Abstract
In the course of a synthesis of the tricyclic sesquiterpene (−)-cameroonan-7α-ol from the acyclic (+)-citronellal, seven aliphatic C-H bonds were converted to C-C bonds, and three rings and four new stereogenic centers were established.
Introduction
Cameroonan-7α-ol (2), a sesquiterpene from the essential oil of the rhizome Echinops giganteus var. lelyi, was originally described by Weyerstahl in 1997.1 While the essential oil is a complex mixture of several sesquiterpenes, (−)-2 is believed to be the major contributor to its strong woody, patchouli-like fragrance. The tricyclic skeleton of (−)-cameroonanol (2) is biogenetically derived from farnesyl pyrophosphate, through polyene cyclization and carbocationic rearrangements.2 Composed of three cis-fused five member rings and five contiguous stereogenic centers in a dense configuration, (−)-2 offers significant synthetic challenges.
 |
(1) |
Previous syntheses of (±)-2 focused on dipolar cycloaddition to a racemic bicyclooctenone.3 We sought an alternative approach, based on ring construction by C-H to C-C bond functionalization (Equation 1). There has been a great deal of attention paid recently to the selective oxidation of aliphatic C-H bonds to C-O bonds.4 The direct conversion of aliphatic C-H bonds to C-C bonds is strategically at least as important.5,6 Herein, we report the first synthesis of enantiopure (−)-cameroonan-7α-ol (2), starting (Scheme 1) from the acyclic (+)-citronellal (3). We envisioned that by the judicious conversion of seven aliphatic C-H bonds to C-C bonds, it could be possible to incorporate the complete carbon skeleton of 1 into (−)-cameroonan-7α-ol (2). Of particular note is a sequence of RhII-catalyzed C-H functionalization followed by MnIIImediated oxidative radical cyclization for the stereocontrolled construction of [3.3.0] fused bicyclooctanes in enantiomerically pure form.
Scheme 1.
Results and Discussion
Cyclization by Rh-carbenoid C-H Functionalization
We planned to establish the absolute configuration of (−)-2 via enantioretentive C-H functionalization7 of the C-4 methine of (+)-citronellal 3. To that end, the α-diazo-β-ketoester 5 was constructed (Scheme 1). The synthesis commenced with a three step sequence of LiAlH4 reduction, tosylation and nucleophilic displacement of (+)-3 to provide the known nitrile 4.8 Homologation to the β-ketoester 1 was accomplished via Blaise reaction9 of the nitrile 4 with BrCH2CO2Et. We found that reproducibly high yields in this reaction could be achieved using activated Zno metal10 and in situ activation with a catalytic amount of TFA.11 Diazo transfer using MsN312 then provided the requisite α-diazo-β-ketoester 5 for C-H functionalization. Using catalytic Rh2(Oct)4, 5 readily cyclized to 6.
MnIII Oxidative Radical Cyclization
We planned next to cyclize the β-ketoester 6 with Mn(OAc)3. As described by Snider,13 oxidative radical generation, followed by subsequent cyclization, further oxidation and deprotonation, would provide the second ring of 2 (Scheme 2). Its application to the synthesis of (−)-cameroonanol (2) immediately raised a question of selectivity, as one ring and at least one additional stereogenic center are formed in the cyclization. We expected preference for the formation of the cis-5/5 fused versus the corresponding trans-5/5 or 6/5-bicyclic products. The relative stereochemical orientation of the pendant alkyl side chain (ie. endo vs. exo selectivity) in the product had yet to be investigated using this method, although radical cyclizations in general proceed with endo selectivity.14
Scheme 2.
In the event (Scheme 2), we observed that the cyclization did proceed with high preference for the cis-5/5 fused products, as expected. Initially, the use of anhydrous acetonitrile solvent led to the endo-alkene 7 as the dominant product. The use of acetic acid as a solvent shortened the reaction time considerably, but also delivered a more complex mixture of products.15 Conveniently, after treatment of that crude reaction material with water, the hemiketal 8 and the endo-alkene 7 emerged as the major products. Furthermore, products 7, 9 or 10 could each be selectively obtained from the crude reaction mixture in high overall yield depending upon the dehydration conditions. This iterative protocol of cyclization followed by dehydration allowed endo or exo cyclization products to be selectively obtained.
Stereoselective Reduction
Each of the three bicycles (7, 9 and 10) from MnIII-mediated cyclization could potentially be useful for the synthesis of (−)-cameroonanol (2). Each was independently investigated, beginning with the endo-alkene 7. Installation of the gem-dimethyl moiety was accomplished using methyl iodide and potassium hydride in paraffin [KH(P)].16a Chemoselective ketone reduction of 7 with NaBH4 provided the secondary alcohol 11 as a single diastereomer (Equation 2). However, alcohol 11 was prone to undesired cyclization to the cyclic ether 12 under mildly acidic conditons. Conversely, when the cis-fused lactone 10 was subjected to identical NaBH4 reduction, the opposite stereoselectivity was observed (Equation 3). Attempts to fragment the protected lactone 13 ultimately resulted in the formation of tetrasubstituted alkenes of type 14. As these derivatives were more directly accessible from 9, we turned our attention to that intermediate.
 |
(2) |
 |
(3) |
We were pleased to observe that reduction of the ketone 9 delivered the desired alcohol 15 with high diastereocontrol (Scheme 3). Before manipulation of the alkyl side chain, we sought to protect the potentially sensitive β-hydroxy ester 15. Benzyl ether formation under Williamson etherification conditions16b provided 16 with complete inversion of the secondary carbinol center, presumably by a retro-aldol/aldol process. After screening a variety of benzylation procedures, we found that the protocol developed by Dudley17 delivered the benzyl ether 17 cleanly and with retention of relative configuration.
Scheme 3.
Alkyl Side Chain Functionalization
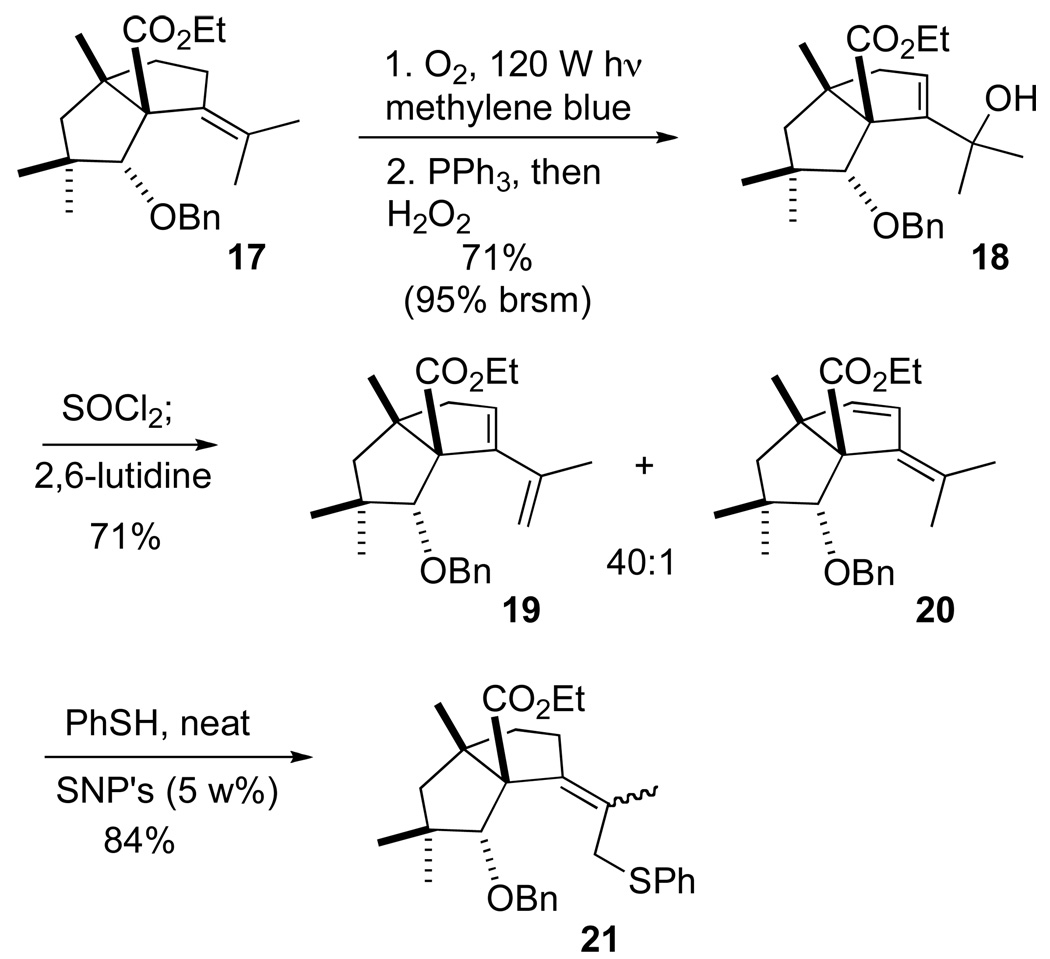
Attempts to directly cyclize 17 (Scheme 4) by direct allylic deprotonation were unsuccessful. We therefore functionalized the allylic C-H of 17 to set the stage for the construction of final ring of (−)-2. Exposure of the alkene 17 to singlet oxygen regioselectively formed the alcohol 18. Optimized dehydration conditions suppressed formation of the undesired 20, giving clean conversion to the diene 19. Initial attempts to functionalize the diene 19 as the thioether under standard radical conditions were low yielding and proceeded with poor regiocontrol. However, the use of Lewis acidic silica nanoparticles (SNP’s)18 allowed the selective functionalization of the terminal alkene. Concomitant (and desired) alkene isomerization provided an inseparable mixture of E/Z allylic thioethers 21 in good yield.
Scheme 4.
Completion of the synthesis
Formation of the final ring of (−)-2 was accomplished by cyclization of the Z-allylic sulfone 24 derived from the thioether 21 (Scheme 5). While direct oxidation of the thioether 21 to the sulfone 24 was possible, an iterative oxidation sequence by way of the sulfoxides 22 and 23 proved useful for two reasons. First, the intermediate Z and E allylic sulfoxide isomers could be chromatographically resolved. Further, we found that the E-allylic sulfoxides 22a, b could be equilibrated to a 3:1 mixture of E:Z allylic sulfoxides under microwave conditions.19 This allowed for increased throughput to the Z-allylic sulfoxides 23a, b, that were oxidized to the sulfone 24. On exposure to LDA20, 24 smoothly cyclized to the enone 25. The corresponding E-allylic sulfone did not cyclize.
Scheme 5.
a) mCPBA, CH2Cl2, −78 °C, 61% 23a,b, 37% 24. b) PhMe, μ-wave, 120 °C, 23a,b:24 = 3:1. c) H2WO4 (0.1 eq), H2O2, RT, 24 h, 76%. d) LDA, −40 °C, 90%.
Having successfully installed the all-cis 5/5 ring fusions, we next turned our attention to the final stereocenter of 2. Specifically, we required a method for introducing hydrogen to the more congested face of enone 25 to establish the C9 stereocenter (Scheme 6). Among the various known protocols for enone reduction, methods based on hydrogenation or nucleophilic hydride delivery were anticipated to deliver the wrong diastereomer (ie. product 27) via approach from the more sterically accessible face of the enone 25. However, dissolving metal reduction had been shown to deliver the thermodynamically more stable products from β-substituted cyclic enones.21
Scheme 6.
SmI2/HMPA/NH4Cl22 proved to be an efficient system for the reduction of the enone 25, providing a 1:1 mixture of desulfonylated C-9 methyl diastereomers (that is, 28 and epi-28, Scheme 6). However, the two ketone diastereomers were not separable. Mg/MeOH effected the chemoselective reduction of the enone 25 to a separable mixture of 26 and 27. The relative configuration of the secondary methyl group was confirmed by the conversion of 26 to C-9-epi-cameroonanol (epi-2).23 It seemed that the combined steric effect of the C-4 methyl group and developing C-2 to C-9 syn-pentane interaction slow protonation from the desired face during the reduction, even when using a small proton source such as NH4Cl.
The ketone 27 was subject to SmI2 mediated desulfonylation to deliver the ketone 28. Wolff-Kishner reduction was followed by deprotection to complete the synthesis of the volatile (−)-cameroonan-7α-ol (2). The spectroscopic data (IR, MS, 1H and 13C NMR) for synthetic (−)-2 fully agreed with those previously reported [αD ref.1 = (−) 34°; obs.= (−) 37°].
Conclusion
The first total synthesis of enantiomerically pure (−)-cameroonan-7α-ol (2) has been accomplished. Central to the synthesis is the specific conversion of aliphatic C-H bonds to C-C bonds. The procedure presented for the stereoselective two-stage construction of the first two rings of (−)-cameroonanol (2) from the acyclic ester 1 exemplifies the strategic efficiency of C-H bond functionalization in natural product synthesis. We anticipate that the sequence of RhII-mediated C-H functionalization followed directly by MnIII-oxidative radical cyclization developed here will become a general method for the construction of bicyclic intermediates with the control of both relative and absolute configuration. In the application described here, this two-step strategy enabled the controlled installation of the two contiguous cyclic quaternary centers of (−)-cameroonan-7α-ol (2).
Experimental Section
General Procedures
1H NMR and 13C NMR spectra were recorded, as solutions in deuteriochloroform (CDCl3) unless otherwise indicated, at 400 MHz and 100 MHz, respectively. 13C multiplicities were determined with the aid of a JVERT pulse sequence, differentiating the signals for methyl and methine carbons as "d" from methylene and quaternary carbons as "u". The infrared (IR) spectra were determined as neat oils. Rf values indicated refer to thin layer chromatography (TLC) on 2.5 × 10 cm, 250 mm analytical plates coated with silica gel GF, unless otherwise noted, and developed in the solvent system indicated. Microwave reactions were performed using a CEM Discover Labmate microwave reactor with vertically-focused IR temperature sensor. All glassware was oven dried and rinsed with dry solvent before use. THF and diethyl ether were distilled from sodium metal/benzophenone ketyl under dry nitrogen. Toluene, dichloromethane and acetonitrile were distilled from calcium hydride under dry nitrogen. CH2Cl2 is dichloromethane, MTBE is methyl-tertbutyl ether and PE is petroleum ether. All reactions were conducted under N2 and stirred magnetically.
(4R)-4,8-Dimethylnon-7-enenitrile (4)
To a slurry of LiAlH4 (11.81 g, 311 mmol, 1.2 equiv) in 450 mL of THF, in a 2 L multi-neck round bottom flask (equipped with a reflux condenser, addition funnel and N2 inlet adapter) submerged in a 0°C ice bath, was added a solution of (R)-citronellal 3 (39.95 g, 259 mmol) in 70 mL of THF dropwise via addition funnel over 30 min. Upon complete addition, the ice bath was removed and the reaction was stirred at room temperature for 1 h. The reaction was quenched according to the method of Micovic23 After stirring at room temperature overnight, 20 g of Na2SO4 was added and the mixture was filtered through Celite to remove the white precipitate. The filter cake was thoroughly washed with Et2O and concentrated in vacuo.
The residue was diluted with 108 mL of anhydrous pyridine and cooled to 0°C under an N2 atmosphere. p-Toluenesulfonyl chloride (76.52 g, 401.5 mmol, 1.55 equiv) was added in three equal portions over 15 min intervals. The reaction was gradually stirred to room temperature with the ice bath. After 4 h at room temperature, the reaction material was partitioned between Et2O, and, sequentially, 1N aqueous HCl and saturated aqueous NaHCO3. The combined organic extracts were dried with Na2SO4 and concentrated in vacuo.
The residue was diluted with 860 mL of 2:1 EtOH:H2O and KCN (50.5 g, 777 mmol, 3.0 equiv) was added. The temperature was increased to 70°C and the mixture was stirred overnight (16 h). After cooling to room temperature, the reaction mixture was concentrated in vacuo (to remove the bulk of the EtOH) and extracted with CH2Cl2. Combined organic phases were dried (Na2SO4) and concentrated in vacuo. The residue was distilled bulb-to-bulb (bath = 100 – 105 °C; 0.05 torr) to afford the known nitrile 4 as a pale yellow oil (36.32 g, 220 mmol, 85 % yield from citronellal). The analytical data for nitrile 4 match those previously reported.8
TLC Rf (PE:MTBE = 20:1) = 0.39; [α]20D = (+) 3.7° (c 2.15 CHCl3). 1H NMR δ 5.10 (1H, m), 2.01 (2H, m), 1.76-1.68 (1H, m), 1.70 (3H, s), 1.62 (3H, s), 1.61-1.56 (1H, m), 1.50 (1H, m), 1.36 (1H, m), 1.21 (1H, m), 0.94 (3H, d, J=6.5 Hz);13C NMR24 δ u 131.7, 120.0, 36.3, 32.2, 25.2, 14.9; d 124.1, 31.6, 25.68, 18.7, 17.6; IR (film, cm−1) 1672, 2247, 2359, 2921. HRMS calcd for C11H20N (M+H) 166.1596, obsd 166.1598.
Ethyl (6R)-6,10-Dimethyl-3-oxoundec-9-enoate (1)
Zn metal (Zn dust, 17.8 g, 271.9 mmol, 3.1 equiv) was washed successively with 3 N aqueous HCl, H2O, EtOH and anhydrous Et2O. After removal of all solvent under high vacuum, the activated Zn metal was added to 50 mL of THF and the slurry was brought to reflux. Trifluoroacetic acid (65 µL, 0.88 mmol, 0.01 equiv) was added, followed by a minor amount of BrCH2CO2Et (ca. 0.5 mL) until the reaction mixture became green. Nitrile 4 (14.47 g, 87.7 mmol) was then added in one portion. After stirring for 20 min, a solution of BrCH2CO2Et (21.4 mL, 193 mmol, 2.2 equiv) in 21 mL of THF was added dropwise via addition funnel over 1 h. Upon complete addition, the reaction was stirred at reflux for 0.5 h. After cooling to room temperature, 100 mL of 3 N aqueous HCl was added and the reaction stirred for 2 h at room temperature. The reaction material was partitioned between EtOAc and, sequentially, saturated aqueous NaHCO3 and brine. The combined organic extracts were dried (Na2SO4) and concentrated in vacuo. The residue was chromatographed to afford β-ketoester 1 as a colorless oil (18.34 g, 72.2 mmol, 82% yield); TLC Rf (PE:MTBE = 20:1) = 0.15; [α]20D = (−) 3° (c 2.7 CHCl3); 1H NMR δ 5.08 (1H, m), 4.20 (2H, q, J=7.1 Hz), 3.44 (2H, s), 2.54 (2H, m), 1.97 (2H, m), 1.68 (3H, s), 1.60 (3H, s), 1.65-1.58 (1H, m), 1.42 (2H, m), 1.28 (1H, m), 1.28 (3H, t, J=7.1 Hz), 1.17 (1H, m), 0.89 (3H, d, J=6.3 Hz); 13C NMR δ u 203.1, 167.3, 131.3, 61.3, 49.3, 40.8, 36.8, 30.3, 25.4; d 124.6, 31.9, 25.7, 19.2, 17.6, 14.1; IR (film, cm−1) 1643, 1743, 2921; HRMS calcd for C15H26O3 (M+Na) 277.1780, obsd 277.1771.
Ethyl (6R)-2-Diazo-6,10-dimethyl-3-oxoundec-9-enoate (5)
To a solution of β-ketoester 1 (10.0 g, 39.37 mmol) and MeSO2N3 (7.15 g, 59.06 mmol, 1.5 equiv) in 79 mL of acetonitrile at room temperature was added NEt3 (11.05 mL, 78.74 mmol, 2.0 equiv) dropwise via syringe. Stirring was continued for 3.5 h at room temperature. The reaction material was diluted with 10% aqueous NaOH and extracted with Et2O. Combined organic extracts were dried (Na2SO4) and concentrated in vacuo. The residue was chromatographed to afford α-diazo-β-ketoester 5 as a colorless oil (10.21 g, 36.5 mmol, 93 % yield); TLC Rf (PE:MTBE = 20:1) = 0.46; [α]20D = (−) 6.1° (c 3.0 CHCl3); 1H NMR δ 5.09 (1H, m), 4.30 (2H, q, J=7.1 Hz), 2.86 (2H, m), 1.98 (2H, m), 1.68 (1H, m), 1.68 (3H, s), 1.60 (3H, s), 1.46 (2H, m), 1.33 (1H, m), 1.33 (3H, t, J=7.1 Hz), 1.18 (1H, m), 0.90 (3H, d, J=6.4 Hz); 13C NMR δ u 193.4, 161.4, 131.2, 75.8, 61.3, 38.0, 36.8, 31.3, 25.5; d 124.7, 32.2, 25.7, 19.3, 17.6, 14.3; IR (film, cm−1) 1658, 1720, 2135, 2921; HRMS calcd for C15H24N2O3 (M+Na) 303.1685, obsd 303.1689.
Ethyl (2R)-2-Methyl-2-(4-methylpent-3-en-1-yl)-5-oxocyclopentanecarboxylate (6)
Rh2(Oct)4 (142 mg, 0.182 mmol, 0.005 equiv) was added to 530 mL of CH2Cl2 and the mixture sonicated to homogeneity over 10 minutes. To the resultant pale green homogenous solution was added a solution of diazo compound 5 (10.2 g, 36.4 mmol) in 200 mL of CH2Cl2 dropwise via addition funnel over 1 h. The reaction was stirred for 1 h at room temperature and then concentrated in vacuo. The residue was chromatographed to afford a mixture β-ketoesters 6 (both α-epimers and enol) as a colorless oil (7.85 g, 31.14 mmol, 85 % yield); TLC Rf (PE:MTBE = 20:1) = 0.40;1H NMR δ 10.98 (0.15H, bs, OH), 5.07 (1H, m), 4.24-4.11 (2H, m), 2.98 (0.52H, s), 2.89 (0.30H, s), 2.51-2.29 (2H, m), 2.20-1.74 (4H, m), 1.67 (3H, m), 1.60-1.40 (5H, m), 1.31-1.23 (3H, m), 1.12 (0.46H, s), 1.10 (0.90H, s), 1.09 (1.54H, s); 13C NMR δ u 213.5, 212.8, 168.8, 168.6, 132.0, 131.7, 60.9, 60.8, 59.6, 45.0, 44.0, 43.8, 41.7, 40.3, 37.5, 36.2, 36.1, 33.9, 33.4, 33.2, 30.9, 23.8, 23.1, 22.9; d 124.9, 124.0, 123.8, 65.8, 64.8, 27.4, 25.7, 25.6, 25.4, 21.2, 17.6, 17.5, 14.2, 14.2, 14.1; IR (film, cm−1) 1649, 1726, 2968; HRMS calcd for C15H25O3 (M+H) 253.1804, obsd 253.1807.
MnIII-Oxidative Radical Cyclization Method A
(1R,5S,8R)-1-Ethoxycarbonyl-5-methyl-8-(prop-1-en-2-yl)bicyclo[3.3.0]octan-2-one (7)
A dry mixture of Mn(OAc)3•2H2O (12.92 g, 48.20 mmol, 2.0 equiv) and Cu(OAc)2•H2O (4.82 g, 24.10 mmol, 1.0 equiv) was purged with argon for 10 min and diluted with 240 mL of acetonitrile. β-Ketoester 6 (6.08 g, 24.10 mmol) was added and the reaction stirred at room temperature overnight (16 h) under an atmosphere of argon. The reaction mixture was filtered through Celite, diluted with saturated aqueous NaHCO3 and extracted with Et2O. The combined organic extracts were dried (Na2SO4) and concentrated in vacuo. The residue was chromatographed to afford endo alkene 7 as a colorless oil (3.57 g, 14.30 mmol, 59 % yield) accompanied by a complex mixture of other isomers (1.81 g, ca. 7 mmol). For 7: TLC Rf (PE:MTBE = 10:1) = 0.31. [α]20D = (+) 61.4° (c 1.52 CHCl3); 1H NMR (400 MHz, CDCl3) δ 4.78 (2H, d, J=48 Hz), 4.20 (2H, m), 3.43 (1H, dd, 11.8, J=12.0 Hz), 2.50 (1H, ddd, J=6.1, 8.7, 18.1 Hz), 2.28 (1H, m), 1.79 (3H, s), 1.64–1.91 (6H, m), 1.27 (3H, t, J=7.1 Hz), 1.17 (3H, s); 13C NMR (100 MHz, CDCl3) δ u 214.1, 171.7, 144.8, 111.4, 71.2, 61.1, 53.6, 40.5, 39.7, 35.1, 29.1; d 52.6, 26.1, 23.7, 14.2; IR (film, cm−1), 1643, 1732, 2958, 3086; HRMS calcd for C15H22O3 (M+Na) 273.1467, obsd 273.1459.
MnIII-Oxidative Radical Cyclization Method B
To a mixture of Mn(OAc)3•2H2O (14.63 g, 54.6 mmol, 2.0 equiv) and Cu(OAc)2•H2O (5.46 g, 27.3 mmol, 1.0 equiv) in 136 mL glacial acetic acid was added β-ketoester 6 (6.88 g, 27.3 mmol) and the reaction mixture purged with argon while stirring rapidly. The heterogeneous solution was stirred at 80 °C under an argon atmosphere. Upon complete consumption of β-ketoester 6, 20 mL of H2O was added and the reaction gradually cooled to room temperature. The reaction mixture was filtered through Celite and concentrated in vacuo to remove the bulk of the acetic acid. The residue was diluted with saturated aqueous NaHCO3 and extracted with Et2O. The combined organic extracts were dried (Na2SO4) and concentrated in vacuo to afford 6.9 g of crude product consisting mainly of hemiketal 8 and endo product 7. This crude mixture was used in smaller portions as needed (and purified when required) to develop and optimize the procedures described below.
Analysis of Product Mixture
A small portion of the crude reaction material (235 mg), prior to treatment with H20, was chromatographed to afford the following pure materials along an additional amount of unresolved material (45 mg):
(1R,5S,8R)-1-Ethoxycarbonyl-5-methyl-8-(prop-1-en-2-yl)bicyclo[3.3.0]octan-2-one (7)
34.5 mg.
(1R,5S,8S)-1-Ethoxycarbonyl-5-methyl-8-(prop-1-en-2-yl)bicyclo[3.3.0]octan-2-one(7b)
7 mg, TLC Rf (PE:MTBE = 6.5:1) = 0.39; 1H NMR (400 MHz, CDCl3) δ 4.88 (2H, m), 4.14 (2H, m), 2.78 (1H, m), 2.52 (2H, m), 2.17 (1H, m), 1.79 (3H, s), 1.98-1.67 (5H, m), 1.25 (3H, t, J=7.2 Hz), 1.17 (3H, s); 13C NMR (100 MHz, CDCl3) δ u 214.9, 169.4, 144.4, 111.6, 71.0, 60.4, 54.8, 38.4, 36.6, 31.2, 30.0; d 53.0, 24.3, 23.4, 14.1; HRMS calcd for C15H22O3 (M+Na) 273.1467, obsd 273.1459.
(1R,4S,7S,10R)-10-Ethoxycarbonyl-1-hydroxy-3,3,7-trimethyl-2-oxatricyclo[2.2.2.1]decane (8)
54 mg; TLC Rf (PE:MTBE = 4:1) = 0.32; [α]20D = (−) 18.5° (c 0.61 CHCl3); 1H NMR (400 MHz, CDCl3) δ 5.08 (OH, s), 4.25 (2H, m), 3.04 (1H, t, J=9.3 Hz), 2.15 (1H, dd, J=6.0, 12.8), 1.90-1.54 (7H, m), 1.34 (3H, t, J=7.1 Hz), 1.33 (3H, s), 1.27 (3H, s), 1.07 (3H, s); 13C NMR (100 MHz, CDCl3) δ u 176.0, 114.5, 83.9, 74.9, 61.1, 56.9, 40.6, 40.3, 34.6, 26.4; d 59.3, 30.1, 23.9, 23.4, 14.3; IR (film, cm−1) 1724, 2967, 3456; HRMS calcd for C15H24O4 (M-OH) 251.1647, obsd 251.1644.
(1S,4S,7S,10R)-10-Ethoxycarbonyl-1-acetoxy-3,3,7-trimethyl-2-oxatricyclo[2.2.2.1]decane (8a)
11 mg; TLC Rf (PE:MTBE = 6.5:1) = 0.26; 1H NMR (400 MHz, CDCl3) δ 4.25 (2H, m), 3.15 (1H, dd, J=1.6, 6.0 Hz), 2.76 (1H, ddd, J=4.5, 7.5, 12.0), 2.25 (1H, dt, J=9.0, 14.3 Hz), 1.97 (3H, s), 1.89-1.67 (6H, m), 1.47 (1H, m), 1.33 (3H, t, J=7.1 Hz), 1.30 (6H, bs), 1.12 (3H, s); 13C NMR (100 MHz, CDCl3) δ u 171.6, 168.9, 119.3, 87.5, 80.1, 60.5, 54.7, 40.6, 37.0, 36.9, 26.9; d 55.3, 29.7, 24.4, 24.3, 22.1, 14.3; HRMS calcd for C17H26O5 (M+Na) 333.1678, obsd 333.1674.
(1R,4S,7S,10R)-1-Ethoxy-10-ethoxycarbonyl-3,3,7-trimethyl-2-oxatricyclo[2.2.2.1]decane (8b)
42 mg, TLC Rf (PE:MTBE = 6.5:1) = 0.61; 1H NMR (400 MHz, CDCl3) δ 4.17 (2H, m), 3.62 (1H, dq, J=7.1, 8.9 Hz), 3.38 (1H, dq, J=7.1, 8.9 Hz), 3.10 (1H, dd, 5.8, 7.7), 2.11-1.97 (2H, m), 1.83-1.64 (5H, m), 1.40 (1H, m), 1.33 (3H, s), 1.28 (3H, t, J=7.1 Hz), 1.24 (3H, s), 1.11 (3H, s), 1.08 (3H, t, J=7.1 Hz); 13C NMR (100 MHz, CDCl3) δ u 172.4, 119.2, 85.4, 79.1, 60.1, 58.0, 54.9, 41.0, 36.8, 34.5, 26.9; d 55.7, 29.6, 24.7, 15.3, 14.2; HRMS calcd for C17H28O4 (M+Na) 319.1885, obsd 319.1880.
(1R,5R,8S)-4,4,8-Trimethyl-3-oxatricyclo[6.3.0.01,5]undecan-2,11-dione (10)
6.5 mg; TLC Rf (PE:MTBE = 4:1) = 0.22. MP = 102 °C; [α]20D = (+) 119.1° (c 1.33 CHCl3); 1H NMR (400 MHz, CDCl3) δ 2.76 (1H, t, J=8.1 Hz), 2.57 (2H, m), 1.91 (4H, m), 1.75 (2H, m), 1.53 (3H, s), 1.39 (3H, s), 1.32 (3H, s); 13C NMR (100 MHz, CDCl3) δ u 214.8, 173.0, 84.4, 73.8, 53.8, 41.2, 37.9, 32.6, 28.0; d 56.9, 31.2, 24.3, 23.1; IR (film, cm−1) 1313, 1462, 1729 (br), 2939; HRMS calcd for C13H19O3 (M+H) 223.1334, obsd 223.1332.
Selective Product Formation
The following is the optimized procedure for the selective formation of the endo alkene product 7 from the β-ketoester 6
(1R,5S,8R)-1-Ethoxycarbonyl-5-methyl-8-(prop-1-en-2-yl)bicyclo[3.3.0]octan-2-one (7)
A mixture of Mn(OAc)3•2H2O (268 mg, 1.0 mmol, 2.0 equiv), Cu(OAc)2•H2O (100 mg, 0.5 mmol, 1.0 equiv) and β-ketoester 6 (126 mg, 0.5 mmol) were subject to the conditions described in Method B above. The residue was chromatographed to afford endo alkene product 7 (47 mg, 0.19 mmol, 38% yield) along with a mixture of isomers (61 mg). This mixture of isomers was diluted with dry toluene (2.5 mL) and p-toluenesulfonic acid monohydrate (9 mg, 0.05 mmol, 0.05 equiv) was added. The reaction mixture was stirred at reflux for 1 h. After cooling to room temperature, the reaction material was diluted with 5% aqueous NaOH and extracted with Et2O. The combined organic extracts were dried (Na2SO4) and concentrated in vacuo. The residue was chromatographed to afford endo alkene 7 (47 mg, 0.19 mmol, 37% yield, 75% overall yield from β-ketoester 6) as a viscous yellow oil and lactone 10 (12 mg, 0.05 mmol, 11% yield). The data for 7 prepared by Method B was identical to the data for 7 prepared by Method A.
The following are the optimized procedures for the selective formation of the lactone 10 or the tetrasubstituted alkene 9 directly from the β-ketoester 6.
(1R,5R,8S)-4,4,8-Trimethyl-3-oxatricyclo[6.3.0.01,5]undecan-2,11-dione (10)
A mixture of Mn(OAc)3•2H2O (268 mg, 1.0 mmol, 2.0 equiv), Cu(OAc)2•H2O (100 mg, 0.5 mmol, 1.0 equiv) and β-ketoester 6 (126 mg, 0.5 mmol) were subject to the conditions described in Method B above.
The resultant crude mixture was then diluted with dry toluene (2.5 mL) and p-toluenesulfonic acid monohydrate (100 mg, 1.05 mmol, 1.05 equiv) was added. The reaction was stirred at reflux for 4 h. After cooling to room temperature, the reaction material was diluted with 5% aqueous NaOH and extracted with Et2O. The combined organic extracts were dried (Na2SO4) and concentrated in vacuo. The residue was chromatographed to afford lactone 10 (97 mg, 0.44 mmol, 87% yield) as a viscous clear oil which crystallized upon standing.
(1S,5S)-1-Ethoxycarbonyl-5-methyl-8-(propan-2-ylidene)bicyclo[3.3.0]octan-2-one (9)
A mixture of Mn(OAc)3•2H2O (268 mg, 1.0 mmol, 2.0 equiv), Cu(OAc)2•H2O (100 mg, 0.5 mmol, 1.0 equiv) and β-ketoester 6 (126 mg, 0.5 mmol) were subject to the conditions described in Method B above. The resultant crude mixture was then diluted with dry toluene (2.5 mL) and p-toluenesulfonic acid monohydrate (9 mg, 0.05 mmol, 0.05 equiv) was added. The reaction was stirred at reflux for 4 h. After cooling to room temperature, the reaction material was diluted with 5% aqueous NaOH and extracted with Et2O. The combined organic extracts were dried (Na2SO4) and concentrated in vacuo. The residue was chromatographed to afford tetrasubstituted alkene 9 (103 mg, 0.44 mmol, 82% yield) as a viscous yellow oil along with lactone 10 (20 mg, 0.09 mmol, 18%). For 9, TLC Rf (PE:MTBE = 6.5:1) = 0.42; [α]20D = (−) 147° (c 1.9 CHCl3); 1H NMR (400 MHz, CDCl3) δ 4.24-4.10 (2H, m), 2.60-2.50 (1H, m), 2.49-2.30 (3H, m), 1.89-1.79 (2H, m), 1.71 (3H, s), 1.68 (3H, s), 1.65-1.55 (2H, m), 1.25 (3H, 7, J = 7.1 Hz), 1.18 (3H, s), 1.01 (3H, s); 13C NMR (100 MHz, CDCl3) δ u 213.9, 170.6, 131.8, 131.3, 72.4, 60.8, 55.4, 37.1, 37.0, 31.2, 30.3; d 23.2, 22.9, 22.5, 14.3; IR (film, cm−1) 1033, 1257, 1378, 1453, 2939; HRMS calcd for C15H22O3 (M+Na) 273.1467, obsd 273.1461.
(1S,2R,5S)-1-Ethoxycarbonyl-3,3,5-trimethyl-8-(propan-2-ylidene)bicyclo[3.3.0]octan-2-ol (15)
To a slurry of KH(P) [2.63 g KH(P), 50 w% KH, 32.88 mmol KH, 3.0 equiv] in 27 mL toluene was added tetrasubstituted alkene 9 (2.74 g, 10.96 mmol) dropwise over 10 min. After stirring for 5 min, CH3I (6.83 mL, 110 mmol, 10 equiv) was added followed by a small amount of non-anhydrous Et2O to initiate the reaction. The reactor was equipped with a condenser and stirred at room temperature for 3 h. The reaction material was cooled to 0°C, diluted with Et2O and then quenched by slow addition of saturated aqueous NH4Cl. After extraction with Et2O, the combined organic extracts were dried (Na2SO4) and concentrated in vacuo. The residue was chromatographed to afford the gem-dimethyl ketone as a colorless oil (2.54 g, 10.16 mmol, 83 % yield), TLC Rf (PE:MTBE = 10:1) = 0.46. [α]20D = (−) 140° (c 1.0 CHCl3); 1H NMR (400 MHz, CDCl3) δ 4.18 (2H, m), 2.50 (1H, m), 2.37 (1H, m), 1.82 (1H, d, J=13.5 Hz), 1.75 (1H, d, J=13.5 Hz), 1.74-1.62 (2H, m), 1.71 (3H, s), 1.69 (3H, s), 1.26 (3H, t, J=7.0 Hz), 1.22 (3H, s), 1.15 (3H, s), 1.11 (3H, s); 13C NMR (100 MHz, CDCl3) δ u 217.5, 171.1, 132.9, 131.4, 73.3, 60.7, 52.6, 47.9, 46.5, 38.7, 30.1; d 28.8, 27.9, 24.8, 22.7, 22.4, 14.3; IR (film, cm−1) 1031, 1731, 2936; HRMS calcd for C17H26O3 (M+H) 279.1960, obsd 279.1967.
To a solution of ketone (2.29 g, 8.24 mmol) in 36 mL 1:1 v/v MeOH: EtOH at 0°C was added NaBH4 (2.73 g, 71.9 mmol, 8.7 equiv) in small portions over 1 h. The reaction was stirred to room temperature over 2 h, diluted with 15 mL of 15% aqueous NaOH and stirred for 3 h at room temperature. The reaction mixture was diluted with H2O and extracted with Et2O. The combined organic extracts were dried (Na2SO4) and concentrated in vacuo. The residue was chromatographed to afford alcohol 15 (2.04 g, 7.29 mmol, 74 % yield from 9) as a colorless oil which solidified upon standing (mp = 38–39 °C), TLC Rf (PE:MTBE = 10:1) = 0.38, [α]20D = (−) 28.2° (c 1.0 CHCl3); 1H NMR (400 MHz, CDCl3) δ 4.47 (1H, d, J=7.3 Hz), 4.15 (2H, m), 2.52 (1H, dd, J=6.6, 14.7 Hz), 2.37 (1H, m), 1.80 (OH, d, J=7.3 Hz), 1.71 (3H, s), 1.66-1.51 (7H, m), 1.24 (3H, t, J=7.1 Hz), 1.13 (3H, s), 1.02 (3H, s), 0.93 (3H, s); 13C NMR (100 MHz, CDCl3) δ u 175.4, 135.7, 128.5, 69.5, 60.4, 53.3, 52.9, 40.3, 39.5, 31.3; d 83.5, 31.3, 26.4, 24.2, 22.8, 22.7, 14.38; IR (film, cm−1) 1206, 1453, 1712, 2942, 3519; HRMS calcd for C17H28O3 (M+H) 281.2117, obsd 281.2121.
(1S,2R,5S)-2-Benzyloxy-1-ethoxycarbonyl-3,3,5-trimethyl-8-(propan-2-ylidene)bicyclo[3.3.0]octane (17)
A mixture of alcohol 15 (2.02 g, 7.20 mmol), benzyloxypyridinium triflate (BnOPT, 3.65 g, 10.44 mmol, 1.45 equiv) and MgO (421 mg, 10.44 mmol, 1.45 equiv) in 14.5 mL anhydrous dichloroethane (freshly distilled from CaH2) was stirred at 80°C for 8 h. Additional BnOPT (1.26 g, 3.6 mmol, 0.5 equiv) and MgO (145 mg, 3.6 mmol, 0.5 equiv) were added and the mixture was stirred at 80°C for an additional 8 h. After cooling to room temperature, the reaction mixture was filtered through Celite to remove precipitates and the filter cake was washed thoroughly with Et2O. The filtrate was concentrated in vacuo and the residue chromatographed to afford benzyl ether 17 (2.02 g, 5.47 mmol, 76 % yield) as an inseparable mixture with Bn2O (607 mg, 3.06 mmol), and residual alcohol 12 (470 mg, 1.68 mmol, 23 % recovered). For 17, TLC Rf (PE:MTBE = 20:1) = 0.63; [α]20D = (−) 12.2° (c 2.3 CHCl3; material is 84 w% benzyl ether 17 and 16 w% Bn2O); 1H NMR (400 MHz, CDCl3) δ 7.38-7.23 (10H, m), 4.88 (1H, d, J=11.8 Hz), 4.68 (1H, d, J=11.8 Hz), 4.56 (1.76H, s, Bn2O), 4.40 (1H, s), 4.15 (2H, dq, J=1.1, 7.3 Hz), 2.49 (1H, dd, J=7.5, 15.5 Hz), 2.38 (1H, m), 1.56–1.63 (5H, m), 1.50-1.44 (5H, m), 1.25 (3H, t, J=7.1 Hz), 1.03 (3H, s), 1.00 (3H, s), 0.95 (3H, s); 13C NMR (100 MHz, CDCl3) δ u 176.3, 139.8, 138.3, 135.0, 127.9, 74.6, 72.1 (Bn2O), 68.1, 60.2, 52.9, 52.2, 39.8, 39.1, 31.2; d 128.4, 128.0, 127.8, 127.7, 127.6, 127.0, 91.8, 32.3, 26.5, 24.9, 23.3, 22.8, 14.4; IR (film, cm−1) 1114, 1207, 1364, 1453, 2361, 2936; HRMS calcd for C24H34O3 (M+H) 371.2586, obsd 371.2600.
(1S,5S,8R)-8-Benzyloxy-1-ethoxycarbonyl-5,7,7-trimethyl-2-(2-hydroxyprop-2-yl)bicyclo[3.3.0]oct-2-ene (18)
A solution of benzyl ether 17 (1.07 g, 2.88 mmol) and methylene blue (photosensitizer, 11 mg, 0.03 mmol, 0.01 equiv) in 7.2 mL MeOH was prepared in a 100 mL Pyrex glass test tube. A smaller diameter test tube was then submerged into the solution to create a thin film annular reactor. The reactor was capped and irradiated with a flood lamp (120 W, 12 inches from reactor) as O2 was bubbled through the solution. A water bath was used to thermostat the reaction at 20 – 25°C as it was irradiated. After 9 h, the reaction was diluted with 10 mL of Et2O, PPh3 (755 mg, 2.88 mmol, 1.0 equiv) was added and the reaction stirred overnight (12 h). Aqueous H2O2 (30 w%, 1.5 mL, 5.0 equiv) was added and the reaction stirred 1.5 h at room temperature. The reaction mixture was partitioned between Et2O and, sequentially, saturated aqueous Na2S2O3 and brine. The combined organic extracts were dried (Na2SO4) and concentrated in vacuo. The residue was chromatographed to afford alcohol 18 (780 mg, 2.02 mmol, 70 % yield) as a colorless oil and residual alkene starting material (298 mg, 0.81 mmol, 28 % recovered). For 18, TLC Rf (PE:MTBE = 20:1) = 0.22; [α]20D = (−) 4.78° (c 1.0 CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.39-7.28 (5H, m), 5.55 (1H, t, J=2.4 Hz), 4.68 (1H, d, J=10.8 Hz), 4.63 (1H, d, J=10.8 Hz), 4.59 (1H, s), 4.50 (OH, s), 4.18 (2H, m), 2.41 (2H, d, J=2.4 Hz), 1.71 (1H, d, J=13.6 Hz), 1.54 (1H, d, J=13.6 Hz), 1.30 (3H, s), 1.29 (3H, s), 1.27 (3H, t, J=7.2 Hz), 1.15 (3H, s), 1.09 (3H, s), 1.04 (3H, s); 13C NMR (100 MHz, CDCl3) δ u 174.6, 153.3, 137.3, 75.4, 73.8, 70.3, 60.7, 55.5, 50.2, 49.2, 40.4; d 128.5, 128.5, 128.4, 128.0, 90.9, 32.0, 31.9, 31.5, 27.2, 22.7, 14.2; IR (film, cm−1) 1227, 1366, 1366, 1455, 1718, 2930, 3456; HRMS calcd for C24H34O4 (M+Na) 409.2355, obsd 409.2351.
(1S,5S,8R)-8-Benzyloxy-1-ethoxycarbonyl-5,7,7-trimethyl-2-(propan-2-ylidene)bicyclo[3.3.0]oct-2-ene (19)
To a solution of alcohol 18 (386 mg; 1.0 mmol) and anhydrous DMF (233 µL, 3.0 mmol, 3.0 equiv) in 10 mL CH2Cl2 at −78 °C was added SOCl2 (183 µL, 2.5 mmol, 2.5 equiv) dropwise. After 10 min at −78 °C, 2,6-lutidine (582 µL, 5.0 mmol, 5.0 equiv) was added dropwise over 10 min. The reaction was gradually stirred to −40°C over 20 min and additional SOCl2 (183 µL, 2.5 mmol, 2.5 equiv) was added. The reaction was warmed to −20°C and quenched with 1N aqueous HCl. The reaction material was partitioned between CH2Cl2 and, sequentially, H2O and saturated aqueous NaHCO3. The combined organic extracts were dried (Na2SO4) and concentrated in vacuo. The residue was chromatographed to afford diene 19 (276 mg, 0.75 mmol, 79 % yield) as a yellow oil and residual alcohol starting material (47 mg, 0.12 mmol, 12 % recovered). For 19, TLC Rf (PE:MTBE = 10:1) = 0.66, [α]20D = (+) 81.6° (c 1.84 CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.34-7.20 (5H, m), 5.84 (1H, t, J=2.4 Hz), 4.78 (2H, d, J=12.5 Hz), 4.70 (1H, d, J=11.6 Hz), 4.60 (1H, d, J=11.6 Hz), 4.41 (1H, s), 4.17 (2H, m), 2.46 (1H, d, J=17.6 Hz), 2.40 (1H, d, J=17.6 Hz), 1.83 (3H, s), 1.72 (1H, d, J=13.4 Hz), 1.52 (1H, d, J=13.4 Hz), 1.25 (3H, t, J=7.0 Hz), 1.08 (3H, s), 1.00 (3H, s), 0.99 (3H, s); 13C NMR (100 MHz, CDCl3) δ u 175.3, 146.0, 140.6, 139.5, 112.1, 74.3, 73.3, 60.6, 55.4, 51.5, 49.7, 41.6; d 130.8, 128.0, 127.5, 127.0, 90.2, 30.7, 27.3, 23.2, 22.5, 14.2; IR (film, cm−1) 1718, 2928, 3418; HRMS calcd for C24H32O3 (M+H) 368.2351, obsd 368.2341.
(1S,2R,5S)-E and Z-2-Benzyloxy-1-ethoxycarbonyl-3,3,5-trimethyl-8-[1-(phenylthio)propan-2-ylidene]bicyclo[3.3.0]octane (21)
A mixture of diene 19 (380 mg, 1.03 mmol), thiophenol (115 µL, 1.13 mmol, 1.1 equiv) and silica nanoparticles (SNP’s, 19 mg, 5 w%) in a small vial were stirred vigorously at room temperature for 18 h. The reaction mixture was filtered through Celite and concentrated in vacuo. The residue was chromatographed to afford a 1:1.5 Z:E mixure of allylic thioethers 21 (412 mg, 0.86 mmol, 84 % yield) as a colorless oil, TLC Rf (PE:MTBE = 100:1) = 0.22; 1H NMR (400 MHz, CDCl3) δ 7.38-6.90 (10H, m), 4.95 (0.4H, d, J=11.5 Hz, Z-isomer), 4.88 (0.6H, d, J=11.7 Hz, E-isomer), 4.77 (0.4H, d, J=11.7 Hz, E-isomer), 4.69 (0.7H, d, J=11.7 Hz, Z-isomer), 4.43 (1H, m), 4.24-4.09 (2H, m), 3.98 (0.4H, d, J=11.5 Hz, Z-isomer), 3.66 (0.6H, d, J=11.1 Hz, E-isomer), 3.44 (0.6H, d, J=11.1 Hz, E-isomer), 3.18 (0.4H, d, J=11.5 Hz, Z-isomer), 2.59-2.37 (2H, m), 1.78 (1.1H, s), 1.64 (1.8H, s), 1.61-1.45 (4H, m), 1.23–1.30 (3H, m), 1.09 (1H, s), 1.04 (3H, s), 1.00 (2H, s), 0.96 (1H, s), 0.94 (2H, s); 13C NMR (100 MHz, CDCl3) δ u 175.7, 175.5, 140.7, 139.7, 139.6, 140.0, 139.0, 137.9, 127.3, 126.6, 74.6, 74.6, 68.6, 68.0, 60.8, 60.5, 53.0, 52.9, 52.1, 51.8, 49.7, 49.5, 41.0, 40.9, 40.4, 40.3, 39.9, 39.4, 39.0, 38.8, 31.8, 31.0; d 129.4, 128.6, 128.5, 128.1, 128.0, 127.6, 127.8, 127.5, 127.1, 127.0, 125.7, 124.7, 91.8, 91.3, 32.5, 32.2, 26.5, 26.4, 25.0, 24.7, 20.8, 20.4, 14.4, 14.2; IR (film, cm−1) 1218, 1452, 1582, 1714, 2945; HRMS calcd for C30H38O3S (M+H) 479.2620, obsd 479.2612.
(1S,2R,5S)-E-2-Benzyloxy-1-ethoxycarbonyl-3,3,5-trimethyl-8-[1-(phenylsulfinyl)propan-2-ylidene]bicyclo[3.3.0]octane (22a, b) and (1S,2R,5S)-Z-2-Benzyloxy-1- ethoxycarbonyl -3,3,5-trimethyl-8-[1-(phenylsulfinyl)propan-2-ylidene]bicyclo[3.3.0]octane (23a, b)
To a solution of allylic thioethers 22 (410 mg, 0.86 mmol) in 4.0 mL CH2Cl2 at −78°C was added a solution of m-CPBA (148 mg, 0.86 mmol, 1.0 equiv) in 4.6 mL CH2Cl2 dropwise via syringe over 10 min. The reaction material was stirred at −78°C for 30 min and diluted with 5 mL of saturated aqueous Na2S2O3. The reaction material was diluted with H2O and extracted with CH2Cl2. The combined organic extracts were dried (Na2SO4) and concentrated in vacuo. The residue was chromatographed to afford a mixture of E-allylic sulfoxides 22a, b (260 mg, 0.526 mmol, 61% yield) and Z-allylic sulfoxides 23a, b (155 mg, 0.314 mmol, 37% yield, dr=1.5:1).
E-allylic sulfoxide 22a (major)
TLC Rf (PE:EtOAc = 5:1) = 0.13; [α]20D = (−) 24.6° (c 1.79 CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.68 (2H, d, J=7.6 Hz), 7.40-7.24 (8H, m), 4.80 (1H, d, J=11.2 Hz), 4.67 (1H, d, J=11.2 Hz), 4.47 (1H, s), 4.20 (2H, dq, J=1.7, 7.0 Hz), 3.81 (1H, d, J=12.2 Hz), 3.43 (1H, d, J=12.2 Hz), 2.52 (1H, m), 2.25 (1H, dd, J=6.8, 16.0), 1.63-1.45 (7H, m), 1.30 (3H, t, J=7.0 Hz), 1.09 (3H, s), 1.04 (3H, s), 1.00 (3H, s); 13C NMR (100 MHz, CDCl3) δ u 175.2, 145.3, 144.4, 139.2, 121.5, 75.1, 69.1, 66.8, 60.7, 53.0, 52.2, 40.1, 39.0, 31.6; d 130.8, 128.9, 128.2, 128.0, 127.3, 92.3, 32.3, 26.4, 25.4, 22.5, 14.4; IR (film, cm−1) 1040, 1724, 2936; HRMS calcd for C30H38O4S (M+H) 495.2569, obsd 495.2564.
E-allylic sulfoxide 22b (minor)
TLC Rf (PE:EtOAc = 5:1) = 0.16; [α]20D = (−) 42.9° (c 0.96 CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.63 (2H, m), 7.45 (3H, m), 7.35-7.29 (4H, m), 7.25 (1H, m), 4.81 (1H, d, 11.5 Hz), 4.67 (1H, d, J=11.5 Hz), 4.43 (1H, s), 4.19 (2H, m), 3.75 (1H, d, J=12.8 Hz), 3.44 (1H, d, J=12.8 Hz), 2.73 (1H, dd, J=7.4, 15.7 Hz), 2.37 (1H, m), 1.65 (1H, td, J=7.4, 11.7 Hz), 1.58-1.47 (6H, m), 1.31 (3H, t, J=7.1 Hz), 1.06 (3H, s), 1.01 (3H, s), 0.90 (3H, s); 13C NMR (100 MHz, CDCl3) δ u 175.2, 145.2, 144.5, 139.3, 121.9, 74.8, 69.1, 66.5, 60.7, 53.0, 52.4, 40.1, 38.9, 31.5; d 131.0, 129.0, 128.1, 127.7, 127.2, 124.4, 92.0, 32.3, 26.4, 25.2, 22.3, 14.4 ; IR (film, cm−1) 1036, 1208, 1452, 1714, 2934; HRMS calcd for C30H38O4S (M+H) 495.2569, obsd 495.2567.
Z-allylic sulfoxides 23a, b
TLC Rf (PE:EtOAc = 5:1) = 0.25; 1H NMR (400 MHz, CDCl3) δ 7.60-6.82 (10H, m), 4.70-4.06 (6H, m), 3.08 (0.6H, d, J=13.8 Hz), 2.97 (0.4H, d, J=13.5 Hz), 2.64 (1H, m), 2.50 (1H, m), 1.94 (1.8H, s), 1.88-1.78 (2.2H, m), 1.62-1.47 (3H, m), 1.32 (3H, m), 1.07 (6H, m), 0.87 (2H, s), 0.85 (1H, s); 13C NMR (100 MHz, CDCl3) δ u 176.0, 176.0, 145.1, 144.9, 144.4, 142.9, 138.8, 138.3, 125.0, 122.4, 74.9, 74.7, 67.8, 67.8, 67.2, 66.0, 61.4, 60.9, 52.9, 52.6, 52.0, 51.9, 39.2, 39.1, 38.6, 38.5, 32.1, 32.1; d 130.7, 130.4, 129.1, 129.0, 128.0, 127.9, 127.7, 127.5, 127.1, 127.0, 124.2, 123.6, 91.6, 91.4, 32.4, 32.3, 26.2, 26.2, 24.9, 24.7, 22.4, 20.2, 14.2, 14.1; IR (film, cm−1) 1242, 1381, 1451, 1713, 2932; HRMS calcd for C30H38O4S (M+H) 495.2569, obsd 495.2566.
Isomerization of E-allylic sulfoxides (22a, b)
A solution of allylic sulfoxides 22a, b (37 mg, 0.075 mmol) in 1.5 mL toluene was irradiated in a μ-wave reactor (P=150 W, Tramp=23–120°C over 10 minutes, Thold=120°C 15 minutes). The reaction material was concentrated in vacuo and the residue chromatographed to afford Z-allylic sulfoxides 23a, b (7.7 mg, 0.016 mmol, 21% yield) and E-allylic sulfoxides 22a, b (25.8 mg, 0.052 mmol, 70% recovered yield).
(1S,2R,5S)-Z-2-Benzyloxy-1-ethoxycarbonyl-3,3,5-trimethyl-8-[1-(phenylsulfonyl)propan-2-ylidene]bicyclo[3.3.0]octane (24)
A mixture of Z-allylic sulfoxides 23a, b (70 mg, 0.142 mmol), aqueous H2O2 (30 w%, 73 µL, 5.0 equiv) and H2WO4 (3.5 mg, 0.014 mmol, 0.1 equiv) in 700 µL 1:1 of MeOH:THF was stirred vigorously at room temperature for 20 h. The reaction mixture was then diluted with saturated aqueous NaHCO3 and extracted with Et2O. The combined organic extracts were dried (Na2SO4) and concentrated in vacuo. The residue was chromatographed to afford Z-allylic sulfone 24 (55 mg, 0.108 mmol, 76% yield), TLC Rf (PE:EtOAc = 6.5:1) = 0.46; [α]20D = (−) 80.6° (c 1.35 CHCl3);1H NMR (400 MHz, CDCl3) δ 7.77 (2H, d, J=7.6 Hz), 7.57 (1H, t, J=7.4 Hz), 7.48 (2H, t, J=7.6 Hz), 7.12 (1H, t, J=7.3 Hz), 7.05 (2H, t, J=7.3 Hz), 6.85 (2H, d, J=7.5 Hz), 4.69 (1H, d, J=10.8 Hz), 4.55-4.40 (3H, m), 4.25 (2H, m), 3.33 (1H, d, J=14.5 Hz), 2.65 (1H, dd, J=7.9, 16.7 Hz), 2.50 (1H, m), 1.92 (3H, s), 1.90 (1H, m), 1.59-1.48 (3H, m), 1.33 (3H, t, J=7.0 Hz), 1.09 (3H, s), 1.08 (3H, s), 0.83 (3H, s); 13C NMR (100 MHz, CDCl3) δ u 175.7, 145.8, 141.6, 138.4, 120.1, 74.8, 67.8, 63.5, 61.1, 52.6, 52.0, 39.0, 38.3, 32.2; d 133.0, 129.0, 128.0, 127.5, 127.5, 127.1, 91.4, 32.4, 26.0, 24.9, 20.9, 14.2; IR (film, cm−1) 1366, 1452, 1730, 2360, 2949; HRMS calcd for C30H38O5S (M+Na) 533.2338, obsd 533.2332.
(1R,5R,8S,11R)-11-benzyloxy-4,8,10,10-tetramethyl-3-phenylsulfonyltricyclo[6.3.0.01,5]undec-3-en-2-one (25)
To a solution of Et2NH (220 µL 1.56 mmol, 14 equiv) in 200 µL THF at −78°C was added n-BuLi (2.09 M, 641 µL, 12.0 equiv). The solution was gradually stirred to −40°C and a solution of Z-allylic sulfone 25 (57 mg, 0.112 mmol) in 300 µL of THF was added dropwise. The reaction was stirred to −20°C and quenched with saturated aqueous NH4Cl. The reaction mixture was diluted with H2O and extracted with Et2O. The combined organic extracts were combined, dried (Na2SO4) and concentrated in vacuo. The residue was chromatographed to afford α-sulfonylenone 25 (47 mg, 0.101 mmol, 90% yield) as a viscous oil. TLC Rf (PE:EtOAc = 5:1) = 0.54; [α]20D = (−) 21.8° (c 0.55 CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.05 (2H, d, J=7.5 Hz), 7.56 (1H, t, J=7.4 Hz), 7.47 (2H, t, J=7.4 Hz), 7.27 (3H, m), 6.93 (2H, m), 4.00 (1H, s), 3.98 (1H, d, J=11.9 Hz), 3.83 (1H, d, J=11.9 Hz), 3.49 (1H, d, J=9.8 Hz), 2.62 (3H, s), 2.18 (1H, m), 1.81 (1H, dd, J=6.7, 13.5 Hz), 1.64-1.54 (2H, m), 1.50 (1H, d, J=14.0 Hz), 1.28 (1H, m), 1.05 (3H, s), 0.99 (3H, s), 0.92 (3H, s); 13C NMR (100 MHz, CDCl3) δ u 202.3, 186.6, 140.5, 138.3, 138.3, 73.0, 71.5, 53.9, 50.2, 40.4, 40.1, 27.0; d 133.6, 128.8, 128.2, 128.1, 127.4, 127.1, 91.4, 53.8, 31.9, 26.4, 24.9, 17.6.; IR (film, cm−1) 2957, 2361, 1707, 1604, 1453, 1320, 1154; HRMS calcd for C28H32O4S (M+H) 465.2100, obsd 465.2090.
(1R,3S,4R,5R,8S,11R)-11-benzyloxy-4,8,10,10-tetramethyl-3-phenylsulfonyltricyclo[6.3.0.01,5]undecan-2-one (26) and (1R,3R,4S,5R,8S,11R)-11-benzyloxy-4,8,10,10-tetramethyl-3-phenylsulfonyltricyclo[6.3.0.01,5]undecan-2-one (27)
A mixture of enone 26 (138 mg, 0.30 mmol, 1.0 equiv) and Mg turnings (154 mg, 6.34 mmol, 20 equiv) in 5 mL of a 1:1 v/v mixture of MeOH:THF was sonicated at room temperature until bubbling from the surface of the Mg turnings was visible and the mixture turned coudy grey. Small portions of NH4Cl (45 mg, 0.85 mmol, 2.8 equiv/portion) were introduced at 15 min intervals with continuous sonication until the reaction mixture became cloudy white (ca. 10 portions, 8.5 mmol NH4Cl). The reaction was quenched with 3 N aqueous HCl (slow addition!) until clear and diluted with H2O. The biphasic mixture was extracted with Et2O, dried (Na2SO4) and concentrated in vacuo. The residue was chromatographed to afford ketones 26 (45.8 mg, 0.098 mmol, 33% yield), 27 (28.7 mg, 0.062 mmol, 21% yield) and residual enone 25 (60.7 mg, 0.131 mmol, 44 % recovered). Ketone 26 (major diastereomer), TLC Rf (PE:EtOAc = 6.6:1) = 0.52; [α]20D = (+) 30° (c = 0.4 CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.00 (dd, J = 8.4, 1.3 Hz, 2H), 7.57 (d, J = 7.4 Hz, 1H), 7.50 (dd, J = 8.1, 6.7 Hz, 2H), 7.36 –7.28 (m, 5H), 4.21 (d, J = 11.6 Hz, 1H), 4.08 (d, J = 11.6 Hz, 1H), 3.82 (s, 1H), 3.72 (d, J = 13.0 Hz, 1H), 3.30 – 3.22 (m, 1H), 2.84 – 2.69 (m, 1H), 1.99 – 1.88 (m, 2H), 1.59 – 1.48 (m, 2H), 1.42 (dd, J = 11.6, 7.3 Hz, 1H), 1.37 (d, J = 6.7 Hz, 3H), 1.30 (d, J = 13.5 Hz, 1H), 1.06 (s, 3H), 0.90 (s, 3H), 0.77 (s, 3H); 13C NMR (100 MHz, CDCl3) δ u 211.8, 138.9, 138.5, 73.8, 73.4, 54.4, 52.1, 42.8, 42.6, 27.6; d 133.8, 129.5, 128.8, 128.2, 127.8, 127.5, 92.7, 73.7, 49.0, 33.1, 30.6, 26.3, 22.9, 16.8; IR (film, cm−1) 2931, 1729, 1455, 1312, 1149, 1084; HRMS calcd for C28H34O4S (M+Na) 489.2076, obsd 489.2054.
Ketone 27 (minor diastereomer)
TLC Rf (PE:EtOAc = 6.6:1) = 0.56; [α]20D = (−) 45.5° (c = 0.22 CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.91 – 7.87 (m, 2H), 7.71 – 7.64 (m, 1H), 7.62 – 7.56 (m, 2H), 7.24 (dd, J = 5.0, 1.9 Hz, 3H), 7.15 (dd, J = 6.8, 2.8 Hz, 2H), 4.48 (d, J = 11.8 Hz, 1H), 4.14 (d, J = 11.8 Hz, 1H), 3.88 (s, 1H), 3.13 (d, J = 11.1 Hz, 1H), 2.60 – 2.54 (m, 1H), 2.45 – 2.33 (m, 1H), 2.14 – 2.01 (m, 1H), 1.74 – 1.66 (m, 1H), 1.62 (d, J = 14.1 Hz, 1H), 1.59 – 1.54 (m, 2H), 1.52 (d, J = 14.1 Hz, 1H), 1.29 (d, J = 6.6 Hz, 3H), 1.10 (s, 3H), 0.99 (s, 3H), 0.98 (s, 3H); 13C NMR (100 MHz, CDCl3) δ u 212.2, 138.8, 138.4, 75.9, 74.2, 55.8, 54.7, 41.8, 41.5, 30.8; d 133.9, 129.4, 128.8, 128.3, 127.6, 127.5, 95.5, 77.2, 49.8, 36.7, 32.1, 27.2, 24.0, 20.9; IR (film, cm−1) 2957, 1730, 1455, 1313, 1149, 1086; HRMS calcd for C28H34O4S (M+Na) 489.2076, obsd 489.2061.
(1R,4S,5R,8S,11R)-11-benzyloxy-4,8,10,10-tetramethyltricyclo[6.3.0.01,5]undecan-2-one (28)
To a solution of α-sulfonyl ketone 27 (22 mg; 0.047 mmol) in 400 µL dry THF at −78°C (thoroughly purged with N2) was added SmI2 (2.35 mL of 0.1 M stock solution in THF, 0.235 mmol, 5.0 equiv) rapidly via syringe. The reaction material was stirred for 10 min at −78°C and brought to room temperature in the presence of O2. The reaction material was then concentrated in vacuo and chromatographed to afford ketone 28 (14.1 mg, 0.043 mmol, 92% yield); TLC Rf (PE:MTBE = 10:1) = 0.67; [α]20D = (+) 6.2° (c = 0.65 CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.35 – 7.23 (m, 5H), 4.46 (q, J = 11.7 Hz, 2H), 4.06 (s, 1H), 2.68 (ddd, J = 7.4, 5.2, 2.1 Hz, 1H), 2.42 (dd, J = 19.5, 10.5 Hz, 1H), 2.19 – 2.08 (m, 1H), 1.87 (dd, J = 19.1, 6.8 Hz, 1H), 1.88 – 1.83 (m, 1H),1.61 (d, J = 13.9 Hz, 1H), 1.70 – 1.52 (m, 3H), 1.47 (d, J = 13.8 Hz, 1H), 1.10 (s, 3H), 1.03 (s, 3H), 0.99 (m, 6H); 13C NMR (100 MHz, CDCl3) δ u 222.7, 138.9, 73.8, 73.7, 54.9, 53.3, 47.6, 42.3, 41.2, 31.6; d 128.2, 127.5, 127.3, 92.3, 51.7, 34.3, 31.9, 27.1, 24.0, 21.2; IR (film, cm−1) 2953, 1727, 1458, 1109; HRMS calcd for C22H30O2 (M+Na) 349.2144, obsd 349.2149.
(1S,2R,5S,8S,9S)-3,3,5,9-tetramethyltricyclo[6.3.0.01,5]undecan-2-ol; C-9-epi-cameroonan-7α-ol (epi-2)
To a solution of α-sulfonyl ketone 26 (4 mg, 8.6 µmol) in 200 µL of dry THF at −78 °C was added SmI2 (0.1 M stock solution in THF, 1.0 mL, 0.1 mmol) rapidly via syringe. The reaction solution was stirred at −78 °C for 10 min and brought to room temperature in the presence of O2. The reaction material was concentrated in vacuo and filtered through a short plug of silica gel.
The crude filtrate was taken up in triethylene glycol (400 uL) and hydrazine monohydrate (100 uL, 0.1 mmol, 12 equiv). The reaction material was stirred at 130 °C for 2 h. KOH (2 mg, 0.04 mmol, 4 equiv) was added and the reaction mixture was stirred at 140 °C for 6 h. After cooling to room temperature, the mixture was diluted with H2O and extracted with Et2O. The combined organic fractions were dried (Na2SO4) and concentrated in vacuo.
The crude concentrate was then diluted with 0.5 mL EtOH and 10% Pd/C (ca. 5 mg) was added. The reaction mixture was stirred vigorously under H2 (balloon) for 2.5 h at room temperature. The reaction material was filtered through Celite and concentrated in vacuo to afford epi-2 (0.5 mg, 26% yield from 26), TLC Rf (PE:MTBE = 20:1) = 0.28; 1H NMR (400 MHz, CDCl3) δ 3.46 (bs, 1H), 2.51 (dd, J = 15.1, 7.4 Hz, 1H), 1.94 – 1.82 (m, 2H), 1.61 (m, 2H), 1.52 – 1.36 (m, 5H), 1.32 (m, 2H), 1.02 (s, 3H), 1.01 (s, 3H), 0.97 (s, 3H), 0.94 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ u 66.7, 53.2, 49.1, 42.9, 39.6, 34.2, 32.6, 24.3; d 89.2, 49.7, 39.3, 31.6, 26.4, 23.3, 15.8. These data were congruent with those previously reported.3b
(1S,2R,5S,8S,9R)-3,3,5,9-tetramethyltricyclo[6.3.0.01,5]undecan-2-ol: (−)-cameroonan-7α-ol (2)
A mixture of ketone 28 (12 mg, 0.037 mmol), anhydrous N2H4 (38 µL, 1.22 mmol, 33 equiv) and KOH (34 mg, 0.61 mmol, 16 equiv) was stirred at 170–180 °C for 18 h in a sealed vial. The reaction material was diluted with 5% aqueous NaOH and extracted with Et2O. The combined organic extracts were dried (Na2SO4), concentrated in vacuo and filtered through a short plug of silica gel.
The crude material was diluted with 200 uL of dry THF and cooled to −78 °C. Liquid NH3 (ca. 1 mL) was condensed into the reactor and a single piece of Na (ca. 50 mg, 2.17 mmol) was added. The blue reaction solution with blue foam was stirred at −78 °C for 10 min and MeOH was slowly added to dissipate the blue color. Excess NH3 was removed by warming the reaction to room temperature. The reaction material was then concentrated in vacuo and chromatographed to afford (−)-cameroonan-7α-ol 2 (2.0 mg, 24% yield); TLC Rf (PE:MTBE = 20:1) = 0.35; [α]20D = (−) 37° (c = 0.10 CHCl3); 1H NMR (400 MHz, CDCl3) δ 3.69 (s, 1H), 1.90 (t, J = 8.0 Hz, 1H), 1.84 – 1.72 (m, 1H), 1.69 – 1.58 (m, 2H), 1.55 (d, J = 14.3 Hz, 1H), 1.52 – 1.47 (m, 1H), 1.44 – 1.38 (m, 3H), 1.41 (d, J = 14.2 Hz, 1H), 1.36 – 1.29 (m, 1H), 1.25 (t, J = 5.3 Hz, 1H), 1.04 (s, 3H), 1.00 (d, J = 6.6 Hz, 3H), 0.97 (s, 3H), 0.91 (s, 3H); 13C NMR (100 MHz, CDCl3) δ u 67.1, 52.6, 47.5, 39.9, 38.6, 36.1, 35.3, 29.0; d 89.7, 51.3, 43.8, 32.5, 25.7, 23.9, 19.4; IR (film, cm−1) 3485, 2932, 2866, 1459; GC-MS (EI, 70 eV) m/z (rel. intensity %): 222 (2), 204 (18), 189 (14), 166 (25), 148 (32), 135 (100), 124 (33), 109 (24); HRMS calcd for C15H25 (MOH) 205.1956, obsd 205.1955.
Supplementary Material
Acknowledgments
We thank Mr. John Dykins for mass spectrometric measurements, supported by the NSF (0541775), Dr. GlennYap for the X-ray analysis, Dr. Shi Bai for NMR assistance (NSF CRIF:MU, CHE 0840401)and the NIH (GM42056) for financial support.
Footnotes
Supporting Information: General experimental procedures and 1H and 13C NMR spectra for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Weyerstahl P, Marschall H, Seelmann I, Jakupovic J. Eur. J. Org. Chem. 1998;1998:1205. [Google Scholar]
- 2.(a) Coates RM, Ho Z, Klobus M, Wilson SR. J. Am. Chem. Soc. 1996;118:9249. [Google Scholar]; (b) Coates RM, Ho JZ, Klobus M, Zhu L. J. Org. Chem. 1998;63:9166. [Google Scholar]; (c) Bohlmann F, Jakupovic J. Phytochem. 1980;19:259. [Google Scholar]; (d) Fitjer L, Monzó-Ohra H. J. Org. Chem. 1993;58:6171. [Google Scholar]
- 3.(a) Davis CE, Duffy BC, Coates RM. Org. Lett. 2000;2:2717. doi: 10.1021/ol0063149. [DOI] [PubMed] [Google Scholar]; (b) Davis CE, Duffy BC, Coates RM. J. Org. Chem. 2003;68:6935. doi: 10.1021/jo0343580. [DOI] [PubMed] [Google Scholar]; (c) Schmidt AW, Olpp T, Schmid S, Goutal S, Jäger A, Knölker H-J. Synlett. 2007;2007:1549. [Google Scholar]; (d) Schmidt AW, Olpp T, Schmid S, Jäger A, Knölker H-J. Tetrahedron. 2009;65:5484. [Google Scholar]
- 4.For recent examples of intramolecular aliphatic C-H to C-O conversion, see: Stang E, White MC. Nature Chem. 2009;1:547. doi: 10.1038/nchem.351. Kasuya S, Kamijo S, Inoue M. Organic Lett. 2009;11:3630. doi: 10.1021/ol901367m. For recent examples of intermolecular aliphatic C-H to C-O conversion, see: Chen MS, White MC. Science. 2007;318:783. doi: 10.1126/science.1148597. Chen K, Baran PS. Nature. 2009;459:824. doi: 10.1038/nature08043. Gomez L, Garcia-Bosch I, Company A, Benet-Buchholz J, Polo A, Sala X, Ribas X, Costas M. Angew. Chem. Int. Ed. 2009;48:5720. doi: 10.1002/anie.200901865. Ishihara Y, Baran PS. Synlett. 2010:1733. For a general overview of recents advances in C-H functionalization see: Lyons T, Sanford MS. Chem. Rev. 2010;110:1147. doi: 10.1021/cr900184e. and the other articles in that themed issue.
- 5.For recent examples of intramolecular aliphatic C-H to C-C bond formation see: Wolckenhauer SA, Devlin AS, DuBois J. Org. Lett. 2007;9:4363. doi: 10.1021/ol701950d. Herrera AJ, Rondon M, Suárez E. J. Org. Chem. 2008;73:3384. doi: 10.1021/jo702663w. Taber DF, Tian W. J. Org. Chem. 2008;73:7560. doi: 10.1021/jo8010683. Bequette JP, Jungong CS, Novikov AV. Tetrahedron Lett. 2009;50:6963. doi: 10.1016/j.tetlet.2009.09.147. McQuaid KM, Sames D. J. Am. Chem. Soc. 2009;131:402. doi: 10.1021/ja806068h. Wasa M, Engle KM, Yu J-Q. J. Am. Chem. Soc. 2009;131:9886. doi: 10.1021/ja903573p. For recent examples of intermolecular aliphatic C-H to C-C bond formation see: Yoshimitsu T, Matsuda K, Nagaoka K, Tanaka T. Org. Lett. 2007;9:5115. doi: 10.1021/ol7023295. Matsuo J, Tanaki Y, Ishibashi H. Tetrahedron Lett. 2007;18:3233. Wang D-H, Wasa M, Giri R, Yu JQ. J. Am. Chem. Soc. 2008;130:7190. doi: 10.1021/ja801355s. Nadeau E, Li Z, Morton D, Davies HML. Synlett. 2009:151. Protti S, Ravelli D, Fagnoni M, Albini A. Chem. Commun. 2009:7351. doi: 10.1039/b917732a. For a review of C-H to C-C bond functionalization see: Taber DF, Stiriba S-E. In: Natural Product Synthesis by Rh-Mediated Intramolecular C-H Insertion, in Organic Synthesis Highlights IV. Schmalz H-G, editor. Weinheim, Germany: Wiley-VCH Verlag GmbH; 2008.
- 6.For the first uses of RhII-mediated C-H to C-C bond formation in natural product synthesis see: Taber DF, Schuchardt JS. J. Am. Chem. Soc. 1985;107:3618. Taber DF, Raman K, Gaul MD. J. Org. Chem. 1987;52:28. For more recent examples see: Taber DF, Frankowski KJ. J. Org. Chem. 2005;70:6417. doi: 10.1021/jo0508752. (d) Ref. 5c. For a review see: Taber DF, Stiriba S-E. Chem. Eur. J. 1998;4:990.
- 7.Taber DF, Petty EH. J. Am. Chem. Soc. 1985;107:196. [Google Scholar]
- 8.Overberger CG, Ozaki S, Braunstein DM. Makromol. Chem. 1966;93:13. [Google Scholar]
- 9.Pospíšil J, Markó IE. J. Am. Chem. Soc. 2007;129:3516. doi: 10.1021/ja0691728. [DOI] [PubMed] [Google Scholar]
- 10.Hannick SM, Kishi Y. J. Org. Chem. 1983;48:3833. [Google Scholar]
- 11.Shin H, Choi BS, Lee KK, Choi H-W, Chang JH, Lee KW, Nam DH, Kim N-S. Synthesis. 2004;2004:2629. [Google Scholar]
- 12.Taber DF, Ruckle RE, Jr, Hennessy MJ. J. Org. Chem. 1986;51:4077. [Google Scholar]
- 13.(a) Kates SA, Dombroski MA, Snider BB. J. Org. Chem. 1990;55:2427. [Google Scholar]; (b) Snider BB, Merritt JE, Dombroski MA, Buckman BO. J.Org. Chem. 1991;56:5544. [Google Scholar]
- 14.(a) Beckwith ALJ, Phillipou BG. J. Am. Chem. Soc. 1974;96:1613. [Google Scholar]; (b) Schwartz CE, Curran DP. J. Am. Chem. Soc. 1990;112:9272. [Google Scholar]; (c) Taber DF, Wang Y, Stachel SJ. Tetrahedron Lett. 1993;34(39):6209. [Google Scholar]
- 15.See Supporting Information for analysis of this complex mixture.
- 16.(a) Taber DF, Nelson CG. J. Org. Chem. 2006;71:8973. doi: 10.1021/jo061420v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Huang H, Nelson CG, Taber DF. Tetrahedron Lett. 2010;51:3545. [Google Scholar]
- 17.(a) Poon KWC, House SE, Dudley GB. Synlett. 2005:3142. [Google Scholar]; (b) Poon KWC, Dudley GB. J. Org. Chem. 2006;71:3923. doi: 10.1021/jo0602773. [DOI] [PubMed] [Google Scholar]
- 18.Banerjee S, Das J, Santra S. Tetrahedron Let. 2009;50:124. [Google Scholar]
- 19.(a) Bickart P, Carson FW, Jacobus J, Miller EG, Mislow K. J. Am. Chem. Soc. 1968;90:4869. [Google Scholar]; (b) Pelc MJ, Zakarian A. Tetrahedron Lett. 2006;47:7519. [Google Scholar]
- 20.Marcantoni E, Cingolani S. J. Org. Chem. 1998;63:3624. [Google Scholar]
- 21.Banerjee AK, Matheud CAP, Hurtado S, Diaz MG. Heterocycles. 1986;24(8):2155. [Google Scholar]
- 22.Girard P, Namy JL, Kagan HB. J. Am. Chem. Soc. 1980;102:2693. [Google Scholar]
- 23.Micovic VM, Mihailovic MLJ. J. Org. Chem. 1953;18:1190. [Google Scholar]
- 24.13C multiplicities were determined with the aid of a JVERT pulse sequence, differentiating the signals for methyl and methine carbons as “d” and for methylene and quaternary carbons as “u”.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.