invasiveness in human hepatobiliary carcinoma. Cancer Sci. 2008;99(9):1785–1792. doi: 10.1111/j.1349-7006.2008.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palmer HG, Larriba MJ, Garcia JM, et al. The transcription factor SNAIL represses vitamin D receptor expression and responsiveness in human colon cancer. Nat Med. 2004;10(9):917–919. doi: 10.1038/nm1095. [DOI] [PubMed] [Google Scholar]
- 30.Larriba MJ, Valle N, Palmer HG, et al. The inhibition of Wnt/beta-catenin signalling by 1alpha,25-dihydroxyvitamin D3 is abrogated by Snail1 in human colon cancer cells. Endocr Relat Cancer. 2007;14(1):141–151. doi: 10.1677/ERC-06-0028. [DOI] [PubMed] [Google Scholar]
- 31.Jurutka PW, Thompson PD, Eichhorst KR, et al. Cell-specific crosstalk between the vitamin D receptor and beta-catenin signal transduction pathways in 1,25(OH)2D3 target tissues. J Bone Miner Res. 2004;19(S1):S101. [Google Scholar]
- 32.Shah S, Islam MN, Dakshanamurthy S, et al. The molecular basis of vitamin D receptor and beta-catenin cross regulation. Mol Cell. 2006;21(6):799–809. doi: 10.1016/j.molcel.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 33.Jurutka PW, Hsieh JC, Remus LS, et al. Mutations in the 1,25-dihydroxyvitamin D3 receptor identifying C-terminal amino acids required for transcriptional activation that are functionally dissociated from hormone binding, heterodimeric DNA binding, and interaction with basal transcription factor IIB, in vitro. J Biol Chem. 1997;272(23):14592–14599. doi: 10.1074/jbc.272.23.14592. [DOI] [PubMed] [Google Scholar]
- 34.Jimenez-Lara AM, Aranda A. Lysine 246 of the vitamin D receptor is crucial for ligand-dependent interaction with coactivators and transcriptional activity. J Biol Chem. 1999;274(19):13503–13510. doi: 10.1074/jbc.274.19.13503. [DOI] [PubMed] [Google Scholar]
- 35.Jurutka PW, Lamb TL, Dominguez CE, et al. Molecular Analysis of Helix-1 and Helix-3 in the Human Vitamin D Receptor Reveals Functional Subdomains Mediating Hormone Binding, Protein Stabilization, Heterodimerization and Interaction with Coactivators. JBMR. 2001;16(Suppl 1):S229. [Google Scholar]
- 36.Jurutka PW, Thompson PD, Whitfield GK, et al. Molecular and functional comparison of 1,25-dihydroxyvitamin D(3) and the novel vitamin D receptor ligand, lithocholic acid, in activating transcription of cytochrome P450 3A4. J Cell Biochem. 2005;94(5):917–943. doi: 10.1002/jcb.20359. [DOI] [PubMed] [Google Scholar]
- 37.Bazzi H, Fantauzzo KA, Richardson GD, Jahoda CA, Christiano AM. The Wnt inhibitor, Dickkopf 4, is induced by canonical Wnt signaling during ectodermal appendage morphogenesis. Dev Biol. 2007;305(2):498–507. doi: 10.1016/j.ydbio.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 38.Morin PJ, Sparks AB, Korinek V, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275(5307):1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 39.Abbas S, Nieters A, Linseisen J, et al. Vitamin D receptor gene polymorphisms and haplotypes and postmenopausal breast cancer risk. Breast Cancer Res. 2008;10(2):R31. doi: 10.1186/bcr1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karami S, Brennan P, Hung RJ, et al. Vitamin D receptor polymorphisms and renal cancer risk in Central and Eastern Europe. J Toxicol Environ Health A. 2008;71(6):367–372. doi: 10.1080/15287390701798685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hubner RA, Muir KR, Liu JF, Logan RF, Grainge MJ, Houlston RS. Dairy products, polymorphisms in the vitamin D receptor gene and colorectal adenoma recurrence. Int J Cancer. 2008;123(3):586–593. doi: 10.1002/ijc.23536. [DOI] [PubMed] [Google Scholar]
- 42.Clendenen TV, Arslan AA, Koenig KL, et al. Vitamin D receptor polymorphisms and risk of epithelial ovarian cancer. Cancer Lett. 2008;260(1–2):209–215. doi: 10.1016/j.canlet.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slattery ML, Wolff RK, Herrick JS, Caan BJ, Potter JD. IL6 genotypes and colon and rectal cancer. Cancer Causes Control. 2007;18(10):1095–1105. doi: 10.1007/s10552-007-9049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lointier P, Wargovich MJ, Saez S, Levin B, Wildrick DM, Boman BM. The role of vitamin D3 in the proliferation of a human colon cancer cell line in vitro. Anticancer Res. 1987;7(4B):817–821. [PubMed] [Google Scholar]
- 45.Pendas-Franco N, Garcia JM, Pena C, et al. DICKKOPF-4 is induced by TCF/beta-catenin and upregulated in human colon cancer, promotes tumour cell invasion and angiogenesis and is repressed by 1alpha, 25-dihydroxyvitamin D3. Oncogene. 2008;27(32):4467–4477. doi: 10.1038/onc.2008.88. [DOI] [PubMed] [Google Scholar]
- 46.Xi Y, Formentini A, Nakajima G, Kornmann M, Ju J. Validation of biomarkers associated with 5-fluorouracil and thymidylate synthase in colorectal cancer. Oncol Rep. 2008;19(1):257–262. [PubMed] [Google Scholar]
- 47.Aguilera O, Fraga MF, Ballestar E, et al. Epigenetic inactivation of the Wnt antagonist DICKKOPF-1 (DKK-1) gene in human colorectal cancer. Oncogene. 2006;25(29):4116–4121. doi: 10.1038/sj.onc.1209439. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez-Sancho JM, Aguilera O, Garcia JM, et al. The Wnt antagonist DICKKOPF-1 gene is a downstream target of beta-catenin/TCF and is downregulated in human colon cancer. Oncogene. 2005;24(6):1098–1103. doi: 10.1038/sj.onc.1208303. [DOI] [PubMed] [Google Scholar]
- 49.McKenna NJ, O’Malley BW. Minireview: nuclear receptor coactivators--an update. Endocrinology. 2002;143(7):2461–2465. doi: 10.1210/endo.143.7.8892. [DOI] [PubMed] [Google Scholar]
- 50.D’Errico I, Moschetta A. Nuclear receptors, intestinal architecture and colon cancer: an intriguing link. Cell Mol Life Sci. 2008;65(10):1523–1543. doi: 10.1007/s00018-008-7552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin R, White JH. The pleiotropic actions of vitamin D. Bioessays. 2003;26(1):21–28. doi: 10.1002/bies.10368. [DOI] [PubMed] [Google Scholar]
- 52.Rochel N, Moras D. Ligand binding domain of vitamin D receptors. Curr Top Med Chem. 2006;6(12):1229–1241. doi: 10.2174/156802606777864926. [DOI] [PubMed] [Google Scholar]
- 53.Shah S, Hecht A, Pestell R, Byers SW. Trans-repression of beta-catenin activity by nuclear receptors. J Biol Chem. 2003;278(48):48137–48145. doi: 10.1074/jbc.M307154200. [DOI] [PubMed] [Google Scholar]
- 54.Song LN, Gelmann EP. Interaction of beta-catenin and TIF2/GRIP1 in transcriptional activation by the androgen receptor. J Biol Chem. 2005;280(45):37853–37867. doi: 10.1074/jbc.M503850200. [DOI] [PubMed] [Google Scholar]
- 55.Ochs-Balcom HM, Cicek MS, Thompson CL, et al. Association of Vitamin D Receptor Gene Variants, Adiposity and Colon Cancer. Carcinogenesis. 2008 doi: 10.1093/carcin/bgn166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ingles SA, Wang J, Coetzee GA, Lee ER, Frankl HD, Haile RW. Vitamin D receptor polymorphisms and risk of colorectal adenomas (United States) Cancer Causes Control. 2001;12(7):607–614. doi: 10.1023/a:1011292002475. [DOI] [PubMed] [Google Scholar]
- 57.Grau MV, Baron JA, Sandler RS, et al. Vitamin D, calcium supplementation, and colorectal adenomas: results of a randomized trial. J Natl Cancer Inst. 2003;95(23):1765–1771. doi: 10.1093/jnci/djg110. [DOI] [PubMed] [Google Scholar]
- 58.Li C, Li Y, Gao LB, et al. Vitamin D Receptor Gene Polymorphisms and the Risk of Colorectal Cancer in a Chinese Population. Dig Dis Sci. 2008 doi: 10.1007/s10620-008-0375-y. [DOI] [PubMed] [Google Scholar]
- 59.Slattery ML, Yakumo K, Hoffman M, Neuhausen S. Variants of the VDR gene and risk of colon cancer (United States) Cancer Causes Control. 2001;12(4):359–364. doi: 10.1023/a:1011280518278. [DOI] [PubMed] [Google Scholar]
- 60.Murtaugh MA, Sweeney C, Ma KN, et al. Vitamin D receptor gene polymorphisms, dietary promotion of insulin resistance, and colon and rectal cancer. Nutr Cancer. 2006;55(1):35–43. doi: 10.1207/s15327914nc5501_5. [DOI] [PubMed] [Google Scholar]
- 61.Shah S, Pishvaian MJ, Easwaran V, Brown PH, Byers SW. The role of cadherin, beta-catenin, and AP-1 in retinoid-regulated carcinoma cell differentiation and proliferation. J Biol Chem. 2002;277(28):25313–25322. doi: 10.1074/jbc.M203158200. [DOI] [PubMed] [Google Scholar]