Abstract
GATA-3 is necessary for the development of MHC II-restricted CD4 T cells, and its expression is increased during positive selection of these cells. TcR signals drive this upregulation, but the signaling pathways that control this process are not well understood. Using genetic and pharmacological approaches we show that GATA-3 upregulation during thymocyte positive selection is the result of additive inputs from the Ras/MAPK and Calcineurin pathways. This upregulation requires the presence of the transcription factor c-Myb. Furthermore, we show that TH-POK can also upregulate GATA-3 in double positive thymocytes, suggesting the existence of a positive feedback loop that contributes to lock in the initial commitment to the CD4 lineage during differentiation.
INTRODUCTION
During T cell development, the organism generates a T cell population with an extended repertoire of antigen specificities. This repertoire is molded in the thymus through signals derived from the interaction of T cell receptors (TCRs) on thymocytes with their ligands, major histocompatibility complex (MHC) molecules with bound peptides. The majority of thymocytes bear TCRs that do not recognize the MHC molecules present in the thymus, and these cells die relatively rapidly (3–4 days). Those cells bearing a TCR able to interact with self-MHC can receive signals that induce either their differentiation into mature T cells (positive selection) or apoptosis (negative selection). Furthermore, those cells that are positively selected develop into two different lineages, CD4 or CD8, depending on the ability of their TCRs to bind MHC class II or I, respectively. The mechanisms that underlie this lineage commitment process have been extensively studied (reviewed in (1, 2)), and although there is not yet a complete understanding of this process, a number of critical players have been identified in the last few years. These include signal transduction molecules like Lck(3), and transcription factors, including GATA3, Th-POK, Runx-1 and Tox(4–9).
GATA3 is a member of the GATA family of transcription factors expressed by immune cells, from hematopoietic stem cells to mature T cells (10, 11). GATA3 is required at multiple stages during T cell development and differentiation, from T cell commitment (12–14), to Th2 differentiation (15–17). During the process of positive selection and CD4/CD8 lineage commitment GATA-3 is upregulated by TCR mediated signals (4), and is required for the generation of CD4 T cells (4, 5).
The molecular mechanisms that control GATA-3 upregulation during positive selection are unknown, although our previous results showed that it was correlated to the strength of the TCR signal, and the contribution of Lck to this signal (4). The only other known factor that contributes to the regulation of GATA-3 expression at this stage is the transcription factor c-Myb (18). In this manuscript we explore the contributions of different signal transduction pathways to TCR-mediated GATA-3 upregulation, as well as the role of c-Myb and TH-POK in this process. Our results show that a) the upregulation of GATA-3 in DP thymocytes is mediated by Ras/MAPK and Calcineurin, and these pathways act in an additive fashion, b) c-Myb is required for TCR-mediated induction of GATA-3, although signaling is normal in c-Myb-deficient DP thymocytes, and c) Th-POK can up-regulate GATA-3 in DP thymocytes.
MATERIAL AND METHODS
Mice
C57BL/6 (B6), MHC−/− (C57BL/6 β2-microglobulin−/−, I-Aβ−/− I-E-null), dnRas(19) and dLGF(3) mice were bred and maintained in the mouse facility at OMRF. Gata-3-GFP knock-in mice have been described (20). They were bred into the MHC−/− background (G3/MHC°). c-Mybf/fcd4Cre were obtained from Dr. Bender (21, 22) Adult mice used were between the ages of 6 and 12 weeks of age.
Retroviral constructs
The MIG (MSCV-IRES-GFP) bicistronic retroviral vector has been previously described (23, 24). MIR (MSCV-IRES-RFP) is a variant generated in our laboratory where GFP has been replaced by dsRed(25). The LckF505 cDNA was provided by Dr. D. Straus, RasV12 by Dr. J. Downward, GST-VIVIT and CA-NFAT by Dr. F. Macian, constitutively active Akt by Dr. D. Cantrell, constitutively active PDK by Dr. A. Weiss, constitutively active Ikkβ by Dr. J. Pomerantz. All cDNAs were cloned in MIR using conventional molecular biology techniques. ThPOK and ThPOK-HD retroviruses were provided by Dr. D. Kappes.
Retroviral production and thymocyte infection
Retroviral constructs in the MIR vector were co-transfected along with the pCL/Eco plasmid into 293 cells as described. For viral transduction, 1 to 2 ×106 thymocytes were mixed with 2 ml of retroviral supernatant plus 20μg/ml Polybrene per well in 24-well plates and centrifuged for 1 hr, at 210 × g, RT, as described (26). Supernatant was replaced with fresh media. and the cells used for experiments 24–48 hours later. In some experiments thymocytes were cultured over OP9Δ cells (27). The efficiency of the infection varied with the construct, oscillating between 3% and 20%. Survival at the time of analysis was generally around 50%
DP thymocyte enrichment
Petri dishes were coated with anti-Rat IgG + IgM antibody (Jackson Immuno Research) in PBS pH 8 (10 mg/ml) for 2h at 37°C or O/N at 4°C. Thymocytes were incubated for 30 min on ice with rat anti-CD53 (clone OX-79, BD Pharmigen) at saturating concentrations (10 mg/ml). Cell were rinsed once resuspended in RPMI and incubated in the coated plates for 1h at 4°C. The negative fraction (CD53−) was recovered by aspiration without disturbing the adherent cells. More than 90% of the recovered cells were DP population, as determined by FACS using CD4 and CD8 markers.
In vitro stimulation of thymocytes
Thymocytes from MHC−/− mice, or enriched DP thymocytes from other strains, were stimulated on anti-CD3 (clone 2C11; 10 μg/ml) (BD Biosciences or eBiosciences) coated plates or with soluble anti-CD4/CD3 F(ab′)2 antibodies (0.2–1 μg/ml) (4, 28). Cells were harvested at the times indicated and counted. In some experiments thymocytes were stimulated overnight with 5 or 10 ng/ml of PDBu and/or 200 or 500 ng/ml Ionomycin.
Intracellular staining
Cells were fixed with 2% paraformaldehyde (Pella) for 10 min at 37°C, washed in P4F (PBSx1, 4% FCS, 0.1% sodium azide). Then cells were permeabilized with 1% Triton X-100 (Calbiochem) in PBS for 15 min at room temperature (RT), washed twice with 1ml P4F and stained with an Alexa Fluor® 647-conjugated anti Gata-3 antibody (eBiosciences) for 1h at RT. Cells were washed and analyzed by flow cytometry.
Real-time RT-PCR
DP thymocytes from Mybf/wcd4Cre and Mybf/f cd4Cre were stimulated overnight and lysed. Total RNA was extracted using Qiagen RNeasy mini kit according to the manufacturer’s protocol. Reverse transcription (RT) was performed on 2μg of RNA using Taqman reverse transcription reagents (Applied Biosystems). 10ng of cDNA was used for each assay. SYBR Green real time PCR was performed using a Biorad CFX96 Real-Time PCR detection system. Gene specific forward (F) and reverse (R) primers were designed across exon-exon junction using Primer Express software (Applied Biosystems) with equivalent efficiency to the β-actin primers. Data were analyzed using the comparative CT method using CFX software (BioRad). The primers used were EGR1 Fw: 5′-AGT GGG AGC CGG CTG GAG AT-3′ RV:5′-GGG CGA TGT AGC AGG TGC GG-3′; EGR2 FW: 5′-TTG ACC AGA TGA ACG GAG TGG-3′ RV 5′-TGC CCA TGT AGG TGA AGG TCT-3′; NUR77 FW: 5′-AGT GGG AGC CGG CTG GAG AT-3′ RV 5′-GGG CAG TGT AGC AGG TGC GG-3′;β-Actin FW 5′-CAA CGA GCG GTT CCG ATG -3′ RV 5′-GCC ACA GGA TTC CAT ACC CA-3′
RESULTS
Use of a GATA-3-GFP “knock-in” allele to follow GATA-3 expression
As a way to follow GATA-3 upregulation at a single cell level we used a genetically modified strain of mice that have GFP knocked into the GATA-3 locus, so that GFP is driven by the endogenous GATA-3 regulatory regions (20). As shown in Fig 1A, the expression levels of GFP in different thymocyte subpopulations correlate perfectly with the expression pattern of GATA-3, as analyzed by RT-PCR, western blot (4), or a lacZ knock-in (29). For most of the experiments described in this manuscript, the GATA3-GFP mice were bred into an MHC null background (G3/MHC°) to ensure that DP thymocytes have not received any signals through their TCR in vivo.
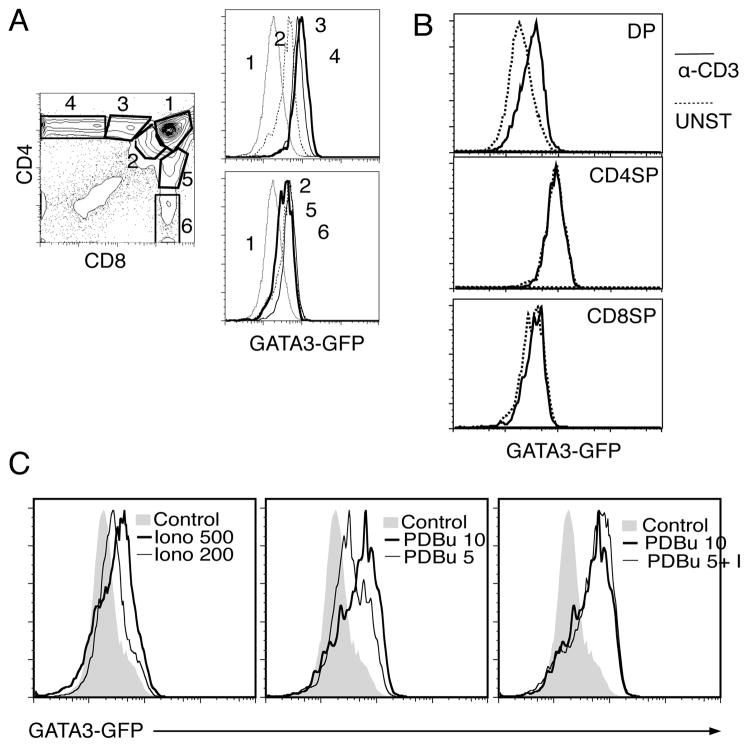
Figure 1.
A. Expression pattern of the GATA3-GFP reporter in different thymocyte subpopulations. Thymocytes from a GATA3-GFP mouse were stained with CD4 and CD8 and GA-TA3-GFP expression analyzed in different subpopulations (1 to 6). The two color histogram shows the different gates, and the histograms show expression within the CD4 development series (1, 2, 3, 4) (top) or CD8 development series (1, 2, 5, 6) (bottom). B. Induction of GATA-3 by TCR signals in different thymic subpopulations. GATA-3 GFP mice were stimulated with plate -bound α-CD3 (10μg/ml) and 18h later stained with CD4 and CD8. Shown are histograms of GATA-3-GFP expression in gated double positive (DP), CD4 single positive (CD4SP) and CD8 single positive (CD8SP) thymocytes, unstimulated (------) and stimulated (____) C. Pharmacological up-regulation of Gata-3. DP G3/MHC° thymocytes were stimulated overnight with different doses of PDBu or Ionomycin, alone or in combination, as indicated. After overnight incubation, Gata-3 levels (GFP) were analyzed by flow cytometry. Shown is one representative experiment out of three.
TCR-regulation of GATA-3 is specific for DP thymocytes
Our previous results had shown that in DP thymocytes TCR stimulation was sufficient to induce maximal GATA-3 upregulation. In contrast, in naive CD4 T cells TCR stimulation alone can only weakly induce GATA-3 transcription, and additional signals mediated by IL-4 are required for maximal induction (30). Using the GATA-3-GFP mice we decided to compare the responsiveness of GATA-3 to TCR crosslinking in different thymocyte subpopulations. Total thymocytes were stimulated on plate-bound α-CD3 for 18h and GATA-3 induction in different subpopulations followed using GFP as a readout. As shown in Fig 1B, DP are the only subpopulation that responds significantly to this stimulus, suggesting that GATA-3 regulation is different in DP vs. more mature T cell populations.
Gata-3 can be upregulated in DP thymocytes by phorbol esters and calcium ionophores
As a first approximation to the signal transduction pathways involved in this process, we decided to use pharmacological agents that are known to activate two of the main signal transduction pathways in T cells, the Ras/MAPK cascade (the phorbol ester PDBu), and intracellular calcium (Ionomycin, a calcium ionophore). GATA3-GFP/MHC° thymocytes were treated with different concentrations of PDBu or Ionomycin, and GATA-3 upregulation was detected 18 hours later by monitoring GFP by flow cytometry. Our results (Fig 1C) show that both PDBu and Ionomycin up-regulate Gata-3 expression in a dose depending manner. Furthermore, a combination of a low dose of both pharmalogical products act additively. These experiments suggested that both Ras/MAPK and calcium are involved in the TCR-induced upregulation of GATA-3 in DP thymocytes.
Activated Lck maximally induces GATA-3 in DP thymocytes
Our previous work showed that in DP thymocytes Gata-3 is upregulated in vitro by TCR signals (4), and suggested that signals that have a stronger Lck component were able to induce a stronger and more sustained upregulation. To test directly the involvement of Lck in GATA-3 upregulation we used a constitutively active form of Lck, LckY505F, cloned in a modified version of the MiG retroviral vector that expresses DsRed instead of GFP (MIR). Thymocytes from Gata3-GPF/MHC° mouse were infected in vitro, cultured over OP9Δ cells and analyzed 48h later by flow cytometry. Culture over OP9Δ improves the viability of the cells, but does not affect the levels of GATA3-GFP in the cultures (data not shown). Analysis was performed by gating on DsRed+ (infected cells expressing the LckY505F) and DsRed− cells (uninfected cells) and comparing the GFP levels. Expression of CD69 was analyzed as a control of the activation. As a negative control the cells were infected with empty MiR virus. As shown in fig 2A, DsRed+ have higher levels of GFP than the DsRed−. Furthermore, the induction level was almost as high as that induced by plate-bound CD3 after 24h. Since activation of Lck is a direct consequence of the binding of CD4/CD8 co-receptors with the MHC molecules, and CD4 co-receptor binds higher amount of Lck, this result is consistent with the idea that CD4 engagement by class II MHC molecules induces higher levels of GATA-3.
Figure 2.
A. Up-regulation of Gata-3 with constitutive active forms of Lck (LckF505), Ras (RasV12) NFAT (CA-NFAT). DP thymocytes from G3/MHC° mice were infected with the indicated constructs cloned in MIR vector, or with empty MIR. 48 hours later the thymocytes were analyzed by flow cytometry, gating on dsRed+ and dsRed−. As a positive control of GATA-3 induction, an aliquot of DP thymocytes was cultured with plate-bound αCD3 (10 μg/ml) overnight. The histograms are representative of 3–5 independent experiments for each construct. B. The same samples were analyzed for CD69 expression. C. Comparison of the effects of constitutive active Lck with other activated mutants in different signal transduction pathways. The experiments were performed as described above, at least three independent times for each construct.
Both Ras and Calcineurin can partially induce GATA-3 in DP thymocytes
To directly test whether the Ras/MAPK pathway could drive Gata-3 up-regulation in DP thymocytes we used a constitutively active Ras mutant (RasV12) cloned into the MIR vector in experiments similar to those described above. As shown in fig 2A, expression of RasV12 can upregulate GATA-3 in DP thymocytes although, contrary to what we observed with the active Lck, the magnitude of this effect was clearly inferior to that induced by direct TCR crosslinking.
Since the best characterized calcium effector during T cell activation is the calcineurin/NFAT pathway, we also tested whether a constitutively active NFAT was sufficient to induce GATA-3 in DP thymocytes using a similar approach. As shown in fig 2A, overexpression of CA-NFAT results also in the upregulation of GATA-3, and, as observed with RasV12, this effect is less pronounced than the upregulation induced by CD3. These data suggest that both pathways are implicated in the Gata-3 up-regulation after TCR stimulation, and also that both pathways have an additive effect on that up-regulation. It must be noted that both mutants can induce maximal levels of other activation markers, such as CD69.
In contrast with the effects of Ras and Calcineurin, constitutive expression of activated mutants in other signal transduction pathways (Akt, PDK, Stat5, IKKβ) did not induce major changes in GATA-3 expression (Fig 2B).
Blockade of the Ras/MAPK pathway and Calcineurin can partially inhibit TCR-induced GATA-3 upregulation in DP thymocytes
In order to confirm the implication of Ras/MAPK and Calcineurin in TCR-induced GATA-3 upregulation, we used a loss of function approach. To study the Ras/MAPK pathway we crossed the GATA3-GFP/MHC° mice to a transgenic line that expresses high levels of a dominant negative form of Ras (RasN17) (19). We stimulated thymocytes from these mice in vitro with increasing doses of αCD3/CD4 bispecific F(ab′)2 and analyzed GFP expression by flow cytometry 18h later. As shown in fig 3A,B, Gata-3 expression (GFP expression) was less up-regulated in the cells expressing dnRas, and this blockade could not be overcome by increasing doses of the antibody (Fig 3C). This partial blocking of Gata-3 up-regulation in the dnRas cells, together with the result with the RasV12 protein, confirm the implication of this Ras pathway in the Gata-3 up-regulation after TCR stimuli.
Figure 3.
Blocking of the Ras pathway partially inhibits TCR-induced Gata-3 up-regulation. A DP thymocytes from dnRas/G3/MHC° mice or G3/MHC° littermates (NLC) were stimulated overnight with 0.5 μg/ml αCD3/CD4 F(ab′)2 or 10 ng/ml of PDBu, and changes in GATA-3 transcription monitored by flow cytometry. B Quantification of the changes in GFP MFI in three independent experiments. C DP thymocytes from dnRas/G3/MHC° mice or G3/MHC° littermates (NLC) were stimulated overnight with increasing concentrations of αCD3/CD4 F(ab′)2, and changes in GATA-3 transcription monitored by flow cytometry (n=3). D. DP thymocytes from G3/MHC° were stimulated overnight with 0.5 μg/ml αCD3/CD4 F(ab′)2 in the presence of decreasing concentrations of U0126 (10, 3, 1, 0.3 and 0 μM). GFP and CD69 expression was monitored by flow cytometry. Shown is the average and SEM of three experiments.
One of the molecules activated downstream of Ras is the Erk MAPK kinase, MEK. We used U0126, a MEK specific inhibitor, to confirm that the Ras/MEK/ERK pathway is implicated in the up-regulation of Gata-3 after TCR stimuli. Thymocytes from GATA3-GFP/MHC° mice were stimulated over night with 0.5 μg/ml αCD3/CD4 F(Ab′)2 plus increasing concentrations of U0126 inhibitor. CD69 was used as a control of cell activation. Gata-3 up-regulation was only partially inhibited by U0126, even at supraoptimal concentrations (Fig 3D), confirming that the Ras/MAPK pathway is implicated in GATA-3 upregulation downstream the TCR, but also that it is responsible only for part of the response.
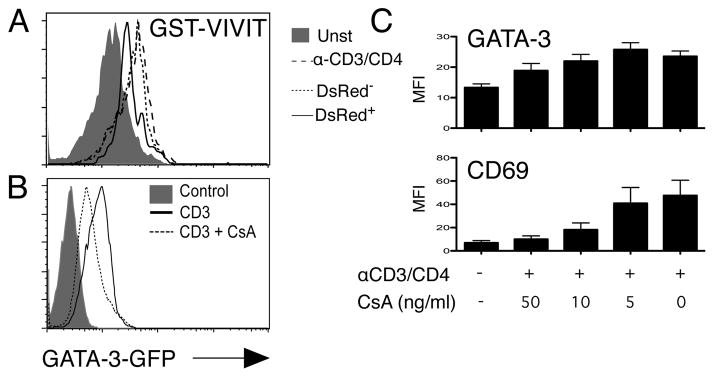
The other pathway implicated in Gata-3 up-regulation in our gain of function experiments, is CaN and NFAT. To study the effects of loss of function of this pathway on Gata-3 response to TCR stimuli, we first used a genetic approach, overexpressing the GST fusion protein GST-VIVIT, which competes with CaN for NFAT (31), using our MIR vector. Thymocytes from GA-TA3-GFP/MHC° were infected with MIR-GST-VIVIT, cultured overnight over OP9Δ and then stimulated for 18h with αCD3/CD4 F(ab′)2. Analysis was performed by gating on DsRed+ and DsRed− cells and comparing the GFP levels in unstimulated and stimulated samples. As shown in fig 4A, thymocytes expressing GST-VIVIT (DsRed+) had a partial block in Gata-3 up-regulation. These results were confirmed using a pharmacological approach with the CaN inhibitor CsA, which also partially blocked this up-regulation in a dose dependent manner (Fig. 4B,C). As observed with the blockade of the Ras/MAPK pathway, we could not completely block TCR-induced GATA-3 upregulation with VIVIT, or with supraoptimal doses of CsA, even though they were sufficient to block other activation markers, such as CD69 (Fig. 4C).
Figure 4.
Blocking of the CaN/NFAT pathway partially inhibits TCR-induced Gata-3 up-regulation. A. DP thymocyte from G3/MHC° mice were infected with GST-VIVIT cloned into MIR. 36h later, the thymocytes were stimulated overnight with plate-bound αCD3, collected and analyzed by flow cytometry. The GFP expression levels of DsRed+ or DsRed− were compared. Uninfected G3/MHC°, stimulated or not overnight were used as a positive and negative control respectively. Shown is one representative experiment out of three B. DP G3/MHC° thymocytes were stimulated overnight with 0.5 μg/ml αCD3/CD4 F(ab′)2 in the presence of CsA (50 ng/ml). Some cells were left unstimulated. Cells were harvested and analyzed by flow cytometry. C. DP G3/MHC° thymocytes were stimulated overnight with 0.5 μg/ml αCD3/CD4 F(ab′)2 in the presence of different concentrations of CsA (50, 10, 3 and 0 ng/ml). Some cells were left unstimulated. Cells were harvested, stained with CD69, and analyzed by flow cytometry. Shown is the average and SEM of three experiments.
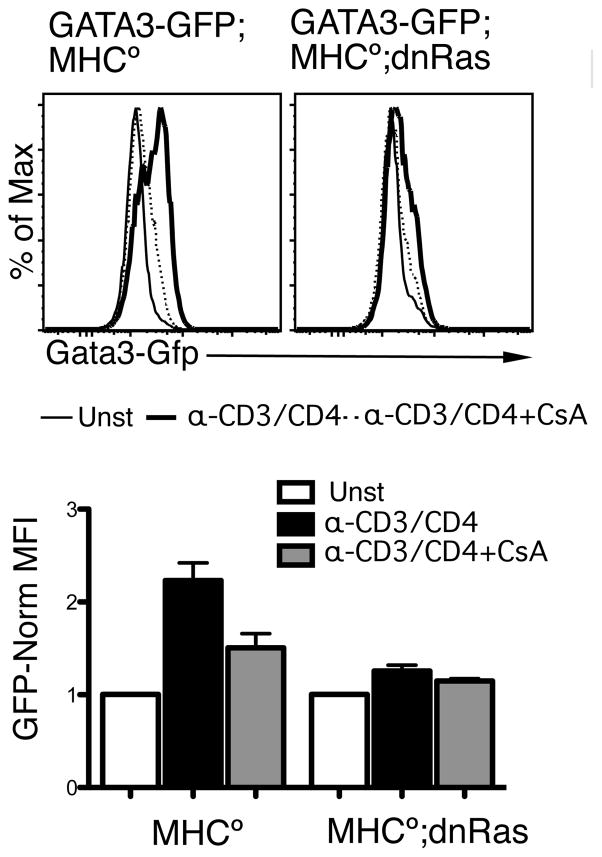
Since these results, and those presented in Fig 1C, suggest that both Ras and CaN partially control TCR-mediated GATA-3 upregulation in DP thymocytes, we tested whether simultaneously blocking both pathways would be sufficient to completely block it. For these experiments we used DP thymocytes from dnRas/MHC°/GATA-3GFP and MHC°/GATA-3GFP mice, and stimulated them in vitro with α-CD3 in the presence or absence of CsA (50 ng/ml). As shown in Fig 5, the combination of the dnRas transgene plus CsA almost completely abrogated CD3-induced GATA-3 upregulation, suggesting that the combined input of these two pathways is responsible for most of the upregulation.
Figure 5.
Simultaneous blocking of the CaN/NFAT and Ras pathways blocks TCR-induced Gata-3 up-regulation. DP thymocyte from G3/MHC° mice or G3/MHC°/dnRas mice were stimulated overnight with 0.5 μg/ml αCD3/CD4 F(ab′)2 in the presence or absence of CsA (50 ng/ml), collected and analyzed by flow cytometry. The GFP expression levels were compared. Shown is the average and SEM of three experiments. A shows a representative histogram of the induction, and B shows the quantification of three independent experiments with a pair of G3/MHC° and G3/MHC°/dnRas mice per experiment.
The effect TCR on GATA-3 expression requires the presence of c-Myb
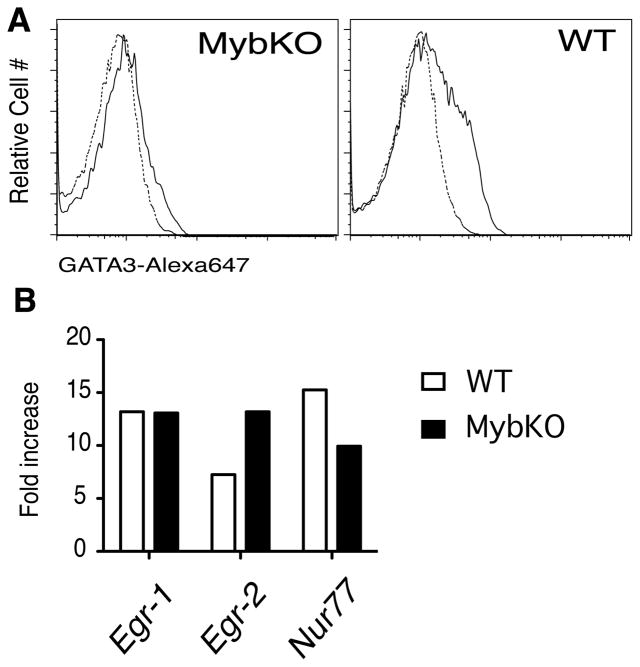
c-Myb is important for CD4 generation, although the loss of CD4 single positive cells is less dramatic in c-Mybf/fcd4Cre mice than in GATA-3f/fcd4Cre mice (21, 22). It has been suggested that this is due to a direct regulation of GATA-3 by c-Myb (18), and, in our hands, overexpression of GATA-3 can rescue the CD4 defect in c-Mybf/fcd4Cre mice (TH and JAI, unpublished data). Therefore, we decided to test whether TCR stimulation could upregulate GATA-3 in c-Mybf/fcd4Cre thymocytes. For these experiments we bred our GATA-3-GFP mice to c-Mybf/fcd4Cre mice, and used enriched CD53− DP thymocytes for the stimulations. Overnight stimulation of GATA-3GFP/c-Mybf/fcd4Cre thymocytes with αCD3/CD4 F(ab′)2 did not induce any increase in GFP expression (Fig 5A) suggesting that in the absence of c-Myb the GATA-3 locus is refractory to stimulation. This is not due to effects of c-Myb on the signal transduction cascades downstream the TCR, because well-characterized downstream effectors the Ras/MAPK (Egr-1) (32), Calcium (Nur77) (33) or MAPK and Calcineurin (Egr-2) (32) are upregulated normally in c-Myb-deficient DP thymocytes (Fig 5B). These results support the idea that c-Myb is an important component of the circuit that regulates GATA-3 expression during positive selection, and that its presence is required to render the GATA-3 locus permissive to TCR induction (18).
Overexpression of Th-POK in DP thymocytes can drive GATA-3 expression
GATA-3 upregulation during CD4 lineage differentiation is sustained, and mature CD4 SP cells express significantly higher levels of GATA-3 than DP or CD8 SP. It is therefore likely that additional factors besides the TCR signals contribute to the regulation of GATA-3 expression during this process. We were interested in a possible role for Th-POK because, like GATA-3, this transcription factor is needed for CD4 commitment and it is up-regulated by TCR signals at later stages than GATA-3 (34). To test whether Th-POK expression could affect GATA-3, we infected 15.5 day old fetal thymocytes from MHC° mice with a vector expressing Th-POK or with empty vector. After 72h of culturing the cells over OP9Δ, they were permeabilized, stained for Gata-3 and analyzed by flow cytometry. Cells infected with Th-POK increased the level of Gata-3 in comparison with uninfected cells, indicating that Th-POK can up-regulate Gata-3 by itself in DP thymocytes. In contrast, we did not observe GATA-3 upregulation in cells infected with the natural point mutation in Th-POK observed in HD mice (6, 35). Similar results were obtained using adult DP cells from MHC° mice.
DISCUSSION
Our data show that GATA-3 expression during CD4/CD8 lineage differentiation is tightly regulated, and controlled at different levels. First, even though GATA-3 is expressed at low levels in pre-selection DP thymocytes, and this basal expression is mostly independent of c-Myb, its induction by TCR signals requires the presence of c-Myb. The mechanism that underlies this requirement is unknown, although our results suggest that it is not related to effects of c-Myb on TCR signaling. It is possible that c-Myb control accessibility of the GATA-3 locus, since it has been shown by chromatin immunoprecipitation that c-Myb is associated to the GATA-3 promoter in DP thymocytes (18). Second, induction of GATA-3 by TCR signals is mediated by at least two different signal transduction pathways downstream of the TCR and Lck, the Ras/MAPK pathway, and Calcineurin. Interestingly, these inputs seem to act additively, and independently. Neither pathway is absolutely required for induction, and neither is able by itself to achieve maximal induction, but when both are simultaneously inhibited TCR-induced GATA-3 upregulation is almost completely blocked. Therefore, GATA-3 transcription functions as an integrative readout of the total signaling output of the TCR and coreceptor-Lck complex, so that stronger and/or more sustained signals result in higher expression of GATA-3, driving those cells towards the CD4 fate. Third, our data show that TH-POK is able to induce GATA-3 expression in DP thymocytes by itself. This observation could have important implications for our understanding of the molecular mechanisms that control CD4 lineage differentiation. Normally, GATA-3 upregulation precedes Th-POK induction, and, in GATA-3-deficient mice, thymocytes are blocked prior to the stage where Th-POK can be induced (5, 36, 37), suggesting that one role of GATA-3 is to drive development up to the stage when Th-POK becomes inducible (2). However, GATA-3 has additional roles in this process, as shown by the fact that overexpression of Th-POK fails to rescue development of CD4 cells in GATA-3-deficient animals (37). This suggests that GATA-3 expression needs to be sustained at high levels during CD4 lineage differentiation. Our results indicate that Th-POK may be important for this maintenance, and that this positive feedback loop may contribute to the irreversibility of the commitment decision, since high levels of GATA-3 expression are incompatible with development into the CD8 lineage (4). However Th-POK is not required for sustained GATA-3 expression in mature CD4 T cells (38), and the population where our experiments have been performed (pre-selection DP thymocytes) is not the exact population where Th-POK effects on GATA-3 expression would occur (CD69+ post-selection CD4+CD8lo thymocytes). Additional experiments will be necessary to confirm that in vivo Th-POK plays a role in the regulation of GATA-3 during the differentiation of CD4 T cells in the thymus.
It is also interesting to contrast what we have learned about the control of GATA-3 expression during CD4/CD8 lineage differentiation with its regulation during differentiation of naïve CD4 T cells into T helper 2 (Th2) cells. The Gata3 gene encodes two transcripts with alternative un-translated first exons, 1a and 1b (30), and the transcription of each one is driven by independent promoters. The proximal promoter drives expression of the transcript containing the exon 1b and is selectively active in thymus, while the distal promoter, which drives the expression of exon 1a, is active in the brain and during Th2 differentiation. Stimulation of naïve CD4+ T cells with anti-CD3 and anti-CD28 antibodies weakly induces exon 1b transcripts, while costimulation with IL-4 strongly induces both 1b and 1a transcripts, and is critical for Th2 differentiation (30). In contrast, in DP thymocytes TCR stimulation by itself maximally activates GATA-3 transcription (4). Furthermore, the distal promoter has binding sites for, and is responsive to Notch, while the proximal promoter is not (39, 40). Accordingly, overexpression of Notch does not alter GATA-3 expression in DP thymocytes (GHH, JAI. unpublished results). Furthermore control of GATA-3 levels in Th2 cells is also regulated by post-transcriptional mechanisms, such as phosphorylation by p38 (41, 42) or Erk (43), and ubiquitination (43), while in thymocytes undergoing positive selection and lineage commitment protein expression seems to correlate with transcriptional activity (4). Additional studies will be necessary to discard the possibility that these post-translational mechanisms play a role in the regulation of GATA-3 levels during CD4 lineage differentiation in the thymus, since our current study was mostly focused on transcriptional readouts. However, it is interesting to point out that in Th2 cells inhibition of activation of Ras/MAPK cascade by PD98059 or a dnRas transgene had no blocking effect on GATA3 mRNA expression, but altered protein stability by increasing its ubiquitination (43). In contrast, our results demonstrate that in DP thymocytes the Ras/MAPK directly controls transcription of the Gata3 gene.
Figure 6.
c-Myb is required for TCR-induced GATA-3 up-regulation. A. Enriched DP thymocytes from c-Mybf/fcd4Cre (MybKO) or c-Mybf/wcd4Cre (WT) thymocytes were stimulated over-night with αCD3/CD4 F(ab′)2 (1 μg/ml), and analyzed for GATA-3 expression by by flow cytometry. The histogram shows GFP expression in unstimulated (----) vs. stimulated (____) thymocytes. Shown is one representative experiment out of three. B. Enriched DP thymocytes from c-Mybf/fcd4Cre (MybKO) or c-Mybf/wcd4Cre (WT) mice were stimulated for three hours with plate-bound (10 μg/ml). Induction of Egr-1, Egr-2 and Nur77 was assessed by quantitative RT-PCR and fold increase versus the unstimulated cells calculated by normalizing to β-actin. Shown are the results of one representative experiment out of two.
Figure 7.
Th-POK up-regulates Gata-3 in DP cells. 15.5 days old fetal DP cells from MHC° mice were infected with MigThPOK or Mig-HD. 24h later cells were permeabilized and stained against Gata-3. Histogram shows Gata-3 protein expression in uninfected (----) or infected (____) thymocytes. Shown is one representative experiment out of three.
Acknowledgments
The authors thank Dr. D. Straus, Dr. J. Downward, Dr. F. Macian, Dr. D. Cantrell, Dr. A. Weiss, Dr. J. Pomerantz, Dr. D. Kappes, and Dr. T Bender for providing reagents and animals, and S. Kovats (OMRF) for discussions.
Footnotes
This work was supported by grants AI059302 (NIH/NIAID) and 0855002F (AHA) to JAI. IG was supported in part by a grant from the Spanish Education and Science Department.
References
- 1.Singer A, Adoro S, Park JH. Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat Rev Immunol. 2008;8:788–801. doi: 10.1038/nri2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He X, Park K, Kappes DJ. The Role of ThPOK in Control of CD4/CD8 Lineage Commitment. Annual Review of Immunology. 2010;28:295–320. doi: 10.1146/annurev.immunol.25.022106.141715. [DOI] [PubMed] [Google Scholar]
- 3.Hernandez-Hoyos G, Sohn SJ, Rothenberg EV, Alberola-Ila J. Lck activity controls CD4/CD8 T cell lineage commitment. Immunity. 2000;12:313–322. doi: 10.1016/s1074-7613(00)80184-3. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez-Hoyos G, Anderson MK, Wang C, Rothenberg EV, Alberola-Ila J. GATA-3 expression is controlled by TCR signals and regulates CD4/CD8 differentiation. Immunity. 2003;19:83–94. doi: 10.1016/s1074-7613(03)00176-6. [DOI] [PubMed] [Google Scholar]
- 5.Pai SY, Truitt ML, Ting CN, Leiden JM, Glimcher LH, Ho IC. Critical roles for transcription factor GATA-3 in thymocyte development. Immunity. 2003;19:863–875. doi: 10.1016/s1074-7613(03)00328-5. [DOI] [PubMed] [Google Scholar]
- 6.He X, He X, Dave VP, Zhang Y, Hua X, Nicolas E, Xu W, Roe BA, Kappes DJ. The zinc finger transcription factor Th-POK regulates CD4 versus CD8 T-cell lineage commitment. Nature. 2005;433:826–833. doi: 10.1038/nature03338. [DOI] [PubMed] [Google Scholar]
- 7.Sun G, Liu X, Mercado P, Jenkinson SR, Kypriotou M, Feigenbaum L, Galera P, Bosselut R. The zinc finger protein cKrox directs CD4 lineage differentiation during intrathymic T cell positive selection. Nat Immunol. 2005;6:373–381. doi: 10.1038/ni1183. [DOI] [PubMed] [Google Scholar]
- 8.Egawa T, Tillman RE, Naoe Y, Taniuchi I, Littman DR. The role of the Runx transcription factors in thymocyte differentiation and in homeostasis of naive T cells. J Exp Med. 2007;204:1945–1957. doi: 10.1084/jem.20070133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aliahmad P, Kaye J. Development of all CD4 T lineages requires nuclear factor TOX. J Exp Med. 2008;205:245–256. doi: 10.1084/jem.20071944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oosterwegel M, Timmerman J, Leiden J, Clevers H. Expression of GATA-3 during lymphocyte differentiation and mouse embryogenesis. Dev Immunol. 1992;3:1–11. doi: 10.1155/1992/27903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hendriks RW, Nawijn MC, Engel JD, van Doorninck H, Grosveld F, Karis A. Expression of the transcription factor GATA-3 is required for the development of the earliest T cell progenitors and correlates with stages of cellular proliferation in the thymus. Eur J Immunol. 1999;29:1912–1918. doi: 10.1002/(SICI)1521-4141(199906)29:06<1912::AID-IMMU1912>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 12.Hattori N, Kawamoto H, Fujimoto S, Kuno K, Katsura Y. Involvement of transcription factors TCF-1 and GATA-3 in the initiation of the earliest step of T cell development in the thymus. J Exp Med. 1996;184:1137–1147. doi: 10.1084/jem.184.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ting CN, Olson MC, Barton KP, Leiden JM. Transcription factor GATA-3 is required for development of the T-cell lineage. Nature. 1996;384:474–478. doi: 10.1038/384474a0. [DOI] [PubMed] [Google Scholar]
- 14.Hosoya T, Kuroha T, Moriguchi T, Cummings D, Maillard I, Lim KC, Engel JD. GATA-3 is required for early T lineage progenitor development. J Exp Med. 2009;206:2987–3000. doi: 10.1084/jem.20090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 16.Zhu J, Min B, Hu-Li J, Watson CJ, Grinberg A, Wang Q, Killeen N, Urban JFJ, Guo L, Paul WE. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat Immunol. 2004;5:1157–1165. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]
- 17.Ho IC, Tai TS, Pai SY. GATA3 and the T-cell lineage: essential functions before and after T-helper-2-cell differentiation. Nat Rev Immunol. 2009;9:125–135. doi: 10.1038/nri2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maurice D, Hooper J, Lang G, Weston K. c-Myb regulates lineage choice in developing thymocytes via its target gene Gata3. EMBO J. 2007;26:3629–3640. doi: 10.1038/sj.emboj.7601801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swan KA, Alberola-Ila J, Gross JA, Appleby MW, Forbush KA, Thomas JF, Perlmutter RM. Involvement of p21ras distinguishes positive and negative selection in thymocytes. Embo J. 1995;14:276–285. doi: 10.1002/j.1460-2075.1995.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grote D, Souabni A, Busslinger M, Bouchard M. Pax 2/8-regulated Gata 3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development. 2006;133:53–61. doi: 10.1242/dev.02184. [DOI] [PubMed] [Google Scholar]
- 21.Bender TP, Kremer CS, Kraus M, Buch T, Rajewsky K. Critical functions for c-Myb at three checkpoints during thymocyte development. Nat Immunol. 2004;5:721–729. doi: 10.1038/ni1085. [DOI] [PubMed] [Google Scholar]
- 22.Hu T, Simmons A, Yuan J, Bender TP, Alberola-Ila J. The transcription factor c-Myb primes CD4+CD8+ immature thymocytes for selection into the iNKT lineage. Nat Immunol. 2010;11:435–441. doi: 10.1038/ni.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Parijs L, Refaeli Y, Lord JD, Nelson BH, Abbas AK, Baltimore D. Uncoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation-induced cell death. Immunity. 1999;11:281–288. doi: 10.1016/s1074-7613(00)80103-x. [DOI] [PubMed] [Google Scholar]
- 24.Laurent MN, Ramirez DM, Alberola-Ila J. Kinase suppressor of Ras couples Ras to the ERK cascade during T cell development. J Immunol. 2004;173:986–992. doi: 10.4049/jimmunol.173.2.986. [DOI] [PubMed] [Google Scholar]
- 25.Bevis BJ, Glick BS. Rapidly maturing variants of the Discosoma red fluorescent protein (DsRed) Nat Biotechnol. 2002;20:83–87. doi: 10.1038/nbt0102-83. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez-Hoyos G, Alberola-Ila J. Analysis of T-cell development by using short interfering RNA to knock down protein expression. Methods Enzymol. 2005;392:199–217. doi: 10.1016/S0076-6879(04)92012-5. [DOI] [PubMed] [Google Scholar]
- 27.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from he-matopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 28.Kostelny SA, Cole MS, Tso JY. Formation of a bispecific antibody by the use of leucine zippers. J Immunol. 1992;148:1547–1553. [PubMed] [Google Scholar]
- 29.Nawijn MC, Ferreira R, Dingjan GM, Kahre O, Drabek D, Karis A, Grosveld F, Hendriks RW. Enforced expression of GATA-3 during T cell development inhibits maturation of CD8 single-positive cells and induces thymic lymphoma in transgenic mice. J Immunol. 2001;167:715–723. doi: 10.4049/jimmunol.167.2.715. [DOI] [PubMed] [Google Scholar]
- 30.Asnagli H, Afkarian M, Murphy KM. Cutting edge: Identification of an alternative GATA-3 promoter directing tissue-specific gene expression in mouse and human. J Immunol. 2002;168:4268–4271. doi: 10.4049/jimmunol.168.9.4268. [DOI] [PubMed] [Google Scholar]
- 31.Aramburu J, Yaffe MB, Lopez-Rodriguez C, Cantley LC, Hogan PG, Rao A. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science. 1999;285:2129–2133. doi: 10.1126/science.285.5436.2129. [DOI] [PubMed] [Google Scholar]
- 32.Shao H, Kono DH, Chen LY, Rubin EM, Kaye J. Induction of the early growth response (Egr) family of transcription factors during thymic selection. J Exp Med. 1997;185:731–744. doi: 10.1084/jem.185.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woronicz JD, Lina A, Calnan BJ, Szychowski S, Cheng L, Winoto A. Regulation of the Nur77 orphan steroid receptor in activation-induced apoptosis. Mol Cell Biol. 1995;15:6364–6376. doi: 10.1128/mcb.15.11.6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He X, Park K, Wang H, He X, Zhang Y, Hua X, Li Y, Kappes DJ. CD4-CD8 lineage commitment is regulated by a silencer element at the ThPOK transcription-factor locus. Immunity. 2008;28:346–358. doi: 10.1016/j.immuni.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Dave VP, Allman D, Keefe R, Hardy RR, Kappes DJ. HD mice: a novel mouse mutant with a specific defect in the generation of CD4(+) T cells. Proc Natl Acad Sci U S A. 1998;95:8187–8192. doi: 10.1073/pnas.95.14.8187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Carr T, Xiong Y, Wildt KF, Zhu J, Feigenbaum L, Bendelac A, Bosselut R. The sequential activity of Gata3 and Thpok is required for the differentiation of CD1d-restricted CD4+ NKT cells. Eur J Immunol. 2010;40:2385–2390. doi: 10.1002/eji.201040534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, Wildt KF, Zhu J, Zhang X, Feigenbaum L, Tessarollo L, Paul WE, Fowlkes BJ, Bosselut R. Distinct functions for the transcription factors GATA-3 and ThPOK during intrathymic differentiation of CD4(+) T cells. Nat Immunol. 2008 doi: 10.1038/ni.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, Wildt KF, Castro E, Xiong Y, Feigenbaum L, Tessarollo L, Bosselut R. The zinc finger transcription factor Zbtb7b represses CD8-lineage gene expression in peripheral CD4+ T cells. Immunity. 2008;29:876–887. doi: 10.1016/j.immuni.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amsen D, Antov A, Jankovic D, Sher A, Radtke F, Souabni A, Busslinger M, McCright B, Gridley T, Flavell RA. Direct regulation of Gata3 expression determines the T helper differentiation potential of Notch. Immunity. 2007;27:89–99. doi: 10.1016/j.immuni.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fang TC, Yashiro-Ohtani Y, Del Bianco C, Knoblock DM, Blacklow SC, Pear WS. Notch directly regulates Gata3 expression during T helper 2 cell differentiation. Immunity. 2007;27:100–110. doi: 10.1016/j.immuni.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen CH, Zhang DH, LaPorte JM, Ray A. Cyclic AMP activates p38 mitogen-activated protein kinase in Th2 cells: phosphorylation of GATA-3 and stimulation of Th2 cytokine gene expression. J Immunol. 2000;165:5597–5605. doi: 10.4049/jimmunol.165.10.5597. [DOI] [PubMed] [Google Scholar]
- 42.Maneechotesuwan K, Xin Y, Ito K, Jazrawi E, Lee KY, Usmani OS, Barnes PJ, Adcock IM. Regulation of Th2 cytokine genes by p38 MAPK-mediated phosphorylation of GATA-3. J Immunol. 2007;178:2491–2498. doi: 10.4049/jimmunol.178.4.2491. [DOI] [PubMed] [Google Scholar]
- 43.Yamashita M, Shinnakasu R, Asou H, Kimura M, Hasegawa A, Hashimoto K, Hatano N, Ogata M, Nakayama T. Ras-ERK MAPK cascade regulates GATA3 stability and Th2 differentiation through ubiquitin-proteasome pathway. J Biol Chem. 2005;280:29409–29419. doi: 10.1074/jbc.M502333200. [DOI] [PubMed] [Google Scholar]