Abstract
We test the hypothesis that quantitative electroencephalogram (qEEG) can be used to objectively assess functional electrophysiological recovery of brain after hypothermia in an asphyxial cardiac arrest rodent model. Twenty-eight rats were randomly subjected to 7-min (n = 14) and 9-min (n = 14) asphyxia times. One half of each group (n = 7) was randomly subjected to hypothermia (T = 33 °C for 12 h) and the other half (n = 7) to normothermia (T = 37 °C). Continuous physiologic monitoring of blood pressure, EEG, and core body temperature monitoring and intermittent arterial blood gas (ABG) analysis was undertaken. Neurological recovery after resuscitation was monitored using serial Neurological Deficit Score (NDS) calculation and qEEG analysis. Information Quantity (IQ), a previously validated measure of relative EEG entropy, was employed to monitor electrical recovery. The experiment demonstrated greater recovery of IQ in rats treated with hypothermia compared to normothermic controls in both injury groups (P < 0.05). The 72-h NDS of the hypothermia group was also significantly improved compared to the normothermia group (P < 0.05). IQ values measured at 4 h had a strong correlation with the primary neurological outcome measure, 72-h NDS score (Pearson correlation 0.746, 2-tailed significance <0.001). IQ is sensitive to the acceleration of neurological recovery as measured NDS after asphyxial cardiac arrest known to occur with induced hypothermia. These results demonstrate the potential utility of qEEG-IQ to track the response to neuroprotective hypothermia during the early phase of recovery from cardiac arrest.
Keywords: Quantitative EEG, Entropy, Neurologic outcome, Hypothermia, Resuscitation, Cardiac arrest
1. Introduction
Cardiac arrest (CA) affects 250,000 to 400,000 people annually and remains the major cause of death in the United States (Myerburg and Castellanos, 2001). Only 17% of patients resuscitated from CA survive to hospital discharge (Morimoto et al., 1993; Safar, 1986). Neurological complications represent the leading cause of disability (Bedell et al., 1983; Berek et al., 1997; Vaagenes et al., 1996). Numerous trials of neuroprotective agents, however, have failed to improve outcomes after CA (BRCT (The Brain Resuscitation Clinical Trial II Study Group), 1991; Longstreth et al., 2002). Recent clinical trials demonstrated that therapeutic hypothermia after CA can improve survival and functional outcomes compared to normothermic controls (Bernard et al., 1997; Bernard et al., 2002; Shankaran et al., 2005; Zeiner et al., 2000). As a result, the International Liaison Committee on Resuscitation made the recommendation to cool unconscious patients resuscitated from out-of-hospital arrest with an initial rhythm of ventricular fibrillation to 32–34 °C for 12–24 h (Nolan et al., 2003).
Ischemic brain injury affects synaptic transmission, axonal conduction, and cellular action potential firing in a sequential manner and plays a critical role in determining characteristics of EEG (Holmes et al., 1983). Cellular mechanisms of neuroprotective hypothermia include retarding the initial rate of ATP depletion (Kramer et al., 1968; Welsh et al., 1990), reduction of excitotoxic neurotransmitter release (Busto et al., 1989), alteration of intracellular messengers (Cardell et al., 1991), reduction of inflammatory responses (Toyoda et al., 1996), and alteration of gene expression and protein synthesis (Kumar et al., 1995; Kumar et al., 1996). Hypothermia reduces the excitatory postsynaptic potential (EPSP) slope in a temperature-dependent manner (Mednikova et al., 2004). A recent study of single unit spike activity using parietal cortex slices subjected to different temperatures found greater spontaneous spike amplitude and frequency in the range of mild hypothermia (32–34 °C) (Mednikova et al., 2004).
The effects of changes in brain temperature on electroencephalogram (EEG) have been described since 1930 s. Hoagland found that hyperthermic patients showed faster alpha rhythms (9–10 Hz) (Hoagland, 1936; Hoagland, 1938; Hoagland, 1949). Deboer demonstrated that temperature changes in animals and humans had an influence on EEG frequencies and that the changes were similar in magnitude in the different species (Deboer and Tobler, 1995; Deboer, 1998). More recently, hypothermia has been demonstrated to improve EEG restoration after reperfusion and the functional tolerance of the brain to impaired oxygen supply (Burger et al., 1998; Burger et al., 2003; Fritz et al., 1999). Most of these results, however, have been based on subjective observation of EEG and clinical parameters.
Using an animal model of global ischemic brain injury and using qEEG analysis, we have shown that the rate of return of EEG activity after CA is highly correlated with behavioral outcome (Geocadin et al., 2000b; Geocadin et al., 2000a; Geocadin et al., 2002). This technique is based on the hypothesis that brain injury reduces the entropy of the EEG, also measured by its information content (defined classically as bits/second of information rate (Shannon, 1948)) in the signal. Further, our technique is based on the hypothesis that neurological recovery can be predicted by monitoring recovery of the entropy, or equivalently, the information quantity (IQ) (Shin et al., in press) in EEG signals. Information can be physically quantified by calculating EEG entropy (Rosso et al., 2001; Shannon, 1948). The EEG signal can be divided into predictable and unpredictable components, with the quantity of information more highly correlated to the latter. Removal of the predictable component (information redundancy) using discrete wavelet transformation (DWT), allows estimation of IQ (Antonini et al., 1992; Mallat, 1989). This qEEG analysis method was recently developed and showed promising results in predicting outcome from CA (Bezerianos et al., 2003; Thakor and Tong, 2004; Tong et al., 2002; Tong et al., 2003).
We have previously established and validated a rodent model for global ischemic brain injury following CA (Geocadin et al., 2000b; Geocadin et al., 2000a). Neurological recovery is monitored using a standardized Neurological Deficit Scale (NDS) which relies on serial performance of a comprehensive behavioral examination (Ao et al., 2001; Xiao et al., 1998; Yli-Hankala et al., 1997; Zeiner et al., 2000). The NDS was patterned after the standard neurologic examination in humans, incorporating some elements from functional outcome scales developed for global cerebral ischemia in rats (Katz et al., 1995), dogs (Vaagenes et al., 1984; Woods et al., 2000), and piglets (Goel et al., 1996; Sherman et al., 1999).
The present study was performed to validate a quantitative analysis of early recovery of EEG as a measure of neurological outcomes in rats. To this end, we examined the impact of hypothermia on NDS and qEEG markers of recovery in rats resuscitated from experimental CA.
2. Results
2.1. Temperature monitoring
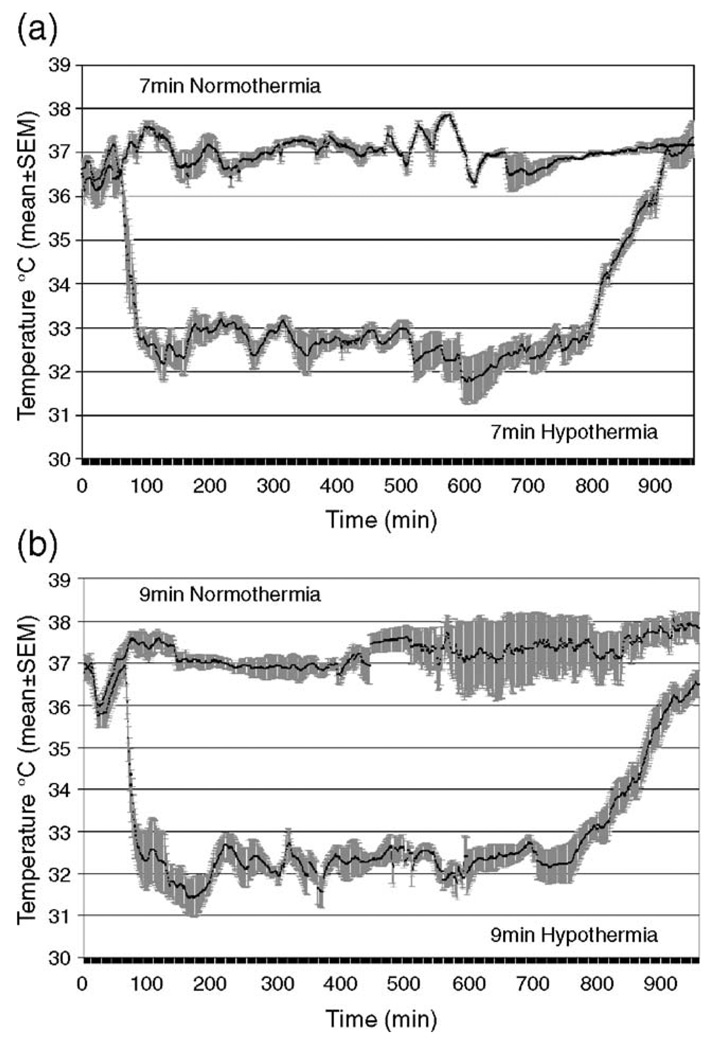
All animals in the normothermia groups (7 and 9 min) were maintained at 37 ± 0.5 °C for 15 h and moved to a neonatal incubator to maintain normothermia during the first 24 h. For the hypothermia groups, the target temperature was reached in 9.0 ± 5.4 min. Rewarming occurred over 119.5 ± 22.5 min to achieve a stable temperature within the target range (36.5–37.5 °C), increased by 1 °C per half hour. All animals were then similarly maintained in the neonatal incubator for the first 24 h to maintain normothermia. The temperature recording for 7- and 9-min groups during the hypothermia experiment is shown in Fig. 1.
Fig. 1.
Temperature recording of normothermic and hypothermic rats with 7-min asphyxia (a) baseline 0–5 min, asphyxial CA period 10–17 min, CPR 17 min, hypothermia period 77–797 min, re-warming period 798–917 min; and 9-min asphyxia (b) baseline 0–5 min, asphyxial CA period 10–19 min, CPR 19 min, hypothermia period 79–799 min, re-warming period 800–919 min. The solid black line is mean temperature and gray field is SEM.
2.2. Baseline characteristics
Baseline characteristics of animals in the hypothermia and normothermia groups were similar, including weight on the day of CA, duration of asphyxia prior to CA, duration of CPR prior to ROSC, and baseline ABG data. No significant difference exists between asphyxial groups for baseline characteristics (P > 0.05).
2.3. NDS analysis
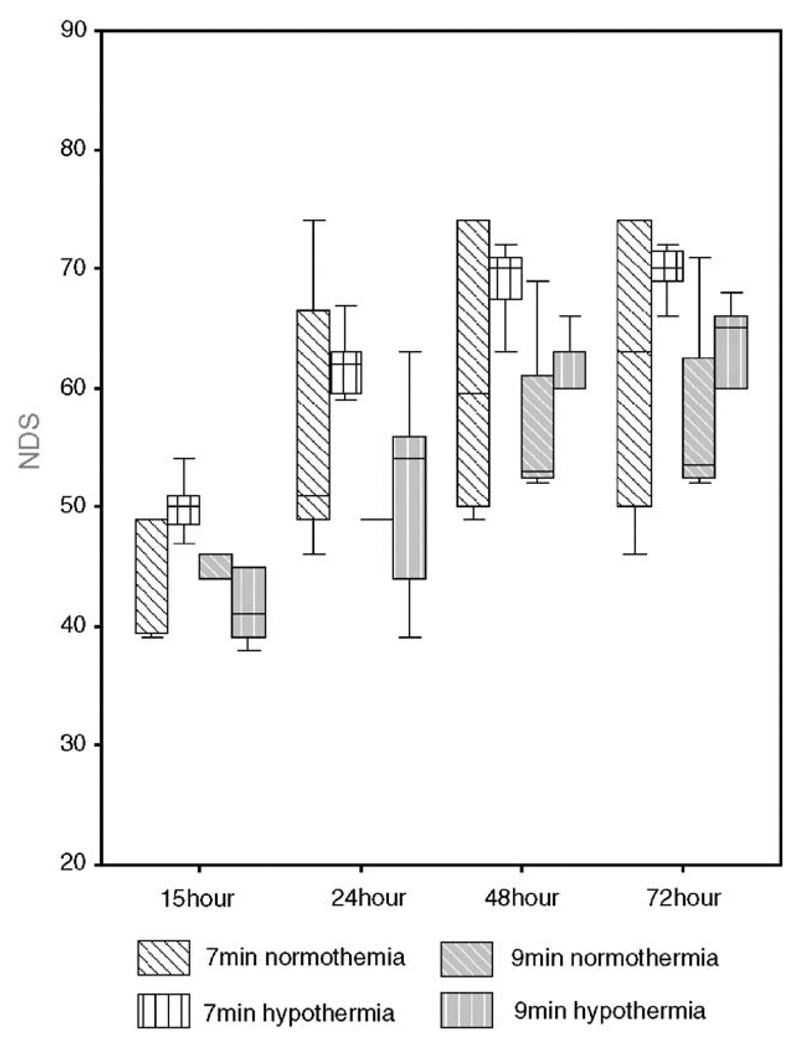
NDS analysis results are shown on Table 1 and Fig. 2. The distribution of total NDS data was normal (P > 0.05). The distribution of NDS scores over the study period was better in hypothermic animals compared to normothermic controls in the 7-min group (P = 0.01) but not the 9-min group (P = 0.255). We noted increased deaths in the 9-min injury group (both normothermia and hypothermia) which reflects the severity of injury in those groups. We believe that this reduction in the animal groups led to the increased variability in the results which is manifested as a lack of statistical significant difference in NDS. We proceeded to combine the NDS scores from both the 7-min and 9-min animals, and noted improvement of NDS scores in the hypothermic animals compared to normothermic controls (P = 0.039). Bivariate analysis revealed significant correlations between the 72-h NDS and NDS values at 15 h (Pearson correlation = 0.711), 24 h (Pearson correlation = 0.800), and 48 h (Pearson correlation = 0.974), with all 2-tailed tests demonstrating statistical significance (P < 0.001).
Table 1.
Survival NDS results by injury and temperature groups [Median (25th–75th percentile)]
| Group | 15 h | 24 h | 48 h | 72 h |
|---|---|---|---|---|
| 7N | 49 (39–49) | 51 (49–72) | 59.5 (49.5–74) | 63 (49–74) |
| 7H | 50 (47–51) | 62 (59–63) | 70 (67–72) | 70 (68–72) |
| 9N | 46 (38.5–46) | 49 (44–55) | 53 (52.25–65) | 53.5 (52.25–66.75) |
| 9H | 41 (38.75–47.25) | 54 (42.75–57.75) | 60 (52–64.5) | 65.5 (61.25–67.5) |
7N: 7-min asphyxia normothermia, 7H: 7-min asphyxia hypothermia, 9N: 9-min asphyxia normothermia, and 9H: 9-min asphyxia hypothermia.
Fig. 2.
NDS score by injury and temperature groups (Box-plot of median and interquartile range (25th and 75th percentile). Statistically significant differences existed between hypothermic and normothermic groups (z = −2.06, P = 0.039) and between 7-min and 9-min group (z = 3.05, P = 0.002).
2.4. Mortality and mean survival
Mortality before 72 h in each group was as follows: 1 rat in the 7N group, 0 rats in the 7 H group, 3 rats in the 9N group, and 2 rats in the 9 H group. No formal investigation was done to determine cause of death. There was a trend toward improved survival rates in rats treated with hypothermia in both the 7-min and 9-min groups, although the data failed to reach statistical significance. There was also no significant difference in mean duration of survival hours between the 7N group (66.9 h) and 7 H group (72.0 h) or the 9N group (48.0 h) and 9 H group (57.1 h) in a Kaplan–Meier analysis.
2.5. ABG Data
ABG data were obtained prior to CA and again 10, 20, and 40 min after ROSC. Arterial pH, HCO3−, PCO2, PO2, Na+, anion gap and glucose concentration were similar in hypothermic and normothermic animals at all time points. There was a trend toward minor metabolic acidosis in the normothermic controls (22.1 ± 0.6 mmol/L) compared to hypothermic rats (24.4 ± 0.6 mmol/L) with a slight but statistically significant difference in bicarbonate concentration at 40 min. Arterial pH, HCO3−, PCO2, PO2, and O2 saturation were similar in all 7N, 7 H, 9N and 9 H animals at all 4-time points (P > 0.05), and no difference existed between the 7- and 9-min asphyxial groups (P > 0.05).
2.6. EEG analysis
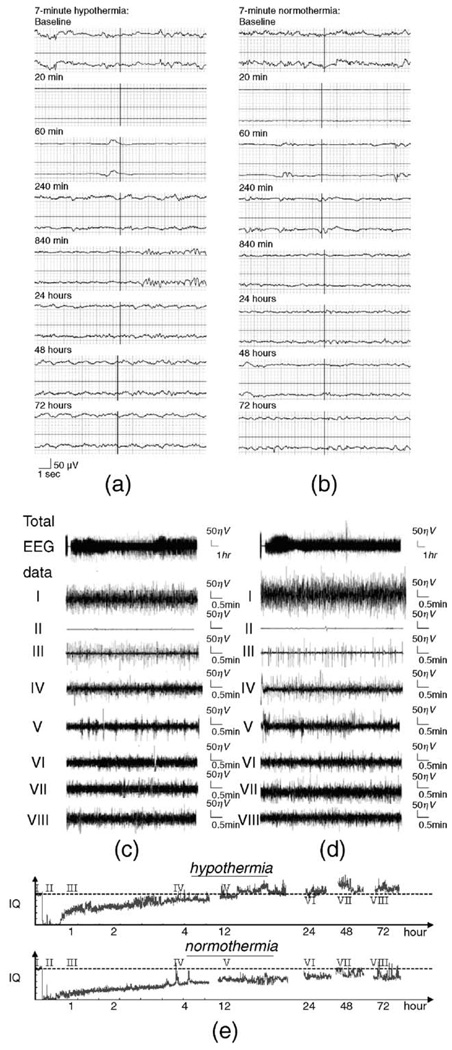
Fig. 3 (a) and (b) illustrate EEG recordings in hypothermic and normothermic animals, respectively. Within seconds of CA, EEG signal amplitude becomes highly suppressed and proceeds quickly to an isoelectric tracing. EEG activity then resumes approximately 16 min after ROSC (16.3 ± 0.3 (Mean ± SEM)) in 7-min asphyxial rats and 15.0 ± 0.7 min after ROSC in 9-min asphyxial rats (No statistical difference existed between 7- and 9-min groups), with an initial periodic pattern resembling burst-suppression of variable duration. Over several hours, there is a gradual increase in the complexity and frequency of EEG bursts and concomitant decrease in EEG suppression. Subjective review of compressed EEG raw data indicated increased frequency of EEG bursts at different periods in the hypothermic rats compared to normothermic controls that was most pronounced at the time of initiation of hypothermia (tracings III and IV). However, comparing their baselines (we set all baseline IQ values as 1), the difference between the two EEG signals in Fig. 3 (a) to (d) is not readily discernable from visualization of the waveform itself; i.e., the effect of hypothermia on the EEG signal recovery was not evident without quantitative analysis.
Fig. 3.
Raw EEG data of representative animals and IQ at various time points with 7 min asphyxia: (a) real-time raw EEG under hypothermia, (b) Real-time raw EEG under normothermia, (c) raw compressed EEG under hypothermia, (I) baseline prior to CA, 0 min, (II) early stage after CA, 20 min, (III) initiation of hypothermia, 60 min, (IV) hypothermia maintenance period, 4 h, (V) initiation of rewarming, 12 h, (VI) late recovery, 24 h, (VII) late recovery, 48 h, (VIII) late recovery, 72 h. (d) Raw compressed EEG under normothermia. (e) IQ plots for hypothermia and normothermia at the same time points, showing the return of the EEG to baseline (hatched line) in hypothermia treated animal.
Using qEEG, the information quantity (IQ) – a marker of EEG entropy relative to baseline – was calculated. A graphical depiction of aggregate IQ data from hypothermic and normothermic animals is shown in Fig. 3 (e). Similar to the raw EEG data, the aggregate IQ decreased from baseline (IQ = 1) to the lowest point rapidly after CA (0.1196 ± 0.0115), then gradually recovered near baseline by 15 h (0.8265 ± 0.0242).IQ continued to improve over the subsequent 72 h, reaching an aggregate peak at 48 h (0.9789 ± 0.0421), and then declining slightly at 72 h (0.9012 ± 0.0389).
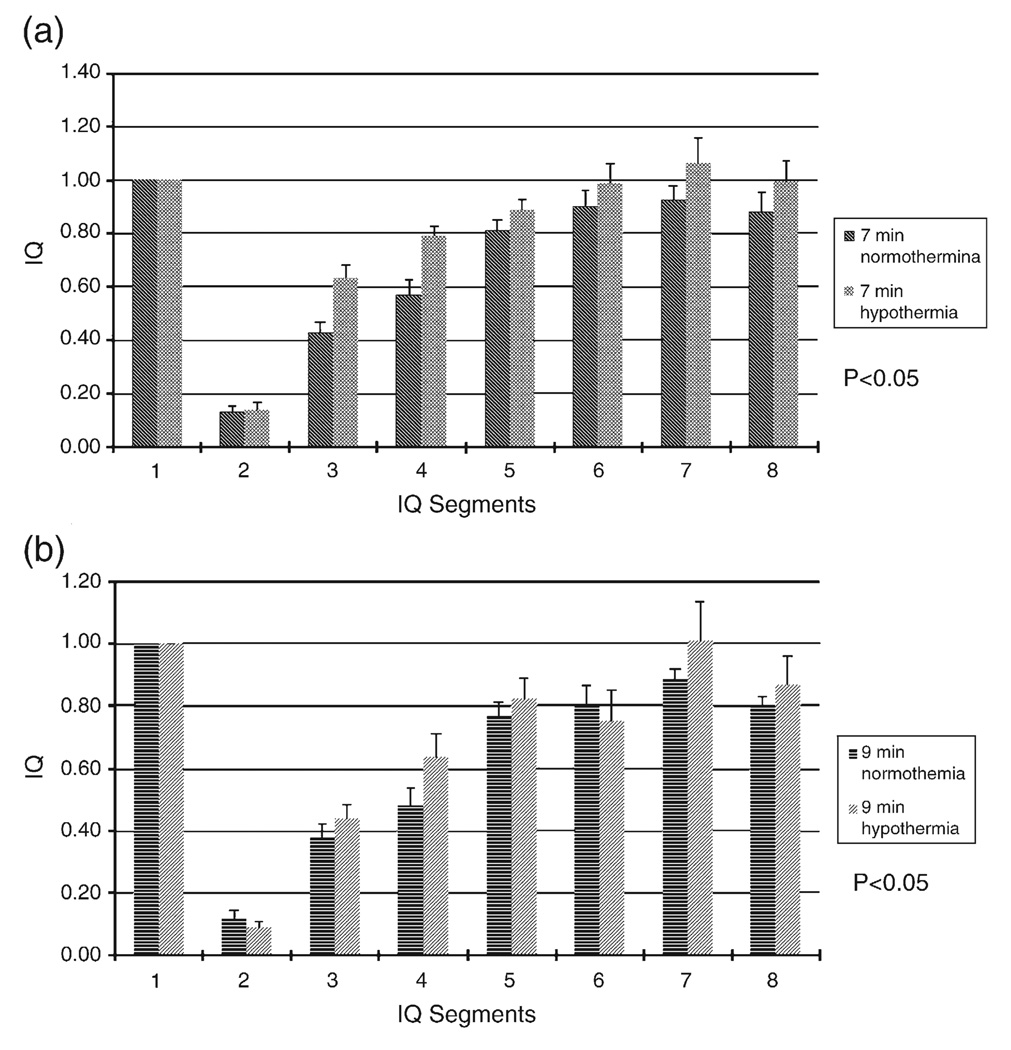
As shown in Figs. 4a and b, the distribution of mean IQ scores was greater in the hypothermia group compared to the normothermic group over the duration of the study in both the 7-min group and the 9-min group. In the 7-min injury group, the aggregate IQ was significantly greater in the hypothermic animals (0.8114 ± 0.0430) compared to normothermic animals (0.6994 ± 0.0421) (P < 0.05). Similarly, the aggregate IQ from hypothermic rats with 9-min asphyxia (0.6755 ± 0.0504) was greater than normothermic controls (0.6176 ± 0.0461) (P < 0.05). IQ was greater in hypothermic rats (0.7524 ± 0.0333) compared to normothermic controls (0.6618 ± 0.0312) at every time point after ROSC in the combined injury groups, with a significant average IQ difference of 0.0906 (P < 0.001). Similar to the NDS data, IQ of the entire 7-min group (0.7564 ± 0.0305) is greater than the entire 9-min group (0.6456 ± 0.0341) (P < 0.001).
Fig. 4.
Comparison of IQ between hypothermic and normothermic rats subjected to 7-min (a) and 9-min (b) ischemia at different time periods: IQ1 baseline, IQ2 CA period, IQ3 hypothermia starting period, IQ4 hypothermia maintenance period, IQ5 re-warming period, IQ6 24 h after ROSC, IQ7 48 h after ROSC, IQ8 72 h after ROSC.
Bivariate analysis reveals significant correlations between the 72-h NDS and IQ values at 60 min (Pearson correlation = 0.692), 4 h (Pearson correlation = 0.746), 14 h (Pearson correlation = 0.742), 24 h (Pearson correlation = 0.709), 48 h (Pearson correlation = 0.937), and 72 h (Pearson correlation = 0.958), with all 2-tailed tests demonstrating statistical significance (P < 0.001). The 4-h IQ measure had a greater correlation with 72-h NDS (Pearson correlation = 0.746) than the earliest NDS score, measured at 15 h (Pearson correlation = 0.711), but the difference between these values was not statistically significant.
3. Discussion
The experiments reported here further validate the utility of the qEEG entropy measure IQ as an early indicator of injury and neurologic recovery after experimental CA. Hypothermia led to better functional outcomes in treated rats and EEG recovery quantified by IQ was significantly greater in rats treated with hypothermia compared to normothermic controls. The separation of IQ values between the treatment and control groups was noticeable within 1 h of ROSC and persisted throughout the 72-h experiment. Better EEG recovery, as manifested by greater IQ values, was associated with significant improvements in neurological function as measured by NDS throughout the experiment. IQ measure was able to detect the acceleration of neurological recovery as measured by NDS in animals treated with hypothermia. IQ also predicted the 72-h functional outcomes as early as 4 h after cardiac arrest and was a comparable outcome predictor to the 15-h NDS. Functional outcome scales such as NDS have previously been validated (Geocadin et al., 2000b; Hickey et al., 2003; Katz et al., 1995; Luft et al., 2002).
Previous attempts to use early qEEG as a measure of neurological recovery after CA have relied on power spectral analysis (Cerchiari et al., 1990). This technique is predicated on analysis of the relative percentage of EEG power confined to specific frequency bands. Power spectral analysis has limited utility for this application, however. After CA, in humans and various animal models, several common EEG patterns are predictive of poor neurological recovery, including generalized EEG suppression, persistent burst-suppression, generalized unreactive alpha or theta activity, and epileptiform discharges (Bassetti et al., 1996; Binnie et al., 1970; Møller et al., 1978; Scollo-Lavizzari and Bassetti, 1987; Synek, 1989; Thomassen et al., 1978). Power spectral analysis is unable to discriminate non-reactive from reactive patterns, epileptiform morphologies from theta waves, or burst-suppression from intermittent slow waves. Because IQ entropy analysis is based on separating predictable and unpredictable patterns of EEG activity, it has much greater potential sensitivity for unfavorable EEG patterns after CA. Seizure activity, burst-suppression, unreactive alpha or theta patterns, and generalized suppression are all periodic – and highly predictable – patterns and, therefore, score low on entropy measures. Reactive patterns appear more chaotic, rendering entropy measures more sensitive to detecting improvements in EEG patterns after CA.
This methodology has the potential for early prognostication after resuscitation. Our experiment demonstrated that IQ values had excellent discriminative power for the 72-h functional outcome measures throughout the study period. It is worth highlighting that an early correlation of NDS and IQ was noted at 4 h, which may provide an opportune time for intervention or injury stratification, and a later, more robust correlation occurred at 72 h. The strong correlation at 4 h likely reflects the great variability between rats destined for good or poor outcomes during this period (Geocadin et al., 2000a). The first 4 h after ROSC are characterized by increasing frequency and complexity of bursts with concomitant decrease in the duration of suppression. Animals that proceed more quickly to a continuous EEG pattern will have higher IQ values and better functional outcomes. The strong correlation between the 72-h IQ and final NDS likely reflects the re-emergence of EEG reactivity during this period in the group with good outcomes, whereas those with poor outcomes tend to have unreactive alpha or theta patterns and lower IQ values.
Most clinical prognostic scales require serial neurological examination over several days, and such clinical observations have poor ability to predict outcomes during the early period when injury amelioration may still be possible. Electrophysiologic tests (EEG and evoked potentials) improve the ability to predict the neurologic outcome at about the third day after injury (Zandbergen et al., 1998). Early EEG after CA in humans, pigs, dogs, and rats is marked by a period of burst-suppression followed by fusion to a continuous EEG pattern (Brechner et al., 1961; Gurvitch et al., 1972; Jørgensen and Malchow-Møller, 1981; Kawai et al., 1992; Lin et al., 1977; Luft et al., 2002; Rosén et al., 1984; Yashon et al., 1970). The interval between ROSC and the return of continuous EEG activity is related with prognosis, but the manual determination of continuous EEG activity is laborious and subjective. Persistence of burst-suppression can be readily monitored with the help of entropy-based qEEG analysis, such as IQ.
Potential limitations of this study include the absence of histopathological data. Using this model, we have previously demonstrated that histopathological markers for ischemic cell death correlate with the degree of asphyxial brain injury, behavioral outcome scores, and EEG restitution (Geocadin et al., 2005; Geocadin et al., 2000a; Luft et al., 2002). Histological markers of injury in rats, however, have been a poor indicator of clinical significance in human trials of the same agents (Gladstone et al., 2002). In a recent review, Gladstone opined that “histological end points cannot tell whether surviving neurons are functional or dysfunctional or will go on to die in a delayed fashion, and they are less predictive of long-term histology than early behavioral assessments”. Similar conclusions have been reached by other authors (Corbett and Nurse, 1998; Hunter et al., 1998). One of the major goals of our group is to develop experimental approaches that can be easily translated clinically. As such, we have moved away from post-mortem markers, such as histological quantification of injury. For this reason, we chose to rely on a functional outcome measure, 72-h NDS, as the primary endpoint.
The development of a non-invasive strategy to track the course of recovery early after resuscitation from CA has a number of readily translatable functions in humans. EEG technology is readily available and familiar in most hospitals, rather than being restricted to tertiary academic centers. Entropy analysis as exemplified by IQ simplifies interpretation of EEG by translating complicated and subjective waveform analysis into an objective measure that can be displayed in real time, allowing physicians to monitor the response to potential neuroprotective strategies.
4. Experimental procedures
4.1. The animal model
4.1.1. Experimental asphyxic-cardiac arrest and resuscitation
The Animal Care and Use Committee of the Johns Hopkins Medical Institutions approved the experimental protocol used in this study. Twenty-eight adult male Wistar rats (350 ± 25 g) were randomly assigned to 7-min asphyxia under normothermia (7N group), 7-min asphyxia under hypothermia (7 H group), 9-min asphyxia under normothermia (9N group), and 9-min asphyxia under hypothermia (9 H group) (n = 7 per group).
Asphyxial cardiac arrest and resuscitation protocol was performed as modified from Katz et al. (Geocadin et al., 2000a; Katz et al., 1995). Several strain-specific life support adjustments, i.e., ventilator settings, anesthesia, and drug requirements, were necessary to stabilize the model for Wistar rats. Anesthesia was induced with 4.5% halothane carried by 50% oxygen and 50% nitrogen gas at 4 L/min, followed by tracheal intubation under direct laryngoscopy with a 14-G plastic catheter. The rat was ventilated at 50 breaths per minute by a rodent ventilator (Harvard Apparatus Model 553438) and maintained with humidified 50% FIO2 and 1.0 halothane (Halocarbon laboratories, River Edge, NJ, USA) at tidal volumes (TV) of 8 ml/kg, positive expiratory end pressure (PEEP) of 3 cm H2O. Body temperature of 37.0 ± 0.5 °C was maintained. The femoral artery and vein were cannulated (Intramedic Non-Radiopaque Polyethylene Tubing PE-50 catheters, PE 50, Becton Dickinson) to monitor mean arterial pressure (MAP), sampling for arterial blood gases (ABG), as well as administration of fluid and drugs.
After preparation, baseline EEG and physiologic measurements were made for 5 min. Halothane 1% by vaporizer (Fluotec MK, serial 316563, from Cyprane Keighley Yorkshire, England) was administered during the baseline recording. A gas washout phase followed the baseline recording with 100% oxygen for 3 min and the rat was paralyzed with vecuronium 2 mg/kg, i.v. (vecuronium bromide for injection, 1 mg/ml, Novaplus, from Abbott Labs, North Chicago, IL 60064, USA), followed by room air for 2 min with unchanged ventilator settings. This was followed by global asphyxia, which was induced for periods of 7 or 9 min by clamping the tracheal tube and stopping and disconnecting the ventilator. Cardiac arrest was defined by pulse pressure <10 mm Hg and was observed with asystolic electrical rhythm, non-pulsatile-pressure wave.
After the predetermined asphyxia time, cardiopulmonary resuscitation (CPR) was initiated with effective ventilation (TV 8 ml/kg, Respiration Rate (RR) 40/min and PEEP 0 cm H2O) and oxygenation (100% O2), epinephrine (0.005 mg/kg, i.v.), sternal chest compression (200 compressions/min) and NaHCO3 (1 mmol/kg i.v.) to normalize arterial pH. Return of spontaneous circulation (ROSC) was defined by achievement of spontaneous MAP > 60 mm Hg. The animal was then hyperventilated (TV 10 ml/kg, RR 65/min and PEEP 6 cm H2O) for 10 min. Subsequent ventilator adjustments were as follows: RR adjusted to 55/min at 10 min after ROSC, RR adjusted to 50/min at 20 min after ROSC, and the O2 concentration adjusted 40 min after ROSC. Adjustments were also slightly changed to avoid respiratory acidosis or alkalosis by serial ABG measurements at 10, 20, and 40 min after ROSC. The animals were allowed to recover spontaneously after resuscitation. To minimize the drug effect on EEG, no anesthesia was provided post-resuscitation. The rats were subsequently weaned off the ventilator after 2 h and then extubated. At all points in the experiment, the rats were monitored and treated accordingly for signs of pain and distress.
4.1.2. Hypothermia
A temperature sensor (G2 E-mitter 870-0010-01, Mini Mitter, Oregon, USA) was used to monitor the core temperature and a rectal temperature sensor was used as a reference. The sensor was implanted into the peritoneal cavity about 1 week before the experiment. Hypothermia was induced 45 min after ROSC by external cooling with a cold water and alcohol mist, aided by an electric fan, to achieve the target temperature of 33 °C within 15 min. An automatic warming lamp (Thermalet TH-5, model 6333, Physiotemp, NJ, USA) was used to prevent precipitous temperature decline. The core temperature was maintained by manual control between 32 and 34 °C for 12 h. Re-warming was initiated 13 h after ROSC. Rats were gradually re-warmed from 33.0° to 37.0 °C over 2 h using a heating pad and heating lamp. For normothermia groups, temperature after CPR was maintained at 36.5–37.5 °C throughout the sham-hypothermia period as a control. To ensure that no temperature fluctuation occurred after the resuscitation, such as the spontaneous hypothermia previously reported (Hickey et al., 2003), all animal were then kept inside a neonatal incubator (Isolette infant incubator model C-86, Air-shields Inc, Pennsylvania, USA) maintained at 28 °C for the first 24 h post-ROSC. In prior experiments, this temperature has been adequate to maintain the animals within the target range (36.5–37.5 °C).
4.1.3. EEG recording
EEG epidural screw electrodes (Plastics One, Roanoke, VA) were implanted 1 week before the experiment. Two channels of EEG were recorded in the right and left parietal areas throughout the experiment using DI700 Windaq system. Using stereotactic guidance, electrodes were placed 2 mm lateral to and 2 mm anterior or posterior to the bregma. A ground electrode was placed 2 mm posterior to the lambda in the midline. Recording continued throughout the hypothermia and re-warming periods and 2 additional recovery hours, with total EEG recording times of 17 h on the day of the experiment. Serial 30-min EEG recordings were then performed in free-roaming, unanesthetized rats at 24, 48, and 72 h after ROSC. The signals were digitized using the data acquisition package CODAS (DATAQ Instruments INC., Akron OH). Sampling frequency of 250 Hz and 12 bit A/D conversion were used.
4.1.4. Arterial blood gas analysis (ABG)
We use Rapidlab 865 blood gas analyzer (Bayer Company) to analyze temperature-corrected ABG, including pH, PaO2, PCO2, HCO3-act, O2 saturation, Na+, anion gap and glucose. We measured the ABG 4 times per animal: at baseline, and 10, 20, and 40 min after ROSC.
4.1.5. Neurological evaluation
The NDS of rats was determined 15 h after ROSC on the first day, and then repeated at 24, 48, and 72 h after ROSC. The standardized NDS examination was performed by a trained examiner blinded to temperature group in all instances except the 15-h NDS. Re-warming at the 15-h time point rendered adequate blinding impossible. The NDS and its components provides a score from 0 (worst) to 80 (best) (Geocadin et al., 2000b). The primary outcome measure of this experiment was defined as the 72-h NDS score.
4.1.6. Quantitative EEG analysis
EEG has been used as a clinical diagnostic and research tool. But the waveform-based EEG analysis is thought to be subjective and laborious because the results depend on the clinicians’ experience and expertise. Quantitative EEG methods and their relative strengths and benefits have been extensively reviewed (Thakor and Tong, 2004). To track and compare the EEG recovery under hypothermia and normothermia, we analyzed EEG signals using quantitative methods. We previously reported the development of this novel, entropy-based EEG analysis which has shown promising results in objectively tracking the EEG recovery after cardiac arrest (Shin et al., in press).
Entropy is a method to quantify the order/disorder of a time series. It is calculated from the distribution of one of the signal parameters, such as amplitude, power or time-frequency representation. The Shannon entropy (SE) gives useful criteria for analyzing and comparing probability distribution and provides a good measure of the information (Shannon, 1948). The classical Shannon entropy is expressed in:
where pm is the probability of finding the system in the mth microstate with 0 ≤ p(m) ≤ 1 and . To analyze nonstationary EEG signals, we need to get a temporal evolution of SE based on application of a sliding temporal window technique.
Such data analysis is done using a sliding window of the sampled EEG, n = 0,1,…,[n/Δ] −w + 1, where sampled data in window W are analyzed using a sliding stepΔ. Next, wavelet analysis of the signal is carried out to decompose the EEG signals into wavelet subbands, which can be interpreted as frequency subbands. Based on the above arguments, we can define the information quantity (IQ). First, the discrete wavelet transform (DWT) coefficients within each window are obtained as:
Next, wavelet coefficients are obtained from the DWT, and the IQ is obtained as:
where pn (m) is an estimated probability that the wavelet-transformed signal belongs to mth bin and M is the number of bin. IQ is calculated from a temporal sliding window block of EEG signal as explained above, for the entire data set. Further information on the algorithm can be obtained from Shin (Shin et al., in press).
We choose 8 segments from hypothermia EEG data in each rat and to derive 8 IQ measurements after qEEG analysis: baseline (0–5 min, IQ1), CA period (10–40 min, IQ2), hypothermia starting period (60–90 min, IQ3), hypothermia maintenance period (3–5 h, IQ4), hypothermia end period (re-warming period) (13–15 h, IQ5), 24 h after CPR (30 min, IQ6), 48 h after CPR (30 min, IQ7), 72 h after CPR (30 min, IQ8). For EEG data under 7 min normothermia, instead of IQ 3–5 segments under hypothermia, we analyzed the corresponding EEG segments under normothermia respectively. A graphical comparison between raw EEG waveforms, compressed waveforms, and IQ data at these time points is shown in Fig. 3.
4.1.7. Statistical methods
Group values that are parametric (i.e., IQ, temperature, ABG results, weight, asphyxia time before CA and ROSC time) are reported as mean ± SEM and non-parametric variables (i.e., NDS) are reported as median (25th–75th percentile). Parametric data were analyzed by one-way analysis of variance (ANOVA) with Student–Newman–Keuls (SNK) analysis used for multiple comparisons. For NDS, we performed statistical tests based on rank order. Non-parametric analysis of variance was used to test for differences in rank order NDS. The factors included in the model were animal group (normothermic versus hypothermic) and time group (7 min and 9 min). The outcome, rank order NDS, was treated as a repeated measure (assessed at 15, 24, 48, and 72 h). The mortality rate was analyzed by Fisher’s Exact Test (crosstabs) and survival was analyzed by a Kaplan–Meier test. Pearson correlation of bivariate analysis was used to analyze the correlation between 72-h NDS score and serial IQ and NDS measurements. For sample size calculations, we used preliminary data from rats subjected to 7-min asphyxia and therapeutic hypothermia. In these preliminary studies the 72-h NDS in the hypothermia-treated animals was greater than normothermic controls by a mean difference of 10.2 ± 5. Given this treatment effect between hypothermia and control group, we computed a sample size for t test and ANOVA to be 7 animals per group in order to have a 95% confidence level and a power of 90%. A level of P < 0.05 was selected in order to consider the differences significant.
Acknowledgments
This research was supported by NIH Grants R01 HL 071568 and R21NS42690. We gratefully acknowledge Dr. Wei Deng at Fudan University in China and Dr. Richard Skolarsky at Johns Hopkins Hospital for assistance with statistical data analysis.
Abbreviations
- EEG
electroencephalogram
- CA
cardiac arrest
- ABG
arterial blood gas
- NDS
Neurological Deficit Score
- qEEG
quantitative EEG
- IQ
Information Quantity
REFERENCES
- Antonini M, Barladu M, Mathieu P, Daubechies I. Image coding using wavelet transform. IEEE Trans. Image Process. 1992;1:205–220. doi: 10.1109/83.136597. [DOI] [PubMed] [Google Scholar]
- Ao H, Tanimoto H, Yoshitake A, Moon JK, Terasaki H. Long-term mild hypothermia with extracorporeal lung and heart assist improves survival from prolonged cardiac arrest in dogs. Resuscitation. 2001;48:163–174. doi: 10.1016/s0300-9572(00)00252-5. [DOI] [PubMed] [Google Scholar]
- Bassetti C, Bomio F, Mathis J, Hess CW. Early prognosis in coma after cardiac arrest: a prospective clinical, electrophysiological, and biochemical study of 60 patients. J. Neurol. Neurosurg. Psychiatry. 1996;61:610–615. doi: 10.1136/jnnp.61.6.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell SE, Delbanco TL, Cook EF, Epstein FH. Survival after cardiopulmonary resuscitation in the hospital. N. Engl. J. Med. 1983;309:569–576. doi: 10.1056/NEJM198309083091001. [DOI] [PubMed] [Google Scholar]
- Berek K, Jeschow M, Aichner F. The prognostication of cerebral hypoxia after out of hospital cardiac arrest in adults. Eur. Neurol. 1997;37:135–145. doi: 10.1159/000117426. [DOI] [PubMed] [Google Scholar]
- Bernard SA, Jones BM, Horne MK. Clinical trial of induced hypothermia in comatose survivors of out-of-hospital cardiac arrest. Ann. Emerg. Med. 1997;30:146–153. doi: 10.1016/s0196-0644(97)70133-1. [DOI] [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N. Engl. J. Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- Bezerianos A, Tong S, Thakor NV. Time-dependent entropy estimation of EEG rhythm changes following brain ischemia. Ann. Biomed. Eng. 2003;31:221–232. doi: 10.1114/1.1541013. [DOI] [PubMed] [Google Scholar]
- Binnie CD, Prior PF, Lloyd DS, Scott DF, Margerison JH. Electroencephalographic prediction of fatal anoxic brain damage after resuscitation from cardiac arrest. Br. Med. J. 1970;4:265–268. doi: 10.1136/bmj.4.5730.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRCT (The Brain Resuscitation Clinical Trial II Study Group) A randomized clinical trial of calcium entry blocker administration to comatose survivors of cardiac arrest, Design, methods, and patient characteristics. The Brain Resuscitation Clinical Trial II Study Group. Control. Clin. Trials. 1991;12:525–545. doi: 10.1016/0197-2456(91)90011-a. [DOI] [PubMed] [Google Scholar]
- Brechner VL, Bethune RWM, Kavan EM, Bauer RO, Phillips RE, Dillon JB. The electroencephalographic effect of arrested circulation in the normothermic human and dog. Curr. Res. Anest. Anal. 1961;40:1–14. [Google Scholar]
- Burger R, Vince H, Meixensberger J, Roosen K. Hypothermia influences time course of intracranial pressure, brain temperature, EEG and microcirculation during ischemia–reperfusion. Neurol. Res. 1998;20 Suppl 1:S52–S60. doi: 10.1080/01616412.1998.11740611. [DOI] [PubMed] [Google Scholar]
- Burger R, Zuechner M, Bendszus M, Vince GH, Roosen K. Moderate hypothermia improves neurobehavioral deficits after an epidural focal mass lesion in rodents. J. Neurotrauma. 2003;20:543–558. doi: 10.1089/089771503767168474. [DOI] [PubMed] [Google Scholar]
- Busto R, Globus MY, Dietrich WD, Martinez E, Valdes I, Ginsberg MD. Effect of mild hypothermia on ischemia-induced release of neurotransmitters and free fatty acids in rat brain. Stroke. 1989;20:904–910. doi: 10.1161/01.str.20.7.904. [DOI] [PubMed] [Google Scholar]
- Cardell M, Boris-Moller F, Wieloch T. Hypothermia prevents the ischemia-induced translocation and inhibition of protein kinase C in the rat striatum. J. Neurochem. 1991;57:1814–1817. doi: 10.1111/j.1471-4159.1991.tb06387.x. [DOI] [PubMed] [Google Scholar]
- Cerchiari EL, Sclabassi RJ, Safar P, Hoel TM. Effects of combined superoxide dismutase and deferoxamine on recovery of brainstem auditory evoked potentials and EEG after asphyxial cardiac arrest in dogs. Resuscitation. 1990;19:25–40. doi: 10.1016/0300-9572(90)90096-w. [DOI] [PubMed] [Google Scholar]
- Corbett D, Nurse S. The problem of assessing effective neuroprotection in experimental cerebral ischemia. Prog. Neurobiol. 1998;54:531–548. doi: 10.1016/s0301-0082(97)00078-6. [DOI] [PubMed] [Google Scholar]
- Deboer T. Brain temperature dependent changes in the electroencephalogram power spectrum of humans and animals. J. Sleep Res. 1998;7:254–262. doi: 10.1046/j.1365-2869.1998.00125.x. [DOI] [PubMed] [Google Scholar]
- Deboer T, Tobler I. Temperature dependence of EEG frequencies during natural hypothermia. Brain Res. 1995;670:153–156. doi: 10.1016/0006-8993(94)01299-w. [DOI] [PubMed] [Google Scholar]
- Fritz H, Bauer R, Walter B, Schlonski O, Hoyer D, Zwiener U, Reinhart K. Hypothermia related changes in electrocortical activity at stepwise increase of intracranial pressure in piglets. Exp. Toxicol. Pathol. 1999;51:163–171. doi: 10.1016/S0940-2993(99)80090-6. [DOI] [PubMed] [Google Scholar]
- Geocadin RG, Muthuswamy J, Sherman DL, Thakor NV, Hanley DF. Early electrophysiological and histologic changes after global cerebral ischemia in rats. Mov. Disord. 2000a;15 Suppl. 1:14–21. doi: 10.1002/mds.870150704. [DOI] [PubMed] [Google Scholar]
- Geocadin RG, Ghodadra R, Kimura T, Lei H, Sherman DL, Hanley DF, Thakor NV. A novel quantitative EEG injury measure of global cerebral ischemia. Clin. Neurophysiol. 2000b;111:1779–1787. doi: 10.1016/s1388-2457(00)00379-5. [DOI] [PubMed] [Google Scholar]
- Geocadin RG, Sherman DL, Hansen HC, Kimura T, Niedermeyer E, Thakor NV, Hanley DF. Neurological recovery by EEG bursting after resuscitation from cardiac arrest in rats. Resuscitation. 2002;55:193–200. doi: 10.1016/s0300-9572(02)00196-x. [DOI] [PubMed] [Google Scholar]
- Geocadin RG, Malhotra AD, Tong S, Seth A, Moriwaki G, Hanley DF, Thakor NV. Effect of acute hypoxic preconditioning on qEEG and functional recovery after cardiac arrest in rats. Brain Res. 2005;1064:146–154. doi: 10.1016/j.brainres.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Gladstone DJ, Black SE, Hakim AM. Heart and Stroke Foundation of Ontario Centre of Excellence in Stroke Recovery, 2002. Toward wisdom from failure: Lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 33:2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- Goel V, Brambrink AM, Baykal A, Koehler RC, Hanley DF, Thakor NV. Dominant frequency analysis of EEG reveals brain’s response during injury and recovery. IEEE Trans. Biomed. Eng. 1996;43:1083–1092. doi: 10.1109/10.541250. [DOI] [PubMed] [Google Scholar]
- Gurvitch AM, Mutuskina EA, Novoderzhkina IS. Quantitative evaluation of brain damage in dogs resulting from circulatory arrest to the central nervous system or the whole animal: 2. Electroencephalographic evaluation during early recovery of the gravity and reversibility of post-ischaemic cerebral damage. Resuscitation. 1972;1:219–228. doi: 10.1016/0300-9572(72)90051-2. [DOI] [PubMed] [Google Scholar]
- Hickey RW, Kochanek PM, Ferimer H, Alexander HL, Garman RH, Graham SH. Induced hyperthermia exacerbates neurologic neuronal histologic damage after asphyxial cardiac arrest in rats. Crit. Care Med. 2003;31:531–535. doi: 10.1097/01.CCM.0000050323.84293.11. [DOI] [PubMed] [Google Scholar]
- Hoagland H. Pacemaker of human brain waves in normal and in general paretics. Am. J. Physiol. 1936;16:R604–R615. [Google Scholar]
- Hoagland H. Brain mechanisms and brain wave frequencies. Am. J. Physiol. 1938;123:R102. [Google Scholar]
- Hoagland H. Brain wave frequencies and brain chemistry. Arch. Neurol. Psychiatry. 1949;62:511–513. [PubMed] [Google Scholar]
- Holmes GL, Rowe J, Hafford J. Significance of reactive burst suppression following birth asphyxia in full-term infants. Clin. Electroencephalogr. 1983;14:138–141. doi: 10.1177/155005948301400308. [DOI] [PubMed] [Google Scholar]
- Hunter AJ, Mackay KB, Rogers DC. To what extent have functional studies of ischaemia in animals been useful in the assessment of potential neuroprotective agents? Trends Pharmacol. Science. 1998;19:59–66. doi: 10.1016/s0165-6147(97)01157-7. [DOI] [PubMed] [Google Scholar]
- Jørgensen EO, Malchow-Møller A. Natural history of global and critical brain ischaemia. Part II: EEG and neurological in patients remaining unconscious after cardiopulmonary resuscitation. Resuscitation. 1981;9:155–174. doi: 10.1016/0300-9572(81)90024-1. [DOI] [PubMed] [Google Scholar]
- Katz L, Ebmeyer U, Safar P, Radovsky A, Neumar R. Outcome model of asphyxial cardiac arrest in rats. J. Cereb. Blood Flow Metab. 1995;15:1032–1039. doi: 10.1038/jcbfm.1995.129. [DOI] [PubMed] [Google Scholar]
- Kawai K, Nitecka L, Ruetzler CA, Nagashima G, Joó F, Mies G, Nowak TS, Saito N, Lohr JM, Klatzo I. Global cerebral ischemia associated with cardiac arrest in the rat: I. Dynamics of early neuronal changes. J. Cereb. Blood Flow Metab. 1992;12:238–249. doi: 10.1038/jcbfm.1992.34. [DOI] [PubMed] [Google Scholar]
- Kramer RS, Sanders AP, Lesage AM, Woodhall B, Sealy WC. The effect of profound hypothermia on preservation of cerebral ATP content during circulatory arrest. J. Thorac. Cardiovasc. Surg. 1968;56:699–709. [PubMed] [Google Scholar]
- Kumar K, Wu X, Evans AT, Marcoux F. The effect of hypothermia on induction of heat shock protein (HSP)-72 in ischemic brain. Metab. Brain Dis. 1995;10:283–291. doi: 10.1007/BF02109359. [DOI] [PubMed] [Google Scholar]
- Kumar K, Wu X, Evans AT. Expression of c-fos and fos-B proteins following transient forebrain ischemia: effect of hypothermia. Brain Res. Mol. Brain Res. 1996;42:337–343. doi: 10.1016/s0169-328x(96)00181-7. [DOI] [PubMed] [Google Scholar]
- Lin SR, Morris TW, Violante MR. Cerebral water content, blood flow, and EEG changes after cardiac arrest in the dog. Invest. Radiol. 1977;12:325–332. doi: 10.1097/00004424-197707000-00005. [DOI] [PubMed] [Google Scholar]
- Longstreth WT, Fahrenbruch CE, Olsufka M, Walsh TR, Copass MK, Cobb LA. Randomized clinical trial of magnesium, diazepam, or both after out-of-hospital cardiac arrest. Neurology. 2002;59:506–514. doi: 10.1212/wnl.59.4.506. [DOI] [PubMed] [Google Scholar]
- Luft AR, Buitrago MM, Paul JS, Hagan J, Ding MC, Thakor NV, Hanley DF. Early restitution of electrocorticogram predicts subsequent behavioral recovery from cardiac arrest. J. Clin. Neurophysiol. 2002;19:540–546. doi: 10.1097/00004691-200212000-00007. [DOI] [PubMed] [Google Scholar]
- Mallat SG. A theory for multiresolution signal decomposition: the wavelet representation. IEEE Trans. Pattern Anal. Mach. Intell. 1989;11:674–693. [Google Scholar]
- Mednikova YS, Pasikova NV, Kopytova FV. Effects of temperature on the spike activity of cortical neurons in guinea pigs. Neurosci. Behav. Physiol. 2004;34:459–465. doi: 10.1023/b:neab.0000022630.53594.99. [DOI] [PubMed] [Google Scholar]
- Møller M, Holm B, Sindrup E, Nielsen BL. Electroencephalographic prediction of anoxic brain damage after resuscitation from cardiac arrest in patients with acute myocardial infarction. Acta Med. Scand. 1978;203:31–37. doi: 10.1111/j.0954-6820.1978.tb14827.x. [DOI] [PubMed] [Google Scholar]
- Morimoto Y, Kemmotsu O, Kitami K, Matsubara I, Tedo I. Acute brain swelling after out of hospital cardiac arrest: pathogenesis and outcome. Crit. Care Med. 1993;21:104–110. doi: 10.1097/00003246-199301000-00020. [DOI] [PubMed] [Google Scholar]
- Myerburg RJ, Castellanos A. Cardiac arrest and sudden cardiac death. In: Fuster V, editor. Hurst’s The Heart. New York: McGraw-Hill; 2001. pp. 1015–1048. [Google Scholar]
- Nolan JP, Morley PT, Vanden Hoek TL, Hickey RW, Kloeck WG, Billi J, Böttiger BW, Okada K, Reyes C, Shuster M, Steen PA, Weil MH, Wenzel V, Carli P, Atkins D. Therapeutic hypothermia after cardiac arrest. An advisory statement by the Advancement Life support Task Force of the International Liaison committee on Resuscitation. Resuscitation. 2003;57:231–235. doi: 10.1016/s0300-9572(03)00184-9. [DOI] [PubMed] [Google Scholar]
- Rosén I, Smith ML, Rehncrona S. Quantitative EEG and evoked potentials after experimental brain ischemia in the rat; correlation with cerebral metabolism and blood flow. Prog. Brain Res. 1984;62:175–182. doi: 10.1016/S0079-6123(08)62175-5. [DOI] [PubMed] [Google Scholar]
- Rosso OA, Blanco S, Yordanova J, Kolev V, Figliola A, Schürmann M, Ba°ar E. Wavelet entropy: a new tool for analysis of short duration brain electrical signals. J. Neurosci. Methods. 2001;105:65–75. doi: 10.1016/s0165-0270(00)00356-3. [DOI] [PubMed] [Google Scholar]
- Safar P. Cerebral resuscitation after cardiac arrest: a review. Circulation. 1986;74:138–153. [PubMed] [Google Scholar]
- Scollo-Lavizzari G, Bassetti C. Prognostic value of EEG in post-anoxic coma after cardiac arrest. Eur. Neurol. 1987;26:161–170. doi: 10.1159/000116329. [DOI] [PubMed] [Google Scholar]
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH National Institute of Child Health and Human Development Neonatal Research Network. Whole-body hypothermia for neonates with hypoxic–ischemic encephalopathy. N. Engl. J. Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- Shannon CE. A mathematical theory of communication. Bell Syst. Tech. J. 1948;27:623–656. [Google Scholar]
- Sherman DL, Brambrink AM, Ichord RN, Dasika VK, Koehler RC, Traystman RJ, Hanley DF, Thakor NV. Quantitative EEG during early recovery from hypoxic–ischemic injury in immature piglets: burst occurrence and duration. Clin. Electroencephalogr. 1999;30:175–183. doi: 10.1177/155005949903000410. [DOI] [PubMed] [Google Scholar]
- Shin HC, Tong S, Yamashita S, Jia X, Geocadin R, Thakor NV. Quantitative EEG and Effect of Hypothermia on Brain Recovery After Cardiac Arrest. IEEE Trans. on Biomedical Eng. doi: 10.1109/TBME.2006.873394. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synek VM. Validity of a revised EEG coma scale for predicting survival in anoxic encephalopathy. Clin. Exp. Neurol. 1989;26:119–127. [PubMed] [Google Scholar]
- Thakor NV, Tong S. Advances in quantitative electroencephalogram analysis methods. Annu. Rev. Biomed. Eng. 2004;6:453–495. doi: 10.1146/annurev.bioeng.5.040202.121601. [DOI] [PubMed] [Google Scholar]
- Thomassen A, Sørensen K, Wernberg M. The prognostic value of EEG in coma survivors after cardiac arrest. Acta Anaesthesiol. Scand. 1978;22:483–490. doi: 10.1111/j.1399-6576.1978.tb01327.x. [DOI] [PubMed] [Google Scholar]
- Tong S, Bezerianos A, Paul J, Zhu Y, Thakor NV. Nonextensive entropy measure of EEG following brain injury from cardiac arrest. Physica, A. 2002;305:619–628. [Google Scholar]
- Tong S, Bezerianos A, Malhotra A, Zhu Y, Thakor NV. Parameterized entropy analysis of EEG following hypoxic–ischemic brain injury. Phys. Lett., A. 2003;314:354–361. [Google Scholar]
- Toyoda T, Suzuki S, Kassell NF, Lee KS. Intraischemic hypothermia attenuates neutrophil infiltration in the rat neocortex after focal ischemia–reperfusion injury. Neurosurgery. 1996;39:1200–1205. doi: 10.1097/00006123-199612000-00024. [DOI] [PubMed] [Google Scholar]
- Vaagenes P, Cantodore R, Safar P, Moossy J, Rao G, Diven W, Alexander H, Stezoski W. Amelioration of brain damage by lidoflazine after prolonged ventricular fibrillation cardiac arrest in dogs. Crit. Care Med. 1984;12:846–855. doi: 10.1097/00003246-198410000-00002. [DOI] [PubMed] [Google Scholar]
- Vaagenes P, Ginsberg M, Ebmeyer U, Ernster L, Fischer M, Gisvold SE, Gurvitch A, Hossmann KA, Nemoto EM, Radovsky A, Severinghaus JW, Safar P, Schlichtig R, Sterz F, Tonnessen T, White RJ, Xiao F, Zhou Y. Cerebral resuscitation from cardiac arrest: pathophysiologic mechanisms. Crit. Care Med. 1996;24:S57–S68. [PubMed] [Google Scholar]
- Welsh FA, Sims RE, Harris VA. Mild hypothermia prevents ischemic injury in gerbil hippocampus. J. Cereb. Blood Flow Metab. 1990;10:557–563. doi: 10.1038/jcbfm.1990.98. [DOI] [PubMed] [Google Scholar]
- Woods RJ, Prueckner S, Safar P, Takasu A, Tisherman SA, Jackson EK, Radovsky A, Kochanek P, Behringer W, Stezoski SW, Hans R. Adenosine by aortic flush fails to augment the brain preservation effect of mild hypothermia during exsanguination cardiac arrest in dogs—An exploratory study. Resuscitation. 2000;44:47–59. doi: 10.1016/s0300-9572(99)00164-1. [DOI] [PubMed] [Google Scholar]
- Xiao F, Safar P, Radovsky A. Mild protective and resuscitative hypothermia for asphyxial cardiac arrest in rats. Am. J. Emerg. Med. 1998;16:17–25. doi: 10.1016/s0735-6757(98)90059-6. [DOI] [PubMed] [Google Scholar]
- Yashon D, White RJ, Taslitz N, Wolin LR, Massopust LC. Experimental cerebral circulatory arrest: effect on electrocerebral potentials. J. Neurosurg. 1970;32:74–82. doi: 10.3171/jns.1970.32.1.0074. [DOI] [PubMed] [Google Scholar]
- Yli-Hankala A, Edmonds HL, Jiang YD, Higham HE, Zhang PY. Outcome effects of different protective hypothermia levels during cardiac arrest in rats. Acta Anaesthesiol. Scand. 1997;41:511–515. doi: 10.1111/j.1399-6576.1997.tb04733.x. [DOI] [PubMed] [Google Scholar]
- Zandbergen EG, de Haan RJ, Stoutenbeek CP, Koelman JH, Hijdra A. Systematic review of early prediction of poor outcome in anoxic–ischaemic coma. Lancet. 1998;352:1808–1812. doi: 10.1016/S0140-6736(98)04076-8. [DOI] [PubMed] [Google Scholar]
- Zeiner A, Holzer M, Sterz F, Behringer W, Schorkhuber W, Mullner M, Frass M, Siostrzonek P, Ratheiser K, Kaff A, Laggner AN. Mild resuscitative hypothermia to improve neurological outcome after cardiac arrest: a clinical feasibility trial. Stroke. 2000;31:86–94. doi: 10.1161/01.str.31.1.86. [DOI] [PubMed] [Google Scholar]