Abstract
Down syndrome (DS) is the most common genetic disease and presents with cognitive impairment, cardiac and gastrointestinal abnormalities, in addition to other miscellaneous clinical conditions. DS individuals may have a high frequency of infections, usually of the upper respiratory tract, characterized by increased severity and prolonged course of disease, which are partially attributed to defects of the immune system. The abnormalities of the immune system associated with DS include: mild to moderate T and B cell lymphopenia, with marked decrease of naive lymphocytes, impaired mitogen-induced T cell proliferation, reduced specific antibody responses to immunizations and defects of neutrophil chemotaxis. Limited evidence of genetic abnormalities secondary to trisomy of chromosome 21 and affecting the immune system is available, such as the potential consequences of gene over-expression, most significantly SOD1 and RCAN1. Secondary immunodeficiency due to metabolic or nutritional factors in DS, particularly zinc deficiency, has been postulated. Non-immunological factors, including abnormal anatomical structures (e.g. small ear canal, tracheomalacia) and gastro-oesophageal reflux, may play a role in the increased frequency of respiratory tract infections. The molecular mechanisms leading to the immune defects observed in DS individuals and the contribution of these immunological abnormalities to the increased risk of infections require further investigation. Addressing immunological and non-immunological factors involved in the pathogenesis of infectious diseases may reduce the susceptibility to infections in DS subjects.
Keywords: antibody response, chemotaxis, Down syndrome, immunodeficiency, otitis media
Introduction
Down syndrome (DS) is the most common chromosomal anomaly among live-born infants, with an incidence of one in 600 to one in 900 in the United States [1,2]. DS is also the most frequent genetic cause of mental retardation and is associated with a high incidence of congenital cardiac and gastrointestinal tract anomalies [3]. Autoimmune phenomena, including hypothyroidism [3] and coeliac disease [4,5], and haematological abnormalities such as acute lymphoblastic leukaemia and transient myeloproliferative disease, occur at much higher frequency compared to non-DS individuals [6].
Infections of the respiratory tract, particularly otitis media, have been identified as one of the most significant health problems in DS children of school age by their parents, with a higher frequency than in the general population [7,8]. This increased susceptibility to infections have been linked to abnormal parameters of the immune system for more than 30 years [9,10], and DS is the most common recognizable genetic syndrome associated with immune defects [11]. Although multiple differences between the immune system of DS children and that of the general population have been described, the clinical relevance of these differences is less clear. Various medical and anatomical co-morbidities commonly associated with DS increase the susceptibility to infections and might also affect the immune responses. We reviewed the infectious disease burden in DS children and the mechanisms of innate and adaptive immunity defective in this condition (Fig. 1).
Fig. 1.
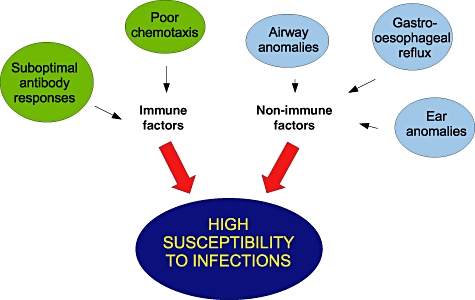
Selected immunological and non-immunological factors that potentially increase the susceptibility to infections in Down syndrome.
Increased morbidity due to recurrent infections
Respiratory tract infections
It is widely accepted that DS children suffer from more frequent infections than normal children, and most studies agree that these are affecting mainly the respiratory tract. Selikowicz [8] used a parent questionnaire and reported that the prevalence of significant lower respiratory illnesses among DS children was 8%. Hilton et al. [12] comprehensively reviewed 232 hospital admissions among DS children over a 6·5-year period, and found that lower respiratory tract pathology was the most common cause for acute hospital admission. This was in contrast to non-DS children, who were most commonly admitted for asthma, chemotherapy administration, fractures, gastroenteritis, bronchiolitis and adeno-tonsillectomy. Based on age groups, the highest percentage of admissions in this study were among 1–5-year-old children (45%), followed by those less than 1 year of age (27%). Both those aged 5–10 years and 10–17 years had the same rate of hospital admissions (each group 14%). Fifty-four per cent of all hospital admissions were for respiratory tract pathology, including infections such as pneumonia (18%), bronchiolitis (7%) and croup (6·5%). The predominant diagnosis of admission to the intensive care unit (ICU) was pneumonia. Interestingly, the co-morbid diagnoses of congenital heart disease and asthma did not influence admission rates to the hospital. Other studies have shown that DS itself is an independent risk factor for the development of bronchiolitis due to respiratory syncitial virus (RSV) infection. Bloemers et al. [13] demonstrated not only increased incidence of hospitalization for RSV lower respiratory tract infection among children with DS, but also a more severe course of infection than non-DS children. RSV is known to be the most important and severe cause of lower respiratory tract infections in all children, and certain groups (e.g. preterm infants) are identified early in infancy to have a high risk of RSV infection and receive immunological prophylaxis against this disease. Of note, a subsequent study [14] showed that hospitalization for RSV-induced lower respiratory tract infection in children with DS did not increase significantly the risk for recurrent wheezing or long-term airway morbidity. This study reported that the incidence of recurrent wheeze was higher among DS children at about 30%, regardless of whether or not they had a history of RSV-induced lower respiratory tract illness. Megged and Schlesinger [15] pointed out that DS infants with RSV are older and require longer hospitalization than non-DS infants, possibly reflecting the association with cardiac disease. More recently, a study of health services utilization by a cohort of DS subjects in Western Australia compared surveys conducted in 1997 and 2004. A reduction of the incidence of overall infections, but mainly upper respiratory infections, were noted. Further analysis of association with other clinical findings showed that the decrease of ear infections was seen only in DS patients without heart disease. Pneumonias, tonsillitis and bronchitis were observed to have a decreasing trend in both groups with and without heart disease, suggesting that cardiac function was not a determinant of the risk of infections.
Streptococcus pneumoniae, Haemophilis influenzae and Moraxella catarrhalis are the three most common bacteria known to cause acute otitis media and pneumonia in children [16,17]. There are few studies on the pathogens causing recurrent respiratory infections or otitis media in DS children, with isolated case reports that describe uncommon aetiologies (i.e. Bordetella bronchiseptica), which probably do not represent the large majority of infections among DS children. Of more relevance, changes in the frequency and microbiology of infections after the introduction of the recommended anti-pneumococcal immunization in 1999 have not been studied in this patient population.
Prolonged course of respiratory infections
Even though some DS children may not present with frequent infections, the course of their infection illnesses might be prolonged and have increased severity compared with non-DS children. In the study by Hilton et al. [12], the median length of stay and cost of admission for DS children was two to three times greater than in non-DS subjects. A higher incidence of acute lung injury secondary to pneumonia was found among DS children when compared to normal control children. A subsequent study examined 24 consecutive children with DS and 317 children without DS who were admitted to the paediatric intensive care unit (ICU) for mechanical ventilation [18]. Fifty-eight per cent of DS children and 13% of non-DS children met criteria for acute lung injury. Similarly, 46% of DS children and 7% of non-DS children were diagnosed with acute respiratory distress syndrome (ARDS). None of the DS children in this cohort with acute lung injury died, whereas others have reported a mortality rate of about 5% of non-DS children with ARDS. These data suggest that children with DS have an increased risk of progressing towards ARDS, although with low mortality, and support the hypothesis of abnormal regulatory mechanisms of inflammation, such as an imbalance of anti-oxidants and oxidative stress [19], which might lead to apoptosis in lung tissue. A review of a large cohort of DS children in Sweden and Denmark [20] revealed a 12-times increased risk for mortality due to infections, especially septicaemia. This excess of mortality was consistent with data from a recent study in which DS children showed a 30% higher risk of fatality secondary to sepsis when compared to other children hospitalized for sepsis [21], after controlling for confounding factors including pathogens and co-morbid conditions.
The above studies highlight the increased frequency and severity of respiratory tract infections in DS children. These are predominantly ear infections; however, pneumonias occur frequently in children younger than 5 years of age and are likely to require hospitalization. Lung disease might be of more prolonged duration and might progress to ARDS. In addition to respiratory tract infections, periodontal disease is another condition of infectious aetiology that occurs frequently between 58% and 96% of individuals with DS [22]. Due to the complexity of the pathophysiology of gingivitis, the contributions of potential determinant factors such as abnormal immunity and poor oral hygiene have not yet been defined clearly.
Immune defects in Down syndrome subjects
Adaptive immunity
Defects in immunological parameters in DS have been described and postulated as explanations for the increased severity of infections seen in DS children [9,10]. Most of these infections are of the respiratory tract, suggesting abnormalities of the humoral immunity. However, differences in several compartments of the immune response have been reported [23–25] (Table 1). Reduced ranges of the different lymphocyte subsets were found to be of most significance in childhood, with subsequent improvement over age. T and B cell subsets are decreased below the 10th percentile of normal in almost 90% of DS children, and below the 5th percentile of normal in 60% of them. The normal early T cell expansion in infancy was not observed. Their thymus size was reported to be smaller than non-DS children, with decreased T cell percentages bearing the T cell receptor (TCR)-αβ and relatively reduced naive T cell percentages [26–28], resulting in mild to moderate lymphopenia. DS children also have decreased T cell receptor excision circles (TREC), which are DNA by-products of TCR recombination that reflect production of new T cells in the thymus [29]. Most of these studies are limited to DS patients who have presented with recurrent infections, and they may not represent the general DS population; however, Kuester et al. [30] reported lymphocyte subsets of 95 DS children visiting their centre for follow-up of their thyroid function and 77% of patients had frequent respiratory infections. In this cohort, 57 (60%) of the children were aged 5–16 years, and only three children were above 16 years of age. The number and percentage of naive T cells were decreased approximately by half across the age-ranges compared to non-DS children, although they did not reach severe immunodeficiency levels. For example, the median naive CD4 T cells in 5–10-year-old children was 280 cells/µl (44% of CD4 T cells) for DS and 730 cells/µl (72% of CD4 T cells) for age-matched controls. There was no association of low T cell counts and the presence of recurrent infections. Memory T cell percentage and count were not significantly different from normal controls, an argument that the study authors used to postulate the presence of an intrinsic immune defect that renders those cells impaired to control infections. In the same DS cohort, the investigators compared several maturation stages of peripheral blood B cells with those of normal children and found decreased numbers of all B cell stages, particularly naive B cells [31]. There was no statistically significant association of low B cell counts and clinical conditions.
Table 1.
Immune defects in Down syndrome
| • Mild to moderately reduced T cell counts |
| • Mild to moderately reduced B cell counts |
| • Absence of normal lymphocyte expansion in infancy |
| • Thymus size is smaller than age-matched controls |
| • Mild to moderately reduced naive T cell percentages, with corresponding reduction of T cell excision circles (Trecs) |
| • Suboptimal antibody responses to immunizations |
| • Decreased total and specific immunoglobulin A in saliva |
| • Decreased neutrophil chemotaxis |
T cell and B cell function have been examined in DS. The lymphocyte proliferative response to phytohaemagglutinin has been reported to be significantly low in DS [8,32]. The abnormalities in immunoglobulin (Ig)G levels do not occur in all DS subjects; while some DS children present with IgG levels under normal ranges for age, particularly IgG2 [8], most DS subjects show adequate levels [33]. In a cohort of 26 DS children, of whom 18 had increased rate of infections, only one child had decreased IgG2 levels [34]. An older cohort of DS individuals, with a mean age of 55 years, showed significantly higher levels of IgG1 and decreased levels of IgG2 subclasses compared to age-matched individuals [35].
The high frequency of periodontal disease in DS might be explained in part by a deficiency of IgA in saliva of DS individuals. A study of young and older adults with DS demonstrated a drastic reduction of both total IgA concentration in saliva and specific IgA to common oral pathogens, compared to controls [36].
Decreased antibody responses to immunizations
The specific antibody responses of DS children to several immunizations have been found defective, although most develop protective IgG titres. Lopez et al. [37] showed that the specific IgG titres to the neoantigen bacteriophage phi174 in DS children were lower than the normal range. Hawkes et al. [38] reported reduced antibody titres to oral polio vaccine of nine DS subjects compared to non-DS subjects, although of statistical significance only for poliovirus type 1, but not types 2 and 3. The enteric viral shedding was similar for DS and non-DS subjects, with large individual variations within the groups. Similar results have been reported for other vaccines, such as acellullar pertussis [39], influenza antigen [40], hepatitis B [41], hepatitis A [42] and pneumococcal vaccines in adults [43] and children [44] with DS. Specific antibody responses are elicited in DS children, although with titres that are lower than in non-DS control individuals, which is consistent with the increase frequency of respiratory tract infections.
Innate immunity defects
The earliest studies of immune function and infection in DS individuals in the late 1970s did not find differences in humoral and cellular immunity, but reported differences in neutrophil chemotaxis [45–47]. Other neutrophil functions such as phagocytosis and oxidative burst responses were not consistently reported to be affected in these studies [48,49]. Studies of the integrin β-2 (CD18) in DS blood cells were conducted when the gene encoding this protein was located to chromosome 21. The initial studies of CD18 expression in DS individuals using lymphoblastoid cells reported increased cell surface expression and cell aggregation [50,51]; however, Novo and others [52,53] showed that this increased expression does not occur in non-transformed cells. They comprehensively studied functions of freshly isolated polymorphonuclear cells and reported integrin surface expression, phagocytoses and oxidative burst responses comparable with controls. They did find significant reduction in chemotaxis activity. The normal oxidative burst responses argue against the hypothesis that the over-expression of the superoxide dismutase (SOD1) gene was responsible for the earlier observation of defective phagocytosis and killing of Candida sp. by neutrophils from DS subjects [54].
Studies using only CD56 as a surface marker for natural killer (NK) cells suggested that these cells were increased in peripheral blood of DS subjects [55]. More recent studies [24] have demonstrated that absolute numbers of NK cells were actually low, and the discrepancy was attributed to the difference of surface markers used. Disturbances of the secretion of cytokines interleukin (IL)-2, IL-7 and IL-10 [56] and deficiency of mannan-binding proteins [57] have also been suggested to contribute to the increased susceptibility to infections.
Down syndrome: primary and secondary immunodeficiency
Kuster et al. [30] summarized the evidence supporting an intrinsic defect of the immune system in Down syndrome children, based on the low naive T and B cell counts, and the increased frequency of infections in DS children with normal numbers of T and B cells. The genetic mechanisms determining the immunological defects associated to DS are not well defined. Over-expression of SOD1 and ITGB2, two genes found in chromosome 21 and of significance to neutrophil functions, have not been shown to impair the immune response significantly. Whether other genes in chromosome 21 can indirectly impair adaptive and innate immune responses is being postulated. For example, the regulator of calcineurin 1 (RCAN1) is a transcription factor that inhibits signal transduction mediated by the nuclear factor of activated T cells (NFAT) [58], and has been shown to reduce inflammatory responses in mice by stabilizing an inhibitor of nuclear factor-kappa B cells (NF-κB) [59].
Two possible causes of secondary immunodeficiency, accelerated ageing and zinc deficiency, have been explored further. Because of the senescence associated to neurological conditions in DS such as premature Alzheimer's disease [60] a similar ageing process in the immune system has been suggested, including mechanisms of increased apoptosis [61,62], that could be responsible for the observed lymphopenia and immune dysfunction. The deficiency of plasma zinc levels observed in some DS subjects and the need of zinc for SOD activity have been proposed as mechanisms of immunological abnormalities. Cocchi and colleagues [25] tested if zinc deficiency might be only transient, and found that plasma levels of zinc decrease over time after 5 years of age. However, observational studies examining zinc levels and immune status and clinical trials of zinc supplementation have failed to show a consistent clinical benefit [63–65].
Allergy is not highly prevalent in DS children
DS children might have symptoms of chronic rhinitis and reactive airway disease, suggesting hypersensitivity to inhaled allergens. A study comparing positivity to skin prick hypersensitivity test between symptomatic DS children and age-matched controls found that 18% of cases had at least one positive allergen in the skin test, which contrasts with 54% of non-DS controls [66]. The authors conclude that allergen sensitization is not a major contributor of respiratory illnesses in DS children. Vestergen et al. [31] found only six of 44 DS patients with elevated IgE, and none of 28 DS individuals tested had an allergen identified as a trigger for allergy symptoms.
Non-immunological factors that increase risk of infections
Despite the multiple immunological abnormalities outlined above, it is still unclear whether these are the major determinants of increased risk of infections in DS children. This susceptibility to infections is probably enhanced by other co-morbidities that weaken mucosal barriers; for example, abnormal airway and ear anatomy, macroglossia, congenital heart disease and reactive airway disease or an inability to handle secretions.
Airway anatomical abnormalities
Anatomical abnormalities of the airways may impair clearance of secretions and facilitate infections. Bertrand et al. [67] described airway anomalies among 75% of DS children and 35% of non-DS children with recurrent respiratory symptoms who underwent fibreoptic bronchoscopy. The most common abnormality seen in both DS and non-DS groups was laryngomalacia, with 50% incidence in the DS group compared to 19% in the non-DS group. Tracheomalacia and tracheal bronchus were also observed. Evidence of pulmonary hypoplasia associated to DS has also been reported [68,69].
Obstructive sleep apnoea
Obstructive sleep apnoea and airway obstruction is extremely common in DS, with an incidence ranging from 63% to almost 80% [70,71]. Predisposing factors that lead to obstructive sleep apnoea in DS include the characteristic mid-face hypoplasia, tongue enlargement and mandibular hypoplasia. This small upper airway, combined with relatively large tonsils and adenoids, contributes to airway obstruction and increases susceptibility to infections. Upper airway obstruction due to adenoids and tonsillar hypertrophy was reported in 30 (6%) of 518 DS children seen consecutively [72]. Those with severe obstructive symptoms, e.g. snoring, were found to be more likely to have tracheobronchomalacia, laryngomalacia, macroglossia and congenital tracheal stenosis. Five patients required tracheostomy because of persistent obstruction.
Gastro-oesophageal reflux and oropharyngeal aspiration
Gastro-oesophageal reflux may result in aspiration of gastric contents into airway causing lung inflammation or a reflex mechanism of the lower oesophagus triggering bronchospasm [73]. It is recommended to rule out gastro-oesophageal reflux in children presenting with recurrent lung disease without other explanation. Recurrent aspiration of thin fluids is well known to be associated with increased incidence of lower respiratory tract infections [74,75]. The hypotonia associated with DS includes poor pharyngeal muscle tone that increases the risk for aspiration [76]. Subclinical aspiration may account for up to 12% of cases of chronic respiratory complaints in non-DS children, and up to 42% in DS children [77,78]. Zarate and collaborators [79] studied oesophagograms of 58 DS subjects and 38 healthy controls, finding 15 of the DS participants with higher tracer retention than the upper limit of the controls' retention. Five were reported definitely abnormal, with achalasia documented in two subjects. Eight had frequent vomiting/regurgitation. DS children would benefit from evaluation of swallowing function [80].
Congenital ear abnormalities
Up to 40–50% of DS newborns may have external ear canal stenosis [81,82] and the Eustachian tube may also be of small width, contributing to the collection of middle ear fluid and chronic otitis media [83]. Otitis media may explain the high incidence of hearing loss and the delayed development of language reported in DS [84].
Discussion
Early health supervision and advances in medical care have lengthened the life expectancy of children with DS. Frequent respiratory tract infections is considered a significant component of the morbidity of DS children; however, few studies help to define the current epidemiology of infections in the DS population. It appears that the incidence of respiratory infections has declined in the last decade, due most probably to the progress in the management of infections and the awareness of the medical problems that are common to DS patients. While the incidence of respiratory infections may not be too high compared to non-DS children, it appears that DS children suffer a prolonged period of illness time and need additional treatment to overcome the same infections compared to non-DS children. Whether or not this is due to an intrinsic defect in the immune system of DS individuals or mainly secondary to the various DS-associated characteristics needs to be investigated further. Chromosome 21 genes that may influence the immune response include SOD1 and RCAN1. Several components of the immune system are variably affected in DS subjects, from which the most consistently reported are defective neutrophil chemotaxis and low humoral immune responses, associated with infections being predominantly of the respiratory tract. Factors that may induce immunodeficiency have been postulated, such as zinc deficiency and accelerated immunosenescence, although their clinical significances have not been established. Common anatomical defects of DS disturb natural barriers and facilitate the infectious disease process and need be considered in the management of infections in these patients.
We recommend investigation of DS children who present with increased frequency of infections for immunological and non-immunological factors that increase the risk of infection. In this evaluation, low specific antibody titres to routine childhood vaccines would suggest the need for additional booster immunization doses.
Acknowledgments
The authors thank Dr Carla Davis and Dr Kathlyn Ostermaier for critical review of this manuscript.
Disclosure
The authors have nothing to disclose.
References
- 1.Yang Q, Rasmussen SA, Friedman JM. Mortality associated with Down's syndrome in the USA from 1983 to 1997: a population-based study. Lancet. 2002;359:1019–25. doi: 10.1016/s0140-6736(02)08092-3. [DOI] [PubMed] [Google Scholar]
- 2.Canfield MA, Honein MA, Yuskiv N, Xing J, Mai CT, Collins JS. National estimates and race/ethnic-specific variation of selected birth defects in the United States, 1990–2001. Birth Defects Res A Clin Mol Teratol. 2006;76:747–56. doi: 10.1002/bdra.20294. [DOI] [PubMed] [Google Scholar]
- 3.van Trotsenburg AS, Heymans HS, Tijssen JG, de Vijlder JJ, Vulsma T. Comorbidity, hospitalization, and medication use and their influence on mental and motor development of young infants with Down syndrome. Pediatrics. 2006;118:1633–9. doi: 10.1542/peds.2006-1136. [DOI] [PubMed] [Google Scholar]
- 4.Zachor DA, Mroczek-Musulman E, Brown P. Prevalence of celiac disease in Down syndrome in the United States. J Pediatr Gastroenterol Nutr. 2000;31:275–9. doi: 10.1097/00005176-200009000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez-Albisua I, Storm W, Wascher I, Stern M. How frequent is celiac disease in Down syndrome. Eur J Pediatr. 2002;161:683–4. doi: 10.1007/s00431-002-1078-6. [DOI] [PubMed] [Google Scholar]
- 6.Goldacre M, Wotton C, Seagroatt V, Yeates D. Cancers and immune related diseases associated with Down Syndrome: a record linkage study. Arch Dis Child. 2004;89:1014–17. doi: 10.1136/adc.2003.046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner S, Sloper P, Cunningham C, Knussen C. Health problems in children with Down's syndrome. Child Care Health Dev. 1990;16:83–97. doi: 10.1111/j.1365-2214.1990.tb00641.x. [DOI] [PubMed] [Google Scholar]
- 8.Selikowitz M. Health problems and health checks in school-aged children with Down syndrome. J Pediatr Child Health. 1992;28:383–6. doi: 10.1111/j.1440-1754.1992.tb02697.x. [DOI] [PubMed] [Google Scholar]
- 9.Burgio GR, Ugazio AG, Nespoli L, Marcioni AF, Bottelli AM, Pasquali F. Derangements of immunoglobulin levels, phytohemagglutinin responsiveness and T and B cell markers in Down's syndrome at different ages. Eur J Immunol. 1975;5:600–3. doi: 10.1002/eji.1830050904. [DOI] [PubMed] [Google Scholar]
- 10.Burgio GR, Lanzavecchia A, Maccario R, Vitiello A, Plebani A, Ugazio AG. Immunodeficiency in Down's syndrome: T lymphocyte subset imbalance in trisomic children. Clin Exp Immunol. 1978;33:298–301. [PMC free article] [PubMed] [Google Scholar]
- 11.Cruz NV, Mahmoud SA, Chen H, Lowery-Nordberg M, Berlin K, Bahna SL. Follow up study of immune defects in patients with dysmorphic disorders. Ann Allergy Asthma Immunol. 2009;102:426–31. doi: 10.1016/S1081-1206(10)60516-9. [DOI] [PubMed] [Google Scholar]
- 12.Hilton JM, Fitzgerald DA, Cooper DM. Respiratory morbidity of hospitalized children with trisomy 21. J Pediatr Child Health. 1999;35:383–6. doi: 10.1046/j.1440-1754.1999.00386.x. [DOI] [PubMed] [Google Scholar]
- 13.Bloemers BL, van Furth AM, Weijerman ME, et al. Down syndrome: a novel risk factor for respiratory syncytial virus bronchiolitis – a prospective birth-cohort study. Pediatrics. 2007;120:1076–81. doi: 10.1542/peds.2007-0788. [DOI] [PubMed] [Google Scholar]
- 14.Bloemers BL, van Furth AM, Weijerman ME, et al. High incidence of recurrent wheeze in children with Down syndrome with and without previous respiratory syncytial virus lower respiratory tract infection. Pedatric Inf Dis J. 2010;29:39–42. doi: 10.1097/INF.0b013e3181b34e52. [DOI] [PubMed] [Google Scholar]
- 15.Megged O, Schlesinger Y. Down syndrome and respiratory sincytial virus infection. Pediatr Infect Dis. 2010;29:672–3. doi: 10.1097/INF.0b013e3181d7ffa5. [DOI] [PubMed] [Google Scholar]
- 16.Casey JR, Pichichero ME. Changes in frequency and pathogens causing acute otitis media in 1995–2003. Pediatr Infect Dis J. 2004;23:824–8. doi: 10.1097/01.inf.0000136871.51792.19. [DOI] [PubMed] [Google Scholar]
- 17.Don M, Canciani M, Korppi M. Community-acquired pneumonia in children: what's old? What's new? Acta Paediatr. 2010;99:1602–8. doi: 10.1111/j.1651-2227.2010.01924.x. [DOI] [PubMed] [Google Scholar]
- 18.Bruijn M, van der Aa LB, van Rijn RR, Bos AP, van Woensel JB. High incidence of acute lung injury in children with Down syndrome. Intens Care Med. 2007;33:2179–82. doi: 10.1007/s00134-007-0803-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jovanovic SV, Clements D, MacLeod K. Biomarkers of oxidative stress are significantly elevated in Down syndrome. Free Radic Biol Med. 1998;25:1044–8. doi: 10.1016/s0891-5849(98)00137-3. [DOI] [PubMed] [Google Scholar]
- 20.Hill DA, Gridley G, Cnattinguius S, et al. Mortality and cancer incidence among individuals with Down syndrome. Arch Intern Med. 2003;163:705–11. doi: 10.1001/archinte.163.6.705. [DOI] [PubMed] [Google Scholar]
- 21.Garrison M, Jeffries H, Christakis D. Risk of death for children with Down syndrome and sepsis. J Pediatr. 2005;147:748–52. doi: 10.1016/j.jpeds.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 22.Morgan J. Why is periodontal disease more prevalent and more severe in people with Down syndrome? Spec Care Dentist. 2007;27:196–201. doi: 10.1111/j.1754-4505.2007.tb00346.x. [DOI] [PubMed] [Google Scholar]
- 23.Kusters MAA, Verstgen RHJ, Gemen EFA, deVries E. Intrinsic defect of the immune system in children with Down syndrome: a review. Clin Exp Immunol. 2009;156:189–93. doi: 10.1111/j.1365-2249.2009.03890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Hingh YC, Van der Vossen PW, Gemen EF, et al. Intrinsic abnormalities of lymphocyte counts in children with Down syndrome. J Pediatr. 2005;147:744–7. doi: 10.1016/j.jpeds.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 25.Cocchi G, Mastrocola M, Capelli M, Bastelli A, Vitali F, Corvaglia L. Immunological patterns in young children with Down syndrome: is there a temporal trend? Acta Paediatr. 2007;96:1479–82. doi: 10.1111/j.1651-2227.2007.00459.x. [DOI] [PubMed] [Google Scholar]
- 26.Murphy M, Epstein L. Down syndrome (trisomy 21) thymuses have a decreased proportion of cells expressing high levels of TCR alpha, beta and CD3. Clin Immunol Immunopathol. 1990;55:453–67. doi: 10.1016/0090-1229(90)90131-9. [DOI] [PubMed] [Google Scholar]
- 27.Murphy M, Epstein L. Down syndrome peripheral blood contains phenotypically mature CD3 TCR alpha beta cells but abnormal proportions of TCR gamma delta, TCR alpha beta and CD4+45RA+ cells: evidence for an inefficient release of mature T cells by DS thymus. Clin Immunol Immunopathol. 1992;62:245–51. doi: 10.1016/0090-1229(92)90079-4. [DOI] [PubMed] [Google Scholar]
- 28.Barrena MJ, Echaniz P, Garcia-Serrano C, Cuadrado E. Imbalance of the CD4 subpopulations expressing CD45RA and CD29 antigens in the peripheral blood of adults and children with Down syndrome. Scand J Immunol. 1993;38:323–6. doi: 10.1111/j.1365-3083.1993.tb01733.x. [DOI] [PubMed] [Google Scholar]
- 29.Prada N, Nasi M, Troiano L, et al. Direct analysis of thymic function in children with Down syndrome. Immun Aging. 2005;2:1–8. doi: 10.1186/1742-4933-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kusters MA, Gemen EF, Vestergen RH, Wever PC, DeVries E. Both normal memory counts and decreased naïve cells favor intrinsic defect over early senescence of Down syndrome lymphocytes. Pediatr Res. 2010;67:557–62. doi: 10.1203/PDR.0b013e3181d4eca3. [DOI] [PubMed] [Google Scholar]
- 31.Verstegen RH, Kusters MA, Gemen EF, deVries E. Down syndrome B-lymphocyte populations, intrinsic defect or decreased T lymphocyte help. Pediatr Res. 2010;67:563–6. doi: 10.1203/PDR.0b013e3181d4ecc1. [DOI] [PubMed] [Google Scholar]
- 32.Rigas D, Elsasser P, Hecht F. Impaired in vitro response of circulating lymphocytes to phytohemagglutinin in Down's syndrome: dose and time–response curves and relation to cellular immunity. Int Allergy Immunol. 1970;39:587–608. doi: 10.1159/000230384. [DOI] [PubMed] [Google Scholar]
- 33.Cetiner S, Dermirhan O, Inal TC, Tastermir D, Sertdemir Y. Analysis of peripheral blood T cell subset, natural killer cells and serum levels of cytokines in children with Down syndrome. Int J Immunogenet. 2010;37:233–7. doi: 10.1111/j.1744-313X.2010.00914.x. [DOI] [PubMed] [Google Scholar]
- 34.Loh RK, Harth SC, Thing YH, Ferrante A. Immunoglobulin G subclass deficiency and predisposition to infection in Down's syndrome. Pediatr Infect Dis J. 1990;9:547–51. doi: 10.1097/00006454-199008000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Mehta PD, Dalton AJ, Mehta SP, Percy ME, Sersen EA, Wisniewski HM. Immunoglobulin G subclasses in older persons with Down syndrome. J Neurol Sci. 1993;117:186–91. doi: 10.1016/0022-510x(93)90172-u. [DOI] [PubMed] [Google Scholar]
- 36.Chaushu S, Chaushu G, Zigmond M, et al. Age-dependent deficiency in saliva and salivary antibodies secretion in Down's syndrome. Arch Oral Biol. 2007;52:1088–96. doi: 10.1016/j.archoralbio.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Lopez V, Ochs HD, Thuline HC, Davis SD, Wedgwood RJ. Defective antibody response to bacteriophage phi 174 in Down syndrome. J Pediatr. 1975;86:207–11. doi: 10.1016/s0022-3476(75)80469-0. [DOI] [PubMed] [Google Scholar]
- 38.Hawkes RA, Philbrook SC, Boughton CR. The response of institutionalized Down's syndrome subjects to enterovirus infections. J Hyg. 1980;84:433–41. doi: 10.1017/s0022172400026978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Volti S, Mattina T, Mauro L, et al. Safety and effectiveness of an acellular pertussis vaccine in subjects with Down's syndrome. Child Nerv Syst. 1996;12:100–2. doi: 10.1007/BF00819505. [DOI] [PubMed] [Google Scholar]
- 40.Epstein L, Phillip R. Abnormalities in the immune response to influenza antigen in Down syndrome (trisomy 21) Prog Clin Biol Res. 1987;246:163–82. [PubMed] [Google Scholar]
- 41.Avanzini M, Monafo V, De Amici M, et al. Humoral immunodeficiency in Down syndrome: serum IgG subclass and antibody response to hepatitis B vaccine. Am J Med Genet. 1990;7:231–3. doi: 10.1002/ajmg.1320370746. [DOI] [PubMed] [Google Scholar]
- 42.Ferreira CT, Leite JC, Taniguchi A, Vieira SM, Pereira-Lima J, da Silveira TR. Immunogenicity and safety of an inactivated hepatitis A vaccine in children with Down syndrome. J Pediatr Gastroenterol Nutr. 2004;39:337–40. doi: 10.1097/00005176-200410000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Nurmi T, Leinonen M, Häivä VM, Tiilikainen A, Kouvalainen K. Antibody response to pneumococcal vaccine in patients with trisomy-21 (Down's syndrome) Clin Exp Immunol. 1982;48:485–90. [PMC free article] [PubMed] [Google Scholar]
- 44.Costa-Carvalho BT, Martinez RM, Dias AT, et al. Antibody response to pneumococcal capsular polysaccharide vaccine in Down syndrome patients. Braz J Med Biol Res. 2006;39:1587–92. doi: 10.1590/s0100-879x2006001200010. [DOI] [PubMed] [Google Scholar]
- 45.Khan AJ, Evans HE, Glass L, Skin YH, Almonte D. Defective neutrophil chemotaxis in patients with Down syndrome. J Pediatr. 1975;87:87–9. doi: 10.1016/s0022-3476(75)80077-1. [DOI] [PubMed] [Google Scholar]
- 46.Barkin RM, Weston WL, Humbert JR, Maire F. Phagocytic function in Down syndrome – I. Chemotaxis. J Ment Defic Res. 1980;24:243–9. doi: 10.1111/j.1365-2788.1980.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 47.Barroeta O, Nungaray L, López-Osuna M, Armendares S, Salamanca F, Kretschmer RR. Defective monocyte chemotaxis in children with Down's syndrome. Pediatr Res. 1983;17:292–5. doi: 10.1203/00006450-198304000-00013. [DOI] [PubMed] [Google Scholar]
- 48.Costello C, Webber A. White cell function in Down's syndrome. Clin Genet. 1976;9:603–5. doi: 10.1111/j.1399-0004.1976.tb01619.x. [DOI] [PubMed] [Google Scholar]
- 49.Barkin RM, Weston WL, Humbert JR, Sunada K. Phagocytic function in Down syndrome – II. Bactericidal activity and phagocytosis. J Ment Defic Res. 1980;24:251–6. doi: 10.1111/j.1365-2788.1980.tb00079.x. [DOI] [PubMed] [Google Scholar]
- 50.Taylor GM, Haugh H, Williams A, D'Souza SW, Harris R. Down's syndrome lymphoid cell lines exhibit increased adhesion due to the over-expression of lymphocyte function-associated antigen (LFA-1) Immunology. 1988;64:451–6. [PMC free article] [PubMed] [Google Scholar]
- 51.Robson AJ, Taylor GM, D'Souza SW. Monoclonal antibodies to CD18 and CD11a distinguish Down's syndrome (trisomy 21) from normal lymphoblastoid cells. Dis Markers. 1989;7:169–80. [PubMed] [Google Scholar]
- 52.Novo E, Garcia MI, Lavergne J. Nonspecific immunity in Down syndrome: a study of chemotaxis, phagocytosis, oxidative metabolism, and cell surface expression of polymorphonuclear cells. Am J Med Genet. 1983;46:384–91. doi: 10.1002/ajmg.1320460408. [DOI] [PubMed] [Google Scholar]
- 53.Barrena MJ, Echaniz P, Garcia-Serrano C, Zubillaga P, Cuadrado E. Differential expression of lymphocyte function-associated antigen (LFA-1) on peripheral blood leucocytes from individuals with Down's syndrome. Clin Exp Immunol. 1992;88:41–4. doi: 10.1111/j.1365-2249.1992.tb03036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feaster WW, Kwok LW, Epstein CJ. Dosage effects for superoxide dismutase-1 in nucleated cells aneuploid for chromosome 21.Am. J Hum Genet. 1977;29:563–70. [PMC free article] [PubMed] [Google Scholar]
- 55.Cossarizza A, Ortolani C, Forti E, et al. Age-related expansion of functionally inefficient cells with markers of natural killer cells activity in Down's syndrome. Blood. 1991;77:1263–70. [PubMed] [Google Scholar]
- 56.Guazzarotti L, Trabattoni D, Castelletti E, et al. T lymphocyte maturation is impaired in healthy young individuals carrying trisomy 21 (Down syndrome) Am J Intellect Dev Disabil. 2009;114:100–9. doi: 10.1352/2009.114.100-109. [DOI] [PubMed] [Google Scholar]
- 57.Nisihara RM, Utiyama SR, Oliveira NP, Messias-Reason IJ. Mannan-binding lectin deficiency increases the risk of recurrent infections in children with Down's syndrome. Hum Immunol. 2010;71:63–6. doi: 10.1016/j.humimm.2009.09.361. [DOI] [PubMed] [Google Scholar]
- 58.Arron JR, Winslow MM, Polleri A, et al. NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21. Nature. 2006;441:595–600. doi: 10.1038/nature04678. [DOI] [PubMed] [Google Scholar]
- 59.Kim YS, Cho KO, Lee HJ, Kim SY, Sato Y, Cho YJ. Down syndrome candidate region 1 increases the stability of the IkappaBalpha protein: implications for its anti-inflammatory effects. J Biol Chem. 2006;281:39051–61. doi: 10.1074/jbc.M604659200. [DOI] [PubMed] [Google Scholar]
- 60.Lott I. Down syndrome, aging and Alzheimer's disease: a clinical review. Ann NY Acad Sci. 1982;396:15–27. doi: 10.1111/j.1749-6632.1982.tb26840.x. [DOI] [PubMed] [Google Scholar]
- 61.Cossariza A, Monti D, Montagnani G, et al. Precocious aging of the immune system in Down syndrome: alteration of B-lymphocyte, T lymphocytes subsets, and cells with natural killer markers. Am J Med Genet Suppl. 1990;7:213–18. doi: 10.1002/ajmg.1320370743. [DOI] [PubMed] [Google Scholar]
- 62.Cuadrado E, Barrena M. Immune dysfunction in Down's syndrome: primary immune deficiency or early senescence of the immune system? Clin Immunol Immunopathol. 1996;78:209–14. doi: 10.1006/clin.1996.0031. [DOI] [PubMed] [Google Scholar]
- 63.Licastro F, Chiricolo M, Mocchegiani E, et al. Oral zinc supplementation in Down's syndrome subjects decreased infections and normalized some humoral and cellular immune parameters. J Intellect Disabil Res. 1994;38:149–62. doi: 10.1111/j.1365-2788.1994.tb00370.x. [DOI] [PubMed] [Google Scholar]
- 64.Stabile A, Pesaresi MA, Stabile AM, et al. Immunodeficiency and plasma zinc levels in children with Down's syndrome: a long-term follow-up of oral zinc supplementation. Clin Immunol Immunopathol. 1991;58:207–16. doi: 10.1016/0090-1229(91)90137-y. [DOI] [PubMed] [Google Scholar]
- 65.Lockitch G, Puterman M, Godolphin W, Sheps S, Tingle AJ, Quigley G. Infection and immunity in Down syndrome: a trial of long-term low oral doses of zinc. J Pediatr. 1989;114:781–7. doi: 10.1016/s0022-3476(89)80136-2. [DOI] [PubMed] [Google Scholar]
- 66.Mannan SE, Yousef E, Hossain J. Prevalence of positive skin prick test results in children with Down syndrome: a case–control study. Ann Allergy Asthma Immunol. 2009;102:205–9. doi: 10.1016/S1081-1206(10)60082-8. [DOI] [PubMed] [Google Scholar]
- 67.Bertrand P, Navarro H, Caussade S, Holmgren N, Sanchez I. Airway anomalies in children with Down syndrome: endoscopic findings. Pediatr Pulmonol. 2003;36:137–40. doi: 10.1002/ppul.10332. [DOI] [PubMed] [Google Scholar]
- 68.Cooney TP, Thurlbeck WP. Pulmonary hypoplasia in Down's syndrome. N Engl J Med. 1982;307:1170–3. doi: 10.1056/NEJM198211043071902. [DOI] [PubMed] [Google Scholar]
- 69.Schloo BL, Vawter GF, Reid LM. Down syndrome: patterns of disturbed lung growth. Hum Pathol. 1991;22:919–23. doi: 10.1016/0046-8177(91)90183-p. [DOI] [PubMed] [Google Scholar]
- 70.Dyken ME, Lin-Dyken DC, Poulton S, Zimmerman MB, Sedars E. Prospective polysomnographic analysis of obstructive sleep apnea in Down syndrome. Arch Pediatr Adolesc Med. 2003;257:655–60. doi: 10.1001/archpedi.157.7.655. [DOI] [PubMed] [Google Scholar]
- 71.Marcus CL, Keens TG, Bautista DB, von Pechmann WS, Ward SL. Obstructive sleep apnea in children with Down syndrome. Pediatrics. 1992;88:132–9. [PubMed] [Google Scholar]
- 72.Jacobs IN, Gray RF, Todd NW. Upper airway obstruction in children with Down syndrome. Arch Otolaryngol Head Neck Surg. 1996;122:945–50. doi: 10.1001/archotol.1996.01890210025007. [DOI] [PubMed] [Google Scholar]
- 73.Sheikh S, Allen E, Shell R, et al. Chronic aspiration without gastroesophageal reflux as a cause of chronic respiratory symptoms in neurologically normal infants. Chest. 2001;120:1190–95. doi: 10.1378/chest.120.4.1190. [DOI] [PubMed] [Google Scholar]
- 74.Sheikh S, Stephen T, Howell L, Eid N. Gastroesophageal reflux in infants with wheezing. Pediatr Pulmonol. 1999;28:181–6. doi: 10.1002/(sici)1099-0496(199909)28:3<181::aid-ppul4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 75.Morton RE, Wheatley R, Minford J. Respiratory tract infections due to direct and reflux aspiration in children with severe neurodisability. Dev Med Child Neurol. 1999;41:329–34. doi: 10.1017/s0012162299000729. [DOI] [PubMed] [Google Scholar]
- 76.Frazier JB, Friedman B. Swallow function in children with Down syndrome: a retrospective study. Dev Med Child Neurol. 1996;38:695–703. doi: 10.1111/j.1469-8749.1996.tb12139.x. [DOI] [PubMed] [Google Scholar]
- 77.Brumbaugh DE, Accurso FJ. Persistent silent aspiration in a child with trisomy 21. Curr Opin Pediatr. 2002;14:231–3. doi: 10.1097/00008480-200204000-00015. [DOI] [PubMed] [Google Scholar]
- 78.Weir K, McMahon S, Barry L, Ware R, Masters IB, Chang AB. Oropharyngeal aspiration and pneumonia in children. Pediatr Pulmonol. 2007;42:1024–31. doi: 10.1002/ppul.20687. [DOI] [PubMed] [Google Scholar]
- 79.Zarate N, Mearin F, Hidalgo A, Malageada JR. Prospective evaluation of esophageal motor dysfunction in Down syndrome. Am J Gastroentrol. 2001;96:1718–24. doi: 10.1111/j.1572-0241.2001.03864.x. [DOI] [PubMed] [Google Scholar]
- 80.Frazier JB, Friedman B. Swallow function in children with Down syndrome: a retrospective study. Dev Med Child Neurol. 1996;38:695–703. doi: 10.1111/j.1469-8749.1996.tb12139.x. [DOI] [PubMed] [Google Scholar]
- 81.Strome M. Down syndrome – a modern otorhinolaryngological perspective. Layngoscope. 1982;41:1581–94. doi: 10.1288/00005537-198110000-00001. [DOI] [PubMed] [Google Scholar]
- 82.Shott SR. Down syndrome: common otolaryngoloic manifestations. Am J Med Genet Part C Semin Med Genet. 2006;142:131–40. doi: 10.1002/ajmg.c.30095. [DOI] [PubMed] [Google Scholar]
- 83.Shibahara Y, Sando I. Congenital anomalies of the Eustachian tube in children with Down syndrome. Ann Otol Rhinol Laryngol. 1989;98:543–7. doi: 10.1177/000348948909800709. [DOI] [PubMed] [Google Scholar]
- 84.Balkany TJ, Mischke RE, Downs MP, Jafek BW. Ossicular abnormalities in Down's syndrome. Otolaryngol Head Neck Surg. 1979b;87:372–4. doi: 10.1177/019459987908700317. [DOI] [PubMed] [Google Scholar]


