Abstract
Complex regional pain syndrome (CRPS) is a chronic pain disorder. Although its pathophysiology is not completely understood, neurogenic inflammation is thought to play a significant role. Microglia and astrocytes are activated following tissue injury or inflammation and have been reported to be both necessary and sufficient for enhanced nociception. Blood-borne monocytes/macrophages can infiltrate the central nervous system (CNS) and differentiate into microglia resulting in hypersensitivity and chronic pain. The primary aim of this study was to evaluate the proportion of the proinflammatory CD14+CD16+ monocytes as well as plasma cytokine levels in blood from CRPS patients compared to age- and gender-matched healthy control individuals. Forty-six subjects (25 CRPS, 21 controls) were recruited for this study. The percentage of monocytes, T, B or natural killer (NK) cells did not differ between CRPS and controls. However, the percentage of the CD14+CD16+ monocyte/macrophage subgroup was elevated significantly (P < 0·01) in CRPS compared to controls. Individuals with high percentage of CD14+CD16+ demonstrated significantly lower (P < 0·05) plasma levels on the anti-inflammatory cytokine interleukin (IL)-10. Our data cannot determine whether CD14+CD16+ monocytes became elevated prior to or after developing CRPS. In either case, the elevation of blood proinflammatoty monocytes prior to the initiating event may predispose individuals for developing the syndrome whereas the elevation of blood proinflammatory monocytes following the development of CRPS may be relevant for its maintenance. Further evaluation of the role the immune system plays in the pathogenesis of CRPS may aid in elucidating disease mechanisms as well as the development of novel therapies for its treatment.
Keywords: central nervous system, immune system, monocytes, neurogenic inflammation
Introduction
Complex regional pain syndrome (CRPS) is a severe chronic pain disorder that often follows an injury to peripheral nerves [1,2]. CRPS demonstrates a 3:1 female to male preponderance and is characterized by pain that is out of proportion to the initial injury and does not respect a nerve or root distribution [3,4]. The signs and symptoms of CRPS cluster into four categories: (1) abnormalities in pain processing; (2) skin colour and temperature changes; (3) sudomotor abnormalities and oedema; and (4) motor dysfunction and trophic changes [5,6].
Although the pathophysiology of CRPS is not completely understood, there is evidence demonstrating that neurogenic inflammation plays a significant role [7,8]. Furthermore, neuroinflammation and neuroimmune activation have been shown to act in concert in persistent pain states [9]. Following injury, mast cells, neutrophils and macrophages are recruited to the involved area and can invade the nerve through a disrupted blood–nerve barrier [10,11]. These cells produce a variety of proinflammatory cytokines that have been implicated in the generation of neuropathic pain either by direct sensitization of nociceptors or indirectly by stimulating the release of agents that act on neurones and glia [12,13].
Glia, in particular microglia and astrocytes, are the immunocompetent cells in the central nervous system (CNS) which become activated following tissue injury or inflammation [14]. Activated glia have been shown to be both necessary and sufficient for enhanced nociception [13]. Specifically, microglia activation is one of the most common and earliest features of most neuroinflammatory disorders [15,16] and CNS pathologies [17–19]. We have reported increased activation of astrocytes and microglia in spinal cord tissue of a CRPS patient when compared to control tissue [20].
In man, CNS microglia is thought to arise during gestation from mesodermal/mesenchymal sources [21]. Normally, CNS microglia can replenish with little or no need of repopulation from circulating bone marrow-derived progenitors [21]. However, in disease conditions, blood-derived monocytes/macrophages are recruited into the CNS and differentiate into microglia [22,23]. A recent study demonstrated that, following nerve injury, blood monocytes/macrophages infiltrate the CNS and differentiate into functional microglia at the involved segmental spinal level, resulting in hypersensitivity and chronic pain [24].
Human peripheral blood monocytes can be subdivided into two subgroups based on their expression of cell surface markers: one expressing CD14, but not CD16 (CD14+CD16-) and the other expressing both CD14 and CD16 (CD14+CD16+) [25]. Both subgroups produce similar levels of proinflammatory cytokines. However, CD14+CD16+ monocytes produce much lower levels of the anti-inflammatory cytokine interleukin (IL)-10, suggesting that these cells constitute a proinflammatory subtype [26]. Increased proportions of the CD14+CD16+ subgroup have been described in disease states including sepsis, acquired immunodeficiency disease syndrome, rheumatoid arthritis, systemic lupus erythematosus and active sarcoidosis [25,27–30].
The primary aim of this study was to evaluate the proportion of proinflammatory CD14+CD16+ monocytes as well as the levels of several plasma cytokines in blood from patients afflicted with CRPS compared to age- and gender-matched healthy control individuals.
Materials and methods
Subjects inclusion and exclusion criteria
All subjects were enrolled after giving informed consent as approved by the Drexel University College of Medicine Institutional Review Board (IRB).
CRPS patients were recruited from the pain clinic of Drexel University School of Medicine and fulfilled the International Association for the Study of Pain (IASP) diagnostic criteria for CRPS [31]. Healthy control subjects were recruited from the general public. The exclusion criteria for all subjects included: pregnancy, recent infection, lupus erythematosus, HIV/AIDS, rheumatoid arthritis, recent extracorporeal circulation (haemodialysis, bypass surgery, plasmapheresis), bone marrow transplant, immunosuppressive therapy, blood disorders (anaemia, leukaemia), thymectomy or sarcoidosis.
Patient evaluation
All CRPS patients received a complete neurological examination and pain evaluation. Overall pain levels were determined on a 0–10 numerical rating scale (NRS) (0 = no pain, 10 = worst possible pain). All CRPS patients were evaluated and blood samples obtained while taking their current medications. Medical history and self-reported values for height and weight were obtained from normal healthy control subjects.
Thermal detection and thermal pain thresholds
Thermal detection thresholds were determined using the TSA-II NeuroSensory Analyzer (Medoc Advanced Medical Systems US, Minneapolis, MN, USA). The device consists of a computer-controlled thermoelectric probe with a surface area of 9 cm2 that is attached using a Velcro strap to the area of skin to be tested (thenar eminence in the hands and the dorsal foot). For each trial the thermal stimulator starts at a thermoneutral baseline temperature of 32°C, and increases for warming thresholds, or decreases for cooling thresholds, linearly at a rate of 1°C per second, until the subject pushes a button that stops and records the temperature and returns the unit to the baseline temperature. Three trials are averaged for cool and warm detection thresholds for each site tested.
Thermal pain thresholds were determined at the same sites and using the same method described above for thermal detection thresholds. The only difference was that for thermal pain trials, the subject was instructed to push the control button (which immediately resets the stimulator back to baseline temperature) when the thermal stimulus (cold or hot) becomes painful. The TSA-II hardware automatically resets if the temperature reaches −10°C (for cooling) or 50°C (for heating) and the control button has not been pushed. This temperature range has been determined to not cause damage to skin or underlying tissue. Normative values for thermal detection and pain thresholds were obtained from published studies [32,33].
Blood sampling and isolation of peripheral blood mononuclear cells
Venous blood samples were collected into ethylenediamine tetraacetic acid (EDTA)-coated vacutainers between 08:00 h and 12:00 h. Following centrifugation, the buffy coat was resuspended in RPMI-1640 (Mediatech Inc, Manassas, VA, USA) and layered onto Histopaque-1077 (Sigma-Aldrich, St Louis, MO, USA) for separation of peripheral blood mononuclear cells (PBMCs) by gradient centrifugation. The plasma was split into 0·25-ml aliquots and stored at −70°C for cytokine level determination.
Determination of PBMC subpopulations
Isolated PBMCs were washed and resuspended in phosphate-buffered saline (PBS) containing combinations of fluorescent-conjugated antibodies (eBioscience, San Diego, CA, USA) to the following cell surface markers: CD4 [fluorescence activated cell sorter (FITC)], CD8 [phycoerythrin-cyanine5 (PE-Cy5)], CD19 (PE), CD56 (PE), CD14 [allophycocyanin (APC)] and CD16 (FITC). PBMCs were incubated in staining cocktails for 30 min on ice in the dark. After multiple washes to minimize random antibody binding, PBMCs were fixed with 1% paraformaldehyde (Sigma-Aldrich). Samples were then acquired on a FACSCanto flow cytometer (BD Biosciences, San Jose, CA, USA) and analysed using FlowJo Software (Tree Star, Ashland, OR, USA). Specific gating techniques were as follows. Lymphocyte gates were set manually according to forward-scatter (FSC) and side-scatter (SSC), and subpopulations were subsequently determined. T cells within the lymphocyte gate were identified as either CD4+CD8- events (T helper cells) or CD4-CD8+ events (T cytotoxic cells). Natural killer (NK) cells and B cells were approximated within the lymphocyte gate as CD56+ and CD19+ events, respectively. To determine the percentage of total monocytes/macrophages, the total live events were first gated and CD14+ events were then plotted versus SSC. Activated monocytes/macrophages were subsequently determined as CD16+ events within the CD14+ population. Therefore the results, reported as CD14+CD16+, represent the percentage of CD14+ cells expressing CD16, not double-positive events within the total live population.
Plasma cytokine determination
Plasma levels of the following interleukins IL-1β, IL-6, IL-8, IL-10 and tumour necrosis factor (TNF)-α were determined using the Milliplex™ MAP high sensitivity human cytokine kit with sensitivities of (0·06, 0·10, 0·11, 0·15 and 0·05 pg/ml), respectively (Millipore Corp. Billerica, MA, USA). The plates were read on a Luminex-200 fluorescent analytical test instrument (Luminex Corp., Austin, TX, USA). All assays were performed in duplicate according to the manufacturers' instructions.
Statistics
For parametric variables, statistical significance between groups was determined by t-test or analysis of variance (anova) using the Tukey–Kramer post-hoc multiple comparison test. The Kruskall–Wallis test was used to compare gender differences between groups. Correlations between parameters were determined using Pearson's correlation. For non-parametric variables, correlations were determined by Spearman's rho. The data was considered significantly different if P < 0·05. Calculations were accomplished with the aid of statistical data analysis software (spss version 17; SPSS Inc., Chicago, IL, USA).
Results
Subject demographics
A total of 46 subjects (25 CRPS, 21 controls) were recruited for this study. The number of subjects in each group, their age, gender, body mass index (BMI), as well as the duration of disease and NRS pain score for the CRPS group are tabulated in Table 1. There were no significant differences in age, gender or BMI (P > 0·05) between the CRPS and control groups. For the CRPS subjects, the location of the initial injury, most prominent signs and symptoms, their overall pain score, the medications they were taking at the time the blood was sampled and other conditions with which the subjects were afflicted are listed in Appendix I.
Table 1.
Subject demographics
| Patient group | n | Age (years) | Male/female | BMI | Duration (years) | NRS (0–10) |
|---|---|---|---|---|---|---|
| Controls | 21 | 42·9 ± 2·9 | 3/18 | 26·3 ± 1·4 | n.a. | n.a. |
| CRPS | 25 | 45·3 ± 2·4 | 2/23 | 27·2 ± 1·5 | 8·5 ± 1·6 | 7·4 ± 0·3 |
The patient groups, the number of patients (n) in each group, their average age in years, gender ratio (males/females), body mass index (BMI), average disease duration (years between initiating event and blood draw) and mean numerical rating scale (NRS) overall pain score for the complex regional pain syndrome (CRPS) patients (0 = no pain, 10 = worst possible pain). Average values are listed ± the standard error of the mean. n.a., not applicable.
Quantitative thermal testing
Eighteen of the 25 CRPS subjects had quantitative thermal tests performed as part of their clinical evaluation. None of the subjects demonstrated low thresholds (hypersensitivity) to cold or warm stimuli. The majority (10 of 18) had cold and heat thresholds within the normal range. Some patients (eight of 18) demonstrated elevated (hyposensitivity) threshold to cold (seven of 18), heat (five of 18) or both (four of 18). Approximately three-quarters of the CRPS patients (13 of 18) demonstrated thermal allodynia. All 13 patients showed cold allodynia, whereas five also demonstrated heat hyperalgesia. None of the patients demonstrated heat hyperalgesia in the absence of cold allodynia.
Percentage of PBMCs subsets
The percentage of PBMCs based on their surface markers are tabulated in Table 2. There were no significant differences (P > 0·05) in the percentage of T helper cells (CD4+CD8-), T cytotoxic cells (CD4-CD8+), NK cells (CD56+), B cells (CD19+) or monocytes/macrophages (CD14+) between the CRPS and control groups. The CRPS group demonstrated increased CD4/CD8 ratios, but the increase was not statistically significant (P = 0·214).
Table 2.
Percentage of peripheral blood mononuclear cells based on surface markers
| Group | CD4+ | CD8+ | CD4/CD8 | CD14+ | CD14+CD16+ | CD19+ | CD56+ |
|---|---|---|---|---|---|---|---|
| Controls | 33·9 ± 1·6 | 20·3 ± 1·4 | 1·78 ± 0·1 | 11·9 ± 1·3 | 8·9 ± 1·7 | 15·8 ± 1·4 | 10·4 ± 1·1 |
| CRPS | 35·9 ± 1·6 | 19·3 ± 1·3 | 2·06 ± 0·2 | 11·1 ± 1·4 | 16·7 ± 2·3* | 15·7 ± 1·2 | 9·8 ± 1·0 |
The percentage of peripheral blood mononuclear cells expressing each cell surface marker. CD4/CD8 is the ratio of CD4+ to CD8+cells. All values are given in percentage ± the standard error of the mean.
P < 0·01. CRPS, complex regional pain syndrome.
Percentage of CD14+CD16+ monocytes in CRPS patients
The CRPS patients demonstrated a significantly (P < 0·01) higher frequency of CD14+CD16+ monocytes compared to controls (Table 2, Fig. 1). There was no correlation between increased number of CD14+CD16+ monocytes in the CRPS group and the patients' overall pain level (r = 0·146, P = 0·487) or duration of disease (r = 0·040, P = 0·848). However, there was a correlation between increased numbers of CD14+CD16+ monocytes in CRPS patients demonstrating cold allodynia. CRPS patients demonstrating cold allodynia showed a significant (P < 0·01) increase in the frequency of CD14+CD16+ monocytes compared to controls. The percentage of CD14+CD16+ monocytes in CRPS patients without cold allodynia was higher than controls and less than the CRPS group with cold allodynia, but not significantly (P > 0·05) different from either group (Fig. 2). Both CRPS and healthy control subjects showed a trend towards an increased percentage of CD14+CD16+ monocytes with increased BMI. However, the correlation was not statistically significant (r = 0·231, P = 0·126).
Fig. 1.
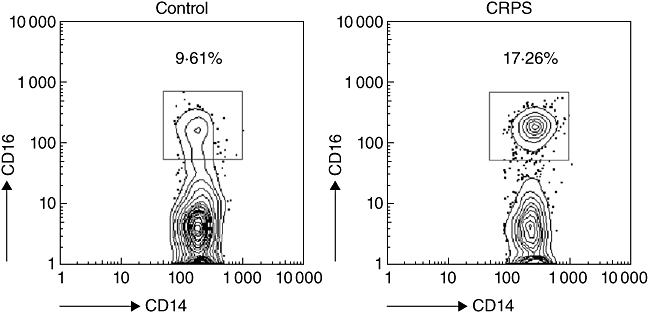
The gating technique for identifying activated monocytes/macrophages from a control subject (left panel) and a subject diagnosed with complex regional pain syndrome (CRPS) (right panel). The numbers shown represent the percentage of CD14+ cells expressing CD16 for each subject.
Fig. 2.
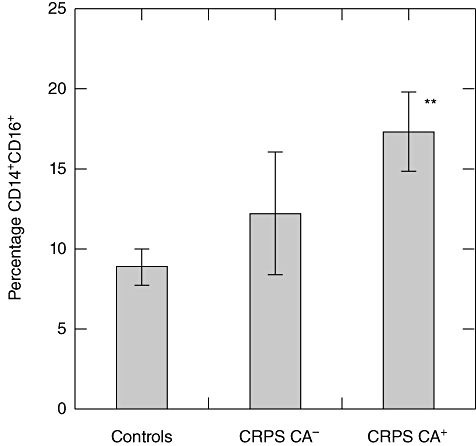
Differences in the percentage of CD14+CD16+ monocytes in complex regional pain syndrome (CRPS) patients with cold allodynia (CRPS CA+), CRPS patients without cold allodynia (CRPS CA-) and healthy controls. The error bars are the standard error of the mean. CRPS patients demonstrating cold allodynia showed a significant (**P < 0·01) increase in the frequency of CD14+CD16+ monocytes compared to controls. The percentage of CD14+CD16+ monocytes in CRPS patients without cold allodynia was higher than controls and less than the CRPS group with cold allodynia, but not significantly (P > 0·05) different from either group.
Plasma cytokine levels
Plasma cytokine levels are tabulated in Table 3. There was a trend for increased levels of the proinflammatory cytokines (IL-6, IL-8, TNF-α) and a decrease of the anti-inflammatory cytokine IL-10 in the CRPS subjects compared to the controls. However, none of the changes reached statistical significance (P > 0·05).
Table 3.
Plasma cytokine levels
| Group | IL-1β | IL-6 | IL-8 | IL-10 | TNF-α |
|---|---|---|---|---|---|
| Controls | 0·86 ± 0·5 | 5·74 ± 1·4 | 2·70 ± 0·3 | 17·54 ± 4·4 | 5·06 ± 0·5 |
| CRPS | 0·68 ± 0·2 | 7·98 ± 1·9 | 6·33 ± 1·9 | 11·37 ± 2·3 | 6·51 ± 0·8 |
The plasma levels of selected cytokines in complex regional pain syndrome (CRPS) patients and healthy control subjects. All values are given in pg/ml ± the standard error of the mean. IL, interleukin; TNF, tumour necrosis factor.
Not all CRPS patients demonstrated an increased percentage of CD14+CD16+ monocytes. High levels of CD14+CD16+ monocytes (control mean plus 1 standard deviation) was found in 9·5% of controls and 40% of CRPS patients. The plasma level of IL-10 was significantly lower (P < 0·05) in individuals with high levels of CD14+CD16+ compared to those with low levels. There was no difference in any of the other cytokines between these two groups (Table 4).
Table 4.
Plasma cytokine comparison
| Group | IL-1β | IL-6 | IL-8 | IL-10 | TNF-α |
|---|---|---|---|---|---|
| Low CD14+CD16+ | 0·84 ± 0·3 | 7·21 ± 1·5 | 4·62 ± 1·4 | 16·07 ± 3·1 | 5·74 ± 0·6 |
| High CD14+CD16+ | 0·53 ± 0·3 | 5·90 ± 1·6 | 4·80 ± 1·1 | 8·84 ± 1·8* | 6·16 ± 1·1 |
The plasma levels of selected cytokines in individuals with high and low percentages of CD14+CD16+ monocytes. All values are given in pg/ml ± the standard error of the mean.
P < 0·05. IL, interleukin; TNF, tumour necrosis factor.
Correlations between CD14+CD16+ monocytes and medications or other medical conditions reported by the subjects
Except for antidepressants, there was no correlation (rho < 0·29, P > 0·16) between the percentage of CD14+CD16+ monocytes in CRPS patients and the medications the subjects were taking. CRPS patients taking antidepressants demonstrated a statistically significant correlation (rho = 0·41, P = 0·042) with elevated CD14+CD16+ monocytes. There was no correlation between other medical conditions reported by the subjects and the percentage of CD14+CD16+ monocytes (rho < 0·27, P > 0·19).
Discussion
Our study is the first to evaluate the percentage of blood monocytes in CRPS patients. Although the percentage of total monocytes (CD14+ peripheral blood mononuclear cells) remained unchanged in CRPS, the percentage of the CD14+CD16+ monocyte subgroup was elevated significantly (P < 0·01) in individuals afflicted with CRPS compared to healthy controls. Previous studies have determined that these cells represent a potent antigen-presenting and proinflammatory subpopulation of monocytes [28] that has been shown to be expanded in inflammatory conditions [34].
Although there was no correlation between the increased number of CD14+CD16+ monocytes in the CRPS group and the patients' overall pain level, there was a correlation between increased numbers of CD14+CD16+ monocytes in CRPS patients demonstrating cold allodynia. This finding suggests that the increased percentage of CD14+CD16+ monocytes may be associated with central sensitization.
As reported previously, there was no difference in plasma levels of TNF-α, IL-10, IL-8, IL-6 and IL-1β between CRPS patients and controls [35,36]. However, individuals with high levels of CD14+CD16+ monocytes demonstrated a significantly lower (P < 0·05) plasma level of IL-10 compared to individuals with low levels of CD14+CD16+. This is consistent with a study showing that CD14+CD16+ monocytes produce similar levels of the proinflammatory cytokines TNF-α, IL-6 and IL-1β and lower levels of the anti-inflammatory cytokine IL-10 [26].
This study also showed that the percentage of lymphocytes (T helper cells, T cytotoxic cells, NK cells or B cells) did not differ between CRPS patients and healthy control individuals. These results are in agreement with the study of Ribbers and colleagues that reported no association between lymphocyte subpopulations and patients with reflex sympathetic dystrophy (currently referred to as CRPS-type 1) [37]. A subsequent study by Kaufmann and colleagues also found no changes in the percentage of T cytotoxic cells, NK cells and B cells in CRPS patients [38]. However, they reported a reduction of T helper cells (CD8+ lymphocytes) as well as an increase in the CD4/CD8 ratio [38] in CRPS patients compared to healthy controls. Although our study also found a small reduction of CD8+ lymphocytes and an increase in the CD4/CD8 ratio, these changes were not statistically significant (P > 0·05).
The elevation in the percentage of CD14+CD16+ monocytes seen in CRPS patients in this study could be due to the syndrome itself or may result from other factors. Factors such as physical inactivity, morbid obesity and sleep have been shown to alter the percentage of CD14+CD16+ monocytes [39–41].
Morbidly obese individuals have been reported to show elevated levels of the CD14+CD16+ monocyte subset [39]. The percentage of obese individuals (BMI > 30) in both the CRPS and control groups was approximately 20%. Obese subjects demonstrated a higher percentage of CD14+CD16+ monocytes than non-obese (10·9% versus 8·7% in controls) and (20·1% versus 15·8% in CRPS). Even though there was no significant difference in BMI (P > 0·05) between the CRPS and control groups in this study, the percentage of CRPS patients in our pain clinic who are either overweight or obese is higher than the general population [42].
Sleep has been shown to decrease the number of CD14+CD16+ monocytes [40], and although acute exercise causes a transient increase in CD14+CD16+ monocytes [43,44], individuals who are physically inactive demonstrate a significantly higher percentage of CD14+CD16+ monocytes compared to those who are physically active [41]. Sleeping difficulties and physical inactivity are reported commonly by individuals afflicted with CRPS [4,45].
In addition, we showed that CRPS patients taking antidepressants demonstrated a positive correlation with elevation of CD14+CD16+ monocytes. Even though other studies have shown that the expression of CD14 and CD16 in monocytes is unchanged in patients with depression compared to normal individuals [46], we cannot rule out that depression or antidepressant use are contributory factors to the increase in CD14+CD16+ monocytes shown by patients with CRPS.
Thus, obesity, sleeping difficulties, physical inactivity and possibly depression may be contributory factors leading to the increase in the percentage of CD14+CD16+ monocytes seen in patients with CRPS.
Following injury, many individuals develop the signs and symptoms of CRPS (swelling, redness, allodynia, hyperalgesia, etc.); however, in most patients, normal healing occurs and these signs and symptoms resolve. The process by which a subject fails to undergo normal healing following an injury and progresses to a chronic pain condition as well as the process by which the pain is maintained with little or no chance of resolving are some of the most important and perplexing questions in CRPS research.
The following observations make our finding of elevated CD14+CD16+ proinflammatory monocytes in patients with CRPS relevant to both the initiation and the maintenance of the disease: (1) the activation of microglia and astrocytes has been shown to be both necessary and sufficient for enhanced nociception [13] and (2) blood-borne monocytes/macrophages infiltrate the CNS and differentiate into fully functional microglia [24]. Our data cannot determine whether CD14+CD16+ monocytes were elevated in the study subjects prior to developing CRPS or became elevated afterwards. In either case, independent of causative mechanism, the elevation of blood proinflammatory monocytes prior to the initiating event may predispose individuals for developing the syndrome, whereas the elevation of blood proinflammatory monocytes following the development of CRPS may be relevant for its maintenance.
The strengths of this study are: (1) that all patients met strictly defined IASP criteria for CRPS and (2) all patients were diagnosed and examined by the same senior clinician. The major limitations of this study are: (1) its small size and (2) neither the control or CRPS groups were evaluated with a standardized depression scale or evaluated with regard to their activity level or sleep. The facts that the pain observed in patients with CRPS can result from multiple mechanisms and that patients with CRPS do not respond equally to the same medications may be due in part to its evolution in time, but it also suggests that CRPS may result from multiple aetiologies. The results of this study demonstrating that a subset of CRPS patients show elevated numbers of the CD14+CD16+ monocyte subgroup may aid in elucidating some of the different mechanisms involved in its pathophysiology. A better understanding of these mechanisms may lead to novel treatments for this very severe, life-altering condition.
Conclusions
This study has demonstrated an increase in the percentage of the CD14+CD16+ monocyte subgroup in individuals afflicted with CRPS. In addition, other investigators have reported mast cell involvement [47], leucocyte accumulation in the affected extremity [48] and impaired neutrophil function [49] in patients with CRPS. Thus, further evaluation of the role the immune system plays in the pathogenesis of CRPS is warranted, and may aid in elucidating disease mechanisms as well as the development of novel therapies for its treatment.
Acknowledgments
We wish to graciously thank Eric B. Wong MS and Jeffrey J. Gerbino for their technical assistance. This study was supported by grants from the Commonwealth of Pennsylvania Department of Health, Drexel University College of Medicine Pain Initiative and gifts from the Tilly Family Foundation and the Sunstein family.
Appendix I
| PatID | Gender/Age | Initiating Event/Duration | Signs/Symptoms/Overall Pain Score NRS(0-10) | Pain Medications | Other Conditions |
|---|---|---|---|---|---|
| CRPS01 | F/68 | Kyphoscoliosis; disc disease at L5-S1/22 years | L5-S1 sensory loss; spontaneous burning pain in both legs; weakness; inability to move toes; severe dystrophic changes. Pain (NRS) 8 | NSAIDs; anti-epileptic drugs (AED), antidepressants; intermittent narcotics; spasmolytics. | L4-L5 bilateral radiculopathy; arthrosclerosis; GERD; osteoporosis; osteoarthritis; IBS; headaches |
| CRPS02 | F/44 | Fall; brachial plexus traction injury (BPTI)/4·5 years | Paresthesias; deep ache; deep muscle joint pain; dynamic and static allodynia; generalized from BPTI; weakness; poor initiation of movement. Pain (NRS) 8 | Intravenous ketamine; intravenous lidocaine; narcotics; AED; antidepressants, lenalidomide. | C5-C6 disk herniation; L4-L5-S1 radiculopathy; mitral valve prolapse; Asthma; headaches. |
| CRPS03 | F/46 | Fall; repetitive strain of right brachial plexus/9 years | Dynamic and static mechano allodynia; cold allodynia right upper quadrant; autonomic dysregulation; neurogenic oedema; dystonia of trunk; weakness. Pain (NRS) 8 | AED; narcotics; spasmolytics. | Hypertension; lumbar radiculopathy; headaches. |
| CRPS04 | F/50 | Fall; BPTI; cervical plexus traction injury/4 years | Positive Tinel sign bilaterally in her brachial plexus; mechano and thermal allodynia; hyperalgesia; weakness; poor initiation of movement; generalized muscle tremor. Pain (NRS) 5 | NSAIDs; AED; antidepressants; narcotics. | Depression; headaches; TMJ |
| CRPS05 | F/24 | Fall; Repetitive strain injury of brachial plexus/7 years | Generalized mechano and thermal allodynia; hyperalgesia; poor initiation of movement; weakness; positive Tinel signs. Pain (NRS) 6·5 | NSAIDs, AED, antidepressants; spasmolytics; antihistamine; narcotics; intravenous lidocaine; intravenous ketamine. | GERD; chronic fatigue; seizure disorder; headaches. |
| CRPS06 | F/39 | BPTI right arm/4 years | Mechano and thermal allodynia; hyperalgesia; severe autonomic dysregulation; oedema. Pain (NRS) 8 | NSAIDs; AED; narcotics; intravenous ketamine. | |
| CRPS07 | F/64 | L5-S1 radiculopathy/36 years | Dynamic, static and thermal allodynia; deep muscle sensitization; neurogenic oedema; weakness; autonomic dysregulation. Pain (NRS) 7 | AED; baclofen; antianxiolytics; intermittent narcotics; NSAIDs; antidepressants | Hypertension; hyperlipidaemia; heart disease; asthma. |
| CRPS08 | F/48 | Ligament injury of left foot/3·5 years | Generalized spread; severe mechano and thermal allodynia; autonomic dysregulation; dystrophy; weakness; spasms, myoclonus. Pain (NRS) 10 | AED; NSAIDs, antidepressants, narcotics; failed ketamine coma; antianxiolytics; failed intravenous lidocaine. | GERD; depression; Panic attacks/anxiety; headaches. |
| CRPS09 | F/55 | Left knee injury; surgery/2·5 years | Symptoms spread to right leg; generalized; primarily pain; autonomic dysregulation; dystrophy; weakness and decreased initiation of movement. Pain (NRS) 8 | AED; antidepressants, NSAIDS; propoxyphene; stellate ganglion blocks. | Migraines |
| CRPS10 | M/29 | Fractured left fibula/3 years | Pin prick hyperalgesia; mechano allodynia; swelling; sweating; erythema; difficulty initiating movement; nail atrophy; cold allodynia. Pain (NRS) 10 | NSAIDs; spasmolytics; antidepressants; antianxiolytics; intravenous ketamine. | GERD; headaches |
| CRPS11 | F/30 | Motor vehicle accident; Fall BPTI/6 years | Autonomic dysregulation; neurogenic oedema; positive Tinel signs; thermal allodynia; weakness; poor initiation of movement; deep muscle pain, joint pain. Pain (NRS) 7 | Antidepressants; NSAIDs; narcotics; spasmolytics. | Chronic Fatigue; seizure disorder; headaches. |
| CRPS12 | F/26 | Broke right ankle/8 years | Spontaneous burning pain; dynamic and static mechano and thermal allodynia; decreased initiation of movement. Pain (NRS) 6·5 | AED; NSAIDs, narcotics; antidepressants; spasmolytics. | GERD; Seasonal Allergies; eating disorders |
| CRPS13 | F/60 | Fell and fractured left wrist 5 years | Cold allodynia; pin prick hypoesthesia; weak; difficulty initiating movement; hyperhidrosis. Pain (NRS) 2 | AED; NSAIDs; antidepressants. | Osteoarthritis; depression. |
| CRPS14 | F/48 | left BPTI/5 years | Dynamic and static mechano allodynia; positive Tinel signs; cold allodynia; weakness; spasms spread to left lower extremity. Pain (NRS) 8 | Intravenous ketamine; AED; NSAIDs; intermittent narcotics; antidepressants. | |
| CRPS15 | F/45 | L5-S1 radiculopathy (disc)/20 years | Dynamic, static mechano allodynia, all extremities; neurogenic oedema of legs; autonomic dysregulation; bilateral BPTI. Pain (NRS) 8 | AED; antianxiolytic; spasmolytics; antidepressants intravenous ketamine | Depression |
| CRPS16 | F/41 | Motor vehicle accident with BPTI on the left/14 years | Spontaneous burning pain; mechano and thermal allodynia; autonomic dysregulation; neurogenic oedema; spread to ipsilateral cervical plexus and contralateral brachial plexus; weakness of hand muscles. Pain (NRS) 8 | Intravenous ketamine; NSAIDs; AED; narcotics; antidepressants. | Migraines; IBS |
| CRPS17 | F/31 | Excision of neuroma of right foot/3 years | Mechano and thermal allodynia; burning spontaneous pain; mirror spread; then to brachial plexus; autonomic dysregulation; neurogenic oedema; weakness. Pain (NRS) 9 | AED; antidepressants; spasmolytics; memantine; narcotics; NSAIDs; intravenous ketamine. | Depression; hypertension; hypercholesterolemia. |
| CRPS18 | F/52 | Motor vehicle accident; BPTI/8·5 years | Generalized mechano allodynia; hyperalgesia; deep sensitization of muscle; weakness; difficulty initiating movement; positive Tinel signs of brachial plexus. Pain (NRS) 7 | NSAIDs; AED; narcotics; antidepressants; intravenous ketamine; intravenous lidocaine; ECT; spasmolytics. | L4-L5-S1 radiculopathy; hypertension; hypercholesterolemia. |
| CRPS19 | F/48 | Fell on outstretched arm; Thoracic outlet surgery/5 years | Autonomic dysregulation; neurogenic oedema; hyperalgesia; positive brachial plexus Tinel signs; poor movement and weakness of the hand; mechano and thermal allodynia. Pain (NRS) 8 | NSAIDs; AED; narcotics; spasmolytics; antidepressants; intravenous ketamine. | GERD; migraine |
| CRPS20 | F/61 | Motor vehicle accident. (flexion/extension neck injury)/5 years | Generalized mechano and thermal allodynia; hyperalgesia; poor initiation of movement and weakness; autonomic dysregulation; oedema generalized from brachial plexus. Pain (NRS) 7 | NSAIDs; AED; antidepressants; spasmolytics; narcotics; intravenous ketamine. | Depression; hypercholesterolemia; Breast Cancer 1998. |
| CRPS21 | M/58 | L4-L5 left radiculopathy; fell from 20 feet/5 years | Sharp stabbing pain; mechano allodynia Left>Right leg; myoclonic jerks; atrophy; weakness; autonomic dysregulation. Pain (NRS) 8 | AED; NSAIDs; narcotics; mexiletine; intravenous lidocaine. | Hypertension; GERD. |
| CRPS22 | F/34 | Fibroadenoma invading the right brachial plexus; two surgical biopsies/7 years | Autonomic dysregulation; neurogenic oedema of right arm; weakness of distal right arm muscles; mechano and thermal allodynia; deep sensitization. Pain (NRS) 6·5 | NSAIDs; AED; narcotics; antidepressants. | Depression/panic attacks. |
| CRPS23 | F/41 | Sprained left ankle and tore tendon; two surgeries/2 years | Dynamic and static mechano allodynia; trouble initiating movement; thermal allodynia; swelling; colour and temperature asymmetries’; weakness. Pain (NRS) 7 | NSAIDs; AED; narcotics. | Heart disease. |
| CRPS24 | F/42 | Motor vehicle accident (MVA); right BPTI; disk at C6-C7; surgery with fusion/5·5 years | Neurogenic oedema; autonomic dysregulation; positive Tinel signs; generalized mechano allodynia; hyperalgesia. Pain (NRS) 8 | NSAIDs; AED; antidepressants; spasmolytics; narcotics. | Depression; hypertension; hypercholesterolemia. |
| CRPS25 | F/49 | L5-S1 disc; fall with BPTI/18 years | Dynamic and static mechano allodynia; thermal allodynia; hyperalgesia; spread from leg to brachial plexus; generalized weakness; decreased initiation of movement. Pain (NRS) 7·5 | NSAIDs; AED; antidepressants; narcotics; intravenous ketamine; intravenous lidocaine. | Hypertension; hypercholesterolemia; L5-S1 radiculopathy; migraine. |
Disclosure
The authors certify that they have no commercial associations that might pose a conflict of interest in connection with this article. All funding sources for this study are listed in the Acknowledgements section.
References
- 1.Galer BS, Schwartz L, Allen RJ. Complex regional pain syndrome – type I: reflex sympathetic dystrophy, and type II: causalgia. In: Loeser JD, editor. Bonica's management of pain. 3rd edn. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. pp. 388–411. [Google Scholar]
- 2.Plewes LW. Sudek's atrophy in the hands. J Bone Joint Surg. 1956;38:195–203. doi: 10.1302/0301-620X.38B1.195. [DOI] [PubMed] [Google Scholar]
- 3.Schwartzman RJ, Alexander GM, Grothusen J. Pathophysiology of complex regional pain syndrome. Exp Rev Neurother. 2006;6:669–81. doi: 10.1586/14737175.6.5.669. [DOI] [PubMed] [Google Scholar]
- 4.Schwartzman RJ, Erwin KL, Alexander GM. The natural history of complex regional pain syndrome. Clin J Pain. 2009;25:273–80. doi: 10.1097/AJP.0b013e31818ecea5. [DOI] [PubMed] [Google Scholar]
- 5.Bruehl S, Harden RN, Galer BS, et al. External validation of IASP diagnostic criteria for complex regional pain syndrome and proposed research diagnostic criteria. IASP Pain. 1999;81:147–54. doi: 10.1016/s0304-3959(99)00011-1. [DOI] [PubMed] [Google Scholar]
- 6.Harden RN, Bruehl S, Galer BS, et al. Complex regional pain syndrome: are the IASP diagnostic criteria valid and sufficiently comprehensive? Pain. 1999;83:211–19. doi: 10.1016/s0304-3959(99)00104-9. [DOI] [PubMed] [Google Scholar]
- 7.Birklein F, Schmelz M, Schifter S, et al. The important role for neuropeptides in complex regional pain syndrome. Neurology. 2001;57:2179–84. doi: 10.1212/wnl.57.12.2179. [DOI] [PubMed] [Google Scholar]
- 8.Schinkel C, Gaertner A, Zaspel J. Inflammatory mediators are altered in the acute phase of posttraumatic complex regional pain syndrome. Clin J Pain. 2006;22:235–39. doi: 10.1097/01.ajp.0000169669.70523.f0. [DOI] [PubMed] [Google Scholar]
- 9.DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- 10.Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6:521–32. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- 11.McMahon SB, Cafferty WB, Marchand F. Immune and glial cell factors as pain mediators and modulators. Exp Neurol. 2005;192:444–62. doi: 10.1016/j.expneurol.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Abbadie C. Chemokines, chemokine receptors and pain. Trends Immunol. 2005;26:529–34. doi: 10.1016/j.it.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Watkins LR, Maier SF. Glia: a novel drug discovery target for clinical pain. Nat Rev Drug Discov. 2003;2:973–85. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- 14.Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in ‘small’ glia. Trends Neurosci. 2005;28:101–7. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Eikelenboom P, Bate C, Van Gool WA, et al. Neuroinflammation in Alzheimer's disease and prion disease. Glia. 2002;40:232–39. doi: 10.1002/glia.10146. [DOI] [PubMed] [Google Scholar]
- 16.Liu B, Gao HM, Hong JS. Parkinson's disease and exposure to infectious agents and pesticides and the occurrence of brain injuries: role of neuroinflammation. Environ Health Perspect. 2003;111:1065–73. doi: 10.1289/ehp.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci. 1999;22:219–40. doi: 10.1146/annurev.neuro.22.1.219. [DOI] [PubMed] [Google Scholar]
- 18.McGeer PL, Kawamata T, Walker DG, et al. Microglia in degenerative neurological disease. Glia. 1993;7:84–92. doi: 10.1002/glia.440070114. [DOI] [PubMed] [Google Scholar]
- 19.Ponomarev ED, Shriver LP, Maresz K, et al. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J Neurosci Res. 2005;81:374–89. doi: 10.1002/jnr.20488. [DOI] [PubMed] [Google Scholar]
- 20.Del Valle L, Schwartzman RJ, Alexander G. Spinal cord histopathological alterations in a patient with longstanding complex regional pain syndrome. Brain Behav Immun. 2009;23:85–91. doi: 10.1016/j.bbi.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Chan WY, Kohsaka S, Rezaie P. The origin and cell lineage of microglia – new concepts. Brain Res Rev. 2007;53:344–54. doi: 10.1016/j.brainresrev.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Mildner A, Schmidt H, Nitsche M, et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10:1544–53. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- 23.Priller J, Flugel A, Wehner T, et al. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med. 2001;7:1356–61. doi: 10.1038/nm1201-1356. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Shi XQ, Echeverry S, et al. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J Neurosci. 2007;27:12396–406. doi: 10.1523/JNEUROSCI.3016-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ziegler-Heitbrock HW. Heterogeneity of human blood monocytes: the CD14+ CD16+ subpopulation. Immunol Today. 1996;17:424–8. doi: 10.1016/0167-5699(96)10029-3. [DOI] [PubMed] [Google Scholar]
- 26.Frankenberger M, Sternsdorf T, Pechumer H, et al. Differential cytokine expression in human blood monocyte subpopulations: a polymerase chain reaction analysis. Blood. 1996;87:373–7. [PubMed] [Google Scholar]
- 27.Cairns AP, Crockard AD, Bell AL. The CD14+ CD16+ monocyte subset in rheumatoid arthritis and systemic lupus erythematosus. Rheumatol Int. 2002;21:189–92. doi: 10.1007/s00296-001-0165-8. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto H, Mizuno K, Horio T. Circulating CD14+ CD16+ monocytes are expanded in sarcoidosis patients. J Dermatol. 2003;30:503–9. doi: 10.1111/j.1346-8138.2003.tb00424.x. [DOI] [PubMed] [Google Scholar]
- 29.Pulliam L, Gascon R, Stubblebine M, et al. Unique monocyte subset in patients with AIDS dementia. Lancet. 1997;349:692–5. doi: 10.1016/S0140-6736(96)10178-1. [DOI] [PubMed] [Google Scholar]
- 30.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–92. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 31.Harden RN, Bruehl S, Stanton-Hicks M, et al. Proposed new diagnostic criteria for complex regional pain syndrome. Pain Med. 2007;8:326–31. doi: 10.1111/j.1526-4637.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 32.Rolke R, Baron R, Maier C, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123:231–43. doi: 10.1016/j.pain.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 33.Schwartzman RJ, Grothusen JR. Brachial plexus traction injury: quantification of sensory abnormalities. Pain Med. 2008;9:950–7. doi: 10.1111/j.1526-4637.2007.00394.x. [DOI] [PubMed] [Google Scholar]
- 34.Ancuta P, Wang J, Gabuzda D. CD16+ monocytes produce IL-6, CCL2, and matrix metalloproteinase-9 upon interaction with CX3CL1-expressing endothelial cells. J Leukoc Biol. 2006;80:1156–64. doi: 10.1189/jlb.0206125. [DOI] [PubMed] [Google Scholar]
- 35.Huygen FJPM, de Bruijn AGJ, de Bruin MT. Evidence for local inflammation in complex regional pain syndrome type 1. Mediat Inflam. 2002;11:47–51. doi: 10.1080/09629350210307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van de Beek WJT, Remarque EJ, Westendorp RGJ, et al. Innate cytokine profile in patients with complex regional pain syndrome is normal. Pain. 2001;91:259–61. doi: 10.1016/S0304-3959(00)00443-7. [DOI] [PubMed] [Google Scholar]
- 37.Ribbers GM, Oosterhuis WP, van Limbeek J, et al. Reflex sympathetic dystrophy: is the immune system involved? Arch Phys Med Rehabil. 1998;79:1549–52. doi: 10.1016/s0003-9993(98)90418-x. [DOI] [PubMed] [Google Scholar]
- 38.Kaufmann I, Eisner C, Richter P, et al. Lymphocyte subsets and the role of TH1/TH2 balance in stressed chronic pain patients. Neuroimmunomodulation. 2007;14:272–80. doi: 10.1159/000115041. [DOI] [PubMed] [Google Scholar]
- 39.Cottam DR, Schaefer PA, Shaftan GW, Velcu L, Angus LD. Effect of surgically induced weight loss on leukocyte indicators of chronic inflammation in morbid obesity. Obes Surg. 2002;12:335–42. doi: 10.1381/096089202321088101. [DOI] [PubMed] [Google Scholar]
- 40.Dimitrov S, Lange T, Nohroudi K, et al. Number and function of circulating human antigen presenting cells regulated by sleep. 2009;30:401–11. doi: 10.1093/sleep/30.4.401. [DOI] [PubMed] [Google Scholar]
- 41.Timmerman KL, Flynn MG, Coen PM, et al. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti-inflammatory influence of exercise? J Leukoc Biol. 2008;84:1271–8. doi: 10.1189/jlb.0408244. [DOI] [PubMed] [Google Scholar]
- 42.Peterlin BL, Rosso AL, Nair S, et al. Migraine may be a risk factor for the development of complex regional pain syndrome. Cephalalgia. 2009;30:214–33. doi: 10.1111/j.1468-2982.2009.01916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong S, Mills PJ. Effects of an exercise challenge on mobilization and surface marker expression of monocyte subsets in individuals with normal vs. elevated blood pressure. Brain Behav Immun. 2008;22:590–9. doi: 10.1016/j.bbi.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steppich B, Dayyani F, Gruber R, et al. Selective mobilization of CD14(+)CD16(+) monocytes by exercise. Am J Physiol Cell Physiol. 2000;279:C578–86. doi: 10.1152/ajpcell.2000.279.3.C578. [DOI] [PubMed] [Google Scholar]
- 45.Galer BS, Henderson J, Perander J, et al. Course of symptoms and quality of life measurement in Complex Regional Pain Syndrome: a pilot survey. J Pain Symptom Manage. 2000;20:286–92. doi: 10.1016/s0885-3924(00)00183-4. [DOI] [PubMed] [Google Scholar]
- 46.Schlatter J, Ortuño F, Cervera-Enguix S. Monocytic parameters in patients with dysthymia versus major depression. J Affect Disord. 2004;78:243–7. doi: 10.1016/S0165-0327(02)00316-6. [DOI] [PubMed] [Google Scholar]
- 47.Huygen FJ, Ramdhani N, van Toorenenbergen A, et al. Mast cells are involved in inflammatory reactions during complex regional pain syndrome type 1. Immunol Lett. 2004;91:147–54. doi: 10.1016/j.imlet.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 48.Tan EC, Oyen WJ, Goris RJ. Leukocytes in complex regional pain syndrome type I. Inflammation. 2005;29:182–6. doi: 10.1007/s10753-006-9015-x. [DOI] [PubMed] [Google Scholar]
- 49.Kaufmann I, Eisner C, Richter P, et al. Psychoneuroendocrine stress response may impair neutrophil function in complex regional pain syndrome. Clin Immunol. 2007;125:103–11. doi: 10.1016/j.clim.2007.07.004. [DOI] [PubMed] [Google Scholar]


