Abstract
Insulin autoantibodies (IAA) can appear in children within months of introducing solid foods to the diet and before clinical type 1 diabetes. The aim of this study was to determine whether infant dietary antigens could be immunizing agents of IAA. To this end, IAA binding to [125I]insulin was competed with food preparations and extracts of foods encountered in the infant diet (milk formulas, bovine milk, wheat flour, fowl meal). Bovine milk powder extracts inhibited IAA-positive samples from six of 53 children (age 0·3–14·0 years) participating in German prospective cohorts. Inhibition in these sera ranged from 23 to 100%. Competition was abolished when hydrolyzed milk powder was used. Competition with protein components of bovine milk found that two of the six milk-reactive sera were inhibited strongly by alpha- and beta-casein; none were inhibited by the milk proteins bovine serum albumin or lactoglobulins. The two casein-reactive sera had high affinity to alpha-casein (1·7 × 109; 3·1 × 109 l/mol), and lesser affinity to beta-casein (4·0 × 108; 7·0 × 107 l/mol) and insulin (2·6 × 108; 1·6 × 108 l/mol). No children with milk-reactive IAA developed autoantibodies to other islet autoantigens or diabetes (median follow-up 9·8 years). These results suggest that autoimmunity to insulin can occur infrequently via cross-reactivity to food proteins, but this form of IAA immunization does not appear to be associated with progression to diabetes.
Keywords: autoimmunity, casein, food components, insulin autoantibodies, type 1 diabetes
Introduction
Type 1 diabetes (T1D) is a T cell-mediated autoimmune disease leading to the destruction of insulin producing beta cells in the pancreatic islets of Langerhans. Autoantibodies against islet beta cell antigens can appear early in life and predict the development of T1D [1–5]. Autoantibodies against insulin (IAA) are often the first autoantibodies that are detected in childhood. Some, but not all children who have IAA also develop antibodies to other beta cell antigens, and subsequently overt T1D [1,2,5,6].
Genetic risk factors predispose, and environmental factors modify, the risk of developing T1D [7]. There are several suggestions that antigen exposure in the gut and through diet may modify the risk of T1D [8]. Food introduction into the human diet varies between countries and ethnicity, but general principles are exclusive breast milk or formula feeding during the first 4–6 months of life, with solid foods such as cereal, vegetables and fruit being introduced between 17 and 26 weeks of age [9]. Exposure to food components such as the wheat protein gluten [10,11] and bovine insulin from cow's milk [12–15], as well as the bovine milk component casein [16–18], have been discussed as causes of T1D, and in the case of bovine insulin as the primary immunizing antigen giving rise to antibodies that are reactive with human insulin. Other evidence of gut-related immunization includes an increased gut permeability in islet autoantibody-positive relatives [19], preferential trafficking of immune cells and antigens from the gastrointestinal tract to the pancreatic lymph nodes [20] and a role of gut commensal bacteria in the development of autoimmune diabetes [21]. Specifically with respect to IAA, we recently identified insulin binding immunoglobulin (Ig)A that has increased affinity to insulin of species other than human [22]. Relevant to a potential immunization through the gut, islet autoantibodies, including IAA, appear from around 6 months of age, which is after the cessation of full breast feeding and the first introduction of food supplements. Here we have investigated whether IAA contain a component of infant food-reactive antibodies. Specifically, we have performed insulin binding competition with infant food ingredients. We report the identification of insulin binding antibodies that are strongly cross-reactive to casein in a minority of subjects.
Materials and methods
Subjects and samples
Samples were obtained from persistent IAA-positive children participating in the BABYDIAB and BABYDIET studies, where children who are genetically at risk for T1D are followed from birth for the development of islet autoantibodies and diabetes [1,6,23]. Human leucocyte antigen (HLA) typing at the HLA DRB1, DQA1 and DQB1 loci is also performed in the children. The studies were carried out in accordance with the Declaration of Helsinki and all families gave written informed consent to participate in the studies. The ethics committee of Bavaria, Germany (Bayerische Landesaerztekammer no. 95357) approved the BABYDIAB study, and the BABYDIET study was approved by the ethics committee of the Ludwig-Maximilian University, Munich, Germany (Ethikkommission der Medizinischen Fakultaet der Ludwig-Maximilians Universität no. 329/00).
In order to preserve serum from precious samples, the current study design had an exploratory phase in which a limited number of sera were tested against multiple food extracts, and an investigation phase where sera from the majority of IAA-positive children in the BABYDIAB and BABYDIET studies were tested against milk proteins. For the exploratory phase, seven IAA-positive children from the BABYDIAB study whose IAA were heterogeneous in eptiope specificity and affinity, as determined previously [22,24], were selected for competition studies against food proteins. These children had IAA that ranged in affinity to human insulin from 3·0 × 105 l/mol to 3·8 × 108 l/mol and epitope specificity representative of the majority of those we had identified previously in the BABYDIAB study [24]. For the subsequent experiments examining competition with milk proteins, an additional 46 children were identified with persistent IAA and sufficient serum available at or soon after IAA seroconversion from the BABYDIAB and BABYDIET studies. The 53 children included five who had a sibling with T1D, 30 with a T1D mother and 18 with a T1D father; 25 were boys. Their median age at IAA seroconversion was 2·0 years (range: 0·3 to 14·0 years) and nine developed IAA prior to age 1 year. Where possible, the earliest available IAA-positive sample of the subjects was used, and for children with IAA that was inhibited by food extracts the findings were confirmed in multiple samples from each child. In 45 of the 53 samples, the IAA affinity was determined previously by competitive inhibition using a protein A/G radio-binding assay, as described previously [24].
Protein extractions
Whole organic bovine milk powder (Bayerische Milchindustrie eG, Landshut, Germany), milk-containing infant formula [regular non-hydrolyzed (REG); Milumil 1; Milupa GmbH, Friedrichsdorf/Ts, Germany], infant formula adapted closely to human breast milk (PRE; Milupa GmbH); hypo-allergic follow-on formula with hydrolyzed milk components (HA; Milumil HA 2; Milupa GmbH), special milk-free infant formula (SOM; Milupa GmbH), wheat flour (type 550; Davert GmbH, Senden, Germany), fowl meal (Altromin GmbH & Co. KG, Lage, Germany) and defined components from bovine milk such as alpha- and beta-casein (Sigma-Aldrich, St Louis, MO, USA), lactoglobulin (Sigma-Aldrich) and bovine serum albumin (BSA; Sigma-Aldrich) were homogenized into assay buffer (50 mM Tris, 1% Tween, pH 8). After removal of undissolved components by centrifugation, the protein solutions were incubated with Protein A Sepharose (6 mg/ml; GE Healthcare, Chalfont St Giles, UK) and GammaBind Plus Sepharose (8 mg/ml; GE Healthcare) overnight to remove components with non-specific binding. The supernatant protein contents after centrifugation were analysed for purity by gel electrophoresis and for concentration using the BCA Protein Assay Kit (Thermo Fisher Scientific Inc., Rockford, IL, USA) before inclusion into the radio-binding assays.
IAA reactivity and affinity to food proteins
Antibody binding to Tyr14A [125I]human insulin (Sanofi-Aventis Deutschland GmbH, Frankfurt, Germany) was competed with either single or increasing concentrations (4·6 × 10−4 to 113·6 µg/ml) of the individual food protein extracts using the method for IAA affinity determination [24]. Half maximal inhibitory concentration (IC50) and Kd values were calculated using the GraphPad Prism 3 program (GraphPad Software Inc., San Diego, CA, USA), and antibody affinity was expressed as reciprocal Kd values (l/mol) as described previously [24].
T lymphocyte proliferative responses
Peripheral blood mononuclear cells were isolated by Ficoll-Hypaque (Lonza, Basel, Switzerland) density gradient centrifugation from sodium-heparinized venous blood. CD4+ T cells were isolated using a CD4-negative isolation kit II (Miltenyi Biotec, Bergisch Gladbach, Germany). The CD4-negative fraction, containing antigen-presenting cells, was mixed 1:1 with the CD4-positive T cell fraction. The cell mixture was labelled with 0·5 µM carboxyfluorescein succinimidyl ester (CFSE; Invitrogen, Carlsbad, CA, USA) [25] and cultured at 2 × 105 cells/well with antigen and 100 ng/ml recombinant human Fas (Sigma-Aldrich) in X-VIVO 15 medium (Lonza) supplemented with 5% pooled human AB serum in 96-well round-bottomed plates in a standard 37°C CO2 incubator for 7 days. Antigens were alpha- and beta-casein, lactoglobulin, BSA (Sigma-Aldrich), all at 5 µg/ml, tetanus toxoid (TT; Sanofi Pasteur MSD, Leimen, Germany) at 1 µl/ml, and recombinant human proinsulin (Eli Lilly, Indianapolis, IN, USA) at 10 µg/ml. Proliferation of live cells was detected via CFSE dilution and surface marker expression by first staining with anti-human CD3 allophycocyanin (APC)-Cy7, anti-human CD4 APC, anti-human CD25 phycoerythrin (PE) and anti-human CD45RO PE-Cy7 (all BD Bioscience, Franklin Lakes, NJ, USA) and subsequently analysing on a BD LSR-II flow cytometer (BD Bioscience); 7AAD (BD Bioscience) was used to exclude dead cells.
Results
IAA binding to food protein extracts
Initial competition experiments were performed on selected sera from seven children identified previously to have atypical and typical IAA binding patterns [22,24] and two sera from insulin-treated T1D patients (Fig. 1). In all sera, binding to human [125I]insulin was inhibited by insulin. None of the food protein extracts inhibited insulin antibodies in the two insulin-treated patients (T1D·1 and T1D·2), and in two of the seven IAA-positive samples (subjects 1 and 4). In contrast, IAA in two of the seven children were inhibited strongly (subject 2, 100%; subject 6, 93%) and three were inhibited weakly (subject 7, 42%; subject 3, 30%; subject 5, 26%) by high concentrations (100 µg/ml) of whole bovine milk powder (marked by arrows in Fig. 1). Competition curves could confirm inhibition by lower concentrations of whole bovine milk powder extract (Fig. 2a and data not shown). IAA binding in the five sera was also inhibited by regular and hydrolyzed milk formula. The two strongly inhibited sera were also inhibited partially by high concentrations of wheat flour extract (64% and 63%), but not by lower concentrations (data not shown). None of the sera were inhibited by fowl meal.
Fig. 1.
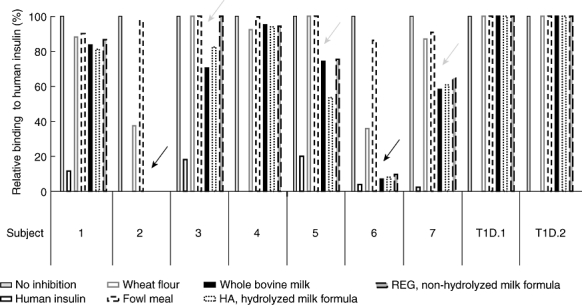
Competition of insulin autoantibody (IAA) binding to human [125I]insulin by addition of different food protein extractions (wheat flour, fowl meal, whole bovine milk, regular non-hydrolyzed (REG) and hydrolyzed (HA) infant milk formula, 100 µg/ml) in comparison to human insulin (2·2 × 10−5 mol/l). Results are shown as binding (cpm) without competition and binding in the presence of the extracts. Results from seven sera selected because of their heterogeneous binding to insulin, as reported previously [22,24], are shown together with sera from two patients with type 1 diabetes. Two of the seven children were inhibited strongly (100%, 93%) and three were inhibited weakly by whole bovine milk powder and milk formulas (marked by arrows).
Fig. 2.
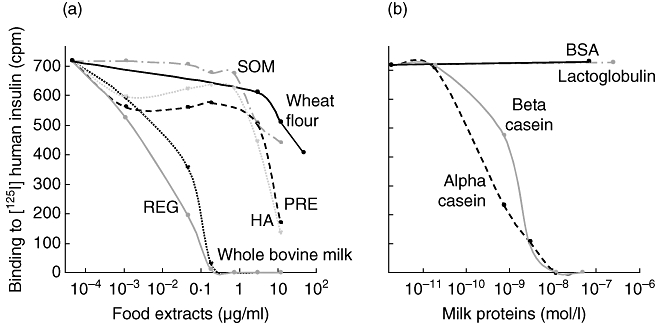
Individual insulin autoantibody (IAA) binding characteristics of one selected sample. Competition of binding to human [125I]insulin by (a) protein extractions from food components [wheat flour, whole bovine milk and four representative infant formulas – regular non-hydrolyzed formula (REG), hypo-allergic hydrolyzed milk formula (HA), infant formula closely adapted to human breast milk (PRE), milk-free infant formula (SOM)] and (b) by milk protein fractions [alpha-casein, beta-casein, lactoglobulin and bovine serum albumin BSA)] are shown. IAA are expressed as bound radioactivity in counts per minute (cpm).
Subsequently, serum samples from a further 46 IAA-positive children representing the majority of the remaining IAA-positive children in the BABYDIAB and BABYDIET studies were screened for milk-reactivity using 9·1 µg/ml whole bovine milk for competition. One of these 46 samples had IAA that could be inhibited partially by milk components (subject 8; 62% inhibition of IAA binding, data not shown). Thus, a total of six children were identified with IAA that could be inhibited partially or fully by whole bovine milk powder.
In contrast to the majority of IAA that were not inhibited by milk proteins which had affinities > 109 l/mol, as reported previously, the six milk-reactive IAA were of low to moderate affinity to insulin (range 3·5 × 105 to 3·8 × 108 l/mol), did not usually bind proinsulin, often bound chicken insulin and/or had an IgA IAA component as determined previously (Table 1). Of note, binding to insulin was shown previously to require the native sequence to residues B28 and B29 in five of the six milk-reactive IAA [24], and this was atypical for IAA associated with progression to T1D [24]. Also, in contrast to other IAA-positive children, none of the six children with milk-reactive IAA developed glutamic acid decarboxylase (GAD) and/or IA-2 autoantibodies or T1D within a median follow-up of 9·8 years [interquartile ratio (IQR) 8·7–11·6].
Table 1.
Subject and sample characteristics of insulin autoantibodies (IAA) that are inhibited by whole bovine milk proteins
| Subject | Age IAA-positive (years)* | IAA affinity (l/mol) | IAA titre (units) | HLA DRB1/DQB1 genotype | Insulin B chain amino acids required for IAA binding | IAA binding to proinsulin | IAA binding to chicken insulin | IgA–IAA component |
|---|---|---|---|---|---|---|---|---|
| 2 | 2·8–8·7 | 3·8 × 108 | 204·4 | 04,08/0302,0402 | B28, B29 | No | Yes | Yes |
| 6 | 1·8–7·5 | 2·2 × 108 | 20·8 | 04,16/not tested | B28, B29 | Yes | No | No |
| 3 | 9·4–11·0 | 3·5 × 105 | 108·0 | 01,07/0501,02 | B28, B29, B30 | No | Yes | Yes |
| 5 | 4·5–8·6 | 3·5 × 107 | 7·5 | 04,16/0302,0502 | B28, B29, B30 | No | No | Not tested |
| 7 | 2·6–3·1 | 2·0 × 107 | 34·6 | 14,16/0502,0503 | B28, B29 | No | No | No |
| 8 | 5·2–12·6 | 4·0 × 105 | 13·5 | 03,13/02,0603 | None identified | Yes | Yes | Yes |
The age range of positivity refers to that range in which samples were measured and tested positive. The earliest sample used for the competition analyses were the first positive sample for all the listed cases except subject 5 (age 5·6-year sample) and subject 8 (age 7·1-year sample). The titre and affinity in the table are for this sample. IgA: immunoglobulin A; HLA: human leucocyte antigen.
IAA binding to bovine milk protein fractions
IAA competition curves were generated for the sample with the strongest binding to bovine milk (indicated as subject 2 in Fig. 1) using multiple concentrations of protein extractions from whole bovine milk powder, four milk-containing infant formula diets and wheat flour (Fig. 2a). The IAA in this sample showed preferred binding to whole bovine milk and to regular (non-hydrolyzed) milk-containing infant formula. The hydrolyzed milk formula extract (HA) and the human breast milk-adapted formula (PRE) were inhibitory only at high concentrations, whereas the milk-free formula extract (SOM) and wheat protein extracts were only partially inhibitory at the highest concentration used. Sample 2 was also used to investigate the ability of selected bovine milk proteins to compete the binding of IAA to human [125I]insulin (Fig. 2b). IAA binding to insulin was inhibited completely by alpha- and beta-casein, and was unaffected by lactoglobulin or BSA, indicating caseins as preferred targets of IAA in subject 2. Identical competition curves were obtained on multiple samples obtained from subject 2 (data not shown).
The IAA affinities to alpha- and beta-casein in comparison to human insulin were determined for the six subjects that were inhibited by whole bovine milk (Fig. 3). For the two strongly milk-reactive IAA (subjects 2 and 6), affinities to the alpha-casein (1·7 × 109 and 3·1 × 109 l/mol) were approximately 1 log higher than to insulin (2·6 × 108 and 1·6 × 108 l/mol, respectively). Affinity to beta-casein was similar to that against insulin in these two sera. The IAA of the remaining four sera tested that had only partial inhibition with whole milk were not inhibited with casein. None of the six sera were inhibited by BSA or lactoglobulin (data not shown).
Fig. 3.
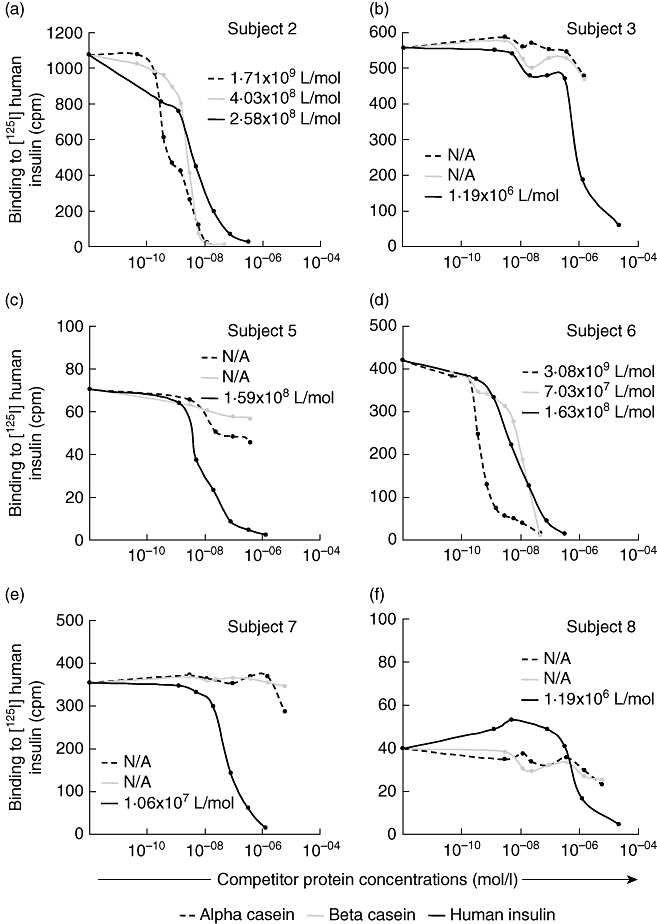
Competition of binding to human [125I]insulin by alpha- (dotted line) and beta-casein (grey line) in comparison to human insulin (black line) from the six children with insulin autoantibodies (IAA) that bind to whole bovine milk; in (a) subject 2, (b) subject 3, (c) subject 5, (d) subject 6, (e) subject 7 and (f) subject 8. The affinities to the competing protein are indicated by the numbers within the figures (l/mol). IAA in (a) and (d) bind better to alpha-casein than to human insulin, and in (b), (c), (e) and (f) bind to human insulin only. IAA are expressed as bound radioactivity in counts per minute (cpm).
T cell responses to casein
Fresh peripheral blood was obtained from subject 2 (strongly casein-reactive IAA), subject 5 (partially milk-reactive, but not casein-reactive IAA) and the two T1D patients (T1D·1 and T1D·2), and T cell proliferative responses to alpha-casein, beta-casein, BSA, proinsulin and TT were examined by the CFSE dilution assay. In this assay we identify the activated proliferated CD4+ T cell population (CSFEdimCD25+CD45RO+CD4+ cells) in the cell culture after 7 days as the antigen responsive cells and express this as a percentage of all CD4+ cells at the end of culture (Fig. 4a). Compared to the medium control (0·02%), a clear response was seen in subject 2 to alpha-casein (0·35%; stimulation index 17·5), beta-casein (0·20%; stimulation index 10) and BSA (0·13%; stimulation index 6·5), as well as the recall antigen TT (0·80%; stimulation index 160), but not to proinsulin (0·04%; stimulation index 2) (Fig. 4b). None of the other subjects had responses to alpha- or beta-caseins (all had stimulation indices <1·5). A response to proinsulin was observed in only patient T1D·2 (0·07%; stimulation index 7·4).
Fig. 4.
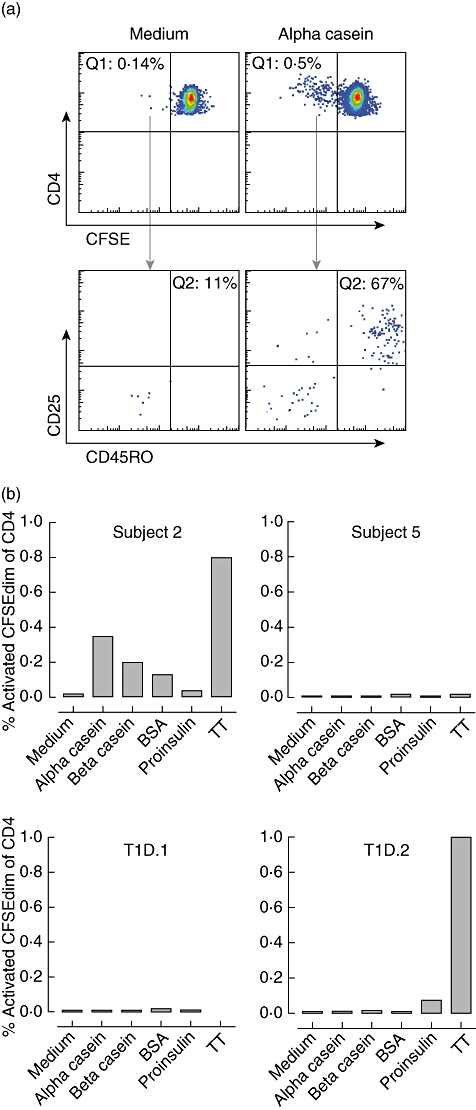
Detection of CD4+ T cell antigen-specific responses. (a) Representative fluorescence activated cell sorter (FACS) plots of carboxyfluorescein succinimidyl ester (CFSE) dilution obtained in subject 2. T cells were labelled with CFSE and incubated with alpha-casein or no antigen (medium). In response to alpha-casein, most of the proliferating CD4+CFSEdim T cells (Q1; upper panel) also up-regulated the activation marker CD25 and memory marker CD45RO (Q2; lower panel) underlining the specificity of the T cell response. (b) The percentage of CD4 T cells which were CFSEdim and CD25+CD45RO+ after 7-day culture with alpha-casein (5 µg/ml), beta-casein (5 µg/ml), bovine serum albumin (BSA) (5 µg/ml), proinsulin (10 µg/ml), tetanus toxoid (TT, 1 µl/ml) or medium only in subjects 2 and 5 and patients T1D·1 and T1D·2 from Fig. 1.
Discussion
Immune responses to dietary constituents that are associated with autoimmunity would provide evidence for causal factors in the pathogenesis of autoimmune disease. In this study we have continued previous efforts to investigate the effects of early dietary food supplements on initial immune reactions to insulin [22,26]. We tested whether autoantibodies to insulin could cross-react with food substances introduced typically into the diet of young infants. Remarkably, we found that the milk protein casein is a high-affinity target of IAA in a minority (4%) of children. However, children with these casein-reactive IAA are not characteristic of prediabetic individuals as they have no evidence of autoimmunity to the other diabetes-associated antigens GAD65, IA-2 and zinc transporter 8, and moderate- to low-affinity IAA. We suggest that autoimmunity can be secondary to immunization against exogenous antigens, but in the case of IAA this rarely leads to disease.
The influence of bovine milk and its constituents on the development of autoimmune diabetes in animal models as well as in humans remains controversial [27,28]. Caseins, beta-lactoglobulin, alpha-lactalbumin, gamma-globulin and BSA are the five major protein fractions from bovine milk [29]. Among those proteins, beta-casein, beta-lactoglobulin and BSA have been implicated as sources of potential diabetes-relevant antigens [30]. Here we identify casein-reactive IAA by screening a panel of previously characterized IAA that were heterogeneous in their affinity and insulin epitope specificity. A number of food homogenates were tested for their ability to inhibit binding of these IAA to insulin. Only infant foods containing milk products were shown to have potent inhibitory effect on the selected IAA. Although the panel of sera selected may not include all IAA specificities, they probably include the vast majority that develop during childhood in an at-risk cohort. Specificity to cow milk proteins was suggested further by the fact that hydrolyzed infant milk formulas and human breast milk-adapted milk formulas were only inhibitory of IAA at high concentrations. In two of six cases containing IAA that were inhibited by the milk-containing preparations, low amounts of the casein proteins but not lactoglobulin or albumin competed antibody binding to insulin. Thus, casein and insulin are the only molecules bound by these antibodies with substantial affinity. Moreover, the affinity of the antibodies to the alpha-casein was up to 1 log higher than that against insulin. These findings suggest that the casein and insulin molecules include portions that share similar conformation, that this conformationally similar region can be a target of humoral immune responses, and that the humoral immune response against this region binds an alpha-casein counterpart preferentially.
There were some similarities among the two children who had casein-reactive IAA. Both developed their IAA at around 2 years of age and both had the HLA DR4 allele. The IAA affinity to insulin was similar for both cases and was between 108 and 109 l/mol. In both children, IAA were confirmed in a second laboratory using a different insulin tracer. IAA were persistently positive for 6·0 and 4·5 years. The best-characterized of the two casein-reactive IAA bound insulin from other species, including the relatively distant chicken and fish insulins with similar affinity [22,24]. In both children, the IAA were strictly dependent on the correct confirmation at positions B28 and B29. Of interest, most of the other milk-reactive IAA were also dependent on having proline and lysine at B chain positions 28 and 29, respectively. Examining the sequences of alpha- and beta-casein shows potential similarities with bovine alpha-S2-casein (NP_776953·1, GI:27806963), where the amino acid sequence proline, lysine and threonine at positions 211 to 213 is identical to positions 28, 29 and 30 of the human insulin B chain. Beta-casein has proline, valine and lysine at these positions, indicating that if this region was involved in the antibody binding, it is likely to be only a portion of the epitope.
Reactivity to multiple antigens by single antibodies has been demonstrated previously [31]. These are the so-called polyreactive antibodies of IgM isotype and include binding to insulin, tetanus, DNA, thyroglobulin and other molecules [32]. The fact that the casein-reactive IAA were of IgG isotype speaks against a form of similar polyreactive antibodies. We sought to find further evidence for a primary immune response to casein by examining T cell responses in one of the children with high titre casein-reactive IAA. We reasoned that if casein was the primary antigen target of the autoantibodies, we were likely to see a T cell response to casein. Relatively pronounced responses to alpha- and beta-casein and BSA, but not proinsulin, were observed in the T cell population from the peripheral blood of this child, suggesting that a pool of T cells with the potential to respond to casein was present. Casein-responding T cells were not observed in subject 5 with milk-reactive IAA that were not against casein and in the patients with type 1 diabetes. We did not, however, determine whether the casein-responding T cells from subject 2 included T cells from the starting memory T cell pool and cannot be certain that the child had a casein-primed T cell pool. Thus, it remains possible that a related or different antigen could be the primary target of the immunity. Further evidence for casein as a driving antigen of the insulin autoimmunity in these children would be obtained by dietary deprivation followed by dietary challenge. This was not considered sufficiently important for the children, as none reported obvious signs of dairy intolerance or hypoglycaemia which has been observed previously in some patients with insulin autoimmune syndrome [33].
In conclusion, we present evidence that IAA can arise secondarily to immunization against other proteins and suggest that one of these could be the alpha- and/or beta-caseins which are derived from dietary sources such as milk. However, in contrast to previous reports that have suggested a causative role for immunity against milk proteins in the pathogenesis of diabetes, we do not find any evidence that these milk-reactive IAA are related to the pathogenesis of T1D, and believe that the children with such IAA are highly unlikely to develop autoimmunity to other beta cell autoantigens or T1D.
Acknowledgments
This study forms part of the dissertation of Daniela B. Mueller at the Technical University of Munich. It was supported by the Deutsche Forschungsgemeinschaft (ZI 310/12-6, ZI 310/14-4, FZ 111), the Juvenile Diabetes Research Foundation (1-2006-665, P. Achenbach: 11-2005-1117) and the NIH/DFG Research Career Transition Award Program (K. Adler: KO 3418/1-1). The authors thank Andreas S. Mueller, Annette Knopff, Evi Wagner and Natalie Grober for excellent technical support.
Disclosure
None of the authors have anything to declare.
Trial registration number
At ClinicalTrials.gov for the BABYDIET study: NCT01115621.
References
- 1.Ziegler AG, Hummel M, Schenker M, Bonifacio E. Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABYDIAB Study. Diabetes. 1999;48:460–8. doi: 10.2337/diabetes.48.3.460. [DOI] [PubMed] [Google Scholar]
- 2.Colman PG, Steele C, Couper JJ, et al. Islet autoimmunity in infants with a type I diabetic relative is common but is frequently restricted to one autoantibody. Diabetologia. 2000;43:203–9. doi: 10.1007/s001250050030. [DOI] [PubMed] [Google Scholar]
- 3.Yu L, Robles DT, Abiru N, et al. Early expression of antiinsulin autoantibodies of humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proc Natl Acad Sci USA. 2000;97:1701–6. doi: 10.1073/pnas.040556697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221–9. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 5.Kimpimaki T, Kulmala P, Savola K, et al. Natural history of beta-cell autoimmunity in young children with increased genetic susceptibility to type 1 diabetes recruited from the general population. J Clin Endocrinol Metab. 2002;87:4572–9. doi: 10.1210/jc.2002-020018. [DOI] [PubMed] [Google Scholar]
- 6.Hummel M, Bonifacio E, Schmid S, Walter M, Knopff A, Ziegler AG. Brief communication: early appearance of islet autoantibodies predicts childhood type 1 diabetes in offspring of diabetic parents. Ann Intern Med. 2004;140:882–6. doi: 10.7326/0003-4819-140-11-200406010-00009. [DOI] [PubMed] [Google Scholar]
- 7.Knip M, Veijola R, Virtanen SM, Hyöty H, Vaarala O, Akerblom HK. Environmental triggers and determinants of type 1 diabetes. Diabetes. 2005;54(Suppl 2):S125–36. doi: 10.2337/diabetes.54.suppl_2.s125. [DOI] [PubMed] [Google Scholar]
- 8.Lefebvre DE, Powell KL, Strom A, Scott FW. Dietary proteins as environmental modifiers of type 1 diabetes mellitus. Annu Rev Nutr. 2006;26:175–202. doi: 10.1146/annurev.nutr.26.061505.111206. [DOI] [PubMed] [Google Scholar]
- 9.Agostoni C, Decsi T, Fewtrell M, et al. Complementary feeding: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2008;46:99–110. doi: 10.1097/01.mpg.0000304464.60788.bd. [DOI] [PubMed] [Google Scholar]
- 10.Norris JM, Barriga K, Klingensmith G, et al. Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA. 2003;290:1713–20. doi: 10.1001/jama.290.13.1713. [DOI] [PubMed] [Google Scholar]
- 11.Ziegler AG, Schmid S, Huber D, Hummel M, Bonifacio E. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA. 2003;290:1721–8. doi: 10.1001/jama.290.13.1721. [DOI] [PubMed] [Google Scholar]
- 12.Vaarala O, Saukkonen T, Savilahti E, Klemola T, Akerblom HK. Development of immune response to cow's milk proteins in infants receiving cow's milk or hydrolyzed formula. J Allergy Clin Immunol. 1995;96:917–23. doi: 10.1016/s0091-6749(95)70229-6. [DOI] [PubMed] [Google Scholar]
- 13.Vaarala O, Paronen J, Otonkoski T, Akerblom HK. Cow milk feeding induces antibodies to insulin in children – a link between cow milk and insulin-dependent diabetes mellitus? Scand J Immunol. 1998;47:131–5. doi: 10.1046/j.1365-3083.1998.00282.x. [DOI] [PubMed] [Google Scholar]
- 14.Vaarala O, Knip M, Paronen J, et al. Cow's milk formula feeding induces primary immunization to insulin in infants at genetic risk for type 1 diabetes. Diabetes. 1999;48:1389–94. doi: 10.2337/diabetes.48.7.1389. [DOI] [PubMed] [Google Scholar]
- 15.Makela M, Vaarala O, Hermann R, et al. Enteral virus infections in early childhood and an enhanced type 1 diabetes-associated antibody response to dietary insulin. J Autoimmun. 2006;27:54–61. doi: 10.1016/j.jaut.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Cavallo MG, Fava D, Monetini L, Barone F, Pozzilli P. Cell-mediated immune response to b-casein in recent-onset insulin-dependent diabetes: implications for disease pathogenesis. Lancet. 1996;348:926–8. doi: 10.1016/S0140-6736(95)12065-3. [DOI] [PubMed] [Google Scholar]
- 17.Elliott RB, Wasmuth HE, Bibby NJ, Hill JP. Seminar on milk protein polymorphism. Brussels, Belgium: IDF; 1997. The role of beta-casein in the induction of insulin-dependent diabetes in the non-obese diabetic mouse and humans; pp. 445–53. In: International Dairy Federation (IDF), ed. Special Issue 9702. [Google Scholar]
- 18.Thorsdottir I, Birgisdottir BE, Johannsdottir IM, et al. Different beta-casein fractions in Icelandic versus Scandinavian cow's milk may influence diabetogenicity of cow's milk in infancy and explain low incidence of insulin-dependent diabetes mellitus in Iceland. Pediatrics. 2000;106:719–24. doi: 10.1542/peds.106.4.719. [DOI] [PubMed] [Google Scholar]
- 19.Bosi E, Molteni L, Radaelli MG, et al. Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia. 2006;49:2824–7. doi: 10.1007/s00125-006-0465-3. [DOI] [PubMed] [Google Scholar]
- 20.Turley SJ, Lee JW, Dutton-Swain N, Mathis D, Benoist C. Endocrine self and gut non-self intersect in the pancreatic lymph nodes. Proc Natl Acad Sci USA. 2005;102:17729–33. doi: 10.1073/pnas.0509006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wen L, Ley RE, Volchkov PY, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–13. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koczwara K, Muller D, Achenbach P, Ziegler AG, Bonifacio E. Identification of insulin autoantibodies of IgA isotype that preferentially target non-human insulin. Clin Immunol. 2007;124:77–82. doi: 10.1016/j.clim.2007.03.545. [DOI] [PubMed] [Google Scholar]
- 23.Schmid S, Buuck D, Knopff A, Bonifacio E, Ziegler AG. BABYDIET, a feasibility study to prevent the appearance of islet autoantibodies in relatives of patients with Type 1 diabetes by delaying exposure to gluten. Diabetologia. 2004;47:1130–1. doi: 10.1007/s00125-004-1420-9. [DOI] [PubMed] [Google Scholar]
- 24.Achenbach P, Koczwara K, Knopff A, Naserke H, Ziegler AG, Bonifacio E. Mature high-affinity immune responses to (pro)insulin anticipate the autoimmune cascade that leads to type 1 diabetes. J Clin Invest. 2004;114:589–97. doi: 10.1172/JCI21307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monti P, Scirpoli M, Rigamonti A, et al. Evidence for in vivo primed and expanded autoreactive T cells as a specific feature of patients with type 1 diabetes. J Immunol. 2007;79:5785–92. doi: 10.4049/jimmunol.179.9.5785. [DOI] [PubMed] [Google Scholar]
- 26.Mueller DB, Koczwara K, Mueller AS, Pallauf J, Ziegler AG, Bonifacio E. Influence of early nutritional components on the development of murine autoimmune diabetes. Ann Nutr Metab. 2009;54:208–17. doi: 10.1159/000220416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wasmuth HE, Kolb H. Cow's milk and immune-mediated diabetes. Proc Nutr Soc. 2000;59:573–9. doi: 10.1017/s0029665100000811. [DOI] [PubMed] [Google Scholar]
- 28.Vaarala O. Is type 1 diabetes a disease of the gut immune system triggered by cow's milk insulin? Adv Exp Med Biol. 2005;569:151–6. doi: 10.1007/1-4020-3535-7_22. [DOI] [PubMed] [Google Scholar]
- 29.Crowther C, Raistrick H. A comparative study of the proteins of the colostrum and milk of the cow and their relations to serum proteins. Biochem J. 1916;10:434–52. doi: 10.1042/bj0100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dosch HM, Becker DJ. Infant feeding and autoimmune diabetes. Adv Exp Med Biol. 2002;503:133–40. doi: 10.1007/978-1-4615-0559-4_15. [DOI] [PubMed] [Google Scholar]
- 31.Rosenstein RW, Musson RA, Armstrong MK, Konigsberg WH, Richards FF. Contact regions for dinitrophenyl and menadione haptens in an immunoglobulin binding more than one antigen. Proc Natl Acad Sci USA. 1972;69:877–81. doi: 10.1073/pnas.69.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casali P, Nakamura M, Ginsberg-Fellner F, Notkins AL. Frequency of B cells committed to the production of antibodies to insulin in newly diagnosed patients with insulin-dependent diabetes mellitus and generation of high affinity human monoclonal IgG to insulin. J Immunol. 1990;144:3741–7. [PubMed] [Google Scholar]
- 33.Ichihara K, Shima K, Saito Y, Nonaka K, Tarui S. Mechanism of hypoglycemia observed in a patient with insulin autoimmune syndrome. Diabetes. 1977;26:500–6. doi: 10.2337/diab.26.5.500. [DOI] [PubMed] [Google Scholar]


