Abstract
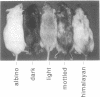
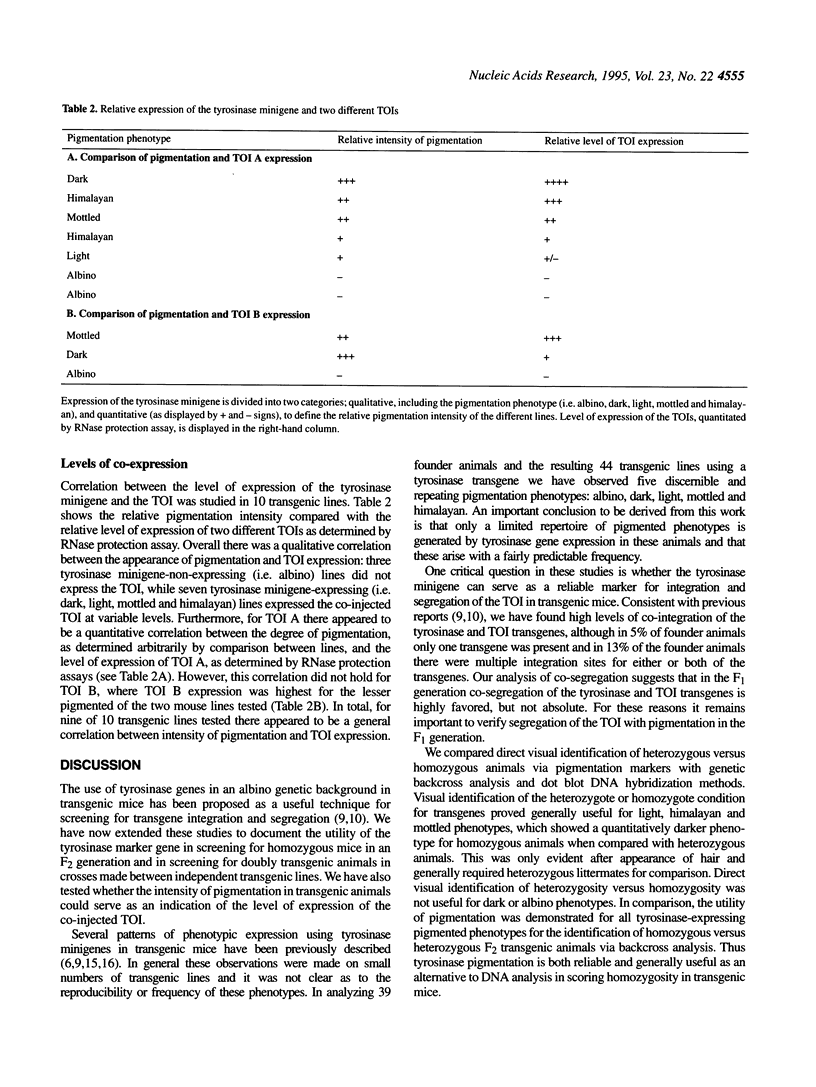
The utility of tyrosinase minigene co-injection was evaluated as a visual marker for the generation and breeding of transgenic mice. In an evaluation of 39 transgenic founder animals and 44 transgenic lines five phenotypic patterns of pigmentation were consistently observed, including albino, dark, light, mottled and himalayan. In these studies co-injection of the tyrosinase minigene along with the transgene of interest (TOI) resulted in genomic integration of the two transgenes in 95% of the F0 generation. Co-segregation of transgenes occurred in 94% of doubly transgenic mice in the F1 generation, without dissociation in subsequent generations. All pigmented phenotypes proved useful for distinguishing homozygous from heterozygous F2 animals via backcross trials, while the light, mottled and himalayan phenotypes proved useful in visually discriminating between homozygous and heterozygous F2 animals. In addition, the light, mottled and himalayan phenotypes proved useful in determining segregation patterns of transgenes in the progeny of crosses between separate transgenic lines. Moreover, there appears to be a correlation between intensity of pigmentation and degree of expression of the co-injected TOI. These studies confirm that tyrosinase co-injection is a useful adjunct in transgenic mouse studies and can serve to reduce routine genetic validation of transgenic lines.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beermann F., Ruppert S., Hummler E., Bosch F. X., Müller G., Rüther U., Schütz G. Rescue of the albino phenotype by introduction of a functional tyrosinase gene into mice. EMBO J. 1990 Sep;9(9):2819–2826. doi: 10.1002/j.1460-2075.1990.tb07470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beermann F., Ruppert S., Hummler E., Schütz G. Tyrosinase as a marker for transgenic mice. Nucleic Acids Res. 1991 Feb 25;19(4):958–958. doi: 10.1093/nar/19.4.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Kwon B. S., Haq A. K., Pomerantz S. H., Halaban R. Isolation and sequence of a cDNA clone for human tyrosinase that maps at the mouse c-albino locus. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7473–7477. doi: 10.1073/pnas.84.21.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon B. S., Wakulchik M., Haq A. K., Halaban R., Kestler D. Sequence analysis of mouse tyrosinase cDNA and the effect of melanotropin on its gene expression. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1301–1309. doi: 10.1016/s0006-291x(88)81370-6. [DOI] [PubMed] [Google Scholar]
- Mintz B., Bradl M. Mosaic expression of a tyrosinase fusion gene in albino mice yields a heritable striped coat color pattern in transgenic homozygotes. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9643–9647. doi: 10.1073/pnas.88.21.9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller G., Ruppert S., Schmid E., Schütz G. Functional analysis of alternatively spliced tyrosinase gene transcripts. EMBO J. 1988 Sep;7(9):2723–2730. doi: 10.1002/j.1460-2075.1988.tb03126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek P. A., Aguilar-Cordova E., Hanten G., Schaffner D. L., Patel P., Lebovitz R. M., Lieberman M. W. Coinjection strategy for visual identification of transgenic mice. Transgenic Res. 1991 Dec;1(1):31–37. doi: 10.1007/BF02512994. [DOI] [PubMed] [Google Scholar]
- Papaioannou V. E., Fox J. G. Efficacy of tribromoethanol anesthesia in mice. Lab Anim Sci. 1993 Apr;43(2):189–192. [PubMed] [Google Scholar]
- Taketo M., Schroeder A. C., Mobraaten L. E., Gunning K. B., Hanten G., Fox R. R., Roderick T. H., Stewart C. L., Lilly F., Hansen C. T. FVB/N: an inbred mouse strain preferable for transgenic analyses. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2065–2069. doi: 10.1073/pnas.88.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Takeuchi T. Expression of tyrosinase gene in transgenic albino mice: the heritable patterned coat colors. Pigment Cell Res. 1992 Nov;5(5 Pt 2):300–303. doi: 10.1111/j.1600-0749.1992.tb00553.x. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Yamamoto H., Takeuchi S., Takeuchi T. Melanization in albino mice transformed by introducing cloned mouse tyrosinase gene. Development. 1990 Feb;108(2):223–227. doi: 10.1242/dev.108.2.223. [DOI] [PubMed] [Google Scholar]
- Yokoyama T., Silversides D. W., Waymire K. G., Kwon B. S., Takeuchi T., Overbeek P. A. Conserved cysteine to serine mutation in tyrosinase is responsible for the classical albino mutation in laboratory mice. Nucleic Acids Res. 1990 Dec 25;18(24):7293–7298. doi: 10.1093/nar/18.24.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]