Abstract
Tuberculosis is a worldwide health problem, and multidrug-resistant (MDR) and extensively multidrug-resistant (XMDR) strains are rapidly emerging and threatening the control of this disease. These problems motivate the search for new treatment strategies. One potential strategy is immunotherapy using cationic anti-microbial peptides. The capacity of l-isoleucine to induce beta-defensin expression and its potential therapeutic efficiency were studied in a mouse model of progressive pulmonary tuberculosis. BALB/c mice were infected with Mycobacterium tuberculosis strain H37Rv or with a MDR clinical isolate by the intratracheal route. After 60 days of infection, when disease was in its progressive phase, mice were treated with 250 µg of intratracheal l-isoleucine every 48 h. Bacillary loads were determined by colony-forming units, protein and cytokine gene expression were determined by immunohistochemistry and reverse transcription–quantitative polymerase chain reaction (RT–qPCR), respectively, and tissue damage was quantified by automated morphometry. Administration of l-isoleucine induced a significant increase of beta-defensins 3 and 4 which was associated with decreased bacillary loads and tissue damage. This was seen in animals infected with the antibiotic-sensitive strain H37Rv and with the MDR clinical isolate. Thus, induction of beta-defensins might be a potential therapy that can aid in the control of this significant infectious disease.
Keywords: anti-microbial peptides, defensins, l-isoleucine, therapy, tuberculosis
Introduction
Tuberculosis (TB) is a worldwide health problem. Reports by the World Health Organization indicate that there are 8 million new cases and 1·6 million deaths yearly due to this disease [1,2]. Moreover, Mycobacterium tuberculosis (Mtb) is highly infectious. It has been reported that nearly one-third of the world's population is latently infected, but only about 10% of these infected individuals will develop active disease [2].
Although TB can be controlled and cured by chemotherapy, treatment requires at least four specific drugs and 6 months of therapy, which produce problems in compliance. The consequence of this is disease relapse, and more importantly the development of multidrug-resistant (MDR) and extensively multidrug-resistant (XMDR) strains. In the last few years these strains have increased in frequency and now afflict approximately 2 million people worldwide [3]. These problems have motivated the search for new treatment strategies. One such strategy is immunotherapy, which requires a better understanding of the immune response against Mtb. Innate immunity has been recognized as a significant participant in the control of mycobacterial growth [4]. In this regard, it is considered that lung epithelial cells and macrophages are the first cells that encounter Mtb during primary infection [4–6]. Interestingly, not only macrophages but also bronchial cells can participate in the elimination of bacilli because epithelial cells can produce molecules of innate immunity such as β-defensins and cathelicidins, which are small cationic anti-microbial peptides [4,7–12]. Defensins contribute directly to defence against pathogens by killing microbes and chemoattracting and activating inflammatory cells in the infection site [8,9,13–15]. Defensins are divided into three subfamilies: α, β and θ, that differ in the position of their disulphide bridges. There are four different human β-defensins (HBD 1–4) that are expressed largely in epithelial cells from different organs, and except for HBD-1, which is expressed constitutively, all β-defensins are induced [16–18]. The expression and up-regulation of these anti-microbial peptides can be induced by pathogen-associated molecular patterns such as lipopolysaccharides (LPS) or by some proinflammatory cytokines [tumour necrosis factor (TNF)-α, interleukin (IL)-1β, interferon (IFN)-γ][19,20]. Interestingly, recent reports demonstrated that amino acids such as l-isoleucine or proteins such as albumin can induce the expression of these peptides [18,21], and a small amount of defensins can control infections efficiently in experimental animal models [22].
In a murine model of progressive pulmonary tuberculosis, we recently showed rapid and stable expression of murine beta defensins (mBD) 3 and 4 by the bronchial epithelium during the early phase of infection, when control of bacterial growth is efficient. Then, during the late progressive phase of the disease when uncontrolled bacillary proliferation occurs, a pronounced decrease of both mBD was detected. These observations provided circumstantial evidence that mBD-3 and mBD-4 provide significant control of bacterial growth during the early phase of experimental tuberculosis [12]. To confirm this, and to determine if mBD could be a novel form of immunotherapy, we sought to determine whether it is possible to reinduce expression of these anti-microbial peptides with l-isoleucine during the late progressive phase of experimental tuberculosis and, if so, whether induction of mBD leads to control of bacterial growth.
Materials and methods
Induction of β-defensins in type II pneumocytes by l -isoleucine in vitro
To determine if l-isoleucine is able to induce β-defensin production in lung epithelial cells in vitro, human type II alveolar pneumocytes [A549; American Type Culture Collection (ATCC) reference number CCL185] were first grown in 75 cm2 culture flasks (Costar, Ontario, Canada) with antibiotic-free RPMI-1640 medium (Gibco BRL, Grand Island, NY, USA) supplemented with 10% fetal calf serum (FCS) (HyClone Laboratories, Logan, UT, USA) at 37°C with 5% CO2. Then A549 cells were seeded into 24-well plates at a concentration of 105 cells per ml of culture medium with 1% of FCS and after 24 h they were stimulated with different concentrations of l-isoleucine (3, 7, 12, 25, 50 and 100 µg/ml) in the presence of 5% CO2 at 37°C. After 1, 6, 12 and 18 h of incubation, A549 cells were collected and lysed in 350 µl RLT buffer (Qiagen, Valencia, CA, USA) for each 105 cells and kept at −70°C until use. Published results have shown that very high concentrations of the enantiomer d-isoleucine are necessary to induce β-defensin production [21]. Thus, we used selected concentrations of d-isoleucine as negative control (50 µg/ml per well).
Human defensin-2 (HBD-2) gene expression was determined by real-time polymerase chain reaction (PCR) following the method described previously [4], and protein production by immunohistochemistry.
For immunohistochemistry, A549 cells were grown to confluence (95%) on four-well chamber slides (Costar) with F-10 medium. Subsequent to l-isoleucine stimuli, as reported above, cells were fixed with formaldehyde 10% for 2 h and stored at 4°C in phosphate-buffered saline solution (PBS). Slides were blocked with 5% goat serum for 20 min, and incubated subsequently with 1 : 5000 dilution of HBD-2 antibody (Peptide International, Osaka, Japan) in 5% goat serum at 4°C for 18 h. Slides were then developed with biotinylated goat anti-rabbit immunoglobulin (Ig)G using Vectastain ABC kit (Vector Laboratories, Burlingame, CA, USA). Then, slides were counterstained with haematoxylin and visualized with a light microscopy axiovert 200 m (Carl Zeiss, Gena, Germany).
Induction of β-defensins in murine lung by l -isoleucine
To test whether l-isoleucine induced β-defensin production in vivo, we dissolved it in physiological saline solution obtaining different concentrations from 25 µg/100 µl to 1 mg/100 µl. These preparations were then administered to male BALB/c mice by the intratracheal route. After 12, 18, 24 and 48 h animals were euthanized and their lungs removed immediately for analysis of defensin production. As control, we used the vehicle (saline solution) and selected concentrations of d-isoleucine (250 µg/100 µl). Due to the high number of samples, conventional reverse transcription (RT)–PCR was used to determine defensin expression. After the mice were euthanized, lungs were removed, hilar lymph nodes and thymus were eliminated and the tissue was frozen immediately by immersion in liquid nitrogen. Three lungs, right or left, from different mice were used to isolate mRNA from each group at each time-point, and the cDNA from the three mice was analysed separately. mRNA was isolated by use of Trizol (Gibco BRL); cDNA was synthesized by use of Maloney murine leukemia virus reverse transcriptase (Gibco BRL) and priming with oligo dT. The PCR products were electrophoresed on 6% polyacrylamide gels, and molecular-weight standards with known DNA mass concentrations (low DNA mass ladder; Gibco BRL) were run. The PCR products were then analysed by use of an image-analysis densitometer linked to a computer program (ID image-analysis software; Kodak Digital Science, Sn Leandro, CA, USA). To determine, in nanograms, the quantity of PCR product the computer program compared the optical densities from the experimental samples with the molecular-marker bands, the DNA content of which was provided by the manufacturer. To correct for errors in the quantity of starting material, the densitometer reading of the glyceraldehyde-3-phosphate dehydrogenase (G3PDH) PCR product was used. Production of mBD was confirmed by immunohistochemistry following the procedure described below.
Experimental model of progressive pulmonary TB in BALB/c mice
The experimental model of progressive pulmonary TB has been described in detail elsewhere [12,23,24]. Briefly, the laboratory drug-sensitive Mtb strain H37Rv (ATCC no. 25618) and MDR strain (clinical isolate, resistant to all first-line antibiotics) were grown in Middlebrook 7H9 broth (Difco Laboratories, Detroit, MI, USA) supplemented with 0·2% (v/v) glycerol, 10% oleic albumin dextrose catalase (OADC) enrichment (Difco) and 0·02% (v/v) Tween-80 at 37°C. Mid log-phase cultures were used for all experiments. Mycobacteria were counted and stored at –80°C until use. Bacterial aliquots were thawed and pulse-sonicated to remove clumps [25].
Male BALB/c mice, 6–8 weeks of age, were anaesthetized in a gas chamber using 0·1 ml per mice of sevofluorane and infected through endotracheal instillation with 2·5 × 105 live bacilli. Mice were maintained in the vertical position until spontaneous recovery. Infected mice were maintained in groups of five in cages fitted with micro-isolators. Animal work was performed in accordance with the national regulations on Animal Care and Experimentation (NOM 062-ZOO-1999).
Treatment of the infected mice with l -isoleucine
After 60 days of infection, survivor animals were allocated arbitrarily into four groups of 20 animals each. l-isoleucine treatment was started 60 days after infection, when advanced progressive disease is well established. The experiments conducted to determine in vivo the efficiency of mBD production by l-isolucine showed that 250 µg/100 µl was the most efficient concentration to induce mBD in non-infected mice. Thus, we used this dose for the therapeutic experiments, performing two separate experiments.
Six animals in each group were euthanized at 15, 30 and 60 days after starting treatment. The first group infected with the drug-sensitive H37Rv strain received l-isoleucine (250 µg/100 µl), dissolved in saline solution, every 48 h by intratracheal instillation. A second group (controls) was infected with H37Rv strain and received only the vehicle (saline solution) by the same route and timing. The third group was infected with the MDR strain, and received l-isoleucine by the same route and schedule. The fourth group was infected with the MDR strain but served as a control, receiving only the vehicle. The efficiency of the l-isoleucine treatment was determined by quantifying the lung bacillary loads by counting colony-forming units (CFU), extent of tissue damage by histopathology and automated morphometry and lung cytokine gene expression by real-time RT–PCR.
Determination of CFU in infected lungs
Right or left lungs from three mice in each time-point in two different experiments were used. Lungs were homogenized with a polytron (Kinematica, Lucerne, Switzerland) in sterile tubes containing 1 ml PBS, Tween-80 at 0·05%. Five dilutions of each homogenate were spread onto duplicate plates containing Bacto Middlebrook 7H10 agar (Difco) enriched with oleic acid, albumin, catalase and dextrose-enriched medium (Becton Dickinson, Sparks, MD, USA). The plates were incubated at 37°C with 5% CO2. The number of colonies was counted 21 days after plating.
Preparation of lung tissue for histology/morphometry and mBD detection by immunohistochemistry
Right or left lungs from three different animals per time-point and group were perfused intratracheally with ethyl alcohol (J:T: Baker, Mexico City, Mèxico). Lungs were dehydrated and embedded in paraffin (Oxford Labware, St Louis, MO, USA), sectioned and stained with haematoxylin and eosin. The percentages of the lung surfaces affected by pneumonia were determined using an automated image analyser (Q Win Leica, Milton Keynes, Cambridge, UK).
For immunohistochemical detection of mBD-3, 5-µm-thick sections were mounted on silane-coated slides, deparaffinized, the endogenous peroxidase quenched with 0·03% H2O2 in absolute methanol and blocked with 2% human serum dissolved in PBS. Lung sections were incubated for 18 h with goat anti-mouse mBD-3 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). After washing, sections were incubated for 2 h with a donkey anti-goat IgG biotin-labelled antibody. Bound antibodies were detected with avidin–biotin peroxidase (Biocare Medical, Concord, CA, USA) and counterstained with haematoxylin.
Expression of anti-microbial peptides and cytokines determined by real-time RT–PCR
Three lungs, right or left, from two different experiments were used for isolating RNA and synthesis of cDNA as described previously [12]. Real-time PCR was performed using the 7500 real-time PCR system (Applied Biosystems, Foster City, CA, USA) and Quantitect SYBR Green Master-mix kit (Qiagen, Hilden, Germany). Standard curves of quantified and diluted PCR product, as well as negative controls, were included in each PCR run. The primers used for the real-time PCR analysis were: mBD-3: 5′- ATC CAT TAC CTT CTG TTT GCA TTT C-3′ and 5′- TGT AGG TGG AGA CAG CAG C-3′; mBD-4: 5′-CAC ATT TCT CCT GGT GCT GCT-3′ and 5′-TGA TAA TTT GGG TAA AGG CTG CA-3′; IFN-γ: 5′-GGTGACATGAAAATCCTGCAG-3′ and 5′-CC TCAAACTTGGCAATACTCATGA-3′; TNF-α: 5′-TGTGG CTTCGACCTCTACCTC-3′, 5′-GCCGAGAAAGGCTGCT TG-3′; and glyceraldehyde-3-phosphate dehydrogenase (G3PDH): 5′-GGC GCT CAC CAA AAC ATC A-3′ and 5′-CCG GAA TGC CAT TCC TGT TA-3′. The specificity of each product was confirmed by PCR in agarose gels. The cell cycling conditions used were initial denaturation at 95°C for 15 min, followed by 40 cycles each at 95°C for 20 s, 60°C or 58°C, respectively, for 20 s, and 72°C for 34 s. Quantities of the specific mRNA in the sample were measured in accordance with the corresponding gene specific standard. The mRNA copy number of each cytokine was related to 1 million copies of mRNA encoding the G3PDH gene.
Statistical analysis
Data from the qPCR assays as well as CFUs and histopathology were analysed as follows. Kolmogorov–Smirnov normality tests were performed for each data set to choose the appropriate group comparison test. For each treatment the dependent variable was the expression, CFUs or variable of interest compared among different time-periods, treatment or not with l-isoleucine and the interaction of both in an interaction model. Results that were significant overall with a regular two-way analysis of variance (anova) (not repeated-measures) were submitted to pairwise comparisons by Bonferroni's post-test. Two-sided P-values of < 0·05 were considered statistically significant. Statistical analyses were performed using the GraphPad Prism version 5·02 for Windows (GraphPad Software, San Diego, CA, USA).
Results
Induction of β-defensin by l -isoleucine in the lung epithelial cell line A549 in vitro
It has been demonstrated that l-isoleucine induces the production of β-defensins efficiently in bovine kidney epithelial cells in vitro, while its enantiomer d-isoleucine required much higher concentrations to achieve this [21]. In order to evaluate if there is a similar effect on human pulmonary epithelial cells, we determined the expression of HBD-2 (of which the homologue in mouse is mBD-3) by real-time RT–PCR using the lung epithelial cell line A549. Our results showed that the expression of this anti-microbial peptide increased with the concentration and time of exposure to l-isoleucine. The highest gene expression was seen after exposure to 25 µg/ml of l-isoleucine for 18 h (Fig. 1a). Immunohistochemistry confirmed the high production of this anti-bacterial peptide induced by l-isoleucine at this time-point (Fig. 1b). In order to confirm the specificity of mBD production by l-isoleucine, A549 cells were incubated during 48 h with 50 µg/ml of d-isoleucine. Figure 1c shows that in these conditions d-isoleucine did not induce the expression of HBD-2.
Fig. 1.
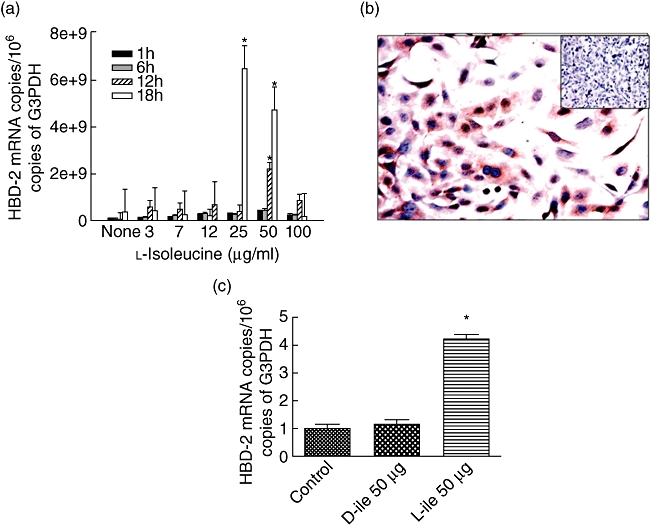
Kinetics of human β-defensin 2 gene expression in the lung epithelial cell line A549 after stimulation with different concentrations of l-isoleucine. (a) A549 cells were stimulated with l-isoleucine using the indicated concentrations. At the indicated time-points the cells were collected, the RNA was isolated and the number of mRNA copies of β-defensin was quantified by real-time reverse transcription–polymerase chain reaction (RT–PCR). All values are mean ± standard deviation of five different experiments. Asterisks represent statistical significance when compared with the control non-stimulated cells. (b) Immunocytochemistry confirmed protein production in the condition in which the cells showed the highest β-defensin expression (25 µg/ml for 18 h), while non-stimulated cells do not show immunostaining (inset). (c) In comparison with l-isoleucine, A549 cells stimulated with 50 µg of d-isoleucine during 48 h did not induce human β-defensin 2 gene expression. Asterisks represent statistical significance when compared with the control non-stimulated cells.
Induction in vivo of β-defensins by l -isoleucine in the lung of non-infected BALB/c mice
After the confirming in vitro that l-isoleucine induced β-defensins efficiently in pulmonary epithelial cells, we tested if the intratracheal instillation of this amino acid had a similar effect in the lung of non-infected mice. We tested multiple concentrations of l-isoleucine at several time-points. Both mBD-3 and mBD-4 were induced efficiently using this treatment (Fig. 2). The highest expression of mBD3 was seen after 12 h of stimulation with 250 µg/100 µl of l-isoleucine, while for mBD4 the peak was at 48 h with the same concentration. In contrast with l-isoleucine, either saline solution or 250 µg/100 µl of d-isoleucine instilled in control animals induced significantly lower expression of both mBD (Fig. 2).
Fig. 2.
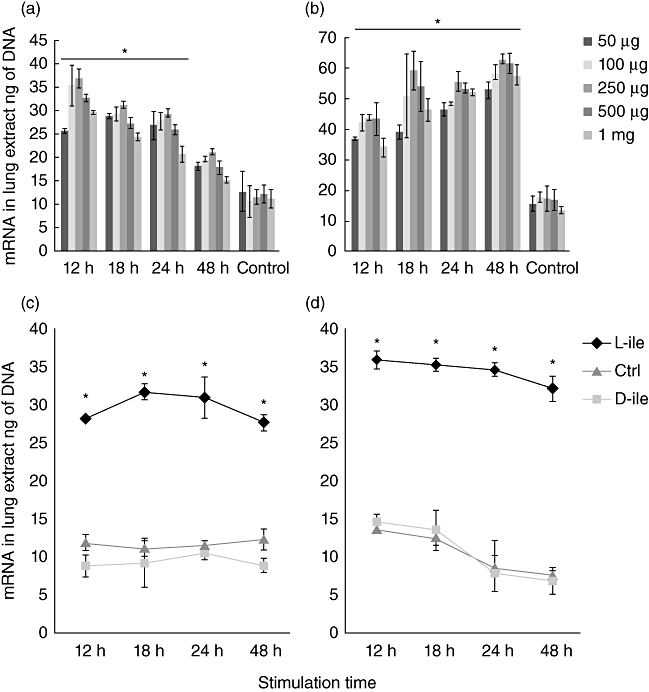
Effect on the β-defensin gene expression in non-infected mice after the administration of l-isoleucine. Groups of BALB/c mice received the indicated amount of l-isoleucine by the intratracheal route, and were euthanized at different time-points and their lungs were used to isolate total mRNA to determine the expression of murine β-defensin 3 (a) and 4 (b). In comparison with l-isoleucine, administration of 250 µg of d-isoleucine or the vehicle saline solution (control group) did not induce gene expression of β-defensin 3 (c) or β-defensin 4 (d). All values are mean ± standard deviation of five mice from three independent experiments. Asterisks represent statistical significance when compared with the control group that received only the vehicle (P < 0·005).
Effect of intratracheal l -isoleucine administration during late progressive tuberculosis produced by the drug-sensitive strain H37Rv
Because 250 µg/100 µl of l-isoleucine administered by intratracheal instillation induced β-defensin production efficiently during 24 to 48 h, this concentration was administered intratracheally every 48 h for 2 months. This treatment was started after 60 days post-infection, when advanced active TB was well established and production of β-defensins decreased. In comparison with control mice, animals treated with l-isoleucine showed significantly higher expression of β-defensins 3 and 4 and lower lung bacillary loads during the whole treatment (Fig. 3). Consistent with these findings, after 4 weeks of treatment, histological examination revealed that the lung areas affected by pneumonia were smaller than in control mice, and the bronchial epithelium in the lungs of treated mice showed strong mBD-3 immunostaining (Figs 3 and 5).
Fig. 3.
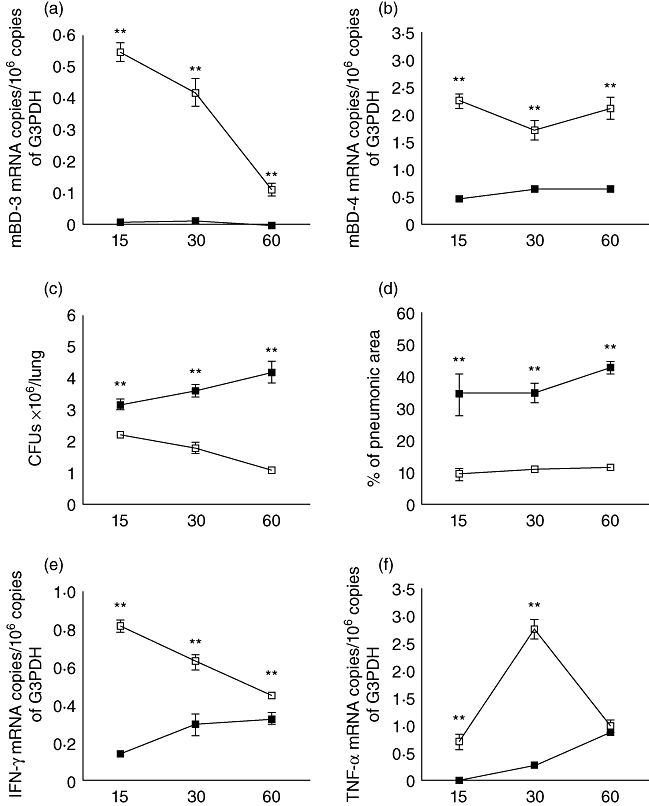
Effect of l-isoleucine administration during advanced disease in the lungs of mice infected with drug-sensitive H37Rv strain. (a) l-isoleucine administration (white symbols) starting 60 days after infection increased mBD3 and mBD4 (b) gene expression when compared with control mice (black symbols). (c) l-isoleucine treatment also decreased pulmonary bacterial loads and (d) the area of pneumonia. (e) In comparison with the control group, l-isoleucine treatment increased the expression of interferon-γ and tumour necrosis factor-α (f). Each point corresponds to the mean and standard deviation of five mice group in one representative experiment. Asterisks represent statistical significance when compared to the control non-treated group (*P < 0·05; **P < 0·01).
Fig. 5.
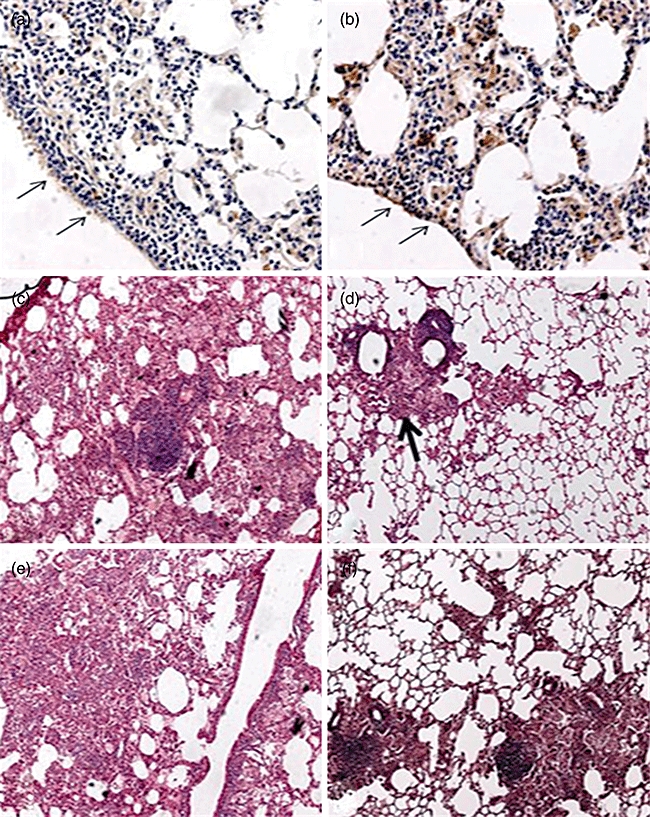
Representative lung histopathology and immunohistochemistry after 2 months of treatment with l-isoleucine and in a control non-treated mouse. (a) Very low expression of mBD3 in the bronchial epithelium (arrows) in the lung of control mouse after 4 months of infection with H37Rv strain. (b) In contrast, there is strong mBD3 immunostaining in the bronchial epithelium (arrows) and in some macrophages in the lung of the mouse infected with strain H37Rv and treated with l-isoleucine. (c) The control animal shows extensive pneumonia after 4 months of infection with drug-sensitive strain H37Rv. (d) In comparison, the l-isoleucine treated mouse shows less lung surface area affected by pneumonia (arrow). (e) Representative micrograph showing extensive pneumonia after 4 months of infection with the multidrug-resistant clinical isolate, while the L-isoleucine-treated mouse shows less lung consolidation (f).
Considering that β-defensins are chemotactic and can activate T helper type 1 (Th1) cells and macrophages [26,27], we determined the expression of IFN-γ and TNF-α in these animals. In comparison with control mice, there was a significant increment of IFN-γ and TNF-α expression in the l-isoleucine-treated group (Fig. 3).
Effect of intratracheal l -isoleucine administration during late progressive tuberculosis produced by multidrug-resistant strain
Due to the emergence of MDR strains, and given the improved course in l-isoleucine-treated mice infected with the drug-sensitive H37Rv strain, we decided to study whether this therapy has the ability to produce similar beneficial effects on mice infected with a clinical isolate resistant to all first-line antibiotics during late active disease. In comparison with control animals, MDR-infected mice treated with l-isoleucine showed a significant increase of mBD-3 and 4 and lower lung bacillary loads (P < 0·01), compatible with the participation of β-defensins in the control of bacillary growth and supporting the beneficial effect of this therapy (Fig. 4). Similarly, improved lung histopathology was seen, with a significant decrease of pneumonia (Figs 4 and 5) at 60 days of treatment (P < 0·01). Determination of cytokine gene expression by real-time PCR showed higher IFN-γ and TNF-α expression in the lungs of l-isoleucine-treated animals (Fig. 4).
Fig. 4.
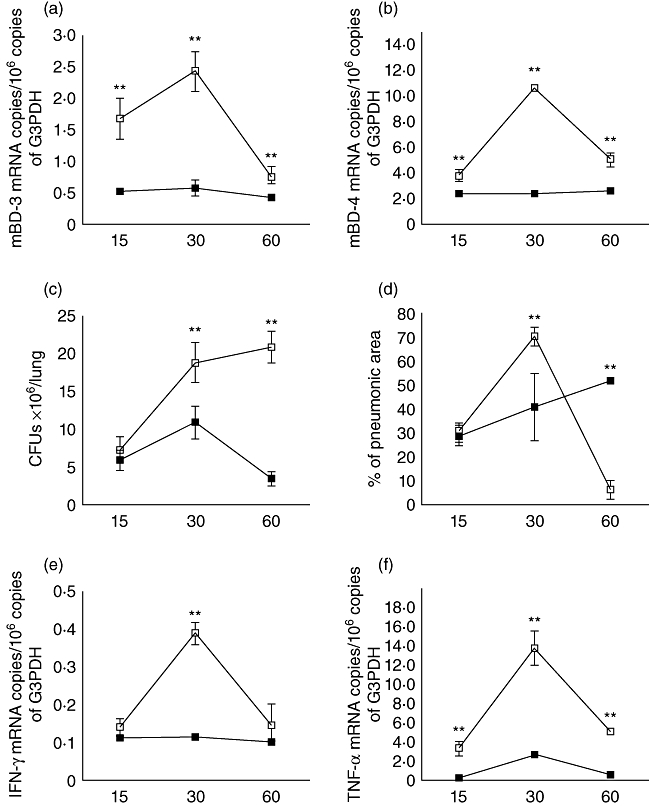
Effect of l-isoleucine administration during advanced disease in the lungs of mice infected with a multidrug-resistant strain. (a) l-isoleucine administration (white symbols) starting 60 days after infection increased mBD3 and (b) mBD4 gene expression when compared with control mice (black symbols). (c) l-isoleucine treatment also decreased pulmonary bacilli burdens, and after 60 days of treatment the lung surface affected by pneumonia (d) when compared with the control group. l-isoleucine treatment increased the expression of interferon-γ (e) and tumour necrosis factor-α (f). Each point corresponds to the mean and standard deviation of five mice group in one representative experiment. Asterisks represent statistical significance when compared to the control non-treated group (*P < 0·05, **P < 0·01).
Discussion
Defensins are anti-microbial peptides that are considered as a prototype family of mediators of innate immunity. They have efficient anti-microbial activity on a broad spectrum of organisms, including Mtb [26,27]. Besides direct bactericidal activity, anti-microbial peptides also have immunoregulatory functions, such as chemotaxis [28], immature dendritic cell activation [29] and activation of other immune cells [30,31]. Mtb induces production of HBD-2 in lung epithelial cells and it seems that these peptides could contribute to bacteria killing [6]. In murine models, expression of mBD-3 and mBD-4 correlates with control of mycobacterial growth in progressive pulmonary tuberculosis [12] and latent infection [10]. Our results confirm and extend these observations by demonstrating that stimulating β-defensin production during late active disease significantly increases control of bacillary growth.
Both mBD-3 and mBD-4 are inducible mainly through pathogen-associated molecular patterns (PAMPs) such as lipoarabinomannan [4] or lipopolysaccharide (LPS) [32] and proinflamatory cytokines [33,34]. However, none of these molecules can be used as therapeutic inducers of defensins because they can produce collateral toxic effects. Conversely, administration of recombinant defensins is not practical due to its high cost and the short half-life of the peptides. Thus, the use of defensin inducers is an attractive and low-cost immunotherapeutical alternative. Interestingly, Fehlbaum and coworkers showed that the essential amino acid l-isoleucine induces β-defensin production efficiently in Madin–Darby bovine kidney epithelial cells through a chiral receptor or enzyme and nuclear factor (NF)-κB/rel species activation, after reaction with a recognition site in an isoleucine-inducible defensin promoter [21]. Here we demonstrate that lung epithelial cells (type II pneumocytes) stimulated with l-isoleucine also produce high amounts of β-defensin. Furthermore, l-isoleucine caused no cytotoxicity even at concentrations as high as 1 mg/ml (data not shown). Similar results were obtained in vivo after intratracheal instillation of this essential amino acid in non-infected mice. In contrast, in the tested concentration of 50 µg/ml or 250 µg/100 µl, d-isoleucine did not induce β-defensin production, respectively, in in-vitro and in-vivo experiments, supporting the specificity of l-isoleucine. Thus, l-isoleucine can be considered as a novel immunostimulant with very limited toxic activity and low cost, which are important attributes considering that TB is a devastating disease in poor countries.
When BALB/c mice are infected by the intratracheal route with a high dose of the drug-sensitive H37Rv strain, there is an early high production of β-defensins and Th1 cytokines which, together with high levels of TNF-α, temporarily controls the infection. After 4 weeks of infection, there is a decrease in the levels of β-defensins, IFN-γ and TNF-α. Gradually, pneumonic areas prevail over granulomas. Extensive pneumonia plus a high burden of bacteria cause death [24]. We started the treatment with l-isoleucine after 8 weeks of infection, when active disease was in course and the expression of β-defensins was very low. This treatment efficiently restored high expression of mBD-3 and -4, and there was a simultaneous decrease in lung bacillary loads and tissue damage. This is compatible with the view that the re-establishment of β-defensin production permitted significant control of bacillary growth. This could be attributable to its direct anti-microbial activity [35], or to blockage of bacterial DNA replication [36], or to its well-known property of immune cell activation [36–38], including production of Th1 cytokines [29], which we confirmed by the high IFN-γ expression observed in l-isoleucine-treated animals. Moreover, alveolar macrophages can take external defensins and use them as anti-microbial effectors to eliminate intracellular mycobacteria [37,38]. Thus, several β-defensin-dependent mechanisms could explain the observed control of murine tuberculosis produced by treatment with l-isoleucine. However, at this stage we cannot assess formally the possible roles of other mediators or biological effects that might be caused by l-isoleucine.
Another important problem in the control of TB is the emergence of MDR strains. Approximately 400 000 new cases of MDR Mtb emerge worldwide each year, and this form of TB has been identified as a significant problem in every region under World Health Organization surveillance. Treatment of MDR strains is resource-intensive and usually requires a combination of second-line drugs that are more expensive, more toxic and less effective than drugs used in standard therapy. Our results showed that intratracheal administration of l-isoleucine in mice infected with MDR bacilli during the advanced phase of infection reduced significantly lung bacillary loads and tissue damage. Similarly to H37Rv strain-infected mice, 1 month after treatment the expression of IFN-γ and TNF-α was higher than in control mice. Thus, l-isoleucine administration was also able to stimulate the production of protective cytokines during MDR progressive disease, reducing disease severity as occurred with the drug-sensitive H37Rv strain infection.
In conclusion, our results show that repeated intrapulmonary administration of l-isoleucine induced β-defensin production in vivo, and that this correlated with improved protective immunity and higher resistance to mycobacterial infection when administered during late progressive disease induced by drug-sensitive or drug-resistant virulent mycobacteria. Although this treatment was not completely curative, these results suggest that continuous administration of l-isoleucine by the respiratory route is a potential therapy that might aid the control of this significant infectious disease. Moreover, there are efficient devices for deep administration of aerosols to human lungs that might reach the infected areas more reliably than the simple intratracheal injection used here.
Acknowledgments
C.R.-S. was supported by the National University of Mexico (UNAM) and a scholarship of the National Council for Science and Technology (CONACyT) Mexico. This study was supported by CONACyT (contract: 84456).
Disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in, or financial conflict with, the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- 1.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–86. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 2.Lonnroth K, Raviglione M. Global epidemiology of tuberculosis: prospects for control. Semin Respir Crit Care Med. 2008;29:481–91. doi: 10.1055/s-0028-1085700. [DOI] [PubMed] [Google Scholar]
- 3.Mitnick CD, Appleton SC, Shin SS. Epidemiology and treatment of multidrug resistant tuberculosis. Semin Respir Crit Care Med. 2008;29:499–524. doi: 10.1055/s-0028-1085702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivas-Santiago B, Schwander SK, Sarabia C, et al. Human {beta}-defensin 2 is expressed and associated with Mycobacterium tuberculosis during infection of human alveolar epithelial cells. Infect Immun. 2005;73:4505–11. doi: 10.1128/IAI.73.8.4505-4511.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korbel DS, Schneider BE, Schaible UE. Innate immunity in tuberculosis: myths and truth. Microbes Infect. 2008;10:995–1004. doi: 10.1016/j.micinf.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 6.van Crevel R, Ottenhoff TH, van der Meer JW. Innate immunity to Mycobacterium tuberculosis. Adv Exp Med Biol. 2003;531:241–7. doi: 10.1007/978-1-4615-0059-9_20. [DOI] [PubMed] [Google Scholar]
- 7.Rivas-Santiago B, Sada E, Hernandez-Pando R, Tsutsumi V. [Antimicrobial peptides in the innate immunity of infectious diseases] Salud Publica Mex. 2006;48:62–71. doi: 10.1590/s0036-36342006000100010. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki T, Chow CW, Downey GP. Role of innate immune cells and their products in lung immunopathology. Int J Biochem Cell Biol. 2008;40:1348–61. doi: 10.1016/j.biocel.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Pazgier M, Hoover DM, Yang D, Lu W, Lubkowski J. Human beta-defensins. Cell Mol Life Sci. 2006;63:1294–313. doi: 10.1007/s00018-005-5540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rivas-Santiago B, Contreras JC, Sada E, Hernandez-Pando R. The potential role of lung epithelial cells and beta-defensins in experimental latent tuberculosis. Scand J Immunol. 2008;67:448–52. doi: 10.1111/j.1365-3083.2008.02088.x. [DOI] [PubMed] [Google Scholar]
- 11.Rivas-Santiago B, Hernandez-Pando R, Carranza C, et al. Expression of cathelicidin LL-37 during Mycobacterium tuberculosis infection in human alveolar macrophages, monocytes, neutrophils, and epithelial cells. Infect Immun. 2008;76:935–41. doi: 10.1128/IAI.01218-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rivas-Santiago B, Sada E, Tsutsumi V, Aguilar-Leon D, Contreras JL, Hernandez-Pando R. Beta-defensin gene expression during the course of experimental tuberculosis infection. J Infect Dis. 2006;194:697–701. doi: 10.1086/506454. [DOI] [PubMed] [Google Scholar]
- 13.Bowdish DM, Davidson DJ, Scott MG, Hancock RE. Immunomodulatory activities of small host defense peptides. Antimicrob Agents Chemother. 2005;49:1727–32. doi: 10.1128/AAC.49.5.1727-1732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funderburg N, Lederman MM, Feng Z, et al. Human-defensin-3 activates professional antigen-presenting cells via Toll-like receptors 1 and 2. Proc Natl Acad Sci USA. 2007;104:18631–5. doi: 10.1073/PNAS.0702130104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang D, Chertov O, Bykovskaia SN, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–8. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 16.Ganz T. Defensins: antimicrobial peptides of vertebrates. C R Biol. 2004;327:539–49. doi: 10.1016/j.crvi.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Ganz T. Defensins and other antimicrobial peptides: a historical perspective and an update. Comb Chem High Throughput Screen. 2005;8:209–17. doi: 10.2174/1386207053764594. [DOI] [PubMed] [Google Scholar]
- 18.Sherman H, Chapnik N, Froy O. Albumin and amino acids upregulate the expression of human beta-defensin 1. Mol Immunol. 2006;43:1617–23. doi: 10.1016/j.molimm.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Oppenheim JJ, Biragyn A, Kwak LW, Yang D. Roles of antimicrobial peptides such as defensins in innate and adaptive immunity. Ann Rheum Dis. 2003;62(Suppl 2):ii17–21. doi: 10.1136/ard.62.suppl_2.ii17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menendez A, Brett Finlay B. Defensins in the immunology of bacterial infections. Curr Opin Immunol. 2007;19:385–91. doi: 10.1016/j.coi.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Fehlbaum P, Rao M, Zasloff M, Anderson GM. An essential amino acid induces epithelial beta-defensin expression. Proc Natl Acad Sci USA. 2000;97:12723–8. doi: 10.1073/pnas.220424597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welling MM, Hiemstra PS, van den Barselaar MT, et al. Antibacterial activity of human neutrophil defensins in experimental infections in mice is accompanied by increased leukocyte accumulation. J Clin Invest. 1998;102:1583–90. doi: 10.1172/JCI3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernandez-Pando R, Orozco H, Arriaga K, Sampieri A, Larriva-Sahd J, Madrid-Marina V. Analysis of the local kinetics and localization of interleukin-1 alpha, tumour necrosis factor-alpha and transforming growth factor-beta, during the course of experimental pulmonary tuberculosis. Immunology. 1997;90:607–17. doi: 10.1046/j.1365-2567.1997.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernandez-Pando R, Orozcoe H, Sampieri A, et al. Correlation between the kinetics of Th1, Th2 cells and pathology in a murine model of experimental pulmonary tuberculosis. Immunology. 1996;89:26–33. [PMC free article] [PubMed] [Google Scholar]
- 25.Jarnagin JL, Luchsinger DW. The use of fluorescein diacetate and ethidium bromide as a stain for evaluating viability of mycobacteria. Stain Technol. 1980;55:253–8. doi: 10.3109/10520298009067249. [DOI] [PubMed] [Google Scholar]
- 26.Sharma S, Verma I, Khuller GK. Antibacterial activity of human neutrophil peptide-1 against Mycobacterium tuberculosis H37Rv: in vitro and ex vivo study. Eur Respir J. 2000;16:112–17. doi: 10.1034/j.1399-3003.2000.16a20.x. [DOI] [PubMed] [Google Scholar]
- 27.Sharma S, Verma I, Khuller GK. Therapeutic potential of human neutrophil peptide 1 against experimental tuberculosis. Antimicrob Agents Chemother. 2001;45:639–40. doi: 10.1128/AAC.45.2.639-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Durr M, Peschel A. Chemokines meet defensins: the merging concepts of chemoattractants and antimicrobial peptides in host defense. Infect Immun. 2002;70:6515–17. doi: 10.1128/IAI.70.12.6515-6517.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biragyn A, Ruffini PA, Leifer CA, et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298:1025–9. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 30.Hancock RE, Brown KL, Mookherjee N. Host defence peptides from invertebrates – emerging antimicrobial strategies. Immunobiology. 2006;211:315–22. doi: 10.1016/j.imbio.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Mookherjee N, Hancock RE. Cationic host defence peptides: innate immune regulatory peptides as a novel approach for treating infections. Cell Mol Life Sci. 2007;64:922–33. doi: 10.1007/s00018-007-6475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang D, Liu ZH, Tewary P, Chen Q, de la Rosa G, Oppenheim JJ. Defensin participation in innate and adaptive immunity. Curr Pharm Des. 2007;13:3131–9. doi: 10.2174/138161207782110453. [DOI] [PubMed] [Google Scholar]
- 33.Beisswenger C, Bals R. Antimicrobial peptides in lung inflammation. Chem Immunol Allergy. 2005;86:55–71. doi: 10.1159/000086651. [DOI] [PubMed] [Google Scholar]
- 34.Beisswenger C, Bals R. Functions of antimicrobial peptides in host defense and immunity. Curr Protein Pept Sci. 2005;6:255–64. doi: 10.2174/1389203054065428. [DOI] [PubMed] [Google Scholar]
- 35.Fattorini L, Gennaro R, Zanetti M, et al. In vitro activity of protegrin-1 and beta-defensin-1, alone and in combination with isoniazid, against Mycobacterium tuberculosis. Peptides. 2004;25:1075–7. doi: 10.1016/j.peptides.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Gera JF, Lichtenstein A. Human neutrophil peptide defensins induce single strand DNA breaks in target cells. Cell Immunol. 1991;138:108–20. doi: 10.1016/0008-8749(91)90136-y. [DOI] [PubMed] [Google Scholar]
- 37.Tan BH, Meinken C, Bastian M, et al. Macrophages acquire neutrophil granules for antimicrobial activity against intracellular pathogens. J Immunol. 2006;177:1864–71. doi: 10.4049/jimmunol.177.3.1864. [DOI] [PubMed] [Google Scholar]
- 38.Martineau AR, Newton SM, Wilkinson KA, et al. Neutrophil-mediated innate immune resistance to mycobacteria. J Clin Invest. 2007;117:1988–94. doi: 10.1172/JCI31097. [DOI] [PMC free article] [PubMed] [Google Scholar]


