Abstract
Background
Sphenoid sinus is the most inaccessible paranasal sinus, enclosed within the sphenoid bone and intimately related to numerous vital neural and vascular structures. Anatomic variation of the sphenoid sinus is well documented and may complicate surgery in such a place.
Objective
To outline the surgically risky anatomic variants of the sphenoid sinus as well as the variable relationships between the sinus and related neurovascular structures, for the safe removal of intrasphenoid and pituitary lesions.
Materials and Methods
We undertook a prospective review of 300 paranasal sinus CT scans of Libyan patients; coronal CT scans were obtained by special parameter techniques. We assessed pneumatization of pterygoid process (PP), anterior clinoid process (ACP), and greater wing of sphenoid (GWS); we also examined protrusion and dehiscence of internal carotid artery (ICA), optic nerve (ON), maxillary nerve (MN), and vidian nerve (VN) into the sphenoid sinus cavity.
Results
Pneumatization of PP, ACP, and GWS were seen in 87 (29%), 46 (15.3%), and 60 patients (20%), respectively. Protrusion of ICA, ON, MN, and VN were noticed in 123 (41%), 107 (35.6%), 73 (24.3%), and 81 patients (27%), respectively; dehiscence of these structures was encountered in 90 (30%), 92 (30.6%), 39 (13%), and 111 patients (37%), respectively. Statistically, there was a highly significant association between ACP pneumatization and ICA protrusion, ACP pneumatization and ON protrusion, PP pneumatization and VN protrusion; and GWS pneumatization and MN protrusion (p-value < 0.001).
Conclusion
The sphenoid sinus is highly variable; this variability necessitates a comprehensive understanding of the regional sphenoid sinus anatomy by a detailed CT scan sinus examination before surgery in and around the sinus. This study indicates the possibility of a racial anatomical variation of the sphenoid sinus in the Libyan population.
Keywords: Sphenoid sinus, variation, internal carotid artery, optic nerve, maxillary nerve, vidian nerve, CT scan, Libyan
Introduction
The sphenoid sinus is deeply seated in the skull and is the most inaccessible paranasal sinus [1–4]. It is surrounded by vital structures, such as the internal carotid artery, optic nerve and cavernous sinus.
The variability in the anatomy of the sphenoid sinus is well documented [5, 6, 7]. Injury to the internal carotid artery or optic nerve is a serious complication of transsphenoidal surgery [8, 9]. A comprehensive knowledge of the variable regional anatomy of the sphenoid sinus will undoubtedly reduce the surgical complications associated with transsphenoidal and functional endoscopic sinus surgery [1–4]. The sphenoid sinuses are irregular cavities, with pneumatization ranging from absent to extensive [10]. Pneumatization occasionally extends into the vomer, palatine bone, ethmoid bones, occipital bone, anterior clinoid processes, the lesser wings, the greater wings, the pterygoid process and plates, posterior clinoid processes, and the clivus [11, 12]. According to the extent of sinus pneumatization, the bone covering the carotid arteries, optic nerves, maxillary nerves, and vidian nerves can be thin or even absent, making these structures susceptible to iatrogenic injury [13].
Computerized tomography is the most precise imaging technique to demonstrate paranasal sinuses [14, 15, 16]. CT screening of paranasal sinuses has the advantages of showing bony details (using wide window settings) and good soft tissue outlines (using narrow window setting). Axial and coronal views may be useful for delineating the anatomical landmarks of the sinonasal cavity, but coronal CT scan provides most of the information required for an endoscopic clearance [17]. Its advantage over axial CT scanning is that it shows progressively deeper structures as they are encountered by the surgeon during the operation (e.g., sphenoid sinus, in an antero-posterior direction).
In spite of the complex anatomy and important surgical relationships of the sphenoid sinus, to our knowledge only a few relevant studies have been reported from North Africa. The aims of the study were to demonstrate the prevalence, clinical significance, and interrelationship of the anatomic variations of sphenoid sinus and related structures, and to determine the ability of coronal CT scan to identify these variations.
Materials and methods
This prospective study comprised 300 paranasal computerized tomography scans of Libyan patients attending Al-Jalla Trauma Hospital, Benghazi, Libya, between May 2006 and May 2007. All the patients underwent a complete medical history and head and neck physical examination. We excluded patients with prior sinus surgery, sinonasal tumors, nasal polyposis, severe cervical arthropathy, or head or neck injury. Patients younger than 16 years were also excluded because, according to Gray, the extension of the nasal cavity into the body of the sphenoid bone to form the sphenoid sinus is present before birth but does not reach its full extension until adolescence [18]. There were equal numbers of male and female patients, and age ranged between 16 and 82 years (mean 34.6 year). For the tomographic studies, systemic studies of the sphenoid sinus region were performed in coronal scans on all the patients. Choosing to scan only in the coronal plan reduces the radiation dose to the patient. Each patient was positioned prone with the head hyper-extended on the scanner bed. The scanner gantry was angled perpendicularly to the hard palate. Contiguous slice CT technique was used with 4-mm section thickness from anterior frontal sinus to anterior sphenoid sinus. To obtain proper evaluation of the neighboring structures and their relation to the sphenoid sinus, 2-mm contiguous slice thickness was used from anterior to posterior sphenoid sinus. For visualization of the complex anatomy of this region, imaging is best centered to the nasal cavity and paranasal sinuses. In all the patients, the existence of the following variants was investigated: pneumatization of pterygoid process (PP), anterior clinoid process (ACP), and greater wing of sphenoid (GWS, i.e. floor of middle cranial fossa), protrusion of internal carotid artery (ICA), optic nerve (ON), maxillary nerve (MN), and vidian nerve (VN), and dehiscence of the walls of ICA, ON, MN, and VN. Dehiscence is defined as absence of visible bone density separating the sinus from the course of the concerned structure. Whenever a clear decision between “very thin bony wall” and “total dehiscence” was not feasible, the results were accepted as dehiscence. Protrusion of ICA and ON was determined by finding any degree of protrusion of the structure into the sinus cavity. We are unaware of any published criteria for protrusion of MN and VN, and presence of air density around these structures is accepted as a clue for the protrusion of MN and VN, at least in a section of coronal investigation. According to our own criteria, PP pneumatization is recognized if it extends beyond a horizontal plane crossing the vidian canal. Likewise, we define GWS pneumatization when it extends beyond a vertical plane crossing the maxillary canal.
The data were analyzed statistically by using Chi-square test and contingency coefficient C. Statistical analysis used Chi-square test to evaluate the association between the anatomic variants (p-values less than 0.01 were accepted statistically significant), and contingency coefficient C to assess the degree of association between the two variables.
Table 1.
Prevalence of anatomic variants of sphenoid sinus and related structures in 300 Libyan patients
| Bilateral | Right side | Left side | Total | |
|---|---|---|---|---|
| Pneumatization | ||||
| ACP | 19 (6.3%) | 17 (5.6%) | 10 (3.3%) | 46 (15.3%) |
| GWS | 26 (8.6%) | 15 (5%) | 19 (6.3%) | 60 (20%) |
| PP | 41 (13.6) | 20 (6.6) | 26 (8.6%) | 87 (29%) |
| Protrusion | ||||
| ICA | 68 (22.6%) | 34 (11.3%) | 21 (7%) | 123 (41%) |
| ON | 61 (20.3%) | 21 (7%) | 25 (8.3%) | 107 (35.6%) |
| MN | 25 (8.3%) | 26 (8.6%) | 22 (7.3%) | 73 (24.3%) |
| VN | 40 (13.3%) | 17 (5.6%) | 24 (8%) | 81 (27%) |
| Dehiscence | ||||
| ICA | 31 (10.3%) | 43 (14.3%) | 16 (5.3%) | 90 (30%) |
| ON | 30 (10%) | 37 (12.3%) | 25 (8.3%) | 92 (30.6%) |
| MN | 10 (3.3%) | 15 (5%) | 14 (4.6%) | 39 (13%) |
| VN | 46 (15.3%) | 31 (10.3%) | 34 (11.3%) | 111 (37%) |
ACP, anterior clinoid process; GWS, greater wing of sphenoid; PP, pterygoid process; ICA, internal carotid artery; ON, optic nerve; MN, maxillary nerve; VN, vidian nerve.
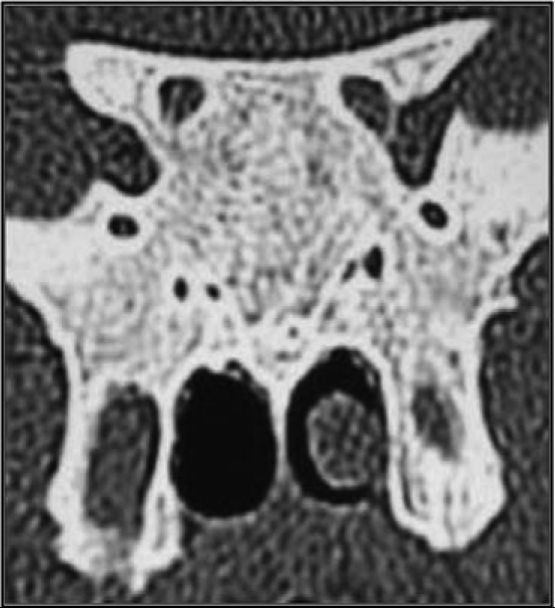
A total of 300 patients fulfilled the study entry criteria. Two incidentally discovered patients with sinus mucocele and sphenoid sinus agenesis were rejected from our series (Figure 1). All patients were Libyan. Ages ranged from 16 to 82 years. There was equal number of men and women. Anatomic variations of sphenoid sinuses were determined in coronal screening.
Fig. 1.
Coronal CT image showing sphenoid sinus agenesis (extremely rare).
Pneumatization of the pterygoid process
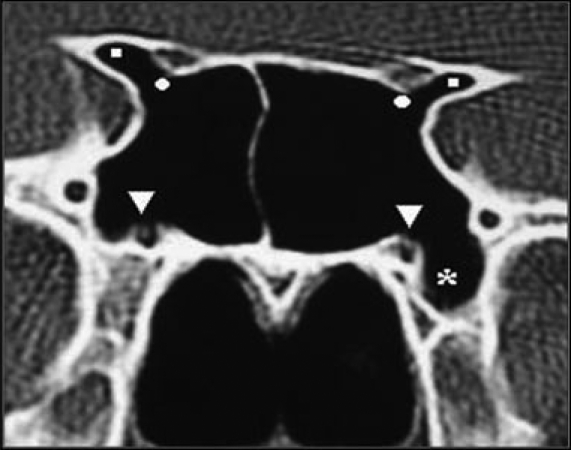
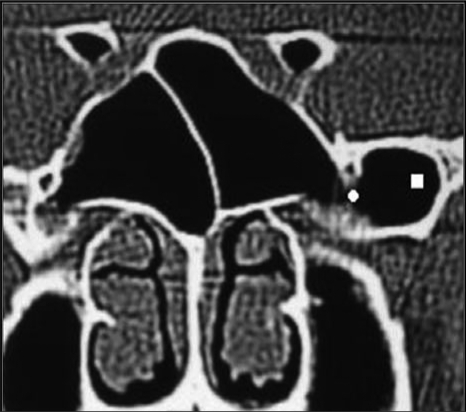
Pneumatization of the pterygoid process was found in 87 patients (29%) of whom 41 (13.7%), 20 (6.6%), and 26 (6.7%) were bilateral, right sided, and left sided, respectively (Figures 3, 5).
Fig. 3.
Coronal CT image showing pneumatization of pterygoid processes (squares), and protrusion of vidian canals (circles).
Fig. 5.
Coronal CT image showing pneumatization of anterior clinoid processes (squares), protrusion and dehiscence of optic nerves (circles), protrusion and dehiscence of vidian nerves (arrowheads), and pneumatization of left pterygoid process (asterisk).
Pneumatization of the anterior clinoid process
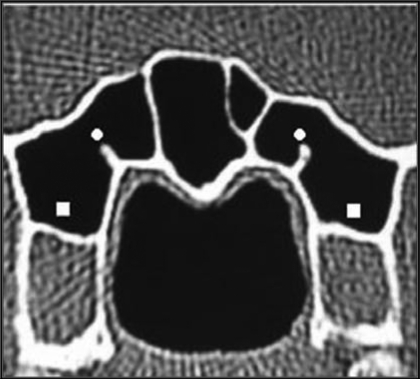
Pneumatization of the anterior clinoid process was encountered in 46 patients (15.3%). It was bilateral in 19 (6.3%), on the right in 17 (5.7%) and on the left in 10 (3.3%) (Figures 2 and 5).
Fig. 2.
Coronal CT image showing dehiscence of internal carotid arteries (asterisks), pneumatization of left anterior clinoid process (arrowhead), and protrusion and dehiscence of right vidian canal (square).
Pneumatization of the greater wing of sphenoid bone
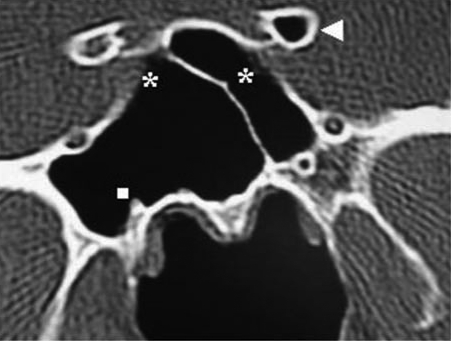
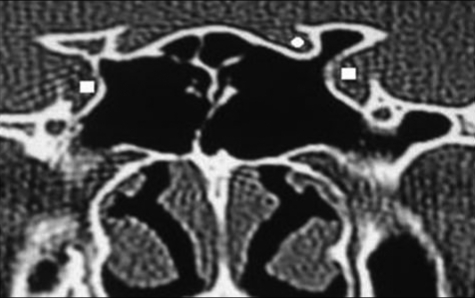
Pneumatization of the greater wing of sphenoid was encountered in 60 patients (20%), of whom 26 (8.6%) were bilateral, 15 (5%) were on the right side, and 19 (6.3%) were on the left side (Figure 6).
Figure 6.
Coronal CT image showing pneumatization of left greater wing of sphenoid (square) associated with protrusion and dehiscence of left maxillary nerve (circle).
Internal carotid artery
A protruding internal carotid artery into the sphenoid sinus was found in 123 patients (41%), of whom 68 (22.7%) were bilateral, 34 (11.3%) were right sided, and 21 (7%) were left sided. Dehiscence of the bony wall on the internal carotid artery was seen in 90 patients (30%) of whom 31 (10.3%) were bilateral, 43 (14.3%) were on the right, and 16 (5.3%) were on the left (Figures 2 and 4).
Fig. 4.
Coronal CT image showing protrusion of internal carotid arteries (squares), and protrusion of left optic nerve (circle).
Optic nerve
The protrusion of the optic nerve was present in 107 patients (35.7%). Protrusions were bilateral, right sided, and left sided in 61 (20.3%), 21 (7%), and 25 (8.3%) of patients, respectively. Dehiscence of the bony wall of the optic canal was observed in 92 (30.7%) patients, of whom 30 (10%) were bilateral, 37 (12.3%) were right sided, and 25 (8.3%) were left sided (Figures 4 and 5).
Maxillary nerve
The protrusion of the maxillary canal (foramen rotundum) was encountered in 73 (24.3%) patients, of whom 25 (8.3%) were bilateral, 26 (8.6%) were on the right side, and 22 (7.3%) were on the left side. Dehiscence of the bony wall of the maxillary canal was seen in 39 (13%) patients, of whom 10 (3.3%) were bilateral, 15 (5%) were right sided, and 14 (4.7%) were left sided (Figure 6).
Vidian nerve
A protruding vidian canal into the sinus cavity was present in 81 (27%) patients of whom 40 (13.3%) were bilateral, 17 (5.7%) were on the right side, and 24 (8%) were on the left side. Dehiscence of the bony wall of the vidian canal was identified in 111 (37%) patients, of whom 46 (15.3%), 31 (10.3%), and 34 (11.3%) were bilateral, right sided, and left sided, respectively (Figures 2, 3, 5)
Relationship between pneumatization of ACP and ICA protrusion
On the right side, ICA protrusion and pneumatization of the ACP were both present in 29 (9.7%) patients and on the left side in 27 (9%). Chi-square test indicated significant association between ACP pneumatization and ICA protrusion (p < 0.001) and contingency coefficient C showed highly significant association between the two variants.
Relationship between pneumatization of ACP and ON protrusion
Concomitant presence of a pneumatized ACP and a protruding ON was encountered on the right and left sides in 27 (9%) and 26 (8.7%) patients, respectively. Chi-square test indicated significant association between ACP pneumatization and ON protrusion (p < 0.001), and contingency coefficient C showed highly significant association.
Relationship between pneumatization of GWS and MN protrusion
Concomitant presence of a pneumatized GWS and a protruding maxillary nerve was found on the right in 35 (11.7%) and on the left in 27 (9%). Chi-square test indicated significant association between GWS pneumatization and MN protrusion (p < 0.001), and contingency coefficient C showed highly significant association.
Relationship between pneumatization of PP and VN protrusion
Concomitant presence of a pneumatized PP and a protruding VN was seen on the right side in 44 (14.7%) patients and on the left side in 55 (18.3%) patients. Chi-square analysis indicated significant association between PP pneumatization and VN protrusion (p < 0.001), and contingency coefficient C showed highly significant association.
Discussion
Pneumatization of the pterygoid process
Pterygoid process pneumatization is recognized if it extends beyond a horizontal plane crossing the vidian canal. We found a pneumatized pterygoid process in 29% of the patients. Without explaining their criterion, Bolger et al. identified pterygoid process pneumatization in 43.6% of patients [19]. This wide range of prevalence may be attributed to the use of different criteria. It is noteworthy that review of CT scan images for the presence of pterygoid process pneumatization is much more sensitive than cadaveric dissection.
Pterygoid process pneumatization, when present, is an important pathway for access to the central skull base. For instance, extended transnasal endoscopic approaches may reach the pterygoid process through the medial part of the posterior maxillary wall [20]. These techniques may provide a route for endoscopic repair of cerebrospinal fluid leaks and endoscopic biopsy of skull base lesions. Such information may be important in preoperative planning for skull base surgery. Pneumatization of pterygoid process thins the bony floor of the scaphoid fossa to as little as 0.2 mm, producing an intimate relation between the sinus and the auditory tube [21]. Sirikci et al., reported pneumatization of the pterygoid process in 29.3% [22]. They recognized pterygoid process pneumatization if it extended beyond a plane tangential to the most inferolateral aspect of the maxillary division of the trigeminal and vidian nerves. Despite the different criterion used to define pneumatization of the pterygoid process, the results reported by Sirikci et al. and those reported here are almost the same. Our statistical analysis revealed a significant relationship between the ipsilateral pneumatized pterygoid process and vidian canal protrusion.
Anterior clinoid process
The prevalence of anterior clinoid process pneumatization has been well documented in the literature. Bolger et al. found anterior clinoid process pneumatization in 13% of 202 paranasal sinus CT scans [19]. These authors reviewed coronal sinus CT scans with a slice thickness of 3 mm. In a review of 150 paranasal sinus CT scans, De Lano et al. found anterior clinoid process pneumatization in only 13 of 300 sides (4%) (7). Of note, these CTs included only coronal images obtained at a slice thickness of 4 mm. Sirikci et al. found anterior clinoid process pneumatization in 29.3% of 92 paranasal sinus coronal CT scans studied at 2.5 mm slice thickness [22]. Birsen et al. encountered pneumatization of anterior clinoid process in 24.1% of 260 patients, for whom coronal sinonasal CT cuts were obtained at 3 mm slice thickness [23]. Our study reports anterior clinoid process pneumatization in 15.3%. Obviously, the reported prevalence rates vary considerably. This may reflect differences among the studied populations; however, it is more likely that thin CT scan sections are substantially more precise. Thus, the previous reports of prevalence of anterior clinoid process pneumatization based on thick-cut CT scan review may underestimate the prevalence of this anatomic variant. Pneumatization of anterior clinoid process forms the opticocarotid recess, i.e. the small space on the lateral wall of the sphenoid sinus, between the optic canal, superiorly, and the carotid prominence, inferiorly. The opticocarotid recess is supposed to concur with ipsilateral optic nerve and/or internal carotid artery protrusion into the sphenoid sinus. That was what we observed in our series as well. Two previous studies suggested that there is a statistically significant relationship between the pneumatization of anterior clinoid process and the protrusion of optic nerve, which is consistent with our results [22, 23]. As far as we know, the association between the pneumatization of anterior clinoid process and the protrusion of internal carotid artery has not been reported. Nevertheless, in the case of hypertrophic mucosa or polyps of the sphenoid sinus, optic nerve and internal carotid artery protrusion may not be clearly recognized by a routine sinus CT scan. Radiological experience reveals that carefully tracing the course of the optic nerve and internal carotid artery seems to underestimate the presence of protrusion. Therefore, as a rule, ipsilateral anterior clinoid process pneumatization is a good indicator of optic nerve and internal carotid artery protrusion.
Greater wing of sphenoid
Pneumatization of greater wing of sphenoid, i.e. floor of middle cranial fossa, is inadequately reviewed in the literature. John Earwaker discovered pneumatization of greater wing of sphenoid in 10.7% of patients [24]. We observed pneumatization of greater wing of sphenoid in 20%. We defined pneumatization of greater wing of sphenoid as extension beyond a vertical line crossing foramen rotundum. Pneumatization of the floor of the middle cranial fossa in the presence of arachnoid granulations along the inner surface of the greater wing of the sphenoid, where this appendage forms the anterior wall of the middle cranial fossa. These granulations form “pit-holes” on the floor of the middle cranial fossa, and although they are not pathologic in and of themselves, enlargement of these pits has been casually implicated in the development of non-traumatic cerebrospinal leaks [25].
Internal carotid artery
We found protrusion of internal carotid artery into the sphenoid in 41% of patients, and dehiscence of the artery in 30%. These obviously were high rates and most likely explained by our criteria for defining protrusion and dehiscence. Fuji et al. studied 25 cadaver sphenoid bones and found 8% of carotid arteries dehiscent of bone in the lateral sphenoid [26]. Kennedy et al. found dehiscence on the bony wall of the internal carotid artery in 25% of patients [27]. Occasionally, they found the artery, only with a mucoperiosteal covering, coursing through the sphenoid sinus. Sareen et al. studied sagittal sections of 20 dried skulls and found dehiscence of the carotid artery in 5% [28]. Sirikci et al. reported protrusion of internal carotid artery in 26.1% of patients and dehiscence of the artery in 23% [22]. Birsen et al. encountered protrusion of internal carotid artery in 30.3% and dehiscence in 5.3% of patients [23]. Both Sirikci and Birsen recognized protrusion of internal carotid artery or optic nerve into the sphenoid sinus as the presence of more than half the circumference of the concerned structures into the sinus cavity. In our present study, presence of the circumference into the sinus cavity, at any degree, was enough to define protrusion. Without explaining their criteria, Sethi et al. identified carotid protrusion in 93% [29] and Elwany et al. observed protrusion of carotid artery in 29% and dehiscence in 4.8% of patients [30]. This wide range of prevalence might indicate that the relationship of internal carotid artery to the sphenoid sinus may be different in the Libyan population as an ethnic group. If the surgeon is unaware of dehiscence or protrusion of the artery, even fatal hemorrhage may happen, because is hardly possible to control bleeding from an injured internal carotid artery within the sphenoid sinus. Even so, neurological sequelae are inevitable. Sphenoid sinus Infection may also make a dehiscent or protruded internal carotid artery vulnerable to damage [22].
Optic nerve
In our study, the protrusion of the optic nerve was found in 35.6%, and dehiscence of the optic nerve was observed in 30.6% of the patients. Likewise, this high prevalence may be explained by the criteria we used to define protrusion and dehiscence. However, previous studies reported a wide range of protrusion rates of 8 to 70% [31, 32]. Fuji et al. found that 4% of optic nerves were dehiscent of bone in the lateral sphenoid [26]. They also reported that most optic nerves were covered by thin bone, measuring 0.5 mm or less in 78% of cases. They attributed the difference in the prevalence of anatomic variations to ethnic background. In the case of protrusion or dehiscence, optic nerve injury can occur due either to surgical trauma or as a complication of sinus disease. The risk of blindness is high if the surgeon damages the nerve within the sinus [33]. Moreover, visual deficits may result from a sphenoid sinus infection or from a mucocele compressing the optic canal or nerve. Compression of the optic nerve can cause ischemia and venous congestion of the nerve. Furthermore, the optic canal is the place where optic nerve is least nourished, which makes it very susceptible to injury [34]. Protrusion of the optic nerve and/or internal carotid artery may coexist with ipsilateral pneumatization of the anterior clinoid process or with migration of the posterior ethmoidal air cells posteriorly into the upper sphenoid (spheno-ethmoidal or Onodi cells) [22]. Our patients were not examined for the presence of spheno-ethmoidal cells.
Maxillary nerve
We observed maxillary nerve protrusion in 24.3% of the patients and dehiscence of the nerve in 13%. Birsen et al. encountered maxillary nerve protrusion in 30.3% and dehiscence in 3.5% [23]. Sareen et al. in their anatomical study found neither of the sinuses with protrusion nor dehiscence of maxillary nerve [28]. The discrepancy between these prevalence rates may be due to different techniques or else it may reflect ethnic differences between the populations. Our study seems to be the first one referring to statistically significant association between them. In endoscopic sphenoid surgery, a protruded or dehiscent maxillary nerve is liable to iatrogenic injury. Furthermore, neuritis of a dehiscent maxillary nerve may result from sphenoid sinusitis and present as trigeminal neuralgia [35].
Vidian nerve
Lang and Keller reported that the vidian canal was protruded into the sinus cavity in 18% [36]. Pandolfo et al. emphasized that there is a variable relationship between the vidian canal and the sphenoid sinus [37]. They also suggested that the vidian nerve can cause a clinical syndrome characterized by pain referred deeply in the nasal cavity (vidian neuralgia). In our study, protrusion of the vidian canal was found in 27% and we suspect that position of the canal within the sinus cavity can favor the involvement of the vidian nerve in sinus diseases. Due to the privations and inadequate information from radiological literatures on the radiographic anatomy of vidian canal, this study may serve as an appendix to radiological authorities in the description of anatomic relationship of the vidian canal to the sphenoid sinus cavity, as well as improving the results and decreasing the complication of the endoscopic transsphenoidal and vidian neurectomy surgery.
Conclusions
The anatomical variations of the sphenoid sinus in a Libyan population were remarkably common. Prevalence of Protrusion and dehiscence of the internal carotid artery and optic nerve were high. The internal carotid artery and optic nerve may not be well protected and thus could be damaged during endoscopic sphenoid surgery. Protrusion of the internal carotid artery andor optic nerve was strongly associated with ipsilateral pneumatization of the anterior clinoid process. Protrusion and dehiscence of the maxillary nerve were less common. Protrusion of the vidian canal into the sinus cavity was strongly associated with pneumatization of the pterygoid process, on the same side. Coronal CT screening should be used in the pre-surgical evaluation of patients under consideration of endoscopic sphenoid sinus surgery to minimize perioperative neural and vascular injury.
Acknowledgement
The authors are grateful to Dr. Yousef El-Gomatti for statistical analysis and to Mrs. Fatma El-Sughaer, CT scan technician, for skillful technical assistance.
References
- 1.Janskowski R, Auque J, Simon C. Endoscopic pituitary tumor surgery. Laryngoscope. 1992;102:198–203. doi: 10.1288/00005537-199202000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Stankiewicz JA. Complications of endoscopic nasal surgery. Occurrence and treatment. Am J Rhinol. 1987;1:45–49. [Google Scholar]
- 3.Buus DR, Tse DT, Farris BK. Ophthalmic complications of sinus surgery. Ophthalmology. 1990;97:612–621. doi: 10.1016/s0161-6420(90)32535-6. [DOI] [PubMed] [Google Scholar]
- 4.Cappabianca P, Cavallo LM, Coloa A, et al. Endoscopic endonasal transsphenoidal approach: outcome analysis of 100 consecutive procedures. Minim Invas Neurosurg. 2002;45:193–200. doi: 10.1055/s-2002-36197. [DOI] [PubMed] [Google Scholar]
- 5.Cheung DK, Attia E, Kirkpatrick DA, et al. An anatomic and CT scan study of the lateral wall of the sphenoid sinus as related to the transnasal transethmoid endoscopic approach. J Otolaryngol. 1993;22:63–68. [PubMed] [Google Scholar]
- 6.Mafee MF, Chow JM, Meyers R. Functional endoscopic sinus surgery: anatomy, CT screening, indications, and complications. Am J Roentgenol. 1993;160:735–744. doi: 10.2214/ajr.160.4.8456654. [DOI] [PubMed] [Google Scholar]
- 7.Delano MC, Fun FY, Zinrich SJ. Relationship of the optic nerve to the posterior paranasal sinuses: a CT anatomic study. Am J Neuroradiol. 1996;17:669–675. [PMC free article] [PubMed] [Google Scholar]
- 8.Ahuja A, Guterman LR, Hopkins LN. Carotid cavernous fistula and false aneurysm of the cavernous carotid artery: complications of transsphenoidal surgery. Neurosurgery. 1992;31(4):774–779. doi: 10.1227/00006123-199210000-00025. [DOI] [PubMed] [Google Scholar]
- 9.Fukushima T, Maroon JC. Repair of carotid artery perforations during transsphenoidal surgery. Surg Neurol. 1998;50:174–177. doi: 10.1016/s0090-3019(96)00416-8. [DOI] [PubMed] [Google Scholar]
- 10.Kinnman J. Surgical aspects of the anatomy of the sphenoidal sinuses and the sella turcica. J Anat. 1977;124:541–53. [PMC free article] [PubMed] [Google Scholar]
- 11.Yune H, Holden R, Smith J. Normal variations and lesions of the sphenoid sinus. Am J Roentgenology. 1975;124:129–38. doi: 10.2214/ajr.124.1.129. [DOI] [PubMed] [Google Scholar]
- 12.Fujioka M, Yung L. The sphenoid sinuses: radiographic patterns of normal development and abnormal findings in infants and children. Radiology. 1978;129:133–139. doi: 10.1148/129.1.133. [DOI] [PubMed] [Google Scholar]
- 13.Liu S, Wang Z, Zhou B. Related structures of the lateral sphenoid wall anatomy studies in CT and MRI. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2002;16:407–409. [PubMed] [Google Scholar]
- 14.Kainz J, Stammberger H. Danger areas of the posterior rhinobasis. An endoscopic and anatomical-surgical study. Acta otolaryngol. 1992;122:852–861. doi: 10.3109/00016489209137484. [DOI] [PubMed] [Google Scholar]
- 15.Arsalan H, Aydinlioglu A, Bozkurt M, et al. Anatomic variations of the paranasal sinuses: CT examination for endoscopic sinus surgery. Auris Nasus Larynx. 1997;26:39–48. doi: 10.1016/s0385-8146(98)00024-8. [DOI] [PubMed] [Google Scholar]
- 16.Zinreich J. Functional anatomy and computed tomography imaging of the paranasal sinuses. Am J Med Sci. 1998;316:2–1. doi: 10.1097/00000441-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Kaluskar SK, Patil NP, Sharkey AN. The Role of CT Scan in Functional Endoscopic Sinus Surgery. Rhinology. 1993;31(2):49–52. [PubMed] [Google Scholar]
- 18.Gray H. Gray's anatomy. 37th edn. Edinburgh: Churchill Livingstone; 1989. pp. 376–377. [Google Scholar]
- 19.Bolger WE, Butzin CA, Parsons DS. Paranasal sinus bony anatomic variations and mucosal abnormalities: CT analysis for endoscopic sinus surgery. Laryngoscope. 1991;101:56–64. doi: 10.1288/00005537-199101000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Lane AP, Bolger WE. Endoscopic transmaxillary biopsy of pterygopalatine space masses: A preliminary report. Am J Rhinol. 2002;16:109–112. [PubMed] [Google Scholar]
- 21.Vidic B. The postnasal development of the sphenoidal sinus and its spread into the dorsum sellae and posterior clinoid processes. Am J Roentgenol Radium Ther Nucl Med. 1968;104:177–183. doi: 10.2214/ajr.104.1.177. [DOI] [PubMed] [Google Scholar]
- 22.Sirikci A, Bayazit YA, Bayram M, et al. Variations of sphenoid sinus and related structures. Eur Radiol. 2000;10:844–848. doi: 10.1007/s003300051016. [DOI] [PubMed] [Google Scholar]
- 23.Birsen U, Gulsah B, Yasemin K, et al. Risky anatomic variations of sphenoid sinus for surgery. Surg Radil Anat. 2006;28:195–201. doi: 10.1007/s00276-005-0073-9. [DOI] [PubMed] [Google Scholar]
- 24.Earwaker J. Anatomic variants in sinonasal CT. Radiographics. 1993;13:381–415. doi: 10.1148/radiographics.13.2.8460226. [DOI] [PubMed] [Google Scholar]
- 25.Lauri C, Carter LC, Pfaffenbach A, et al. Hyperaeration of the sphenoid sinus: cause for concern? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:506–510. doi: 10.1016/s1079-2104(99)70071-5. [DOI] [PubMed] [Google Scholar]
- 26.Fuji K, Chambers A, Rhoton J. Neurosurgical relationships of the sphenoid sinus: A microsurgical study. J. Neurosurg. 1979;50:31–39. doi: 10.3171/jns.1979.50.1.0031. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy D, Zinrich H, Hassab M. The internal carotid artery as it relates to endoscopic sheno-ethmoidectomy. Am J Rhinol. 1990;4:7–12. [Google Scholar]
- 28.Sareen D, Agarwal AK, Kaul JM, et al. Study of sphenoid sinus anatomy in relation to endoscopic surgery. Int. J. Morphol. 2005;23(3):261–266. [Google Scholar]
- 29.Sethi DS, Stanley RE, Pillay PK. Endoscopic anatomy of sphenoid sinus and sella turcica. J. larynol. Otol. 1995;109:951–955. doi: 10.1017/s0022215100131743. [DOI] [PubMed] [Google Scholar]
- 30.Elwany S, Elsaeid I, Thabet H. Endoscopic anatomy of sphenoid sinus. J. Laryngol. Otol. 1999;113:122–126. doi: 10.1017/s0022215100143361. [DOI] [PubMed] [Google Scholar]
- 31.Dessi P, Moulin G, Castro F, et al. Protrusion of the optic nerve into the ethmoid and sphenoid sinus: prospective study of 150 studies. Neuroradiology. 1994;36:515–516. doi: 10.1007/BF00593511. [DOI] [PubMed] [Google Scholar]
- 32.Teatini G, Simonetti G, Masala W, et al. Computed tomography of the ethmoid labyrinth and adjacent structures. Ann Otol Rhinol Laryngol. 1987;96:239–250. doi: 10.1177/000348948709600301. [DOI] [PubMed] [Google Scholar]
- 33.Maniglia AJ. Fatal and major complications secondary to nasal and sinus surgery. Laryngoscope. 1989;99:276–283. doi: 10.1288/00005537-198903000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Sofferman RA, Harris P. The recovery potential of the optic nerve. Laryngoscope. 1995;105(suppl):1–38. [PubMed] [Google Scholar]
- 35.Chong VF, Fan YF, Lau DP. Imaging the sphenoid sinus. Austrlas Radiol. 1994;29:47–54. [Google Scholar]
- 36.Lang J, Keller H. The posterior opening of the pterygopalatine fossa and the position of the pterygopalatine ganglion. Gegenbaurs Morphol Jahrb. 1978;124:207–214. [PubMed] [Google Scholar]
- 37.Pandolfo I, Gaeta M, Blandino I, et al. The Radiology of Pterygoid Canal: Normal and Pathologic Findings. AJNR. 1987;8:497–483. [PMC free article] [PubMed] [Google Scholar]
- 38.Batra PS, Citardi MJ, Gallivan RP. Software-enabled CT analysis of optic nerve position and paranasal sinus pneumatization patterns. Otolaryngol Head Neck Surg. 2004;131:940–945. doi: 10.1016/j.otohns.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 39.Stammberger H, Kopp W. Functional Endoscopic Sinus Surgery: The Messerklinger Technique. Laryngoscope. 1991;210:67–68. [Google Scholar]
- 40.Driben JS, Bolger WE, Robles HA. The reliability of computerized tomographic in detection of the Onodi (sphenoethmoid cell) Am J Rhinol. 1998;12:105–111. doi: 10.2500/105065898781390325. [DOI] [PubMed] [Google Scholar]