Abstract
Increased vascular synthesis of 20-HETE is associated with increased vascular contraction, endothelial dysfunction and endothelial activation; all are believed to account for 20-HETE pro-hypertensive properties. We previously demonstrated that the 20-HETE-dependent inhibition of NO production is mediated through IκB kinase (IKK) suggesting a cross talk between 20-HETE-mediated endothelial dysfunction and activation. In this study, we examined the temporal relationship among blood pressure, endothelial dysfunction and endothelial activation and the role of IKK in the rat model of androgen-driven 20-HETE-mediated hypertension. In Sprague-Dawley rats treated with 5α-dihydrotestosterone (DHT), renal vascular 20-HETE levels increased by day 2 of treatment from 17.7±2.4 to 57.7±9.7 ng/mg, while blood pressure elevation reached significance by day 3 (132.7±1.7 vs 117.2±0.8 mmHg). In renal interlobar arteries, when compared to vehicle, DHT treatment increased the sensitivity to phenylephrine-induced vasoconstriction by 3.5-fold, decreased acetylcholine-induced vasorelaxation and increased NF-kB activity, all of which were attenuated by treatment with the 20-HETE antagonist, 20-HEDE. Co-treatment with parthenolide, an IKK inhibitor, attenuated the androgen-dependent 20-HETE-mediated elevation in blood pressure (from 133.7±3.1 to 109.8±3.0 mmHg). In addition, parthenolide treatment negated 20-HETE-mediated inhibition of the relaxing response to acetylcholine and 20-HETE-mediated increase in vascular NF-kB activity. These findings suggest that inhibition of IKK attenuates the androgen-dependent 20-HETE-mediated increase in blood pressure by inhibiting both 20-HETE-dependent endothelial activation and dysfunction.
Keywords: Cytochrome P450, 20-HETE, IKK, NF-kB, Androgen
INTRODUCTION
Gender differences are observed in many aspects of mammalian cardiovascular physiology and pathology. In particular, hypertension is more common in men than in premenopausal women of the same age. 1, 2 Epidemiological, clinical, and experimental studies have shown that androgens may be an important determinant of sex-specific differences in arterial blood pressure (BP). 3, 4 Androgens have been hypothesized to modulate BP at the cardiac and the vascular levels. 4, 5 Sex-specific differences in BP are also observed in various animal models: male spontaneously hypertensive rats (SHR),6–8 Dahl salt-sensitive rats,9 deoxycorticosterone (DOCA) acetate-salt hypertensive rats,10, 11 New Zealand genetically hypertensive rats 12 and Cyp4a14 (−/−) knockout mice. 13 In all, males demonstrated higher BP than females. Treatment of Sprague Dawley (SD) rats with androgen increased BP in both male and female rats. 14 Recent studies in our laboratory implicated a role for vascular CYP4A-derived 20-Hydroxy-5, 8, 11, 14-eicosatetraenoic acid (20-HETE) in the development of androgen-induced hypertension and suggested that 20-HETE is a key mediator of androgen-induced vascular resistance and hypertension. 14–16
20-HETE is a primary arachidonic acid metabolite in the renal microcirculation that is generated by enzymes of the CYP4 gene family and participates in the regulation of vascular tone via its actions on both vascular smooth muscle and endothelial cells. In vascular smooth muscle cells, 20-HETE inhibits the large conductance Ca2+-activated K+ channel (Kca), leading to depolarization and elevation in cytosolic [Ca2+]. 17 This inhibitory action is believed to underlie the effect of 20-HETE in enhancing responsiveness of arterial vessels to constrictor stimuli including pressure, oxygen, phenylephrine, and endothelin, as well as inhibition of NO synthesis. 18 In vascular endothelial cells, 20-HETE impairs endothelial function through eNOS uncoupling, stimulation of superoxide production and activation of a pro-inflammatory program. 15, 19, 20 In renal microvessels, 20-HETE attenuates the relaxing responses to acetylcholine; 20 this endothelial dysfunction is reversed by 20-HETE synthesis inhibitors or 20-HETE antagonists. 16, 21, 22 Thus, enhanced production of 20-HETE within the vasculature, as in the SHR, 23, 24 the androgen-dependent hypertensive rats 16, 25 and mice, 13 or in CYP4A2-transduced rats, 21, 22 contributes to hypertension and inhibition of 20-HETE synthesis or action lowers blood pressure in these models. In humans, increased urinary excretion of 20-HETE has been correlated with hypertension and endothelial dysfunction. 26, 27 These studies clearly implicated an important role for vascular 20-HETE in the development of hypertension.
In addition to its actions on eNOS, 20-HETE potently stimulates endothelial activation by activating the NF-κB and MAPK-ERK1/2 pathways, leading to increased levels of pro-inflammatory cytokines and adhesion molecules. 19 NF-κB is activated upon phosphorylation of IκB by IκB kinase (IKK), thus setting NF-κB free and marking the IκB proteins for proteasomal degradation. 28 A recent study by Cheng et al., 20 demonstrated that 20-HETE-mediated eNOS uncoupling is mediated through activation of IKK, suggesting the existence of a cross talk between 20-HETE-mediated endothelial dysfunction and activation. The present study was undertaken to determine the temporal relationship between vascular 20-HETE and NF-kB activation in the androgen-treated rats and examined whether inhibition of IKK, a key signaling step in 20-HETE-mediated endothelial dysfunction and endothelial activation, attenuates androgen-induced hypertension, a model of 20-HETE-dependent hypertension. 16
MATERIALS AND METHODS
A detailed description of experimental protocols, methods and materials is included in a supplemental material (please see http://hyper.ahajournals.org).
RESULTS
Time course of increases in blood pressure and vascular 20-HETE in DHT-treated rats
Administration of DHT to normotensive rats significantly increased systolic blood pressure by day 3 (132.7±1.7 vs 117.2±0.8 mmHg in DHT- and vehicle-treated rats, respectively). By day 7, blood pressure reached its maximal levels (Figure S1A). The time course of the increase in renal vascular levels of 20-HETE paralleled that of blood pressure, demonstrating a 3-fold increase on day 3 of treatment (45.7±12.3 vs 12.6±1.8 ng/mg in DHT- and vehicle-treated rats, respectively) and increasing thereafter to levels 4 times greater than those measured prior to treatment (Figure S1B). Additional experiments indicated that levels of 20-HETE significantly increased as early as day 2 of DHT treatment (57.7±9.7 vs 17.7±2.4 ng/mg at day 0) while a significant increase in systolic blood pressure was observed at day 3 of treatment (Figure S2).
Inhibition of 20-HETE synthesis or action prevents and attenuates androgen-dependent 20-HETE-mediated hypertension
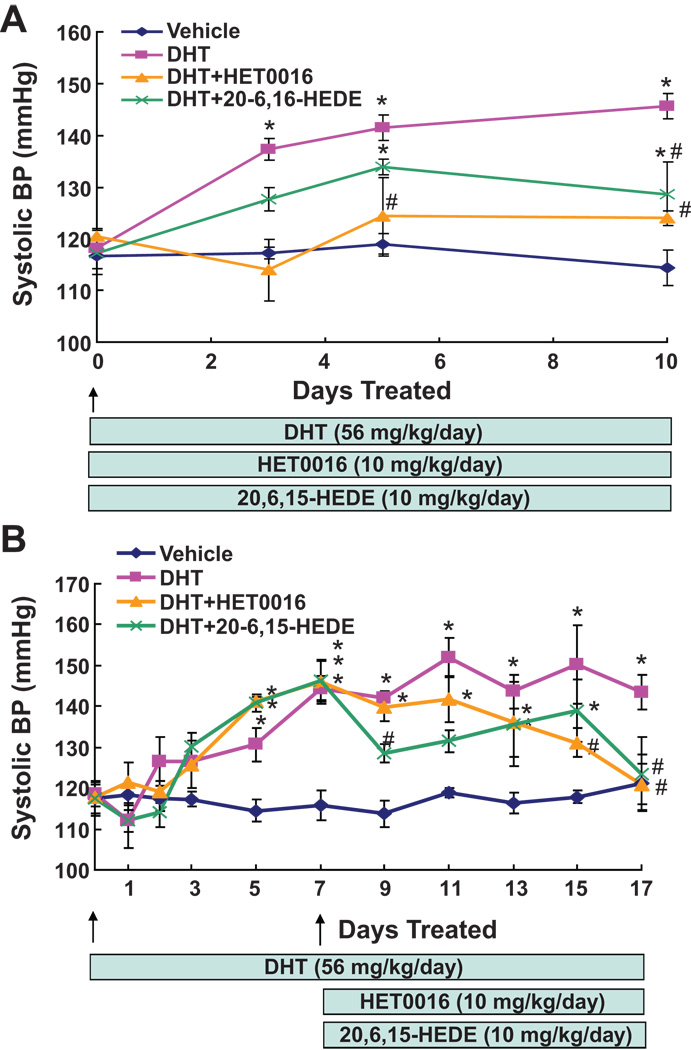
To determine whether the hypertensive effect of androgen is 20-HETE dependent, the 20-HETE synthesis inhibitor, HET0016, or the 20-HETE antagonist, 20-6,15-HEDE, were either co-administered with DHT or administered to rats on day 7 of DHT treatment. As seen in Figure 1A, co-treatment of DHT with HET0016 prevented the blood pressure increase to DHT. Moreover, the DHT-induced increase in blood pressure was greatly attenuated by co-treatment with 20-6,15-HEDE; at day 10 of co-treatment, blood pressure decreased from 145.7±2.4 mmHg in rats treated with DHT alone to 128.7±6.2 mmHg in rats co-treated with 20-6,15-HEDE (Figure 1A). Administration of 20-6,15-HEDE after 7 days of DHT treatment resulted in a rapid decrease in blood pressure. At day 2 of 20-6,15-HEDE administration, blood pressure decreased from 141.9±1.8 to 128.5+2.2 mmHg (Figure 1B) and remained significantly lower than that of the DHT-treated rats thereafter. In contrast, administration of HET0016 on day 7 of DHT treatment, resulted in a gradual decrease in blood pressure that was significantly different than that of the DHT-treated rats on day 15 of the experiment (Figure 1B).
Figure 1. Inhibition of 20-HETE synthesis or activity attenuates androgen-dependent 20-HETE-mediated hypertension.
DHT-treated rats were co-administered either HET0016 or 20-6,15-HEDE at the start of the experiment (A) or after systolic blood pressure plateaued at 7 days of treatment (B). Results are mean±SE (N=4) *p<0.05 vs vehicle treated rats; #p<0.05 vs DHT treated rats.
Increased renal arterial vascular reactivity to DHT is 20-HETE-dependent
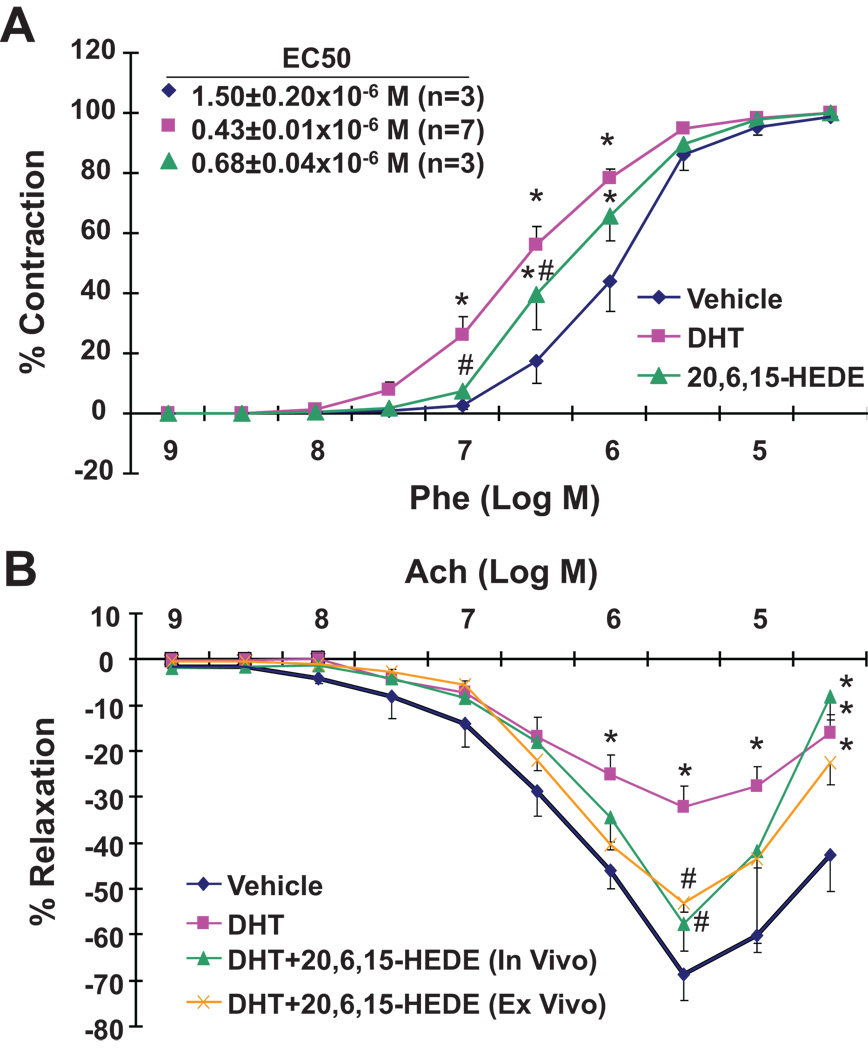
The increases in blood pressure and vascular 20-HETE occurred within the first 3 days of DHT treatment. No differences in phenylephrine sensitivity were observed between arteries from vehicle- and DHT-treated rats at day 2 of treatment (data not shown). However, arteries from rats treated with DHT for 3 days displayed a significant increase in sensitivity to phenylephrine-induced vasoconstriction as evidenced by a 3–5-fold increase in the EC50 to phenylephrine (Figure 2A). This increase in sensitivity was significantly attenuated in arteries from rats co-treated with 20-6,15-HEDE (Figure 2A).
Figure 2. DHT-induced changes in vascular function are 20-HETE-dependent.
DHT-treated rats were co-administered 20-6,15-HEDE for three days. The constrictor responses to phenylephrine (A) and the relaxing responses to acetylcholine (B) of renal interlobar arteries were measured. Results are mean±SE (N=3–7); *p<0.05 vs vehicle treated rats; #p<0.05 vs DHT treated rats.
Impared acetylcholine-mediated vasorelaxation to DHT is 20-HETE-dependent
Similar to vascular reactivity, endothelial dysfunction measured as impaired relaxing responses to acetylcholine was observed as early as 3 days of DHT treatment. As seen in Figure 2B, arteries from DHT-treated rats reached a maximum relaxation of 32.3±5.7% vs 68.8±5.4% in arteries from vehicle-treated rats. This decrease was attenuated in rats co-treated with 20-6,15-HEDE in vivo (57.6±6.0% relaxation). Interestingly, a similar effect (53.0±2.1% relaxation) was observed when 20-6,15-HEDE was added to the chamber of arteries from DHT treated rats (Figure 2B).
DHT treatment leads to endothelial activation that is 20-HETE-dependent
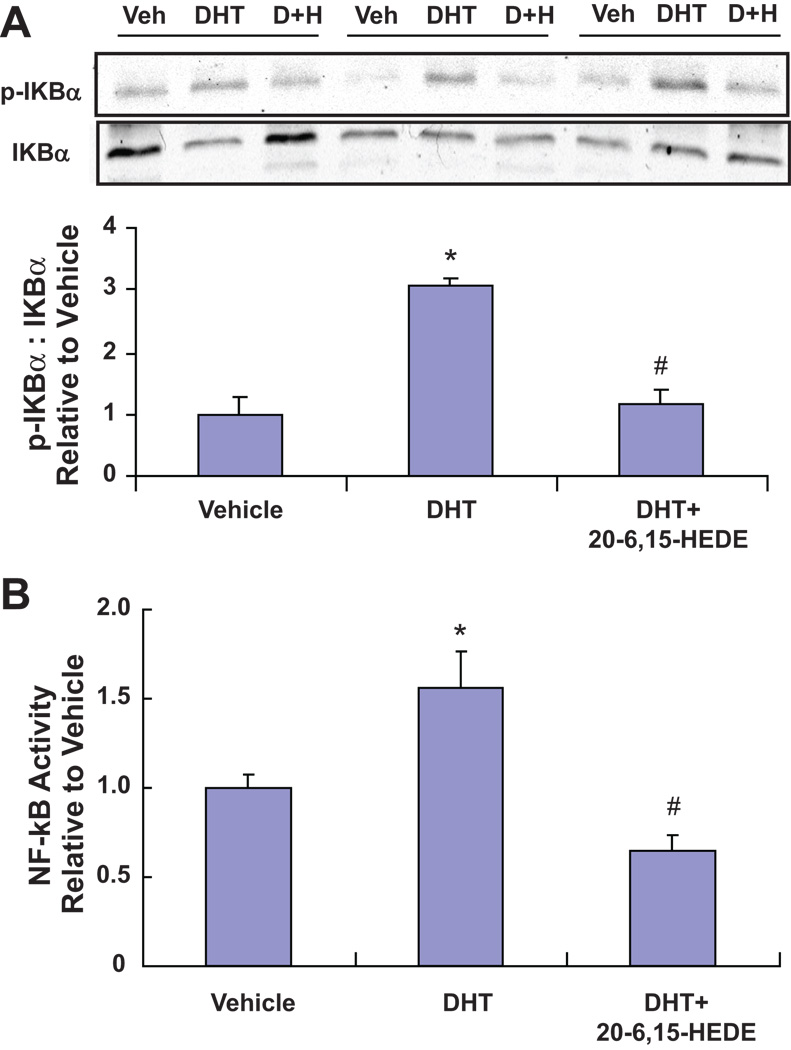
20-HETE has been shown to stimulate vascular endothelial NF-κB activation and cytokine production in vitro, effects that may contribute to the development of vascular inflammation that frequently accompanies endothelial dysfunction as well as hypertension. Along with increasing vascular synthesis of 20-HETE, treatment with DHT also increased NF-kB activation. Thus, arteries from 3-day DHT treatment displayed a 3-fold increase in phosphorylated IκBα when compared to arteries treated with the vehicle (Figure 3A). Moreover, the increase in phosphorylated IκB concurred with an increase in NF-κB transcriptional activity since nuclear protein isolated from interlobar arteries of DHT showed a 50% increase in NF-κB (p65). Importantly, the increase in NF-κB (p65) was completely negated in arteries from rats treated with DHT and 20-6,15-HEDE (Figure 3B). NF-κB activation was not seen in interlobar arteries from rats treated with DHT for two days (data not shown).
Figure 3. DHT treatment leads to NF-κB activation that is 20-HETE-dependent.
(A) Representative Western blots and densitometry analysis of p-IκBα (n=3–6; *p<0.05 vs vehicle) and (B) nuclear NF-κB activity (n=6–18; *p<0.05 vs vehicle treated rats; #p<0.05 vs DHT treated rats) in renal interlobar arteries from from rats treated for 3 days with vehicle, DHT and DHT+20-6,15-HEDE (n=6–18; *p<0.05 vs vehicle treated rats; #p<0.05 vs DHT treated rats).
Inhibition of NF-kB activation attenuates androgen-dependent 20-HETE-mediated hypertension
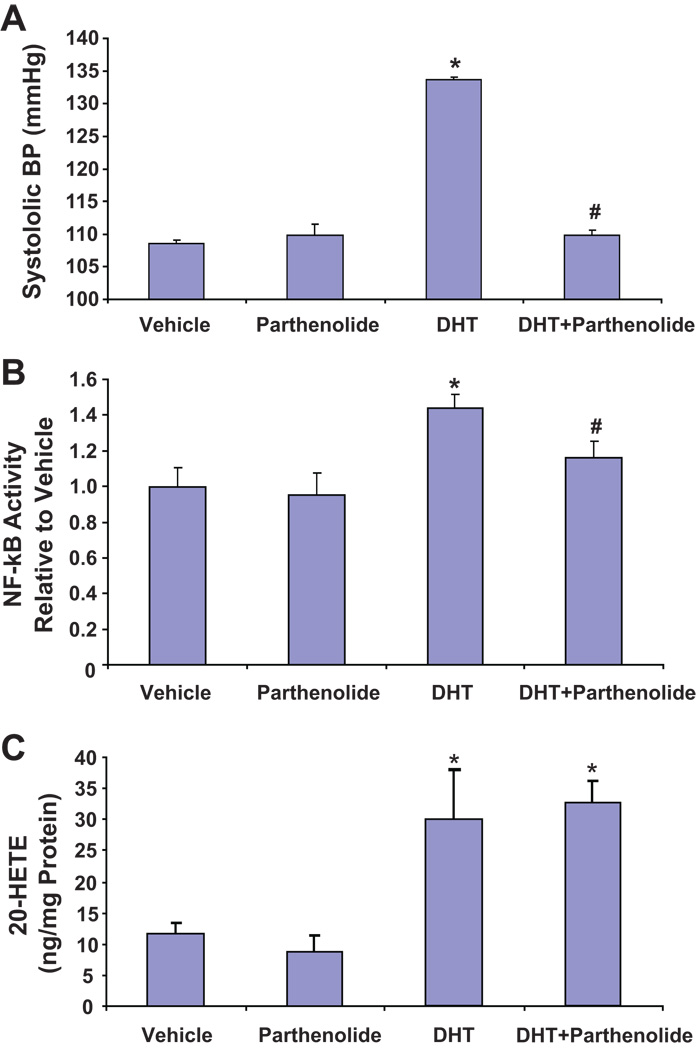
Parthenolide has been used to selectively inhibit IKK activation. As seen in Figure 4A, parthenolide alone had no effect on blood pressure; however, when administered together with DHT, it prevented the blood pressure increase to DHT. At day 3 of the experiment, blood pressure was 108.6±0.5, 109.8±1.7, 133.7±0.4 and 109.8±0.8 mmHg in vehicle-, parthenolide-, DHT- and DHT+parthenolide-treated rats, respectively. NF-kB activity in renal interlobar arteries showed a similar pattern; DHT treatment increased NF-kB activity by 45% when compared to vehicle treatment and co-treatment with parthenolide inhibited DHT-induced NF-kB activation (Figure 4B). Importantly, treatment with parthenolide did not affect the basal or the DHT-stimulated production of 20-HETE in the renal interlobar arteries (Figure 4C).
Figure 4. Inhibition of NF-kB activation attenuates androgen-dependent 20-HETE-mediated hypertension.
(A) Systolic blood pressure (N=8), (B) NF-κB activity in nuclear extract of renal interlobar arteries (N=8–10) and (C) 20-HETE levels in interlobar arteries from rats treated with vehicle, DHT, parthenolide and DHT+parthenolide. Results are mean ± SE. *P<0.05 vs vehicle-treated rats; #P<0.05 vs DHT-treated rats.
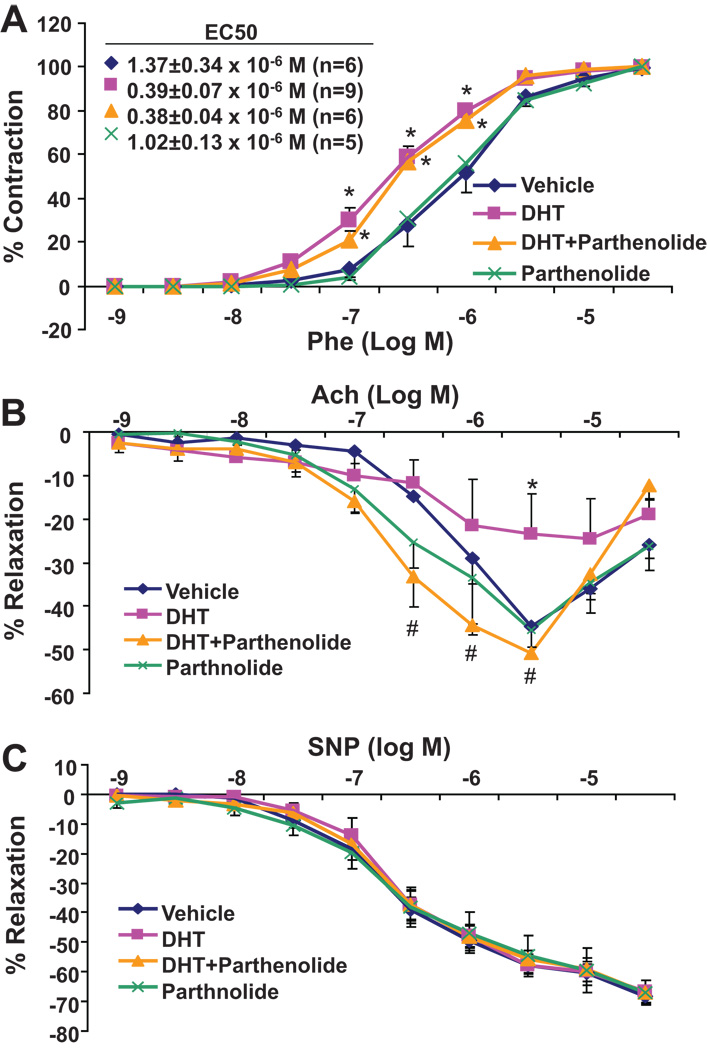
Parthenolide had a differential effect on vascular reactivity and relaxation. While treatment with DHT increased vascular reactivity by 2.6 and 3.5 fold when compared to vehicle or parthenolide alone (Figure 5A), administration of parthenolide to DHT-treated rats did not significantly alter the vascular reactivity to phenylephrine (Figure 5A). In contrast, the relaxing response to acetylcholine, which was greatly diminished in arteries from rats treated with DHT alone, was fully restored in arteries from rats treated with DHT and parthenolide (from 23.4±7.4% to 50.9±1.3 % relaxation at 5×10−6 M Ach) (Figure 5B). The relaxing response to nitroprusside was not different among the groups indicating that the vascular smooth muscle component of NO action is not altered by DHT and/or parthenolide (Figure 5C).
Figure 5. Effect of parthenolide on DHT-induced changes in vascular function.
DHT-treated rats were co-administered Parthenolide for three days. The constrictor responses to phenylephrine (A) and the relaxing responses to acetylcholine (B) and SNP (C) of renal interlobar arteries were measured. Results are mean±SE (N=6–8) *p<0.05 vs vehicle treated rats; #p<0.05 vs DHT treated rats.
DISCUSSION
This study demonstrates a close temporal relationship between increases in blood pressure and vascular 20-HETE synthesis in the rat model of androgen-induced hypertension. Moreover, based on a recent study that identified IKK activation as the common signaling step in 20-HETE-mediated endothelial dysfunction and activation in vitro, 20 this study is the first to demonstrate 20-HETE-mediated NF-κB activation in the renal vasculature of androgen-treated rats and to show that administration of an IKK inhibitor prevents the androgen-mediated 20-HETE-dependent increase in blood pressure.
Recent studies from our laboratory suggested that androgen-driven hypertension is mediated, in part, by increased vascular production of 20-HETE and that 20-HETE-mediated endothelial dysfunction contributes to the hypertension in this model. 16 This conclusion was based on the demonstration that the 20-HETE synthesis inhibitor, HET0016, prevented the blood pressure increase to androgen. However, a direct relationship between hypertension and the vascular actions of 20-HETE, including increased vascular reactivity, impaired endothelial-dependent relaxations and activated endothelium, in the androgen model has not been established. To this end, the use of the 20-HETE antagonist and the focus on the initial response to androgen in the present study provided a thorough examination of this relationship. Hence, the increase in blood pressure in response to androgen paralleled the increase in renal vascular synthesis of 20-HETE; both increased at day 3 and were at their maximum increase at 5–7 days of treatment. However, a closer analysis revealed that the increase in 20-HETE appeared to precede that of blood pressure, although this assumption needs further substantiation by radiotelemetry. Nevertheless, this suggested that 20-HETE may act as an initiating factor of androgen-driven hypertension. Androgen has been identified as a potent inducer of CYP4A enzymes including CYP4A2 and CYP4A8, which are the major 20-HETE producing enzymes in the rat vasculature. 29, 30 It is possible that induction of CYP4A within the vasculature brings about an increase in 20-HETE levels which in turn acts on the smooth muscle to increase vasoconstriction and on the endothelium to impair NO-dependent relaxation and to initiate the inflammatory program; all of these events, including the blood pressure increase, appeared to follow the initial 2.5-fold increase in 20-HETE levels at day 2 of androgen treatment. This order of events, together with the ability of 20-HETE inhibitor or antagonist to prevent/reverse their occurrence, suggests that 20-HETE is a significant causative factor in this model. However, it does not rule out the possibility that the observed changes can occur as independent events influenced by multiple factors. Androgen can modulate blood pressure through multiple pathways. Along with increasing levels of 20-HETE, androgen can activate the renin-angiotensin-aldosterone System (RAAS) by increasing levels of angiotensinogen hence modulating both kidney function and vascular function. 5, 31, 32 In addition, androgen increases levels of phenylephrine, neuropeptide Y, endothelin-1 and the expression of thromboxane A2 receptors, all of which contribute to a pressor effect. These pressor mechanisms may be amplified by the presence of increased levels of 20-HETE. They may also explain the lack of a complete inhibition of the blood pressure increase to DHT in the presence of a 20-HETE synthesis inhibitor or a 20-HETE activity blocker.
In a previous study, we showed that 20-HETE stimulates IkB phosphrylation and NF-κB translocation in endothelial cells. 19 The demonstration that activation of NF-κB, in the form of increased IκB phosphroylation and NF-κB transcriptional activity, was observed as early as 3 days of DHT treatment and was negated by administration of the 20-HETE antagonist, not only substantiated the in vitro data but also has pathophysiological implications. NF-κB is a pro-inflammatory transcription factor that plays an important role in vascular inflammation. Activation of NF-κB is a series of phosphorylation-de-phosphorylation and cellular translocation processes that in the end turn on the transcription machinery of physiological and pathological mediators, including immune, acute phase and pro-inflammatory genes.33 With the increasing evidence of a role for inflammation in the pathogenesis of hypertension, 34 NF-κB-mediated signaling has emerged as a potential target for treatment of hypertension. Inhibitors of NF-κB activation, including pyrrolidinedithiocarbamate (PDTC) and parthenolide, have been shown to lower blood pressure in several models of hypertension, including Ang II-treated rats, spontaneously hypertensive rats (SHR) and DOCA-salt hypertensive rats. 35–38 In these models, NF-κB inhibition was associated with an attenuation of renal interstitial inflammation and oxidative stress as well as amelioration of renal injury.
Endothelial activation presents a pro-inflammatory phenotype in which the endothelium typically displays upregulation of cellular adhesion molecules, endothelial-leukocyte interaction and alterations in the secretion of autocrine and/or paracrine factors, all of which contribute to inflammation. A recent study by Cheng et al., 20 suggested that 20-HETE-mediated endothelial activation and endothelial dysfunction are interdependent through IKK by demonstrating that inhibition of IKK activation in endothelial cells abrogated 20-HETE-mediated inhibition of NO. Our results indicate that vascular dysfunction and activation occur in the model of androgen-dependent hypertension; DHT treatment was associated with increased vascular reactivity, impaired relaxation to acetylcholine and activation of NF-kB in response to DHT. That these were mediated by 20-HETE was evident from the ability of HET0016 or 20-HEDE to reverse and prevent the effect of DHT. Thus, endothelial dysfunction and activation seen in endothelial cells treated with 20-HETE in vitro seemed to occur in vivo likely a result of increasing vascular levels of 20-HETE in response to androgen. Our study also indicates that IKK activation may be the link between these two processes in vivo. Administration of the IKK inhibitor parthenolide to DHT-treated rats not only reversed the increase in blood pressure but also inhibited vascular NF-kB activation as well as abrogated the increase in vascular reactivity and the impaired relaxation to acetylcholine. These results suggest that IKK activity may be the trigger that mediates both 20-HETE-mediated endothelial activation and dysfunction. However, since the parthenolide treatment is systemic, we cannot rule out the potential role of systemic NF-kB signaling and other IKK downstream signaling pathways, which may affect blood pressure and vascular function. To this end, a study by Henke et al., 39 demonstrated that endothelial-specific NF-kB-suppressed mice placed on a high-salt diet and given angiotensin II together with L-NAME displayed reduced hypertension-induced renal damage despite high blood pressure. Additional studies using vascular (endothelial)-specific suppression or deletion of IKK are important to better characterize the role of IKK in 20-HETE-mediated hypertension. Specific targeting is also important when considering the finding that while parthenolide treatment prevented DHT-mediated 20-HETE-dependent increase in blood pressure and decrease in relaxation to acetylcholine, it did not affect the DHT-mediated increase in vascular reactivity to phenylephrine which was fully negated by 20-HEDE. This suggested that the 20-HETE-dependent increase in vascular reactivity to phenylephrine is independent of IKK activation. This does not exclude the possibility that IKK is involved in 20-HETE-mediated increase in sensitivity to other constrictor stimuli.
In summary, this study strongly supports the role of 20-HETE in mediating androgen-dependent hypertension and provides substantial evidence for its underlying pro-hypertensive vascular mechanisms. These mechanisms include IKK-dependent endothelial dysfunction and activation that are manifested by increased vascular reactivity and impaired endothelial-dependent relaxations that are sensitive to IKK inhibition.
Perspective
20-HETE has been identified as a primary microcirculatory eicosanoid whose biological activities are conducive of a hypertensive factor. Numerous studies have documented a direct relationship between its vascular synthesis and blood pressure in animal models of hypertension and recent clinical studies suggested its involvement in human hypertension. 27, 40 Androgen has been implicated as a contributing factor to gender-specific differences in blood pressure and cardiovascular morbidity, postmenopausal hypertension and in the pathogenesis of polycystic ovary diseases. 41 We have shown that 20-HETE plays a role in the hypertensive activity of androgen. In this study, we showed that 20-HETE constitutes an initial step in the development of androgen-induced hypertension. Moreover, the androgen-mediated increase in vascular reactivity and endothelial dysfunction and activation was largely dependent on the initial increase in vascular 20-HETE’s synthesis. This study is also the first to implicate 20-HETE-mediated IKK activation, a step that links 20-HETE effects on the eNOS-NO and NF-kB pathways in endothelial cells, in the development of androgen-induced hypertension, thus raising the importance of inflammation in this process. All together, this study sets forth a novel paradigm suggesting that inhibition of 20-HETE can be a potential target for diseases that are regulated by androgen-induced hypertension. Inhibition of 20-HETE-mediated signaling in the vasculature or its downstream signaling via IKK may be a therapeutic approach to treating androgen-induced hypertension.
Supplementary Material
Acknowledgments
Sources of Funding
This study was supported by NIH grants HL034300 (MLS), F30 HL097402 (CCW), and GM31278 (JRF), and by the Robert A. Welch Foundation (JRF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
REFERENCES
- 1.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–1208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 2.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 3.Coylewright M, Reckelhoff JF, Ouyang P. Menopause and hypertension: an age-old debate. Hypertension. 2008;5:952–959. doi: 10.1161/HYPERTENSIONAHA.107.105742. [DOI] [PubMed] [Google Scholar]
- 4.Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocrine reviews. 2003;24:313–340. doi: 10.1210/er.2003-0005. [DOI] [PubMed] [Google Scholar]
- 5.Kienitz T, Quinkler M. Testosterone and blood pressure regulation. Kidney & blood pressure research. 2008;31:71–79. doi: 10.1159/000119417. [DOI] [PubMed] [Google Scholar]
- 6.Chen YF, Meng QC. Sexual dimorphism of blood pressure in spontaneously hypertensive rats is androgen dependent. Life sciences. 1991;48:85–96. doi: 10.1016/0024-3205(91)90428-e. [DOI] [PubMed] [Google Scholar]
- 7.Reckelhoff JF, Zhang H, Srivastava K. Gender differences in development of hypertension in spontaneously hypertensive rats: role of the renin-angiotensin system. Hypertension. 2000;35:480–483. doi: 10.1161/01.hyp.35.1.480. [DOI] [PubMed] [Google Scholar]
- 8.Reckelhoff JF, Zhang H, Granger JP. Testosterone exacerbates hypertension and reduces pressure-natriuresis in male spontaneously hypertensive rats. Hypertension. 1998;31:435–439. doi: 10.1161/01.hyp.31.1.435. [DOI] [PubMed] [Google Scholar]
- 9.Crofton JT, Ota M, Share L. Role of vasopressin, the renin-angiotensin system and sex in Dahl salt-sensitive hypertension. J hypertension. 1993;11:1031–1038. doi: 10.1097/00004872-199310000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Crofton JT, Share L. Gonadal hormones modulate deoxycorticosterone-salt hypertension in male and female rats. Hypertension. 1997;29:494–499. doi: 10.1161/01.hyp.29.1.494. [DOI] [PubMed] [Google Scholar]
- 11.Ouchi Y, Share L, Crofton JT, Iitake K, Brooks DP. Sex difference in the development of deoxycorticosterone-salt hypertension in the rat. Hypertension. 1987;9:172–177. doi: 10.1161/01.hyp.9.2.172. [DOI] [PubMed] [Google Scholar]
- 12.Ashton N, Balment RJ. Sexual dimorphism in renal function and hormonal status of New Zealand genetically hypertensive rats. Acta endocrinologica. 1991;124:91–97. doi: 10.1530/acta.0.1240091. [DOI] [PubMed] [Google Scholar]
- 13.Holla VR, Adas F, Imig JD, Zhao X, Price E, Jr, Olsen N, Kovacs WJ, Magnuson MA, Keeney DS, Breyer MD, Falck JR, Waterman MR, Capdevila JH. Alterations in the regulation of androgen-sensitive Cyp 4a monooxygenases cause hypertension. Proc Natl Acad Sci U S A. 2001;98:5211–5216. doi: 10.1073/pnas.081627898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh H, Schwartzman ML. Renal vascular cytochrome P450-derived eicosanoids in androgen-induced hypertension. Pharmacol Rep. 2008;60:29–37. [PubMed] [Google Scholar]
- 15.Cheng J, Ou JS, Singh H, Falck JR, Narsimhaswamy D, Pritchard KA, Jr, Schwartzman ML. 20-Hydroxyeicosatetraenoic acid causes endothelial dysfunction via eNOS uncoupling. Am J Physiol Heart Circ Physiol. 2008;294:H1018–H1026. doi: 10.1152/ajpheart.01172.2007. [DOI] [PubMed] [Google Scholar]
- 16.Singh H, Cheng J, Deng H, Kemp R, Ishizuka T, Nasjletti A, Schwartzman ML. Vascular Cytochrome P450 4A Expression and 20-Hydroxyeicosatetraenoic Acid Synthesis Contribute to Endothelial Dysfunction in Androgen-Induced Hypertension. Hypertension. 2007;50:123–129. doi: 10.1161/HYPERTENSIONAHA.107.089599. [DOI] [PubMed] [Google Scholar]
- 17.Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, Roman RJ. 20-HETE is an endogenous inhibitor of the large-conductance Ca2+-activated K+ channel in renal arterioles. Am J Physiol. 1996;270:R228–R237. doi: 10.1152/ajpregu.1996.270.1.R228. [DOI] [PubMed] [Google Scholar]
- 18.Miyata N, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. J Smooth Muscle Res. 2005;41:175–193. doi: 10.1540/jsmr.41.175. [DOI] [PubMed] [Google Scholar]
- 19.Ishizuka T, Cheng J, Singh H, Vitto MD, Manthati VL, Falck JR, Laniado-Schwartzman M. 20-Hydroxyeicosatetraenoic acid stimulates nuclear factor-kappaB activation and the production of inflammatory cytokines in human endothelial cells. J Pharmacol Exp Ther. 2008;324:103–110. doi: 10.1124/jpet.107.130336. [DOI] [PubMed] [Google Scholar]
- 20.Cheng J, Wu CC, Gotlinger KH, Zhang F, Narsimhaswamy D, Falck JR, Schwartzman ML. 20-HETE Mediates Endothelial Dysfunction Via Ikk-Dependent Enos Uncoupling. J Pharmacol Exp Ther. 2009;332:57–65. doi: 10.1124/jpet.109.159863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang JS, Singh H, Zhang F, Ishizuka T, Deng H, Kemp R, Wolin MS, Hintze TH, Abraham NG, Nasjletti A, Laniado-Schwartzman M. Endothelial dysfunction and hypertension in rats transduced with CYP4A2 adenovirus. Circ Res. 2006;98:962–969. doi: 10.1161/01.RES.0000217283.98806.a6. [DOI] [PubMed] [Google Scholar]
- 22.Inoue K, Sodhi K, Puri N, Gotlinger KH, Cao J, Rezzani R, Falck JR, Abraham NG, Laniado-Schwartzman M. Endothelial-specific CYP4A2 overexpression leads to renal injury and hypertension via increased production of 20-HETE. Am J Physiol Renal Physiol. 2009;297:F875–F884. doi: 10.1152/ajprenal.00364.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang F, Wang MH, Krishna UM, Falck JR, Laniado-Schwartzman M, Nasjletti A. Modulation by 20-HETE of phenylephrine-induced mesenteric artery contraction in spontaneously hypertensive and Wistar-Kyoto rats. Hypertension. 2001;38:1311–1315. doi: 10.1161/hy1201.096116. [DOI] [PubMed] [Google Scholar]
- 24.Imig JD, Falck JR, Gebremedhin D, Harder DR, Roman RJ. Elevated renovascular tone in young spontaneously hypertensive rats: Role of cytochrome P450. Hyperten. 1993;22:357–364. doi: 10.1161/01.hyp.22.3.357. [DOI] [PubMed] [Google Scholar]
- 25.Nakagawa K, Marji JS, Schwartzman ML, Waterman MR, Capdevila JH. Androgen-mediated induction of the kidney arachidonate hydroxylases is associated with the development of hypertension. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1055–R1062. doi: 10.1152/ajpregu.00459.2002. [DOI] [PubMed] [Google Scholar]
- 26.Ward NC, Rivera J, Hodgson J, Puddey IB, Beilin LJ, Falck JR, Croft KD. Urinary 20-hydroxyeicosatetraenoic acid is associated with endothelial dysfunction in humans. Circulation. 2004;110:438–443. doi: 10.1161/01.CIR.0000136808.72912.D9. [DOI] [PubMed] [Google Scholar]
- 27.Ward NC, Tsai IJ, Barden A, van Bockxmeer FM, Puddey IB, Hodgson JM, Croft KD. A single nucleotide polymorphism in the CYP4F2 but not CYP4A11 gene is associated with increased 20-HETE excretion and blood pressure. Hypertension. 2008;51:1393–1398. doi: 10.1161/HYPERTENSIONAHA.107.104463. [DOI] [PubMed] [Google Scholar]
- 28.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 29.Imaoka S, Yamazoe Y, Kato R, Funae Y. Hormonal regulation of rat cytochrome P450s by androgen and pituitary. Arch Biochem Biophys. 1992;299:179–184. doi: 10.1016/0003-9861(92)90260-4. [DOI] [PubMed] [Google Scholar]
- 30.Muller DN, Schmidt C, Barbosa-Sicard E, Wellner M, Gross V, Hercule H, Markovic M, Honeck H, Luft FC, Schunck WH. Mouse Cyp4a isoforms: enzymatic properties, gender- and strain-specific expression, and role in renal 20-hydroxyeicosatetraenoic acid formation. Biochem J. 2007;403:109–118. doi: 10.1042/BJ20061328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen YF, Naftilan AJ, Oparil S. Androgen-dependent angiotensinogen and renin messenger RNA expression in hypertensive rats. Hypertension. 1992;19:456–463. doi: 10.1161/01.hyp.19.5.456. [DOI] [PubMed] [Google Scholar]
- 32.Reckelhoff JF, Granger JP. Role of androgens in mediating hypertension and renal injury. Clin Exp Pharmacol Physiol. 1999;26:127–131. doi: 10.1046/j.1440-1681.1999.02996.x. [DOI] [PubMed] [Google Scholar]
- 33.Baeuerle PA. IkappaB-NF-kappaB structures: at the interface of inflammation control. Cell. 1998;95:729–731. doi: 10.1016/s0092-8674(00)81694-3. [DOI] [PubMed] [Google Scholar]
- 34.Savoia C, Schiffrin EL. Inflammation in hypertension. Curr Opin Nephrol Hypertens. 2006;15:152–158. doi: 10.1097/01.mnh.0000203189.57513.76. [DOI] [PubMed] [Google Scholar]
- 35.Elks CM, Mariappan N, Haque M, Guggilam A, Majid DS, Francis J. Chronic NF-{kappa}B blockade reduces cytosolic and mitochondrial oxidative stress and attenuates renal injury and hypertension in SHR. Am J Physiol Renal Physiol. 2009;296:F298–F305. doi: 10.1152/ajprenal.90628.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez-Iturbe B, Ferrebuz A, Vanegas V, Quiroz Y, Mezzano S, Vaziri ND. Early and sustained inhibition of nuclear factor-kappaB prevents hypertension in spontaneously hypertensive rats. J Pharmacol Exp Ther. 2005;315:51–57. doi: 10.1124/jpet.105.088062. [DOI] [PubMed] [Google Scholar]
- 37.Ozawa Y, Kobori H. Crucial role of Rho-nuclear factor-kappaB axis in angiotensin II-induced renal injury. Am J Physiol Renal Physiol. 2007;293:F100–F109. doi: 10.1152/ajprenal.00520.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beswick RA, Zhang H, Marable D, Catravas JD, Hill WD, Webb RC. Long-term antioxidant administration attenuates mineralocorticoid hypertension and renal inflammatory response. Hypertension. 2001;37:781–786. doi: 10.1161/01.hyp.37.2.781. [DOI] [PubMed] [Google Scholar]
- 39.Henke N, Schmidt-Ullrich R, Dechend R, Park JK, Qadri F, Wellner M, Obst M, Gross V, Dietz R, Luft FC, Scheidereit C, Muller DN. Vascular endothelial cell-specific NF-kappaB suppression attenuates hypertension-induced renal damage. Circ Res. 2007;101:268–276. doi: 10.1161/CIRCRESAHA.107.150474. [DOI] [PubMed] [Google Scholar]
- 40.Ward NC, Puddey IB, Hodgson JM, Beilin LJ, Croft KD. Urinary 20-hydroxyeicosatetraenoic acid excretion is associated with oxidative stress in hypertensive subjects. Free Radic Biol Med. 2005;38:1032–1036. doi: 10.1016/j.freeradbiomed.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 41.Reckelhoff JF. Sex steroids, cardiovascular disease, and hypertension: unanswered questions and some speculations. Hypertension. 2005;45:170–174. doi: 10.1161/01.HYP.0000151825.36598.36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.