Abstract
We report a rare association of Henoch-Schönlein Purpura with recurrent endocarditis in a 36 year old male patient presenting with rash and renal failure. Bacterial endocarditis can be complicated by renal failure of various etiologies. Biopsy may distinguish these and guide therapy as seen in this case. Here, timely diagnosis of Henoch-Schönlein Purpura in the setting of recurrent methacillin sensitive staphylococcus endocarditis, led to steroid therapy and renal recovery. This is a rare reported case of Henoch-Schönlein Purpura during an episode of recurrent adult endocarditis that also highlights the complex interplay between genetic susceptibility and immune responses.
CASE REPORT
A 36 year old male with a history of intravenous drug use initially presented with three weeks of fevers, chills, night sweats and malaise. He had a history of remote native tricuspid valve endocarditis with methacillin sensitive staphylococcus aureus (MSSA) six years earlier. Then, septic embolic complications required debridement of his tricuspid valve and several months of antibiotic therapy. He had recovered and was in good health until his current illness began sub-acutely with daily fevers greater than >101 degrees Fahrenheit. At his first clinic evaluation a white blood count of 22,000 prompted an injection of intramuscular ceftriaxone followed by oral cephalexin. No blood cultures were obtained. The next day he developed a diffuse, tender, purple raised rash on his arms and legs and then abdomen. Four days later, at a local Emergency department, cephalexin was stopped, based upon possible drug induced vasculitis, and he was given a short course of dexamethasone. He had had no previous history of cephalosporin allergy, purpura, or leukocytoclastic vasculitis. A week later, with ongoing fevers and rash, he was admitted to the hospital. Then the patient reported diffuse arthralgias and abdominal pain without hematochezia. Steroids were stopped. A urinalysis showed hematuria and pyuria, thus he was treated with levofloxacin for presumed urosepsis. Biopsy of a rash lesion was sent for pathology. On hospital day two, blood cultures returned positive for MSSA. Antibiotic coverage included vancomycin, followed by daptomycin. Computed tomography of the chest showed multiple abscesses. A transesophageal echocardiogram showed a1.6 cm vegetation on the lateral leaflet of the tricuspid valve and severe tricuspid regurgitation.
Upon rheumatology consult after transfer, the patient reported improving arthralgias, ongoing abdominal pain and remaining rash on his extremities without evolution. His exam was notable for a pansystolic murmur heard best over the left lower sternal border, lack of synovitis, and numerous 1-3mm, non-blanchable, dark-red macules and voilaceous papules. About 90% of his body surface area was involved with the extremities being more severely affected, and sparing of the head, neck, palms and soles. Laboratory data at the time is provided in Table 1.
Table 1.
Laboratory data upon inpatient transfer
| Test | Patient Value | Normal Range |
|---|---|---|
| WBC (K/uL) | 4.9 | 3.8-10.5 |
| Hemoglobin (g/dL) | 8.7 | 13.6-17.2 |
| Hematocrit (%) | 28 | 40-52 |
| Platelet (K/uL) | 160 | 160-370 |
| Creatinine (mg/dL) | 2.0 | 0.6-1.3 |
| Albumin (g/dL) | 1.7 | 3.3-4.7 |
| CRP (mg/dL) | 4 | 0-1 |
| ESR (mm/Hr) | 94 | 0-15 |
| UA | 6-10 RBC | 0-2 |
| 2-5 WBC | 0-2 | |
| Protein/creatinine ratio | 1.6 | 0 |
| C3 (mg/dL) | 139 | 90-180 |
| C4 (mg/dL) | 17 | 10-40 |
| IgA (mg/dL) | 586 | 50-450 |
| c-ANCA, p-ANCA | Negative | Negative |
| Cryoglobulin | Negative | Negative |
| ANA | Negative | Negative |
| HIV 1 and 2 antibody | Negative | Negative |
| Hepatitis B surface antigen | Negative | Negative |
| Hepatitis C antibody | Negative | Negative |
WBC=white blood count; CRP=C-Reactive Protein; ESR=erythrocyte sedimentation rate; UA=urinalysis; C3=complement component 3; C4=complement component 4; ANCA=anti-neutrophilic cytoplasmic antibody; ANA=anti-neutrophilic antibody.
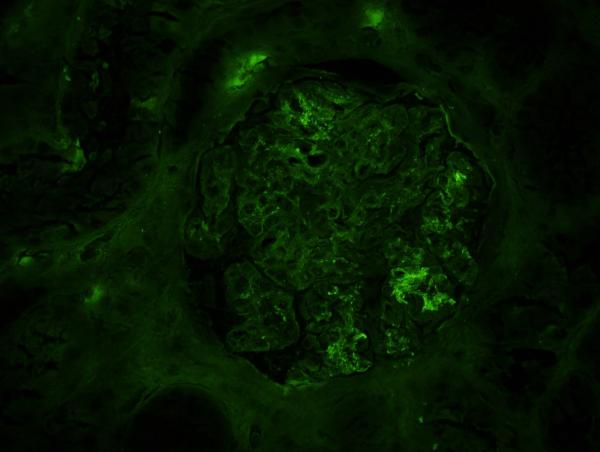
Review of the prior skin pathology report described leukocytoclastic vasculitis with IgA deposition on immunoflourescence. Nevertheless, given worsening renal function and potential therapeutic implications, a renal biopsy was obtained. This demonstrated focal, segmental proliferative glomerulonephritis with IgA and C3 staining and few subendothelial deposits (Figure 1). Staining was negative for immune complexes, and no thrombi or crescents were noted on light microscopy with H+E staining. The patient's picture and biopsy results confirmed a diagnosis of Henoch-Schönlein Purpura (HSP) with renal involvement in the setting of recurrent native tricuspid valve endocarditis with MSSA.
Figure 1.
Immunoflourescence of the patient's renal biopsy showing a glomerulus with diffuse and segmental IgA staining.
DISCUSSION
Herein we presented a rare association of Henoch-Schönlein Purpura with endocarditis in an adult patient. As in this case, renal failure may accompany 1/3 of endocarditis cases and pathology is often imperative, particularly in light of the broad differential diagnosis (Table 2)1-3. Renal pathology was instrumental in this case to unify the skin pathology, renal disease, and reach a definitive HSP diagnosis. When HSP was confirmed, this case was particularly interesting in light of his prior MSSA endocarditis episode that did not precipitate vasculitis. HSP is characterized by the triad of arthritis, purpura (Figure 2) and colicky GI symptoms4. Histological evidence of granulocytes in the walls of small arterioles or venules plus IgA-dominant immune deposits confirms the diagnosis in appropriate clinical settings. HSP is primarily a disease of children that is typically self-limited, but 10% of cases occur in adults where features and outcomes may vary. Like in children, in adults, HSP often spontaneously resolves. More severe renal disease imposes significant morbidity and is often an indication for steroid treatment. When compared to children, adults with HSP have a lower frequency of abdominal pain and fever, a higher frequency of joint symptoms, but more frequent and severe renal involvement 5. Adults are thereby more likely to require aggressive therapy including steroids or cytotoxic agents. The prognosis for adult patients with HSP nephritis is also worse than in children. In a large cohort of 250 cases of HSP nephritis in adults, 11% reached end-stage renal failure and 13% had severe renal failure defined by creatinine clearance <30 ml/min6. Full renal recovery was achieved in only 20%. Survival was only 74% at the end of follow-up after a median of 14.8 years.
Table 2.
Causes of kidney disease in infective endocarditis.
| Immune Complex Mediated Glomerulonephritis: |
| Post streptococcal |
| Other post infectious |
| Cryoglobulinemia |
| Drug-induced: |
| Acute interstitial nephritis (e.g. penicillin) |
| Acute tubular necrosis (e.g. aminoglycosides) |
| Embolic disease: |
| Renal infarction |
| Renal abscess |
| Primary Vasculitis: |
| Henoch-Schönlein Purpura |
| ANCA-associated vasculitis |
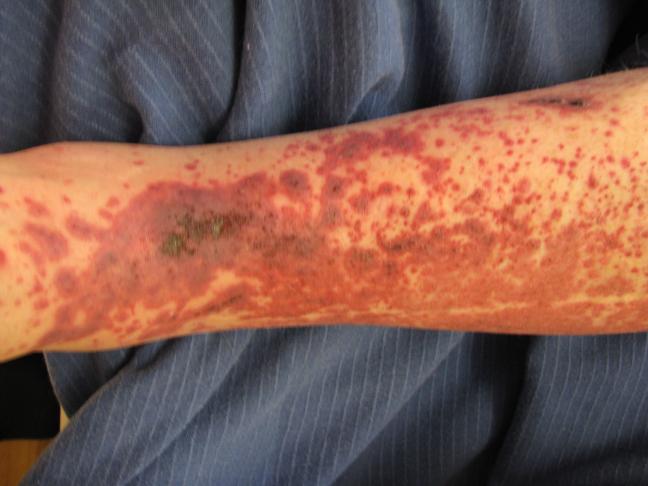
Figure 2.
Classic ankle distribution voilaceous palpable rash seen in Henoch-Schönlein Purpura.
The underlying pathogenesis of HSP remains unknown. Streptococcal infections, staphylococcal infections, vaccinations, medications and even insect bites have all been implicated as possible triggers, although some cases lack a clear precipitating event5. In cases with an identifiable trigger, upper respiratory infections and medications, including beta-lactam or cephalosporin antibiotics, are most frequently culprit. One study of 6 cases with staphylococcus associated HSP nephritis demonstrated a unique profile of T-cell receptor activation and cytokine production that normalized following clearance of infection7. The outcome of these six patients was poor including two deaths, and two patients requiring maintenance hemodialysis.
To date, only two cases of adult endocarditis-associated HSP have been described in the English literature, and no cases involved recurrent endocarditis. The first case report described a 21 year old male with history of IV drug use and right sided staphylococcal endocarditis who developed skin and renal biopsy proven HSP8. Initially when the endocarditis was diagnosed, prior to the onset of HSP, he received cloxacillin and netilmicin , thus it was impossible to know whether the drug or the infection was the precipitating event. Like our patient, he had multiple pulmonary cavitary embolic lesions and large bilateral effusions. At discharge that patient had mild proteinuria, but did not require steroids, though he was lost to follow up. The second report described a 41 year old female patient with left-sided streptococcus sanguis subacute bacterial endocarditis who presented with a purpuric rash prior to the diagnosis of endocarditis9. The rash was biopsy-proven to be consistent with HSP. She was started on ampicillin and gentamicin, and eventually underwent mitral valve replacement. Due to renal failure, she received methylprednisolone, although a kidney biopsy was not performed. Renal recovery status was not reported.
Our patient went on to develop more severe renal failure with a peak creatinine of 5.3mg/dL. He required a short course of hemodialysis and a prolonged steroid taper, but subsequently regained his renal function. He received a prolonged course of antibiotics and his tricuspid valve was ultimately replaced successfully.
CONCLUSION
Henoch-Schönlein Purpura is a vasculitis that is frequently triggered by upper respiratory infections, though less commonly specifically linked to endocarditis. In contrast to the classic pediatric presentation, in adults HSP is characterized by more severe renal disease that often requires renal replacement and steroid therapies. Our case was unique given that the patient did not develop HSP during his first episode of MSSA endocarditis, but developed biopsy-proven HSP during his subsequent episode years later. This case highlights the complex interplay between genetic susceptibility and infection specific immune triggering in HSP.
ACKNOWLEDGEMENTS
The authors would like to thank Nadia Naderi, MD for assistance in obtaining pathology images from this case, and Jessica Zmolik, MD for the cutaneous teaching image.
Funding: Dr. Bartels receives support from University of Wisconsin Institute for Clinical and Translational Research (UW-ICTR), grant 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health.
Footnotes
Disclosures: none
REFERENCES
- 1.Majumdar A, Chowdhary S, Ferreira MAS, Hammond LA, Howie AJ, Lipkin GW, Littler WA. Renal pathological findings in infective endocarditis. Nephrol Dial Transplant. 2000;15:1782–1787. doi: 10.1093/ndt/15.11.1782. [DOI] [PubMed] [Google Scholar]
- 2.Conlon PJ, Jefferies F, Krigman HR, Corey GR, Sexton DJ, Abramson MA. Predictors of prognosis and risk of acute renal failure in bacterial endocarditis. Clin Nephrol. 1998;49:96. [PubMed] [Google Scholar]
- 3.Kishimoto N, et al. Cytoplasmic antineutrophil cytoplasmic antibody positive pauci-immune clomerulonephritis associated with infectious endocarditis. Clin Nephrol. 2006;66:447–54. doi: 10.5414/cnp66447. [DOI] [PubMed] [Google Scholar]
- 4.Szer I. Henoch-Schönlein Purpura. In: Hochberg M, editor. Rheumatology. Elsevier Limited; Philedelphia: 2004. pp. 1571–1578. [Google Scholar]
- 5.Blanco R, Martínez-Taboada VM, Rodríguez-Valverde V, García-Fuentes M, González-Gay MA. Henoch-Schönlein purpura in adulthood and childhood: two different expressions of the same syndrome. Arthritis Rheum. 1997;40:859–864. doi: 10.1002/art.1780400513. [DOI] [PubMed] [Google Scholar]
- 6.Pillebout E, Thervet E, Hill G, Alberti C, Vanhille P, Nochy D. Henoch-Schönlein Purura in Adults: Outcome and Prognostic Factors. J Am Soc Nephrol. 2002;13:1271–1278. doi: 10.1097/01.asn.0000013883.99976.22. [DOI] [PubMed] [Google Scholar]
- 7.Hirayama K, Kobayashi M, Muro K, Yamagata K, Koyama A. Specific T-cell receptor usage with cytokinemia in Henöch-Schonlein purpura nephritis associated with Staphylococcus aureus infection. J Intern Med. 2001;249:289–295. doi: 10.1046/j.1365-2796.2001.00815.x. [DOI] [PubMed] [Google Scholar]
- 8.Montoliu J, Miró J, Campistol J, Trilla A, Mensa J, Torras A, Revert L. Henöch-Schonlein Purpura Complicating Staphylococcal Endocarditis in a Heroin Addict. Am J Nephrol. 1987;7:136–139. doi: 10.1159/000167450. [DOI] [PubMed] [Google Scholar]
- 9.Galaria NA, Lopressti NP, Magro Henöch-Schonlein purpura secondary to subacute bacterial endocarditis. Cutis. 2002;69:269–273. [PubMed] [Google Scholar]