Abstract
Background
Nosocomial transmission has been described in extensively drug resistant tuberculosis (XDR-TB) and HIV co-infected patients in South Africa. However, little is known about rates of drug-resistant TB among healthcare workers (HCWs) in TB and HIV endemic settings.
Objective
To estimate rates of multi-drug resistant tuberculosis (MDR-TB) and XDR-TB hospitalizations among HCWs in KwaZulu-Natal (KZN), South Africa.
Design
Retrospective study of drug-resistant TB patients admitted for the initiation of drug-resistant TB therapy between 2003 and 2008.
Setting
A public TB referral hospital in KZN, South Africa.
Participants
HCWs admitted with MDR-TB (N=203) or XDR-TB (N=28) were compared with non-HCWs admitted with MDR-TB (N=3807) or XDR-TB (N=344).
Measurements
Hospital admission rates, hospital admission incidence rate ratios.
Results
Estimated incidence of MDR-TB hospitalization was 64.8/100,000 for HCWs versus 11.9/100,000 for non-HCWs (I.R.R. 5.56 95% C.I. 4.87–6.35). Estimated incidence of XDR-TB hospitalizations was 7.2/100,000 among HCWs versus 1.1/100,000 in non-HCWs (I.R.R. 6.69 95% C.I. 4.38–10.20). A higher percentage of HCWs than non-HCWs with MDR-TB or XDR-TB were female (78% vs. 47%, p<0.001) and fewer HCWs reported previous TB treatment (41% vs. 92%, p<0.001). Prevalence of HIV infection did not differ between HCW and non-HCW (55% vs. 57%, p=0.71), but a higher percentage of HIV infected HCWs were on antiretroviral medications (63% vs. 47%, p<0.001).
Conclusion
HCWs in this HIV-endemic area were substantially more likely to be hospitalized with either MDR-TB or XDR-TB compared to non-HCWs. The increased risk may be explained by occupational exposure and not by other risk factors, underlining the urgent need for TB infection control programs.
Primary Funding Source
No funding was received for this study
Background
Mycobacterium tuberculosis (MTB) is among the leading causes of global mortality by an infectious agent (1). It is well documented that health care workers (HCWs), including those in developing countries, are at increased risk for occupational exposure to tuberculosis (TB) (2–4). In developed countries, outbreaks of drug-resistant TB among HCWs have been described in hospitals, and settings where HIV/AIDS patients are treated (5,6). These multidrug-resistant TB (MDR-TB) outbreaks in industrialized countries were controlled after the implementation of comprehensive infection control policies. In South Africa, however, drug-resistant TB among HCWs has not been well characterized, even as endemic HIV has contributed to the nosocomial spread of drug-resistant TB (3,7).
KwaZulu-Natal (KZN) province, the epicenter of the South African HIV/AIDS epidemic (8), has high rates of MDR-TB and extensively drug-resistant TB (XDR-TB) (9). Recent studies indicate nosocomial transmission is a potentially important factor in the spread of both MDR- and XDR-TB in KZN province (7,10). A systematic review of occupational risk for drug susceptible TB infection and disease among HCWs in low and middle income countries found that TB rates among HCWs were substantially higher than the general population (4). Reports of an XDR-TB outbreak (9) and an XDR-TB treatment cohort in KZN (11) both included relatively high percentages of HCWs (5%). In KZN, those who work in health care settings face occupational risks due to the high incidence of TB (3), the increased susceptibility to TB due to HIV infection (12), and more recently the emergence of drug-resistant strains of MTB (13).
We undertook a retrospective chart review at a public TB referral hospital in KZN in order to identify cases of MDR-TB and XDR-TB among HCWs. Our study sought to address the following questions: 1) Was the incidence of hospitalization for MDR-TB and XDR-TB more common among HCWs than among non-HCWs in KZN? 2) Was increased risk of MDR-TB and XDR-TB among HCWs associated with HIV infection? 3) Were HCWs more likely than non-HCWs to have been previously treated for TB?
Methods
Study Design
Our study was a retrospective chart review of patients admitted for initiation of treatment for MDR-TB or XDR-TB to King George Vth Hospital (KGV) a public TB referral hospital in KZN. During the time of this study KGV was the only public hospital in the province authorized to initiate therapy for MDR-TB or XDR-TB and all therapy for MDR-TB and XDR-TB was initiated on an inpatient basis. All patients admitted to KGV with either MDR-TB or XDR-TB between January 1, 2003 and December 31, 2008 for initial therapy for drug-resistant TB were included in this study.
All participants had culture confirmed TB with MTB drug susceptibility testing. Drug susceptibility to isoniazid, rifampin, ethambutol, streptomycin, ethionamide, ofloxacin, and kanamycin and/or amikacin was determined using the modified proportional growth method on 7H11 agar according to standard techniques (14,15). MDR-TB was defined as resistance to isoniazid and rifampicin. XDR-TB was defined as resistance to isoniazid, rifampicin, any fluoroquinolone, and one of three injectable second-line anti-TB agents: capreomycin, kanamycin, and amikacin (16).
All MDR-TB and XDR-TB admissions during the time period were identified using a hospital-based database. This database has been maintained by the MDR-TB service at King George Hospital since 1998. The charts on all MDR-TB and XDR-TB health care workers were reviewed in detail. Data was cleaned by removing patients who did not meet the case definition for MDR-TB or XDR-TB based on drug susceptibility test results. For patients with repeat admissions to KGV, subsequent admissions were excluded. The admitting physician asked participants at the time of hospital admission to self-identify as health care workers as part of an occupational history. On the basis of this self-reported health care worker status MDR-TB and XDR-TB patients were classified as HCWs or non-HCWs. These participants served as the numerators for incident hospitalization rate calculations.
In order to estimate denominators for incident hospitalization rates, we assumed that the population base for HCWs was all HCWs in KZN, and for non-HCWs, the general adult population in KZN excluding HCWs, during the study period. Annual KZN provincial adult population was obtained from 2001 South African national census data and 2007 population estimates from a national community survey (17,18). Numbers of HCWs in the province were estimated using enrollment in registering bodies for professional HCWs and numbers of filled posts in the provincial public health system for non-professional HCWs for each study year (19). Analysis was restricted to participants’ age ≥ 20 years, since HCW is an adult occupation and comparison with non-adults might skew estimated rate ratios. The estimate of the proportion of the population age ≥ 20 years was taken from the KZN 2001 census data. Incidence rates were calculated by dividing the number of cases of TB in the group of interest by the population of that group. Further information on these estimates is included in an online supplement.
Statistical Analysis
HCWs with MDR-TB and XDR-TB were compared to non-HCWs using Chi-square for categorical variables and medians were compared for continuous variables, using the Wilcoxon–Mann–Whitney U test. Incidence rate ratios and confidence intervals were calculated using Poisson regression analysis. Statistical analysis was performed with SAS version 9.1 software (SAS Institute, Inc., Cary, North Carolina).
Role of the Funding Source
There was no funding source for this study.
The study protocol was approved by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal, the Research Ethics Committee of the University of Cape Town, and the Institutional Review Board of Boston University Medical Center.
Results
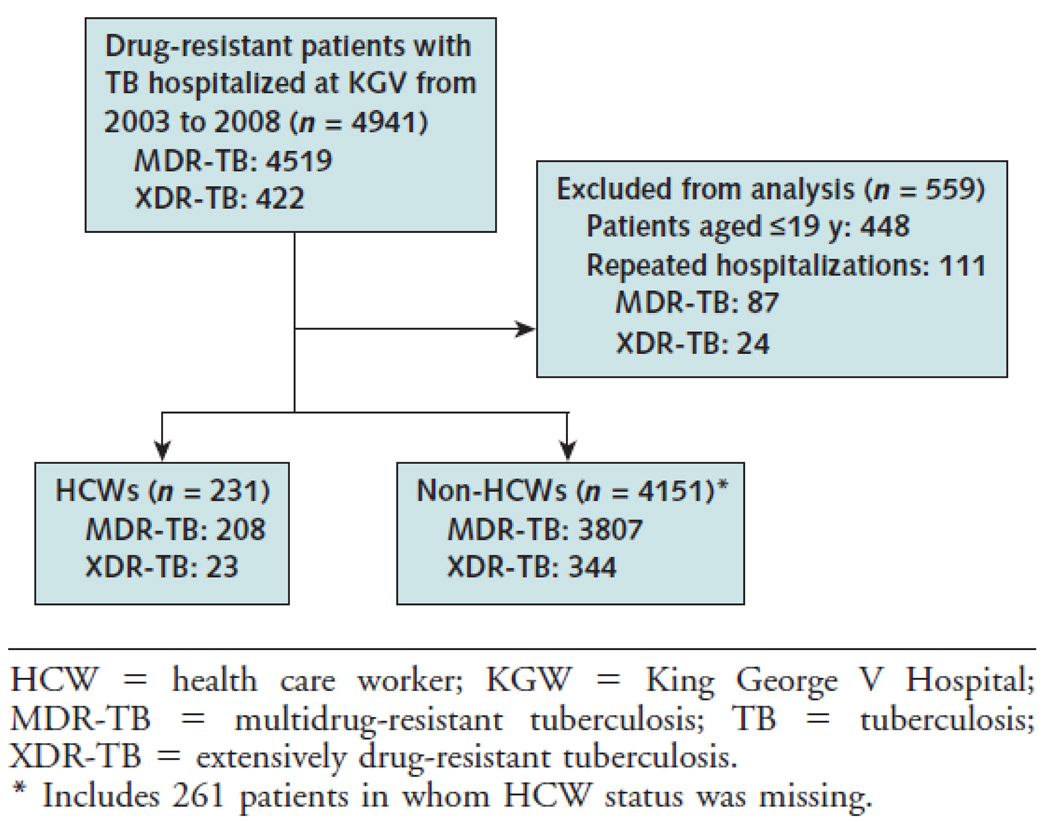
From 2003 to 2008, there were 4941 admissions for MDR-TB or XDR-TB to KGV. Approximately 5% of these self-identified as HCW. When patients less than age 20 (N=448) and repeat admissions were excluded (N=111), there were 231 HCW and 4151 adult members of the general population (non-HCWs) admitted to KGV for initiation of treatment for MDR-TB (N=4015) or XDR-TB (N=367). 261/4382 (6%) MDR-TB or XDR-TB patients were missing data on HCW status. These 261 patients were categorized as non-HCWs for purposes of analysis. Dates of treatment initiation for MDR-TB or XDR-TB were available for 67% (2,771/4,151) of non-HCWs and 98% (227/231) of HCWs. All patients for whom data was available were initiated on treatment during KGV admission.
During the study period 231 patients who self-identified as HCWs were admitted to KGV with either MDR-TB (N=208) or XDR-TB (N=23). HCWs with MDR-TB or XDR-TB were mostly female (78%), young (median age 35), with a high percentage HIV infected among those tested (67%). (Table 1) There were no significant differences between MDR-TB and XDR-TB infected HCWs except for the percentage who reported previous TB treatment (38% vs. 65%, p=0.01). HCWs were referred from a mix of public (138/231, 60%), private (31/231, 13%), and public TB specialist (56/231, 24%) hospitals. In some cases this facility was the workplace of the HCW; in other cases it was a facility where the HCW was seen prior to referral to KGV. Specific HCW occupation was not recorded in the medical record. During treatment 65% of MDR-TB infected HCWs converted their TB cultures to negative as compared to 13% of XDR-TB HCWs (p<0.001). While we do not have sufficient follow up data to characterize survival or cure rates, in hospital mortality was similar among MDR-TB and XDR-TB infected HCWs (32% vs. 30%).
Table 1.
Demographic and Treatment Characteristics of Health Care Workers and Non–Health Care Workers With MDR-TB and XDR-TB Hospitalized at King George V Hospital, 2003 to 2008*
| Characteristic | Health Care Workers |
Non-Health Care Workers |
||
|---|---|---|---|---|
| MDR-TB (n = 208) | XDR-TB (n = 23) | MDR-TB (n = 3807) | XDR-TB (n = 344) | |
| Admission year, n (%) | ||||
| 2003 | 24 (11.5) | 1 (4.4) | 416 (10.9) | 5 (1.5) |
| 2004 | 19 (9.1) | 0 (0.0) | 388 (10.2) | 5 (1 5) |
| 2005 | 22 (10.6) | 2 (8.7) | 482 (12.7) | 34 (9.9) |
| 2006 | 33 (15.9) | 3 (13.0) | 578 (15.2) | 72 (20.9) |
| 2007 | 67 (32.2) | 5 (21.7) | 958 (25.2) | 126 (36.6) |
| 2008 | 43 (20.7) | 12 (52.2) | 985 (25.9) | 102 (29.7) |
| Women, n (%) | 162 (77.9) | 18 (78.3) | 1754 (46.1) | 196 (57.0) |
| Men, n (%) | 46 (22.1) | 5 (21.7) | 2053 (53.9) | 148 (43.0) |
| Median age (IQR), y | 35 (31–42) | 38 (28–43) | 34 (28–42) | 35 (30–42) |
| HIV status, n (%) | ||||
| Positive | 113 (54. 3) | 15 (65.2) | 2112 (55.5) | 240 (69.8) |
| Negative | 58 (27.9) | 6 (26.1) | 845 (22.2) | 66 (19.2) |
| Unknown | 37 (17.8) | 2 (8.7) | 850 (22.3) | 38 (11.1) |
| HIV-positive and receiving antiretroviral drugs, n (%) | ||||
| Yes | 69 (61.1) | 12 (80.0) | 971 (46.0) | 135 (56.3) |
| No | 44 (38.9) | 3 (20.0) | 1141 (54.0) | 105 (43.8) |
| Previous treatment of tuberculosis, n (%) | ||||
| Yes | 80 (38.5) | 15 (65.2) | 3565 (93.6) | 336 (97.7) |
| No | 20 (9.6) | 0 (0.0) | 185 (4.9) | 7 (2.0) |
| Unknown | 108 (51.9) | 8 (34.8) | 57 (1.5) | 1 (0.3) |
| Median drugs resistant to tuberculosis at baseline (IQR), n | 3 (2–7) | 6 (5–8) | NA† | NA† |
| Median drugs in initial treatment regimen (IQR), n | 6 (3–7) | 6 (4–7) | NA† | NA† |
| Type of health care facility, n (%) | ||||
| Private | 30 (14.4) | 1 (4.3) | NA† | NA† |
| Public | 125 (60.1) | 13 (56.5) | – | – |
| Tuberculosis specialist | 53 (25 5) | 3 (13.0) | – | – |
| Unknown | – | 6 (26.1) | – | – |
| Type of tuberculosis, n (%)‡ | ||||
| Pulmonary | 97 (58.8) | 7 (63.6) | 1494 (52.9) | 141 (58.3) |
| Extrapulmonary | 62 (37.6) | 4 (36.4) | 1265 (43.8) | 101 (41.7) |
| Missing | 6 (3.6) | 0 (0.0) | 123 (4.4) | 0 (0.0) |
IQR = interquartile range; MDR-TB = multidrug-resistant tuberculosis; NA = not available; XDR-TB = extensively drug-resistant tuberculosis.
Percentage totals may sum to greater than 100% because of rounding.
These data were nor available for non–health care workers.
Data from 2003 to 2007.
Compared to the non-HCWs with either MDR-TB or XDR-TB, the population of HCWs with MDR-TB or XDR-TB was similar in terms of median age, HIV prevalence, TB type (pulmonary vs. extra-pulmonary), and proportions of XDR-TB cases. However, a higher percentage of HCWs were female (78% vs. 47% p<0.001). Although the percentages of HIV infected patients were similar (55% vs. 57%), more HCWs tested negative for HIV (28% vs. 22%, p=0.04), and a higher percentage of HIV infected HCWs were on antiretroviral medications at time of admission (63% vs. 47%, p<0.001) compared to non-HCWs. Significantly fewer HCWs admitted with drug-resistant TB reported previous treatment for TB (38% vs. 92%, p>0.001) compared to non-HCWs, but a substantial number of HCWs were missing data on previous treatment (116/231, 50%).
Using cases of HCWs and non-HCWs admitted to KGV as the numerator, and age-adjusted total numbers of HCWs and provincial population as the denominator, HCWs had an annual estimated incidence of hospital admissions for MDR-TB of 64.8/100,000, compared to an annual incidence of MDR-TB hospital admissions among the adult general population of 11.9/100,000 for non-HCWs (I.R.R. 5.56 95% C.I. 4.87–6.35). Average annual incidence of XDR-TB hospital admissions was 7.2/100,000 for HCW and 1.1/100,000 for the general KZN population (I.R.R. 6.69 95% C.I. 4.38–10.20).
Discussion
The most striking finding in our study is the high incidence of hospital admission for drug-resistant tuberculosis among HCWs in KZN, South Africa. HCWs were found to have a 5 to 6 fold increased rate of hospital admission with MDR-TB or XDR-TB compared with non-HCWs. This burden of disease is particularly concerning since HCWs are also front line care providers for TB and HIV patients in the province.
The higher rate of hospitalization for drug-resistant TB among HCWs in our study was not explained by higher percentages of HIV infection or previous TB treatment among HCWs compared to non-HCWs. It is likely that the increased risk for HCWs seen in our study is due to increased occupational exposure to drug-resistant MTB within health care settings. (20) The majority of HCWs with MDR-TB and XDR-TB were young, female, with a significant burden of HIV-infection, reflecting the epidemiology of the HIV epidemic in KZN (21). Female nurses are known to be a high-risk group for nosocomial TB (4) because of their close and prolonged risk of contact with TB patients, and it is plausible that many young females in our HCW cohort were nurses or nursing trainees.
In South Africa, XDR-TB/HIV co-infection was highlighted by the well-publicized 2006 outbreak at Tugela Ferry, KZN (10). The authors raised the concern that the XDR-TB outbreak was nosocomial, and therefore South African HCWs could be at high risk for primary acquisition of XDR-TB (20). Although we lack molecular epidemiologic data on TB isolates, we assume that many of the drug-resistant-TB cases among HCWs reported in our study represent primary drug-resistant TB. This assumption is supported by the fact that HCWs in our study were less likely to report previous TB treatment compared to non-HCWs, and is consistent with other studies of risk for drug-susceptible TB among HCWs in Africa (3,4,22). From an infection control standpoint, it is important to note that the majority of HCWs with MDR-TB and XDR-TB were referred from, and may have worked at, non-TB specialist facilities. This implies that it is not enough to focus infection control efforts at specialist TB hospitals in South Africa in order to prevent nosocomial transmission of drug-resistant TB.
Drug-resistant TB among health care workers in the developing world has been underreported. To date, there has been one published case report of a single health care worker infected with nosocomial XDR-TB in India (23). In reports from the U.S. in the 1990s there have been case series reporting nosocomial MDR-TB transmission to HCWs in hospital (5), a dental clinic (24) and HIV/AIDS hospital wards (6). The increased risk for XDR-TB and MDR-TB hospitalization we report is similar in magnitude to the estimated increased risk for drug-susceptible TB among HCWs in a hospital based study (25) and in a systematic review of TB risk for HCWs in low income countries (26). The elevated risk for XDR-TB among HCWs is a critical public health concern because of low cure rates, increased mortality, and potential nosocomial transmission to patients and other HCWs (25–28). The high proportion of HIV co-infection we observed in HCWs admitted for initiation of TB therapy is particularly alarming as in two recent studies approximately 40% of HIV and XDR-TB co-infected patients died within 12 months of therapy, and only 18 – 20% achieved culture conversion (11, 29).
Our study has several limitations many of which reflect challenges in collecting retrospective data in resource-constrained settings. First, stigma may have prevented persons from seeking or accepting HIV testing (30) therefore HIV status may be misclassified and/or underreported. Second, we lacked details regarding HCWs occupational classifications, and so could not identify degree or duration of exposure to drug-resistant TB. Third, HCWs with drug-resistant TB may have been more likely to obtain microbiologic diagnosis, seek specialized referral, or access treatment at KGV compared to non-HCWs leading to referral bias. For example, HCWs who were more likely to get ARVs may have been more likely to develop symptomatic TB (or immune reconstitution inflammatory syndrome) leading to increased TB diagnosis. On the other hand, HCWs are potentially less likely to seek care in a public health facility or self-identify as HCWs, leading to an underestimate of MDR-TB or XDR-TB hospitalization incidence. Fourth, the HCW workforce in KZN was estimated using professional registering bodies for professional workers and filled posts in the public health sector for non-professional workers (19). This undercounted private sector non-professional HCWs and could lead to an overestimate of hospitalization rates drug-resistant TB among HCWs. On the other hand, the estimate may inflate the numbers of professional HCWs since registering bodies include HCWs who have retired, emigrated, died or who no longer work in the health care sector leading to an underestimate of rates (19). Fifth, it is possible that patients may have come from other provinces in South Africa or other countries such as Leostho or Swaziland causing an overestimate in the numerator for population-based rate calculations. Given the large distances between treatment centers (several hundred miles) and known health-seeking patterns it is unlikely there was substantial misclassification of cases. Finally, to estimate the incidence of drug resistant TB the numerators of our reported rates are based on admission to a TB referral hospital rather than all diagnosed MDR-TB and XDR-TB in KZN. A recently published community-based study of MDR-TB and XDR-TB in KZN report nearly 50% 30 day mortality after collection of sputum and prior to admission for appropriate second line TB treatment. This suggests that our study of hospital admissions might underestimate the incidence of both MDR-TB and XDR-TB in KZN (31).
Future research directions include identifying specific practices and occupational classifications that place HCWs at risk for drug-resistant TB. We are developing studies to identify obstacles to the implementation of best practices in infection control in KZN health care institutions.
The results of this study have implications for health policy in countries with endemic HIV and drug-resistant tuberculosis. HCWs are likely at substantially higher risk for drug-resistant TB compared with the general population and this should be addressed by occupational risk reduction and infection control policy. A recently updated evidence-based guideline on TB infection control for HCWs in hospitals and congregate settings (26) published in 2009 by the WHO recommends prioritizing TB control on the national level, including developing national infection control policies in member states, implementing administrative and environmental controls, implementing ongoing surveillance for TB disease among HCWs, monitoring and evaluating infection control measures, and conducting operational research. Policies that prioritize reducing HCWs occupational risk for drug-resistant TB are urgently needed to reduce the numbers of MDR-TB and XDR-TB infected HCWs and nosocomial transmission of drug-resistant TB in South Africa.
Figure 1.
Study flow diagram
Table 2.
Average Annual Incidence of Hospitalization for Treatment of Drug-Resistant Tuberculosis Among Health Care Workers and General Population Patients Hospitalized at King George V Hospital for Drug-Resistant Tuberculosis, 2003 to 2008*
| Variable | Incidence in Health Care Workers (per 100 000) | Incidence in General Population Patients (per 100 000) | Hospital Admission Incidence Rate Ratio (95% CI)† |
|---|---|---|---|
| MDR or XDR-TB incidence | 71.9 | 12.9 | 5.56 (4.87–6.35) |
| MDR-TB incidence | 64.8 | 11.9 | 5.46 (4.75–6.28) |
| XDR-TB incidence | 7.2 | 1.1 | 6.69 (4.38–10.20) |
MDR-TB = multidrug-resistant tuberculosis; XDR-TB = extensively drug-resistant tuberculosis.
Restricted to persons aged ≥20 y.
P < 0.001 for all.
Acknowledgments
Grant support: Dr O’Donnell was supported by the National Institute of Health (NIH) T32 AI52074 (NIAID), and an American Thoracic Society Career Development Award. Drs. O’Donnell and Padayatchi were supported by the Centre for AIDS Programme of Research (CAPRISA). CAPRISA is funded by the NIH and the US Department of Health and Human Services (DHHS); grant# A1069469). Dr Padayatchi was also supported by Columbia University-Southern African Fogarty AIDS International Training and Research Programme (AITRP) funded by the Fogarty International Center, National Institutes of Health (grant # D43TW00231). Dr M Pai is supported by a New Investigator Award from the Canadian Institutes of Health Research (CIHR). These funding sources played no role in the study design or data analysis.
References
- 1.WHO. Global tuberculosis control - surveillance, planning, financing. Geneva Switzerland: World Health Organization; 2008. [Google Scholar]
- 2.Menzies D, Joshi R, Pai M. Risk of tuberculosis infection and disease associated with work in health care settings. Int J Tuberc Lung Dis. 2007;11(6):593–605. [PubMed] [Google Scholar]
- 3.Naidoo S, Jinabhai CC. TB in health care workers in KwaZulu-Natal, South Africa. Int J Tuberc Lung Dis. 2006;10(6):676–682. [PubMed] [Google Scholar]
- 4.Joshi R, Reingold AL, Menzies D, Pai M. Tuberculosis among health-care workers in low- and middle-income countries: a systematic review. PLoS Med. 2006;3(12):e494. doi: 10.1371/journal.pmed.0030494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearson ML, Jereb JA, Frieden TR, Crawford JT, Davis BJ, Dooley SW, Jarvis WR. Nosocomial transmission of multidrug-resistant Mycobacterium tuberculosis. A risk to patients and health care workers. Ann Intern Med. 1992;117(3):191–196. doi: 10.7326/0003-4819-117-3-191. [DOI] [PubMed] [Google Scholar]
- 6.Dooley SW, Villarino ME, Lawrence M, Salinas L, Amil S, Rullan JV, Jarvis WR, Bloch AB, Cauthen GM. Nosocomial transmission of tuberculosis in a hospital unit for HIV-infected patients. JAMA. 1992 May 20;267(19):2632–2634. [PubMed] [Google Scholar]
- 7.Basu S, Andrews JR, Poolman EM, et al. Prevention of nosocomial transmission of extensively drug-resistant tuberculosis in rural South African district hospitals: an epidemiological modelling study. Lancet. 2007;370(9597):1500–1507. doi: 10.1016/S0140-6736(07)61636-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdool-Karim Q, Abdool-Karim SS. The evolving HIV epidemic in South Africa. Int J Epidemiol. 2002;31(1):37–40. doi: 10.1093/ije/31.1.37. [DOI] [PubMed] [Google Scholar]
- 9.Buthelezi S. Situational analysis of TB drug resistance in KwaZulu-Natal Province, Republic of South Africa. Program and abstracts of the 2nd Global XDR-TB Task Force Meeting; April 9–10, 2008; Geneva, Switzerland. [Google Scholar]
- 10.Gandhi NR, Moll A, Sturm AW, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368(9547):1575–1580. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 11.O'Donnell MR, Padayatchi N, Master I, Osburn G, Horsburgh CR. Improved early results for patients with extensively drug-resistant tuberculosis and HIV in South Africa. Int J Tuberc Lung Dis. 2009;13(7):855–861. [PMC free article] [PubMed] [Google Scholar]
- 12.Wells CD, Cegielski JP, Nelson LJ, et al. HIV infection and multidrug-resistant tuberculosis: the perfect storm. J Infect Dis. 2007;196 Suppl 1:S86–S107. doi: 10.1086/518665. [DOI] [PubMed] [Google Scholar]
- 13.Raviglione MC, Smith IM. XDR tuberculosis--implications for global public health. N Engl J Med. 2007;356(7):656–659. doi: 10.1056/NEJMp068273. [DOI] [PubMed] [Google Scholar]
- 14.Reisner BS, Gatson AM, Woods GL. Evaluation of mycobacteria growth indicator tubes for susceptibility testing of Mycobacterium tuberculosis to isoniazid and rifampin. Diagn Microbiol Infect Dis. 1995;22(4):325–329. doi: 10.1016/0732-8893(95)00147-7. [DOI] [PubMed] [Google Scholar]
- 15.Rusch-Gerdes S, Pfyffer GE, Casal M, Chadwick M, Siddiqi S. Multicenter laboratory validation of the BACTEC MGIT 960 technique for testing susceptibilities of Mycobacterium tuberculosis to classical second-line drugs and newer antimicrobials. J Clin Microbiol. 2006;44(3):688–692. doi: 10.1128/JCM.44.3.688-692.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CDC. Notice to Readers: Revised Definition of Extensively Drug-Resistant Tuberculosis. MMWR. Morbidity and mortality weekly report. 2006;55(43):1176. [Google Scholar]
- 17. [Accessed Nov 15th, 2009];Statistics, South, Africa. South African National Popultaion Census 2001. 2001 http://www.statssa.gov.za/census01/html/default.asp.
- 18. [Accessed Nov 15th, 2009];Statistics, South, Africa. South African Community survey 2007. 2007 http://www.statssa.gov.za/community_new/content.asp.
- 19.Health, Systems, Trust. HST, editor. [Accessed Nov. 15th, 2009];South African Health Review 2007. 2007 http://www.hst.org.za/publications/711.
- 20.Basu S, Galvani AP. The transmission and control of XDR TB in South Africa: an operations research and mathematical modelling approach. Epidemiol Infect. 2008:1–14. doi: 10.1017/S0950268808000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdool Karim SS, Churchyard GJ, Abdool Karim Q, Lawn SD. HIV infection and tuberculosis in South Africa: an urgent need to escalate the public health response. Lancet. 2009;374(9693):921–933. doi: 10.1016/S0140-6736(09)60916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galgalo T, Dalal S, Cain KP, et al. Tuberculosis risk among staff of a large public hospital in Kenya. Int J Tuberc Lung Dis. 2008;12(8):949–954. [PubMed] [Google Scholar]
- 23.James P, Christopher DJ, Balamugesh T, Gupta R. Death of a health care worker with nosocomial extensively drug-resistant tuberculosis in India. Int J Tuberc Lung Dis. 2009;13(6):795–796. [PubMed] [Google Scholar]
- 24.Cleveland JL, Kent J, Gooch BF, Valway SE, Marianos DW, Butler WR, Onorato IM. Multidrug-resistant Mycobacterium tuberculosis in an HIV dental clinic. Infect Control Hosp Epidemiol. 1995;16(1):7–11. doi: 10.1086/646995. [DOI] [PubMed] [Google Scholar]
- 25.Sotgiu G, Arbore AS, Cojocariu V, Piana A, Ferrara G, Cirillo DM, Matteelli A, Castiglia P, Ditiu L, Spanevello A, Zellweger J-P, Mihaescu T, Migliori GB. High risk of tuberculosis in health care workers in Romania. Int J Tuberc Lung Dis. 2008;12(6):606–611. [PubMed] [Google Scholar]
- 26.WHO. WHO policy on TB infection control in health-care facilities, congregate settings and households. Geneva Switzerland: World Health Organization; 2009. [PubMed] [Google Scholar]
- 27.Askew GL, Finelli L, Hutton M, et al. Mycobacterium tuberculosis transmission from a pediatrician to patients. Pediatrics. 1997;100(1):19–23. doi: 10.1542/peds.100.1.19. [DOI] [PubMed] [Google Scholar]
- 28.Dimitrova B, Hutchings A, Atun R, et al. Increased risk of tuberculosis among health care workers in Samara Oblast, Russia: analysis of notification data. Int J Tuberc Lung Dis. 2005;9(1):43–48. [PubMed] [Google Scholar]
- 29.Dheda K, Shean K, Alimuddin Z, et al. Early Treatment Outcomes of Extensively Drug-Resistant Tuberculosis in South Africa are poor regardless of HIV status. Lancet. 2010;375(9728):1798–1807. doi: 10.1016/S0140-6736(10)60492-8. [DOI] [PubMed] [Google Scholar]
- 30.Zelnick J, O'Donnell M. The impact of the HIV/AIDS epidemic on hospital nurses in KwaZulu Natal, South Africa: nurses' perspectives and implications for health policy. J Public Health Policy. 2005;26(2):163–185. doi: 10.1057/palgrave.jphp.3200021. [DOI] [PubMed] [Google Scholar]
- 31.Gandhi N, Shah NS, Andrews JR, Vella V, Moll AP, Scott M, Weissman D, Marra C, Lalloo UG, Friedland GH. HIV coinfection in multidrug- and extensively drug-resistant tuberculosis results in high early mortality. Am J Respir Crit Care Med. 2010;181(1):80–86. doi: 10.1164/rccm.200907-0989OC. [DOI] [PubMed] [Google Scholar]