Abstract
OBJECTIVE
To estimate the effect of bacterial vaginosis (BV) on mid-trimester cervical length (CL) in women at increased risk for recurrent spontaneous preterm birth (PTB).
STUDY DESIGN
Secondary analysis of pre-randomization data from a multicenter trial of ultrasound-indicated cerclage. Women with prior spontaneous PTB <34 weeks underwent initial CL assessment and vaginal fluid collection at 16–216/7 weeks. Gram stains were scored with Nugent criteria. With serial scans, a shortest CL was observed.
RESULTS
949 women had complete data. In unadjusted regression models, Nugent score (p=0.003) and vaginal fluid pH (p=0.008) were inversely related to CL. Women with BV based on Nugent score ≥7 (p=0.04) or pH ≥5 (p=0.016), had significnatly lower CL than unaffected women; however, all of these effects were null after covariate adjustment.
CONCLUSION
Nugent score, pH, and BV are inversely associated with CL; however, these relationships become null after adjusting for relevant covariates.
Introduction
Preterm birth (PTB) is the leading cause of perinatal morbidity and mortality (1); most occur spontaneously and not for maternal-fetal indications (2). The incidence of PTB continues to rise largely due our poor understanding of the pathophysiology and the paucity of effective interventions (2). There are multiple plausible etiologies for spontaneous PTB, including asymptomatic upper genital tract infection (3). A Cochrane review estimated that one-third of all PTB is associated with largely asymptomatic intrauterine infection resulting directly from genital tract colonization (4). Numerous micro-organisms are associated with asymptomatic infection, and the most common associated condition is bacterial vaginosis (BV) (6).
The diagnosis of BV can be made using clinical Amsel criteria (5), but vaginal pH testing is a valuable screening tool due to its efficiency and cost-effectiveness (6). Vaginal-fluid Gram stain with quantitative assessment of the microbial flora has high sensitivity and specificity and is also widely accepted as an alternate diagnostic technique; using these later two methodologies, BV is diagnosed by a Nugent score ≥ 7 or vaginal pH ≥ 5 (7).
Recent studies have confirmed an association between BV and spontaneous PTB, with adjusted odds ratios between 1.4 and 6.9 (8, 9). Multiple putative mechanisms by which BV leads to PTB exist, however the exact pathway remains speculative. A possible mechanism for the link between asymptomatic genital infection and PTB is the bacterial stimulation of prostaglandin release or bacterial endotoxin introduced into the amniotic fluid leading to cytokine release (10, 11) and spontaneous labor. In women at high risk for spontaneous PTB, the detection and treatment of asymptomatic BV appeared to markedly reduce the rate of preterm premature rupture of membranes and low birth weight, although the rate of subsequent preterm birth was not statistically different (4). We hypothesized that the pathophysiologic effect of BV could operate through changes in the cervical matrix and result in shortened midtrimester cervical length which is widely recognized as a harbinger of spontaneous PTB (12, 13).
The aim of this study was to determine if BV, diagnosed by either Nugent score or vaginal pH, would predict mid-trimester cervical length in women at increased risk for recurrent PTB.
Methods
Recruitment and protocol
This is a planned, secondary analysis of prerandomization data from the NICHD-sponsored randomized trial of cerclage for PTB prevention (14). Sixteen U.S. Clinical Centers enrolled patients between January 2003 and November 2007. Healthy, multiparous women who entered prenatal care were screened to identify those with at least one prior spontaneous preterm birth between 17 0/7 and 33 6/7 weeks’ gestation.
Exclusion criteria were fetal anomaly, planned history-indicated cerclage for a clinical diagnosis of cervical insufficiency, and clinically significant maternal-fetal complications which would increase the risk of an indicated preterm birth. Qualifying women were invited to consent for the ultrasound screening phase of the study. Further details of the study protocol are described elsewhere (14).
Consenting women underwent serial transvaginal ultrasound examinations to measure cervical length, the first of which was scheduled in the gestational age window 16 0/7 to 21 6/7 weeks’ gestation. Follow-up scans were scheduled every 2 weeks unless the cervical length was observed to be 25-29 mm, after which scan frequency was increased to weekly. Women with a cervical length that remained at least 25 mm by the final sonographic evaluation, scheduled to be no later than 22 6/7 weeks, were ineligible for randomization and resumed their obstetric care. If on any evaluation the cervical length was < 25 mm, the woman became eligible for the randomization. Cervical length was obtained using the standard technique as described by Iams (12). The shortest observed cervical length from the serial evaluations was chosen for this analysis.
Before the initial sonographic cervical length evaluation, a sterile speculum examination was performed to collect vaginal fluid for pH and Gram stain. Using a dry, cotton-tipped applicator or Dacron swab, a vaginal secretion specimen from the upper third of the vaginal sidewalls was obtained. The swab was touched to the reagent block on the ColorpHast™ indicator strip. The pH was read after the color stabilized, but before the paper dried completely. The vaginal pH was recorded. If the color fell between two values on the chart, the pH was rounded to the higher value. The pH was not made available to managing physicians. Using a second sterile cotton or Dacron swab, vaginal fluid was obtained from the upper third of the vaginal sidewalls. The vaginal fluid was spread on a glass slide, allowed to air dry and transported to the laboratory for a Nugent score determination. Only a thin layer of vaginal secretion was used. The swab was gently rolled across the entire glass slide, from end to end, as evenly as possible; “blobbing” was avoided. After the slide was allowed to air dry, the slide was labeled on the frosted part of the slide with the center number, the patient's study ID, and the date of collection; no fixative was used.
After trial completion, the slides underwent Gram staining in batch and were analyzed. Five Oil Immersion fields were examined by a single trained investigator (MP) for the presence of lactobacillus, gardnerella vaginalis, Bacteroides, and Mobiluncus morphotypes using the depicted microscopic scoring scale (Table 1). The Gram stains were then scored using Nugent criteria (Table 2) (7). A Nugent score between 0-10 was assigned to each slide.
Table 1.
Microscopic Scoring
| 0 | morphotypes = 0 |
| < 1 | morphotypes = 1+ |
| 1-4 | morphotypes = 2+ |
| 5-30 | morphotypes = 3+ |
| >30 | morphotypes = 4+ |
Table 2.
Nugent Gram stain scoring
| Morphotype | Quantity | Points |
|---|---|---|
| Lactobacilli | 4+ | 0 |
| 3+ | 1 | |
| 2+ | 2 | |
| 1+ | 3 | |
| 0 | 4 | |
| G.vaginalis/Bacteroides | 0 | 0 |
| 1+ | 1 | |
| 2+ | 2 | |
| 3+ | 3 | |
| 4+ | 4 | |
| Mobiluncus | 0 | 0 |
| 1+/2+ | 1 | |
| 3+/4+ | 2 |
Statistical Analysis
Linear regression was used to model the effects of both Nugent score and pH on the shortest observed cervical length. Similar analyses examined the effect of the diagnosis of BV based on both criteria. Multivariable linear regression models were used to evaluate these effects in the presence of covariates predictive of PTB. (See Table 4 for predictors included in the model for coefficients and p values). SAS 9.2 (SAS Institute, Cary, NC) was used for all statistical analyses. A level of 0.05 was selected to indicate statistical significance.
Table 4.
Covariate-adjusted models for prediction of shortest observed cervical length. Models are adjusted for race, maternal age, smoking history, GA of earliest prior preterm birth, and GA and CL observed at first study sonogram. Models illustrate the lack of statistical significance for Nugent Score and pH.
| Covariate adjusted models for Nugent score | Covariate adjusted models for pH | ||||
|---|---|---|---|---|---|
| Variable | Coefficient (95% CI) | p | Variable | Coefficient (95% CI) | p |
| Nugent Score | -0.10 (-0.28, 0.07) | 0.25 | pH | -0.73 (-1.48, 0.03) | 0.06 |
| Race | Race | ||||
| Black | 0.77 (-0.62, 2.17) | 0.28 | Black | 0.76 (-0.62, 2.14) | 0.28 |
| Hispanic | 2.95 (1.43, 4.47) | <0.01 | Hispanic | 2.86 (1.34, 4.38) | <0.01 |
| Asian/Other | 2.82 (1.13, 4.50) | <0.01 | Asian/Other | 2.80 (1.12, 4.48) | <0.01 |
| White | Referent | -- | White | Referent | -- |
| Age | 0.08 (-0.02, 0.17) | 0.11 | Age | 0.07 (-0.02, 0.17) | 0.12 |
| GA of Earliest Prior Preterm Birth | 0.16 (0.05, 0.26) | <0.01 | GA of Earliest Prior Preterm Birth | 0.16 (0.05, 0.26) | <0.01 |
| GA at 1st Sonogram | 1.20 (0.81, 1.58) | <0.01 | GA at 1st Sonogram | 1.17 (0.79, 1.56) | <0.01 |
| CL at 1st Sonogram | 0.45 (0.41, 0.49) | <0.01 | CL at 1st Sonogram | 0.45 (0.41, 0.49) | <0.01 |
| Cigarette Use | 0.93 (-0.53, 2.39) | 0.21 | Cigarette Use | 0.95 (-0.50, 2.40) | 0.20 |
Results
Of the 1044 women who were determined to have a qualifying prior preterm birth, 1014 (99%) were consented and underwent their initial sonographic assessment of cervical length. Of these 1014, 949 (94%) had vaginal fluid samples for both pH and Gram stain collected. Selected baseline characteristics of the study population are shown in Table 3: 117 (12.3%) women had a Nugent score ≥ 7, while 323 (34%) had a pH ≥ 5.
Table 3.
Patient characteristics by BV diagnosis (Nugent Score ≥ 7). Mean SD are presented unless otherwise noted. P-values from independent sample t-tests (for continuous measures) and chi-square tests (for categorical measures) are presented unless otherwise noted.
| Overall (n = 949) | BV (n=117) | No BV (n=832) | p-value | |
|---|---|---|---|---|
| Nugent Score | 2.7 ± 2.8 | 7.6 ± 0.8 | 2.0 ± 2.3 | <0.01 |
| pH | 4.7 ± 0.7 | 5.2 ± 0.6 | 4.6 ± 0.6 | <0.01 |
| BV (by pH ≥ 5) - no. (%) | 323 (34%) | 88 (75%) | 235 (28%) | <0.01 |
| Shortest observed CL (mm) | 29.7 ± 9.7 | 28.0 ± 9.5 | 29.9 ± 9.7 | 0.04 |
| Race/ethnicity* - no. (%) | ||||
| Black (non-Hispanic) | 370 (39%) | 65 (56%) | 305 (37%) | <0.01 |
| White (non-Hispanic) | 176 (19%) | 12 (10%) | 164 (20%) | |
| Hispanic | 248 (26%) | 17 (15%) | 231 (28%) | |
| Asian | 10 (1%) | 2 (2%) | 8 (1%) | |
| Other | 145 (15%) | 21 (18%) | 124 (15%) | |
| Cigarette use - no. (%) | 132 (14%) | 28 (24%) | 104 (13%) | <0.01 |
| Maternal age (y) | 26.5 ± 5.3 | 25.3 ± 4.3 | 26.7 ± 5.4 | <0.01 |
| Drug use - no. (%) | 32 (3%) | 5 (4%) | 27 (3%) | 0.58* |
| Prior abortion - no. (%) | 104 (11%) | 11 (9%) | 93 (11%) | 0.56 |
| Gestational age of earliest prior PTB (wks) | 25.9 ± 4.7 | 26.1 ± 4.5 | 25.9 ± 4.7 | 0.71 |
| Weeks of gestation 1st sonogram (wks) | 17.6 ± 1.3 | 17.5 ± 1.3 | 17.6 ± 1.3 | 0.30 |
| Cervical length at 1st sonogram (mm) | 37.0 ± 12.4 | 34.5 ± 12.3 | 37.4 ± 12.3 | 0.02 |
| Total number of vaginal sonograms (N) | 2.6 ± 1.1 | 2.5 ± 1.2 | 2.7 ± 1.1 | 0.26 |
| Weeks of gestation last sonogram (wks) | 19.7 ± 2.0 | 19.6 ± 2.0 | 19.7 ± 2.0 | 0.76 |
| Number of women with CL < 25 mm | 298 (31.4%) | 47 (40.2%) | 251 (30.2% | 0.03 |
Fisher's exact test
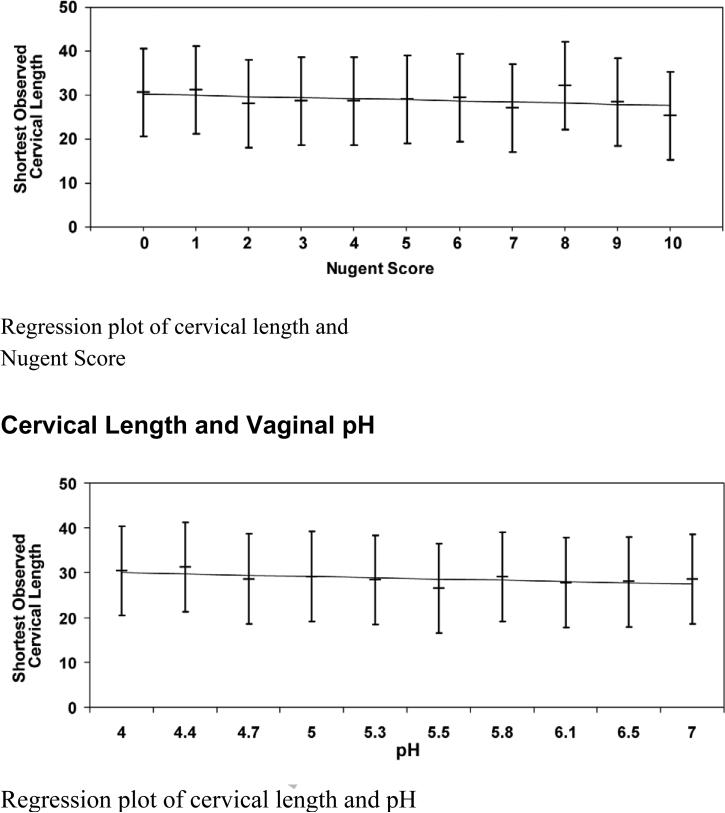
Linear regression was used to independently model the shortest observed cervical length as a function of both Nugent score and pH. The possibility of an interaction between the two criteria was also examined. Multivariable linear regression models included as possible confounders, maternal age, race, smoking, gestational age of earliest prior preterm birth, cervical length, and gestational age at initial ultrasound. There was no significant interaction between Nugent score and pH in the regression model (p = 0.74). In a simple linear regression model, Nugent score was inversely related to the shortest observed cervical length (linear regression coefficient = -0.33, p = 0.003) . Furthermore, women with a high Nugent score > 7, diagnostic of BV, had a cervical length significantly shorter than those with lower scores (28.0 mm vs. 29.9 mm, p = 0.04). However, in the covariate-adjusted models, Nugent score was no longer statistically significant (p = 0.25, Table 4) and statistically similar cervical lengths were observed between those with high Nugent score > 7 versus those with lower scores (29.8 mm vs. 30.2 mm). Vaginal pH was inversely related to shortest observed cervical length in the unadjusted model (linear regression coefficient = -1.28, p = 0.008). However, this effect was also null in the covariate-adjusted model (p = 0.06, Table 4). The mean cervical length for those with high pH > 5 was significantly lower than those with lower pH (28.6 mm vs. 30.2 mm; p = 0.016), but this effect also became null after adjusting for covariate effects (29.7 mm vs. 30.4 mm, p = 0.21).
Comment
Bacterial vaginosis is characterized by an overgrowth of anaerobic bacteria, predominantly Gardnerella vaginalis and Mycoplasma hominis (9) which replace the normal lactobacillus-dominant vaginal flora. BV is the most common cause of vaginal discharge in women of reproductive age (15) and has a worldwide prevalence ranging from 11-48% (16). This change in the vaginal ecosystem has been associated with PTB in several populations (3, 17). The mechanism by which BV is associated with PTB remains speculative. One hypothesis is that the pathologic effect of BV could operate through changes in the cervical matrix and result in shortened mid-trimester cervical length, a well-recognized risk factor for spontaneous PTB (12).
In linear regression models we observed a significant relationship between Nugent score, pH, and BV as diagnosed by either of these criteria, and shortest observed cervical length. But, after adjusting for relevant confounders, these relationships became null. Similarly, BV as diagnosed by Nugent score ≥ 7 and by pH ≥ 5 was not significantly associated with cervical length in covariate-adjusted analyses.
In the above mentioned studies, women with BV are at increased risk for PTB. The majority of these considered symptomatic infection and women were screened and treated based on the clinical diagnosis of BV. In our study population, women were asymptomatic, although we lack information regarding those women who may have presented to their managing physicians with complaints of vaginal discharge and subsequently underwent treatment of symptomatic BV.
We observed that women of black race were more likely to have asymptomatic BV which is consistent with other studies (18). The association between tobacco use and slightly younger age was observed. Without knowing specifics, the covariates could be explained by the number of sexual partners. We cannot determine from our data why this association was observed.
Additionally, the sonographic cervical length data was censored at 22 6/7 weeks’ gestation. Thus we do not have later information regarding cervical length or BV status. It is possible that expanding the gestational age limit of sonographic surveillance would identify women who later developed cervical length shortening associated with BV. Nevertheless, based on our findings in this large population of women at risk for recurrent PTB, we conclude that BV is not an independent predictor of a shortened cervical length at this restricted gestational age.
These results have significant clinical implications as screening for asymptomatic BV does not have an effect on short cervical length by 22 6/7 weeks in high risk women. This lack of association between BV and cervical length might explain the lack of relationship between asymptomatic BV and preterm birth (19) which is why treatment is unsuccessful.
Figure 1.
Cervical Length and Nugent Score
Acknowledgements
We wish to acknowledge other members of the Vaginal Ultrasound Trial Consortium: Gary Hankins , MD, Jay D. Iams, MD , Jeanne S. Sheffield, MD , Annette Perez-Delboy, MD, Vincenzo Berghella, MD, Debora A. Wing, MD, Edwin R. Guzman, MD, Susan Ramin, MD, Mark Tomlinson, MD, Eric Knudtson, MD, Robert Egerman, MD, Richard Silver, MD, Helen How, MD, Mike Gordon, MD.
Source of funding: The Eunice Kennedy Shriver National Institute of Child Health and Development provided funding via grant U01 HD039939; from the same agency, Dr. Owen also received support via grant 5K24 HD43314-5.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in abstract form at the annual Society of Maternal-Fetal Medicine Meeting, February, 2009 in San Diego, California.
CONDENSATION
The inverse relationship between mid-trimester cervical length and bacterial vaginosis, diagnosed by either Nugent score or vaginal pH becomes null after adjusting for relevant covariates.
References
- 1.Surbek DV, Hoeslie IM, Holzgreve W. Morphology assessed by transvaginal ultrasonography differs in patients in preterm labor with vs. without bacterial vaginosis. Ultrasound Obstet Gynecol. 2000;15:242–245. doi: 10.1046/j.1469-0705.2000.00102.x. [DOI] [PubMed] [Google Scholar]
- 2.Spong C. Prediction and Prevention of Recurrent Spontaneous Preterm Birth. Obstetrics and Gynecology. 2007;110:415. doi: 10.1097/01.AOG.0000275287.08520.4a. [DOI] [PubMed] [Google Scholar]
- 3.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007 Jan;25(1):21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldenberg RL, Klebanoff MA, Nugent RP, Krohn MA, Hillier SL, Andrews WW. Bacterial colonization of the vagina in four ethnic groups: Vaginal Infections and Prematurity Study Group. Am J Obstet Gynecology. 1996;174:1618–21. doi: 10.1016/s0002-9378(96)70617-8. [DOI] [PubMed] [Google Scholar]
- 5.Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Amer J Med. 1983;74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 6.Gjerdingen D, Fontaine P, Bixby M, et al. The impact of regular vaginal pH screening on the diagnosis of bacterial vaginosis in pregnancy. J Fam Pract. 2000 Jan;49(1):39–43. [PubMed] [Google Scholar]
- 7.Nugent RP, Krohn MA, Hillier SL. Reliability of Diagnosing Bacterial Vaginosis is Improved by a Standardized Method of Gram Stain Interpretation. J Clin Microbiol. 1991 Feb;29(2):297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hay PE, Lamont RF, Taylor-Robinson D, et al. Abnormal bacterial colonization of the genital tract and subsequent preterm delivery and late miscarriage. Brit Med J. 1994;308:295–8. doi: 10.1136/bmj.308.6924.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hillier SL, Nugent RP, Eschenbach DA, et al. Association Between Bacterial Vaginosis and Preterm Delivery of a Low-Birth-Weight Infant. New Engl J Med. 1995 Dec;333(26):1737–42. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 10.Bejar R, Curbelo V, Davis C, Gluck L. Premature labor II. Bacterial sources of phospholipase. Obstet Gynecol. 1981 April;57(4):479–482. [PubMed] [Google Scholar]
- 11.Yost NP, Cox SM. Infection and preterm labor. Clin Obstet Gynecol. 2000 Dec;43(4):759–767. doi: 10.1097/00003081-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Iams JD, Goldenberg RL, Meis PJ, Mercer BM, Moawad A, Das A, Thom E, McNellis D, Copper RL, Johnson F, Roberts JM, the National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med. 1996;334:567–72. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 13.Owen J, Yost N, Berghella V, Thom E, Swain M, Dildy GA, Miodovnik M, Langer D, Sibai BM, McNellis D, the National Institute for Child Health and Human Development Maternal Fetal Medicine Unit Network Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286:1340–8. doi: 10.1001/jama.286.11.1340. [DOI] [PubMed] [Google Scholar]
- 14.Owen J, Hankins G, Iams JD, Berghella V, Sheffield JS, Perez-Delboy A, Egerman RS, Wing DA, Tomlinson M, Silver R, Ramin SM, Guzman ER, Gordon M, How HY, Knudtson EJ, Szychowski JM, Cliver S, Hauth JC. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009 Oct;201(4):375.e1–8. doi: 10.1016/j.ajog.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joesoef MR, Karundeng A, Runtupalit C, Moran JS, Lewis JS, Ryan CA. High rate of bacterial vaginosis among women with intrauterine devices in Manado, Indonesia. Contraception. 2001 Sep;64(3):169–72. doi: 10.1016/s0010-7824(01)00246-3. [DOI] [PubMed] [Google Scholar]
- 16.Tolosa JE, Chaithongwongwathana S, Daly S, Maw WW, Gaitan H, Lumbiganon P, Festin M, Chipato T, Sauvarin J, Goldenberg RL, Andrews WW, Whitney CG. The International Infections in Pregnancy (IIP) study: variations in the prevalence of bacterial vaginosis and distribution of morphotypes in vaginal smears among pregnant women. Am J Obstet Gynecol. 2006 Nov;195(5):1198–204. doi: 10.1016/j.ajog.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Meis PJ, Goldenberg RL, Mercer B, et al. The preterm prediction study: Significance of vaginal infections. Amer J Obstet Gynecol. 1995 Oct;173(4):1231–5. doi: 10.1016/0002-9378(95)91360-2. [DOI] [PubMed] [Google Scholar]
- 18.Klatt TE, Cole DC, Eastwood DC, et al. Factors associated with recurrent bacterial vaginosis. J Reprod Med. 2010 Jan-Feb;55(1-2):55–61. [PubMed] [Google Scholar]
- 19.Figueroa D, Mancuso M, Paden MM, Szychowski J, Owen J. Does Mid-trimester Nugent score or vaginal pH predict gestational age at delivery in women at risk for preterm birth? Am J Obstet Gynecol. 2008 Dec;199(6):S215. doi: 10.1016/j.ajog.2010.08.029. [DOI] [PubMed] [Google Scholar]