Abstract
Recent evidence suggests that angiotensin II (Ang II) upregulates phosphodiesterase (PDE)-1A expression. We hypothesized that Ang II augmented PDE1 activation, decreasing the bioavailability of cyclic cyclic guanosine 3', 5'-monophosphate (cGMP), contributing to increased vascular contractility. Male Sprague-Dawley rats received mini-osmotic pumps with Ang II (60 ng.min−1) or saline for 14 days. PE-induced contractions were increased in aorta (Emax168±8 vs. 136±4%) and small-mesenteric arteries [(SMA), Emax170±6 vs. 143±3%] from Ang II infused rats compared to control. PDE1 inhibition with vinpocetine (10µM) reduced PE-induced contraction in aortas from Ang II rats (Emax94±12%) but not in control (154±7%). Vinpocetine decreased the sensitivity to PE in SMA from Ang II rats compared to vehicle (pD2 5.1±0.1 vs. 5.9±0.06), but not in control (6.0±0.03 vs. 6.1±0.04). Sildenafil (10µM), a PDE5 inhibitor reduced PE-induced maximal contraction similarly in Ang II and control rats. Arteries were contracted with PE (1µM) and concentration-dependent relaxation to vinpocetine and sildenafil was evaluated. Aortas from Ang II rats displayed increased relaxation to vinpocetine compared to control (Emax82±12 vs. 44±5%). SMA from Ang II rats showed greater sensitivity during vinpocetine-induced relaxation, compared to control (pD2 6.1±0.3 vs. 5.3±0.1). No differences in sildenafil-induced relaxation were observed. PDE1A and PDE1C expressions in aorta and PDE1A expression in SMA were increased in Ang II rats. cGMP production, which is decreased in arteries from Ang II rats, was restored after PDE1 blockade. We conclude that PDE1 activation reduces cGMP bioavailability in arteries from ANG II, contributing to increased contractile responsiveness.
Keywords: PDE1, angiotensin II, cGMP, hypertension, vinpocetine
Introduction
A variety of cellular functions, including mechanical and metabolic events, are regulated by cyclic nucleotides such as cyclic guanosine 3', 5'-monophosphate (cGMP). Cyclic nucleotides acts as second messengers and are responsible for short and long-term responses in the smooth muscle cells (SMCs) [1, 2]. They are synthesized by different isozymes (cyclases) and cGMP formation causes SMCs relaxation by inhibiting RhoA translocation, by lowering intracellular calcium and by activating myosin phosphatase [3–5]. Moreover, phosphodiesterases (PDEs) regulate the amplitude, duration, degradation and compartmentalization of intracellular cyclic nucleotide signaling [4, 6], thereby contributing to vascular tone and cellular proliferation [7].
Calcium (Ca2+)/calmodulin-dependent PDE isoforms (CaM-PDE) contain two Ca2+/calmodulin binding domains and the binding of both Ca2+ and calmodulin is required for full activation of these PDEs [8–10]. Three different isoforms of CaM-PDEs have been reported: PDE1A and PDE1B, which display higher affinity to hydrolyze cGMP, compared to cAMP and, PDE1C which has similar ability to hydrolyze cGMP and cAMP [1, 11].
Vascular changes in hypertension, including increased contractile activity, are closely associated with humoral factors such as angiotensin II (Ang II) [12]. Vascular SMC contraction is initiated by a biphasic Ca2+ elevation in the cytoplasm. The initial transient increase is attributed to inositol triphosphate-mediated release of Ca2+ from the sarcoplasmic reticulum. The subsequent prolonged increase requires extracellular Ca2+ influx through various pathways. When Ca2+ is high, PDE1 is activated, resulting in lower levels of cGMP, which theoretically facilitates the SMC contraction.
Recently, it has been reported that chronic infusion of Ang II induces increased PDE1A expression in ventricular tissues [6]. In addition, it has been demonstrated that CaM-PDEs mediate hypertrophy in cardiomyocyte [6]. Furthermore, the inhibition of PDE1 in SMCs isolated from normal aorta or from atherosclerotic lesions resulted in suppression of SMC proliferation [13].
In view of this next information regarding the functionality of CaM-PDEs, along with the fact that Ang II is able to increase PDE1 expression in vascular SMCs, we hypothesized that hypertension induced by chronic infusion of Ang II stimulates PDE1 expression in vascular SMCs, results in reduced bioavailability of cGMP and increased vascular contractile responsiveness.
Since Ang II also induces PDE5 expression in vitro [14], we adopted a comparative strategy between PDE1 and PDE5. These two isoforms are expressed in SMCs and they have distinct mechanisms of activation. We have used the specific pharmacologic inhibitors vinpocetine (a PDE1 inhibitor) and sildenafil (a PDE5 inhibitor) to better understand the effects of Ang II chronic infusion in the vascular expression and activation of PDEs isoforms.
METHODS
Animals and blood pressure measurement
Ten week-old male Sprague-Dawley rats (230–250g; Harlan Laboratories, Indianapolis, IN), maintained on a 12:12-h light-dark cycle with rat chow and water ad libitum, were used in these studies. All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and were reviewed and approved by the Institutional Animal Care and Use Committee of the Medical College of Georgia.
Rats were anesthetized with isoflurane via a nose cone for surgical procedures (initially with 5% and then maintained at 2.5% in 100% oxygen). Osmotic mini pumps (0.5µl per hour - 14 days - model 2002, Alzet Co., Cupertino, CA) were implanted subcutaneously. Animals were divided into two groups: a control group infused with vehicle only (saline, 8.33ηl.min-1), and the other infused with Ang II (60 ηg.min-) for a period of 14 days. Systolic blood pressure was measured in nonanesthetized animals by tail cuff using a RTBP1001 blood pressure system (Kent Scientific Corporation).
Vascular functional studies
After euthanasia by CO2, the mesentery and aorta were rapidly excised and placed in a 4°C cold physiological salt solution (PSS), containing (mM): NaCl, 130; NaHCO3, 14.9; KCl, 4.7; KH2PO4, 1.18; MgSO4·7H2O 1.18; CaCl2·2H2O, 1.56; EDTA, 0.026; glucose, 5.5. Second-order branches of mesenteric artery (≅ 2 mm in length with internal diameter ≅ 150 to 200µm) and aortic segments were carefully dissected and mounted as rings. The arteries were mounted in an isometric Mulvany-Halpern myograph (model 610 DMT-USA, Marietta, GA) and recorded by a PowerLab 8/SP data acquisition system (ADInstruments Pty Ltd., Colorado Springs, CO). Both dissection and mounting of the vessels were carried out in cold (4°C) PSS. The second-order mesenteric arteries were adjusted to maintain a passive force of 3mN and the aortic ring was placed at a passive force of 30mN. Arteries were equilibrated for 45 min in PSS at 37°C, and continuously bubbled with 5% CO2 and 95% O2. Endothelium was mechanically removed with rat hair in small-mesenteric arteries and with a metallic bar in the aortic rings. Arterial integrity was assessed first by stimulation of vessels with 120 mM KCl. After washing and a new stabilization, the absence of endothelium was assessed by contracting the segments with phenylephrine (PE; 1µM) followed by acetylcholine (ACh; 10 µM). The absence of a relaxation-response to ACh stimulation was taken as evidence of endothelium removal. Concentration-response curves to PE (1nM to 100µM) were performed, in the presence and absence of vinpocetine (10µM, for 30 minutes) or sildenafil (10µM, for 30 minutes), to evaluate vascular contractility. Relaxation-response curves to vinpocetine (PDE1 inhibitor, 1nM to 100µM),sildenafil (PDE5 inhibitor, 10nM to 100µM) and 8-Bromo cGMP (membrane-permeable cGMP analogue, 100nM to 10µM) were performed in aortas contracted with PE (1µM) and in small-mesenteric arteries contracted with U46619 (1µM), a thromboxane A2 analogue. Vinpocetine, a synthetic alkaloid derivative, is one of the most selective PDE1 inhibitors currently available [15–19]. In another set of experiments, vascular segments were incubated with Ca2+-free plus EGTA buffer for 30 minutes, followed by three subsequent washes in Ca2+-free without EGTA and incubation of the vessels in this buffer for 30 minutes, in the presence or in the absence of vinpocetine (10µM, for 30 minutes). Arteries were stimulated with PE (aorta, 1µM; small-mesenteric arteries, 10µM) and concentration-response curves to CaCl2 (10µM to 10mM) were performed.
Western blot for detection of vascular PDE1A, PDE1B, PDE1C and PDE5 isoforms
Proteins (40 µg) extracted from small-mesenteric arteries and aorta were separated by electrophoresis on a 10% polyacrylamide gel and transferred to a nitrocellulose membrane. Nonspecific binding sites were blocked with 5% skim milk in Tris-buffered saline solution with Tween for 1 hour at 24°C. Membranes were incubated with antibodies overnight at 4°C. Selective antibodies for the following were used: PDE1A [(1:500, Abcam) predicted size: 61 kDa; multiple bands from 65 to 72 kDa], PDE1B [(1:500, Abcam) predicted size: 65 kDa; multiple bands from 65 to 72 kDa], PDE1C [(1:500, Abcam) predicted size: 70 kDa; multiple bands from 70 to 87 kDa], PDE5 [(1:500, Abcam) predicted size: 105 kDa; multiple bands from 88 to 99 kDa], and β-actin [(1:1000; Sigma) predicted size: 42kDa]. After incubation with secondary antibodies, signals were revealed with chemiluminescence and quantified by density profile extraction and multiple band analysis for entire lines. Results were normalized to β -actin protein and expressed as arbitrary units. Each value of the experiments from both groups was normalized by the average of the control group, which was assumed as being equal “1”.
Determination of cGMP levels
Aortic rings and small-mesenteric arteries were equilibrated for 10 minutes in oxygenated Krebs’ solution. Vinpocetine (10µM) or vehicle was added to the preparations for 20 minutes. Arteries were collected immediately and segments were frozen in liquid nitrogen. cGMP was extracted and quantified using a cGMP enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI) as previously described [20, 21]. The weights of the dried pellets were used to standardize the different samples.
Data Analysis
The results are shown as mean ± SEM where “n” represents the number of rats used in the experiments. Contractions were recorded as changes in the displacement (millinewton) from baseline, normalized by KCl contraction and are represented as percentage of KCl-induced contraction. Relaxation is expressed as percent change from the PE contracted levels. Concentration–response curves were fitted using a nonlinear interactive fitting program (Graph Pad Prism 3.0; GraphPad Software Inc., San Diego, CA, U.S.A.). Values of P<0.05 were considered a statistically significant difference. Statistical analysis was performed using two-way analysis of variance plus Newman-Keuls post hoc analysis to compare the concentration-responses curves between all the groups. Western blot data were analyzed by 1-sample t test comparing control and Ang II, and the P value was computed from the t ratio and the numbers of degrees of freedom. Values of P<0.05 were considered statistically significant.
Chemicals
Acetylcholine chloride, phenylephrine hydrochloride, vinpocetine and 8-Bromo cGMP were purchased from Sigma Aldrich (St. Louis, MO). Angiotensin II was purchased from Phoenix Pharmaceutical Inc. (Burlingame, CA). Sildenafil was kindly provided by Pfizer.
RESULTS
Blood pressure data
After 14 days of infusion, Ang II treated rats displayed increased systolic blood pressure compared to control rats (166±2 vs. 130±4 mmHg, respectively; n=6).
Effects of chronic infusion of Ang II on vascular contractility
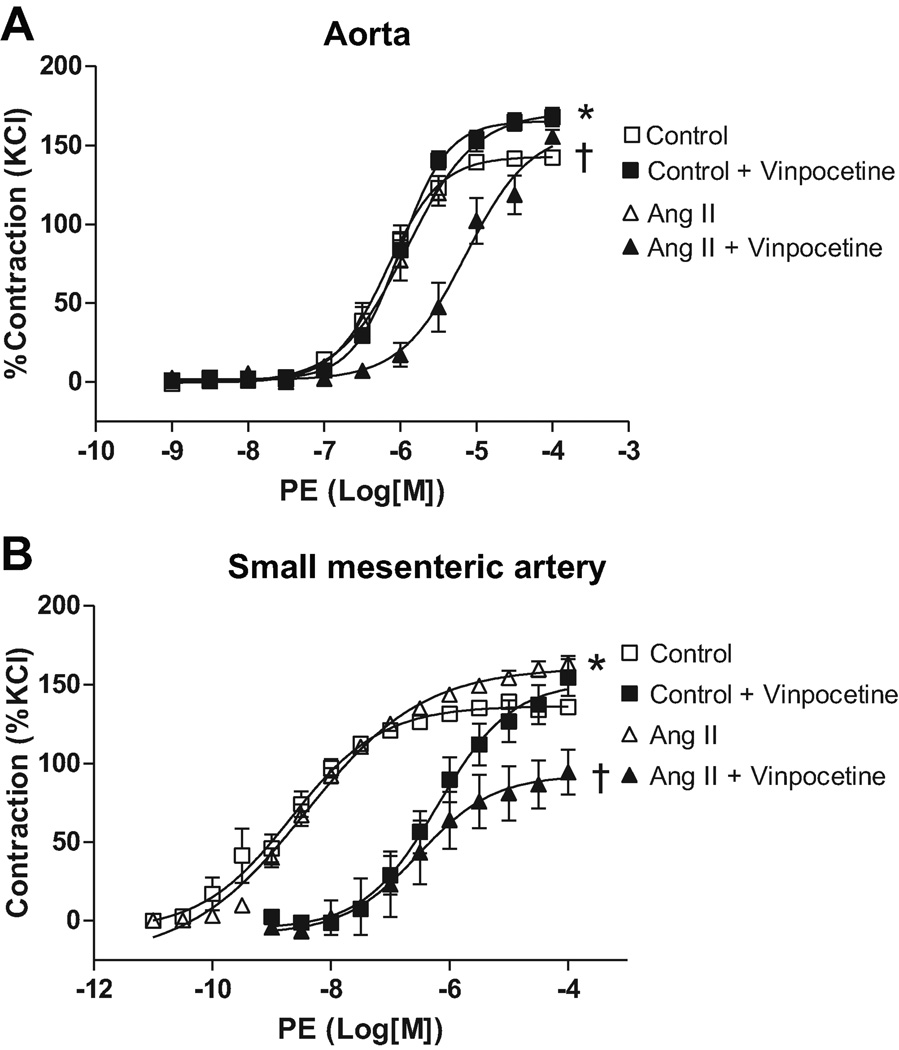
Aortas from Ang II-infused rats displayed increased maximum contraction to PE compared to control values (168±8 vs. 136±4%, respectively; n=6, p=0.0017, Figure 1A). No differences were observed in the pD2 values between hypertensive and control rats (Table 1).
Figure 1. PDE-1 inhibition decreases vascular contraction in arteries from Ang II hypertensive, but not control, rats.
Concentration-response curves to PE were performed in (A) aorta and (B) small-mesenteric arteries from Ang II hypertensive (▲) and control (■) rats, in the absence (open symbols) or in the presence (filled symbols) of vinpocetine (10µM, 30 minutes). Data are mean ± SEM (n=6). Symbols represents the results from two-way ANOVA, and express differences in the Emax. * P<0.05 vs. control. † P<0.05 vs. Ang II.
Table 1. pD2 values for PE-induced contraction in the presence or absence of vinpocetine and sildenafil.
Concentration-response curves to PE were performed in aorta and small-mesenteric arteries from Ang II hypertensive and control rats, in the absence or in the presence of vinpocetine or sildenafil (10µM, 30 minutes).
| Aorta | Small-mesenteric artery | |||
|---|---|---|---|---|
| Incubation | Control | Ang II | Control | Ang II |
| Vehicle | 8.4±0.1 | 8.5±0.1 | 6.1±0.04 | 5.9±0.06 |
| Vinpocetine | 6.2±0.1 † | 6.5±0.2 † | 6.0±0.03 | 5.1±0.10 * † |
| Sildenafil | 7.05±0.1 † | 7.1±0.1 † | 5.3±0.03 † | 5.1±0.10 † |
Data are mean ± SEM (n=6).
p<0.05 vs. respective control group.
p<0.05 vs. respective vehicle group.
Small-mesenteric arteries from Ang II-infused rats displayed augmented contraction to PE compared to control values (170±6 vs. 143±3%, respectively; n=6, p=0.0006, Figure 1B). No differences were observed in the pD2 values between hypertensive and control rats (Table 1).
Effect of PDE1 inhibition on vascular contractility
After vinpocetine incubation (10µM, 30 minutes), aortas from Ang II-infused rats displayed a reduction in the contractile response to PE (94±12%; n=6, p<0.0001), when compared to Ang II aortas without PDE1 inhibition (Figure 1A). PE-induced contraction in aortas from control rats were not affected by vinpocetine incubation (154±7% – Figure 1A). Vinpocetine incubation reduced pD2 values both in Ang II rats and control rats, compared to their respective vehicle (p<0.0001), indicating that PDE1 inhibition decreases sensitivity to PE-induced contractile response in aorta (Table 1).
In small-mesenteric arteries, the PE-induced maximum contraction was reduced by vinpocetine incubation in Ang II (151.7±5.6%; n=6, p=0.0025), but not in control rats (153.3±3.6%; n=6, Figure 1B). The pD2 value was reduced in small-mesenteric arteries from Ang II rats (p<0.0001), but not in control rats, when compared to their respective vehicle groups (Table 1). These data indicate that PDE1 inhibition decreases sensitivity to PE in small-mesenteric arteries from Ang II rats. Together, these results suggest that PDE1 contribution to increased contractile response is greater in aorta and small-mesenteric arteries from Ang II-infused rats, compared to control rats. Presumably, these results indicate a greater reduction in cGMP by PDE1 in blood vessel from Ang II hypertensive rats.
Effect of PDE5 inhibition on vascular contractility
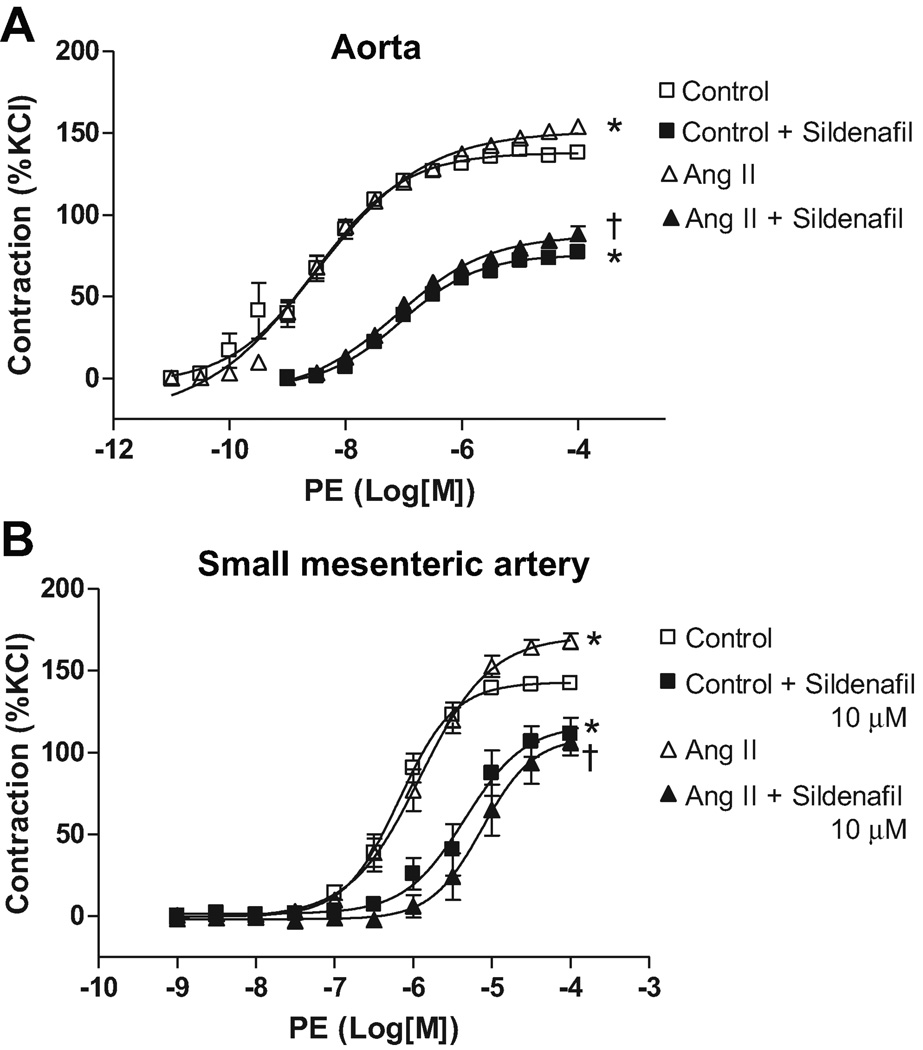
After sildenafil incubation (10µM, 30 minutes), aortas from both Ang II-infused (88.5±4.3%; n=6, p#x0003C;0.001) and control rats (79.1±4.5%; n=6, p<0.001) showed reduction in the maximum contractile response to PE compared to their respective groups without incubation (Figure 2A). Additionally, no differences in PE-induced contraction were observed between Ang II and control aortas after sildenafil treatment.
Figure 2. PDE-5 inhibition decreases vascular contraction similarly in arteries from Ang II hypertensive and control rats.
Concentration-response curves to PE were performed in (A) aorta and (B) in small-mesenteric arteries from Ang II hypertensive (▲) and control (■) rats, in the absence (open symbols) or in the presence (filled symbols) of sildenafil (10µM, 30 minutes). Data are mean ± SEM (n=6). Symbols represents the results from two-way ANOVA, and express differences in the Emax. * P<0.05 vs. control. † P<0.05 vs. Ang II+ vehicle.
The pD2 values were decreased after sildenafil incubation, both in Ang II rats (p<0.0001) and control rats (p<0.0001), indicating that PDE5 similarly contributes to PE-sensitivity in aorta from both groups (Table 1).
PE-induced maximum contraction was reduced by sildenafil incubation in small-mesenteric arteries from Ang II (117±9.7%; n=6, p=0.0002) and control rats (109±10.1%; n=6, p=0.0007), compared to their respective groups without incubation (Figure 2B). The pD2 values were reduced both in small-mesenteric arteries from Ang II rats and control rats (p<0.0001), showing that PDE5 similarly contributes to increased sensitivity to PE in small-mesenteric arteries in both groups (Table 1). The current results show that PDE5 activation contributes to increased contractile response both in arteries from Ang II and control rats.
Effect of PDE1 inhibition on Ca2+-induced contraction
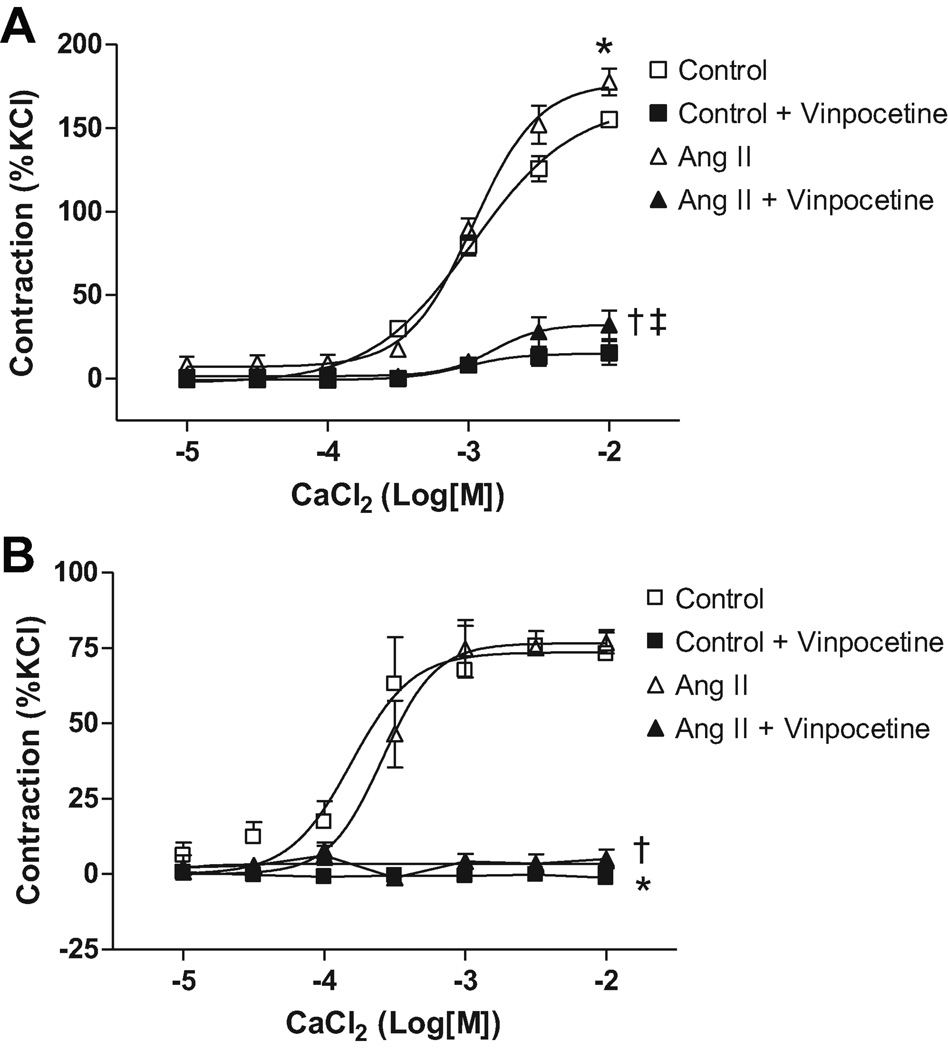
Aortas from Ang II-infused rats displayed increased maximum contraction to Ca2+ (CaCl2) compared to control values (177±7.4 vs. 155±5.42%, respectively; n=6, p=0.037). Vinpocetine incubation reduced maximum contraction both in aorta from Ang II and control rats (15.6±3.2 vs. 32.3±7.5%, respectively; n=6) and abolished differences between the groups (Figure 3A).
Figure 3. Ca2+-induced vascular contraction is increased in aortas from Ang II hypertensive rats.
Concentration-response curves to Ca2+ (CaCl2) were performed in (A) aorta and (B) small-mesenteric arteries from Ang II hypertensive (▲) and control (■) rats, in the absence (open symbols) or in the presence (filled symbols) of vinpocetine (10µM, 30 minutes). Symbols represents the results from two-way ANOVA, and express differences in the Emax. Data are mean ± SEM (n=6). * P<0.05 vs. control. † P<0.05 vs. Ang II. ‡ p<0.05 vs. control + vinpocetine.
Small-mesenteric arteries from Ang II rats displayed similar contraction to Ca2+ (CaCl2) compared to control values (76.6±6.1 vs. 75.6±3.5%, respectively; n=6). PDE1 inhibition abolished contraction to Ca2+ (CaCl2) in both groups (Figure 3B).
Effect of PDE1 inhibition on vascular relaxation
In this set of experiments, concentration-response curves to vinpocetine and sildenafil, PDE1 and PDE5 inhibitor respectively, were performed in arteries from Ang II and control rats, contracted with PE (10µM, aorta) or U-46619 (10µM, small-mesenteric arteries).
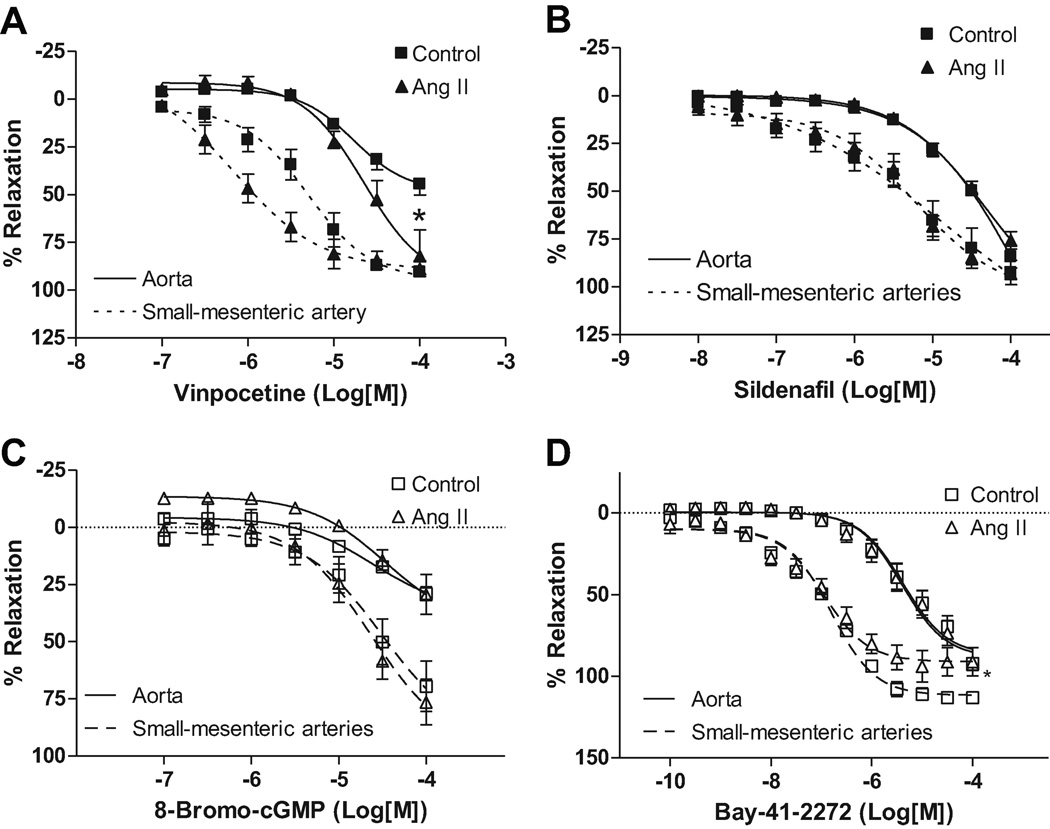
Cumulative concentrations of vinpocetine resulted in greater relaxation response in aortas from Ang II rats, compared to control rats (82.3±6.1 vs. 44.5±5.2%, respectively – n=6 – p<0.0028 – Figure 4A). In this case, no differences were observed in pD2 values between aorta from Ang II and control rats, showing that aorta from both groups display similar sensitivity to vinpocetine (Table 2). In small-mesenteric arteries, no differences in the vinpocetine-induced maximum relaxation response were observed between Ang II (89.9±3.5%; n=6), and control rats (90.6±1.5%; n=6 – Figure 4A). pD2 value was increased in small-mesenteric arteries from Ang II rats compared to control rats (p<0.029), which indicates that small-mesenteric arteries from Ang II rats have augmented sensitivity to PDE1-inhibition, (upon addition of vinpocetine), compared to control rats (Table 2).
Figure 4. Vinpocetine induced-relaxation is increased in arteries from Ang II hypertensive rats.
Concentration-response curves to A) vinpocetine, B) sildenafil, C) 8-Bromo cGMP and D) Bay 41-2272 were performed in aortas (continuous trace) contracted with PE (1µM) or small-mesenteric arteries (dashed trace) contracted with U-46619 (1µM), from Ang II hypertensive (▲) and control (■) rats. Data are mean ± SEM (n=6). Symbols represents the results from two-way ANOVA, and express differences in the Emax. * P<0.05 vs. control.
Table 2. pD2 values for cumulative concentration response (relaxation) curve to vinpocetine, sildenafil, 8-Bromo-cGMP and Bay-41-2272.
Concentration-response curves to vinpocetine, sildenafil, 8-Bromo cGMP and Bay-41-2272 were performed in aortas contracted with PE (1µM) or small-mesenteric arteries contracted with U-46619 (1µM), from Ang II hypertensive and control rats.
| Aorta | Small-mesenteric artery | |||
|---|---|---|---|---|
| Incubation | Control | Ang II | Control | Ang II |
| Vinpocetine | 4.8±0.1 | 4.6±0.2 | 5.3±0.1 | 6.1±0.3* |
| Sildenafil | 4.3±0.3 | 3.8±0.6 | 5.2±0.2 | 4.75±0.6 |
| 8-Bromo cGMP | 4.6±0.2 | 4.6±0.2 | 4.5±0.2 | 4.6±0.2 |
| Bay-41-2272 | 5.3±008 | 5.4±0.09 | 6.8±0.06 | 6.94±0.11 |
Data are mean ± SEM (n=6).
p<0.05 vs. respective control group.
p<0.05 vs. respective vehicle group
Effect of PDE5 inhibition on vascular relaxation
When sildenafil relaxation-curves were performed in aortas, no differences in the maximum response were observed in Ang II compared to control rats (75.8±4.3 vs. 71.7±12.5%, respectively – n=6 – Figure 4B). The pD2 values were similar between Ang II and control rats (Table 2), indicating that aorta from both groups display similar sensitivity to sildenafil.
In small-mesenteric arteries, sildenafil cumulative-curve resulted in similar relaxation response in Ang II (93.2±2.4%; n=6) and control (92.6±6.25%, n=6 – Figure 4B) rats. No differences in the pD2 values were found between Ang II (5.2±0.2) and control rats (Table 2), showing that small-mesenteric arteries from both groups display similar sensitivity to sildenafil.
These results suggest that PDE1 activation plays a role in augmented contractile responsiveness in arteries from Ang II compared to control rats.
Effect of soluble-permeable cGMP analogue and cGMP ctivator
When concentration-response curves to 8-Bromo-cGMP, a membrane-permeable cGMP analogue, were performed in arteries from control or Ang II rats, no significant differences were observed between the groups (Figure 4C). These results indicate that the overall response of the arteries to cGMP is not different between the groups. No differences in the relaxation responses were observed when concentration-response curves to Bay 41-2272, a potent cGMP activator was evaluated in aortas from control or Ang II rats (Figure 4D). However, a smaller relaxation response was observed in small-mesenteric arteries from Ang II rats, compared to control (93.9±7.3vs. 113±2.9%, respectively; p=0.027, Figure 4D) when concentration-response curves to Bay 41-2272 were performed. These results suggest that guanylyl cyclase is differently activated in small-mesenteric arteries, but not aorta, from Ang II rats.
Vascular expression of PDE1s and PDE5 isoforms
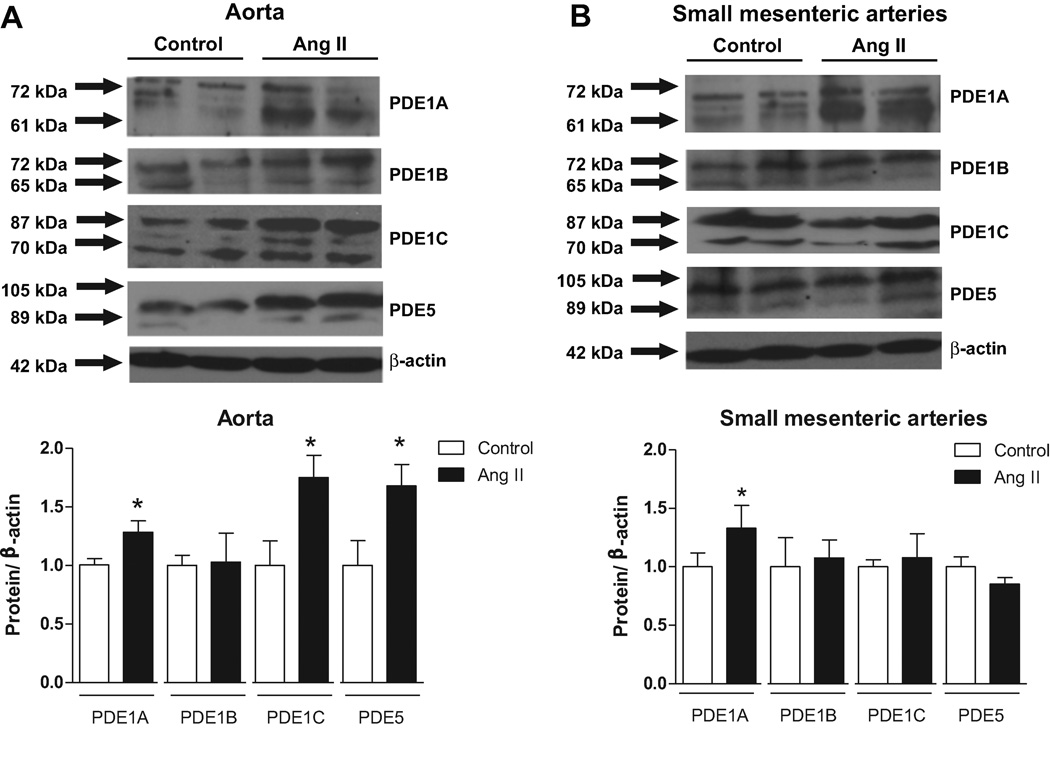
The vascular expression of PDE1s and PDE5 isoforms was accessed by western blot technique. Aortas from Ang II-infused rats displayed increased expression of PDE1A, PDE1C and PDE5 isoforms, compared to control rats (Figure 5A). Small-mesenteric arteries displayed increased expression of PDE1A, compared to control rats (Figure 5B).
Figure 5. PDE1 vascular expression is increased in arteries from Ang II hypertensive rats.
Above, representative western blot images of PDE1A, PDE1B, PDE1c and PDE5. Bellow, corresponding graphs showing the relative expression of PDEs isoforms in (A) aorta and (B) small-mesenteric arteries from Ang II hypertensive (black bars) and control (white bars) rats. Data are mean ± SEM (n=6). * P<0.05 vs. respective control.
Effects of PDE1 inhibition on vascular cGMP production
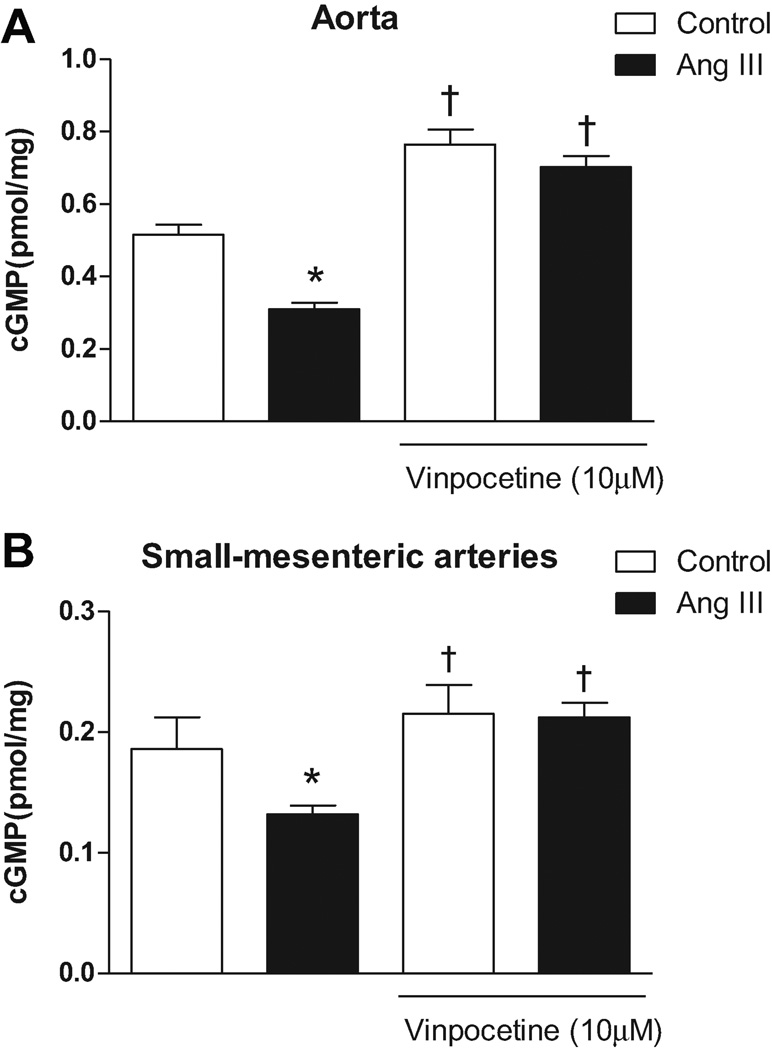
Basal cGMP production was decreased in aortas from Ang II rats compared to control (11.3±0.5 vs. 20.0±1.4pmol/ml, respectively; n=6 - p<0.0001). When aortas were incubated with vinpocetine (10µM), cGMP production was increased both in Ang II (27.5±1.9pmol/ml; n=6 – p<0.0001) as in control (31.1±1.1pmol/ml; n=6 – p<0.0001), and differences between the groups were abolished (Figure 6A). Similar results were observed in small-mesenteric arteries where those from Ang II rats had reduced cGMP levels compared to control (17.4±0.6 vs. 25.5±0.8pmol/ml, respectively; n=6 – p<0.001). Again, the PDE1 inhibitor increased cGMP levels in small-mesenteric arteries from both Ang II (29.7±0.9 pmol/ml; n=6 – p<0.0001) and control rats (26.8±0.9 pmol/ml; n=6 – p<0.0001), and differences between the groups were abolished after vinpocetine (Figure 6B).
Figure 6. PDE-1 inhibition restores cGMP bioavailability in arteries from Ang II hypertensive rats.
cGMP production was evaluated in the presence or absence of vinpocetine (10 µM) in (A) aorta and (B) small-mesenteric arteries from Ang II hypertensive (black bars) and control (white bars) rats. Data are mean ± SEM (n=6). * P<0.05 vs. control. † P<0.05 vs. respective vehicle group.
These data show that augmented activation of PDE1 isoforms in arteries from Ang II rats are contributing to decreased cGMP levels. In addition, PDE1 inhibition was able to abolish differences in cGMP levels between the groups.
DISCUSSION
Our major findings are that arteries from Ang II-hypertensive rats, compared to control rats, display increased PDE1 expression and activation, resulting in augmented PE-induced contractile maximal response, increased sensitivity to PE-stimuli, and impaired cGMP levels. Additionally, PDE1 inhibition abolished differences in the contractile responsiveness between the groups and improved cGMP levels. We found that PDE5 is increased in aorta from hypertensive rats. However, PDE5 inhibition decreased contractile response to PE in arteries from Ang II and control rats in a similar fashion. We speculate that PDE1 may represent a new mechanism by with Ang II enhances vasoconstriction, via cGMP-dependent pathways.
Ang II is critical for the regulation of vascular tone, blood pressure, and volume homeostasis. High levels of circulating Ang II lead to increased contractile activity of vascular SMC to agonistic stimuli, vascular growth, migration, apoptosis and extracellular matrix deposition, which are hallmarks for vascular changes observed during hypertension [22, 23].
cGMP functions as an antagonist of Ang II actions, by counteracting the Ang II signaling pathway at different steps [24, 25]. For example, cGMP has been shown to block Ang II-stimulated Ca2+ mobilization [26] and inhibit several proteins kinase that are activated by Ang II [14, 27].
Indeed, the functional interplay between Ang II and cGMP are determined by the mutual regulation of Ang II and cGMP signaling pathways at different levels, including (1) nitric oxide (NO) production, (2) guanylyl cyclase(GC) activation, (3) cGMP mediated PKG-activation and (4) PDE activation [25].
NO directly stimulates GC, which cause to GTP to be converted into cGMP [28]. Additionally, Ang II can stimulate eNOS (endothelial nitric oxide synthase) expression [29, 30]. In vivo experiments showed that Ang II infusion decreases NO production, but this is mainly due to the fact that eNOS is mainly in the uncoupled form, generating superoxide rather than NO [25, 31]. Hence, Ang II can negatively mediate GC expression [31] and enzymatic activity [32], due to superoxide [33] and peroxynitrite [34] related mechanisms. Moreover, Ang II decreases PKG activity, which is the principal target for cGMP [28].
As first mentioned, PDEs are important proteins that modulate cGMP bioavailability, by hydrolyzing and therefore inactivating the cyclic nucleotide. It has been reported that Ang II is able to increase PDE1 [35] and PDE5 [14] expression in vascular SMCs, resulting in lower cGMP level. Therefore, it seems that the global reduction of cGMP levels caused by Ang II infusion is due to the concomitant activation of PDEs and inactivation of GC, which impairs cGMP signaling pathway.
Our working hypothesis was driven by the observation that Ang II directly modulates PDE1, and its effect on cGMP production. We observed increased expression levels of PDE1A, PDE1C and PDE5 isoforms in arteries from Ang II rats. However, our functional data supported an important role for PDE1 as a contributor to increase vascular responsiveness and sensitivity to contractile stimuli in the arteries from the hypertensive rats, since inhibition of PDE5 decreased the contractile response similarly in both experimental groups.
Considering that these enzymes are coupled to distinct intracellular cGMP-dependent pathways, these results may reflect differential regulatory mechanisms for PDE5 and PDE1. PDE5 is primarily stimulated by NO induced cGMP whereas PDE1 is stimulated by increased intracellular Ca2+ and in this case, PDE1 is hydrolyzing cGMP predominantly during elevations in intracellular Ca2+ in response to agonist stimulation [6]. Our data showed that aortas from Ang II rats displayed increased contractile response to Ca2+, suggesting that this may be one possible mechanism that explains increased PDE1 activity.
Ang II stimulated Ca2+ signaling is complex and occurs via multiple pathways to elicit an integrated Ca2+ signal. It is well described that Ang II mediates augmented Ca2+ signaling in vascular SMC primarily by IP3-induced mobilization of intracellular Ca2+ and secondary by increasing Ca2+ entry [36]. Considering that PDE1 activation depends on Ca2+, it seems plausible that arteries from Ang II-rats may display increased PDE1 activity. Indeed, it was observed that nitrate-relaxation, which is mediated by NO/cGMP, is a process that can be desensitized, leading to nitrate-tolerance. This occurs by augmented expression of PDE1A associated with Ca2+ supersensitized cells [35].
Additionally, we showed that PDE1 specific inhibition was able to increase cGMP levels and to abolish the augmented contractile activity difference between arteries from hypertensive and normotensive rats. These are very exciting data, if we consider a previous report were vinpocetine, a PDE1 inhibitor, in the presence of inhalated nitric oxide, was able to enhance pulmonary vasodilation and transpulmonary cGMP without generating a systemic vasodilation [18]. Additionally, 8-methoxymethyl-3-isobutyl-1-methylxanthine, another PDE1 inhibitor, further reduced systemic arterial pressure induced by iloprost, a long-acting prostacyclin analogue [37]. Therefore, a combination of current therapies with PDE1 inhibition may be useful. Most recently, it was shown that vinpocetine is able to inhibit inflammation induced by tumor necrosis factor alpha (TNF-α), by PDE-independent mechanisms [38]. Given the importance of TNF-α in Ang II-induced inflammatory pathways, it seems interesting that a drug which acutely restores vascular contraction during Ang II-induced hypertension may play additional roles in vascular function. For that purpose, chronic studies using PDE1 inhibitors, such as vinpocetine, should be addressed.
It has been shown that smooth muscle cells express mainly three PDE isoforms, including PDE1 and PDE5 [39]. Our data show that Ang II increased PDE1A expression in both aorta and small-mesenteric arteries whereas PDE1C was only increased in aorta. PDE1C isoform is closely related to vascular remodeling and proliferation of human vascular SMCs, and it was suggested that this enzyme could be a target for treatment of atherosclerosis or restenosis after angioplasty [13, 40, 41]. However, whether Ang II exclusively regulates human SMCs proliferations is still unclear. Of importance, differential subcellular localization of the PDEs isoforms may account for differential cGMP regulation. Taking this into consideration, this new concept predicts that PDEs act as cyclic nucleotide diffusion barriers through their spatially confined zones of enzymatic activity, contributing to the subcellular compartmentalization of distinct signaling cascades [4]. In this regard, data from our laboratory propose that sGC is compartmentalized in the caveolae, in the cavernosal endothelium, and its spatial organization facilitates NO actions [42].
In conclusion, we propose that increased PDE1 expression and its activation, as a result of chronic Ang II infusion, contribute to impaired cGMP levels resulting in increased vascular response and sensitivity to contractile stimuli.
PERSPECTIVES
Regardless of the fact that there is a variety of pharmacological preparations available for therapy, a large number of hypertensive patients are refractory to these treatments. Therefore, new strategies to find new targets to treat hypertensive disease should be encouraged. Considering that PDE1 inhibition resulted in reduction of the maximum contraction response occurred in arteries from Ang II hypertensive rats, it seems that this may be an interesting target to improve vascular functionality in hypertensive subjects. Therefore, we aim to investigate how long-term PDE1 inhibition may change during hypertension. In addition, we would like to see whether PDE1 activation can interfere with other signaling pathways that are modified in hypertension, such as mitogen activated protein kinase activation.
Acknowledgments
Sources of Funding:
This study was supported by grants from National Institutes of Health (NIH - HL71138 and DK83685).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict(s) of interest(s)/Disclosure(s) statement:
The authors state no conflict(s) of interest(s)/disclosures(s).
REFERENCES
- 1.Rybalkin SD, Yan C, Bornfeldt KE, Beavo JA. Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circ Res. 2003;93:280–291. doi: 10.1161/01.RES.0000087541.15600.2B. [DOI] [PubMed] [Google Scholar]
- 2.Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther. 2006;109:366–398. doi: 10.1016/j.pharmthera.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Surks HK, Mochizuki N, Kasai Y, Georgescu SP, Tang KM, Ito M, Lincoln TM, Mendelsohn ME. Regulation of myosin phosphatase by a specific interaction with cGMP- dependent protein kinase Ialpha. Science. 1999;286:1583–1587. doi: 10.1126/science.286.5444.1583. [DOI] [PubMed] [Google Scholar]
- 4.Beavo JA, Brunton LL. Cyclic nucleotide research -- still expanding after half a century. Nat Rev Mol Cell Biol. 2002;3:710–718. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- 5.Schlossmann J, Ammendola A, Ashman K, Zong X, Huber A, Neubauer G, Wang GX, Allescher HD, Korth M, Wilm M, Hofmann F, Ruth P. Regulation of intracellular calcium by a signalling complex of IRAG, IP3 receptor and cGMP kinase Ibeta. Nature. 2000;404:197–201. doi: 10.1038/35004606. [DOI] [PubMed] [Google Scholar]
- 6.Miller CL, Oikawa M, Cai Y, Wojtovich AP, Nagel DJ, Xu X, Xu H, Florio V, Rybalkin SD, Beavo JA, Chen YF, Li JD, Blaxall BC, Abe J, Yan C. Role of Ca2+/calmodulin-stimulated cyclic nucleotide phosphodiesterase 1 in mediating cardiomyocyte hypertrophy. Circ Res. 2009;105:956–964. doi: 10.1161/CIRCRESAHA.109.198515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koyama H, Bornfeldt KE, Fukumoto S, Nishizawa Y. Molecular pathways of cyclic nucleotide-induced inhibition of arterial smooth muscle cell proliferation. J Cell Physiol. 2001;186:1–10. doi: 10.1002/1097-4652(200101)186:1<1::AID-JCP1012>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 8.Sonnenburg WK, Rybalkin SD, Bornfeldt KE, Kwak KS, Rybalkina IG, Beavo JA. Identification, quantitation, and cellular localization of PDE1 calmodulin-stimulated cyclic nucleotide phosphodiesterases. Methods. 1998;14:3–19. doi: 10.1006/meth.1997.0561. [DOI] [PubMed] [Google Scholar]
- 9.Huang CY, Chau V, Chock PB, Wang JH, Sharma RK. Mechanism of activation of cyclic nucleotide phosphodiesterase: requirement of the binding of four Ca2+ to calmodulin for activation. Proc Natl Acad Sci U S A. 1981;78:871–874. doi: 10.1073/pnas.78.2.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wells JN, Baird CE, Hardman YJ, Wu JG. Cyclic nucleotide phosphodiesterase activities of pig coronary arteries. Biochim Biophys Acta. 1975;384:430–442. doi: 10.1016/0005-2744(75)90044-3. [DOI] [PubMed] [Google Scholar]
- 11.Schermuly RT, Pullamsetti SS, Kwapiszewska G, Dumitrascu R, Tian X, Weissmann N, Ghofrani HA, Kaulen C, Dunkern T, Schudt C, Voswinckel R, Zhou J, Samidurai A, Klepetko W, Paddenberg R, Kummer W, Seeger W, Grimminger F. Phosphodiesterase 1 upregulation in pulmonary arterial hypertension: target for reverse-remodeling therapy. Circulation. 2007;115:2331–2339. doi: 10.1161/CIRCULATIONAHA.106.676809. [DOI] [PubMed] [Google Scholar]
- 12.Touyz RM. Recent advances in intracellular signalling in hypertension. Curr Opin Nephrol Hypertens. 2003;12:165–174. doi: 10.1097/00041552-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Rybalkin SD, Rybalkina I, Beavo JA, Bornfeldt KE. Cyclic nucleotide phosphodiesterase 1C promotes human arterial smooth muscle cell proliferation. Circ Res. 2002;90:151–157. doi: 10.1161/hh0202.104108. [DOI] [PubMed] [Google Scholar]
- 14.Kim D, Aizawa T, Wei H, Pi X, Rybalkin SD, Berk BC, Yan C. Angiotensin II increases phosphodiesterase 5A expression in vascular smooth muscle cells: a mechanism by which angiotensin II antagonizes cGMP signaling. J Mol Cell Cardiol. 2005;38:175–184. doi: 10.1016/j.yjmcc.2004.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahn HS, Crim W, Romano M, Sybertz E, Pitts B. Effects of selective inhibitors on cyclic nucleotide phosphodiesterases of rabbit aorta. Biochem Pharmacol. 1989;38:3331–3339. doi: 10.1016/0006-2952(89)90631-x. [DOI] [PubMed] [Google Scholar]
- 16.Hagiwara M, Endo T, Hidaka H. Effects of vinpocetine on cyclic nucleotide metabolism in vascular smooth muscle. Biochem Pharmacol. 1984;33:453–457. doi: 10.1016/0006-2952(84)90240-5. [DOI] [PubMed] [Google Scholar]
- 17.Souness JE, Brazdil R, Diocee BK, Jordan R. Role of selective cyclic GMP phosphodiesterase inhibition in the myorelaxant actions of M&B 22,948, MY-5445, vinpocetine and 1-methyl-3-isobutyl-8-(methylamino)xanthine. Br J Pharmacol. 1989;98:725–734. doi: 10.1111/j.1476-5381.1989.tb14599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evgenov OV, Busch CJ, Evgenov NV, Liu R, Petersen B, Falkowski GE, Petho B, Vas A, Bloch KD, Zapol WM, Ichinose F. Inhibition of phosphodiesterase 1 augments the pulmonary vasodilator response to inhaled nitric oxide in awake lambs with acute pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2006;290:L723–L729. doi: 10.1152/ajplung.00485.2004. [DOI] [PubMed] [Google Scholar]
- 19.Montorsi F, Corbin J, Phillips S. Review of phosphodiesterases in the urogenital system: new directions for therapeutic intervention. J Sex Med. 2004;1:322–336. doi: 10.1111/j.1743-6109.04047.x. [DOI] [PubMed] [Google Scholar]
- 20.Priviero FB, Jin LM, Ying Z, Teixeira CE, Webb RC. Up-regulation of the RhoA/Rho-kinase signaling pathway in corpus cavernosum from endothelial nitric-oxide synthase (NOS), but not neuronal NOS, null mice. J Pharmacol Exp Ther. 333:184–192. doi: 10.1124/jpet.109.160606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teixeira CE, Priviero FB, Webb RC. Effects of 5-cyclopropyl-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-yl]pyrim idin-4-ylamine (BAY 41-2272) on smooth muscle tone, soluble guanylyl cyclase activity, and NADPH oxidase activity/expression in corpus cavernosum from wild-type, neuronal, and endothelial nitric-oxide synthase null mice. J Pharmacol Exp Ther. 2007;322:1093–1102. doi: 10.1124/jpet.107.124594. [DOI] [PubMed] [Google Scholar]
- 22.Touyz RM. Molecular and cellular mechanisms in vascular injury in hypertension: role of angiotensin II. Curr Opin Nephrol Hypertens. 2005;14:125–131. doi: 10.1097/00041552-200503000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt-Ott KM, Kagiyama S, Phillips MI. The multiple actions of angiotensin II in atherosclerosis. Regul Pept. 2000;93:65–77. doi: 10.1016/s0167-0115(00)00178-6. [DOI] [PubMed] [Google Scholar]
- 24.Kemp-Harper B, Schmidt HH. cGMP in the vasculature. Handb Exp Pharmacol. 2009:447–467. doi: 10.1007/978-3-540-68964-5_19. [DOI] [PubMed] [Google Scholar]
- 25.Yan C, Kim D, Aizawa T, Berk BC. Functional interplay between angiotensin II and nitric oxide: cyclic GMP as a key mediator. Arterioscler Thromb Vasc Biol. 2003;23:26–36. doi: 10.1161/01.atv.0000046231.17365.9d. [DOI] [PubMed] [Google Scholar]
- 26.Komalavilas P, Lincoln TM. Phosphorylation of the inositol 1,4,5-trisphosphate receptor by cyclic GMP-dependent protein kinase. J Biol Chem. 1994;269:8701–8707. [PubMed] [Google Scholar]
- 27.Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Smolenski A, Lohmann SM, Bertoglio J, Chardin P, Pacaud P, Loirand G. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J Biol Chem. 2000;275:21722–21729. doi: 10.1074/jbc.M000753200. [DOI] [PubMed] [Google Scholar]
- 28.Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev. 2000;52:375–414. [PubMed] [Google Scholar]
- 29.Saito S, Hirata Y, Emori T, Imai T, Marumo F. Angiotensin II activates endothelial constitutive nitric oxide synthase via AT1 receptors. Hypertens Res. 1996;19:201–206. doi: 10.1291/hypres.19.201. [DOI] [PubMed] [Google Scholar]
- 30.Olson SC, Dowds TA, Pino PA, Barry MT, Burke-Wolin T. ANG II stimulates endothelial nitric oxide synthase expression in bovine pulmonary artery endothelium. Am J Physiol. 1997;273:L315–L321. doi: 10.1152/ajplung.1997.273.2.L315. [DOI] [PubMed] [Google Scholar]
- 31.Mollnau H, Wendt M, Szocs K, Lassegue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Forstermann U, Meinertz T, Griendling K, Munzel T. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res. 2002;90:E58–E65. doi: 10.1161/01.res.0000012569.55432.02. [DOI] [PubMed] [Google Scholar]
- 32.Haneda M, Kikkawa R, Maeda S, Togawa M, Koya D, Horide N, Kajiwara N, Shigeta Y. Dual mechanism of angiotensin II inhibits ANP-induced mesangial cGMP accumulation. Kidney Int. 1991;40:188–194. doi: 10.1038/ki.1991.199. [DOI] [PubMed] [Google Scholar]
- 33.Kim SM, Byun JS, Jung YD, Kang IC, Choi SY, Lee KY. The effects of oxygen radicals on the activity of nitric oxide synthase and guanylate cyclase. Exp Mol Med. 1998;30:221–226. doi: 10.1038/emm.1998.32. [DOI] [PubMed] [Google Scholar]
- 34.Weber M, Lauer N, Mulsch A, Kojda G. The effect of peroxynitrite on the catalytic activity of soluble guanylyl cyclase. Free Radic Biol Med. 2001;31:1360–1367. doi: 10.1016/s0891-5849(01)00706-7. [DOI] [PubMed] [Google Scholar]
- 35.Kim D, Rybalkin SD, Pi X, Wang Y, Zhang C, Munzel T, Beavo JA, Berk BC, Yan C. Upregulation of phosphodiesterase 1A1 expression is associated with the development of nitrate tolerance. Circulation. 2001;104:2338–2343. doi: 10.1161/hc4401.098432. [DOI] [PubMed] [Google Scholar]
- 36.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52:639–672. [PubMed] [Google Scholar]
- 37.Schermuly RT, Inholte C, Ghofrani HA, Gall H, Weissmann N, Weidenbach A, Seeger W, Grimminger F. Lung vasodilatory response to inhaled iloprost in experimental pulmonary hypertension: amplification by different type phosphodiesterase inhibitors. Respir Res. 2005;6:76. doi: 10.1186/1465-9921-6-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeon KI, Xu X, Aizawa T, Lim JH, Jono H, Kwon DS, Abe J, Berk BC, Li JD, Yan C. Vinpocetine inhibits NF-kappaB-dependent inflammation via an IKK-dependent but PDE-independent mechanism. Proc Natl Acad Sci U S A. 107:9795–9800. doi: 10.1073/pnas.0914414107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- 40.Rybalkin SD, Bornfeldt KE, Sonnenburg WK, Rybalkina IG, Kwak KS, Hanson K, Krebs EG, Beavo JA. Calmodulin-stimulated cyclic nucleotide phosphodiesterase (PDE1C) is induced in human arterial smooth muscle cells of the synthetic, proliferative phenotype. J Clin Invest. 1997;100:2611–2621. doi: 10.1172/JCI119805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palmer D, Maurice DH. Dual expression and differential regulation of phosphodiesterase 3A and phosphodiesterase 3B in human vascular smooth muscle: implications for phosphodiesterase 3 inhibition in human cardiovascular tissues. Mol Pharmacol. 2000;58:247–252. doi: 10.1124/mol.58.2.247. [DOI] [PubMed] [Google Scholar]
- 42.Linder AE, Leite R, Lauria K, Mills TM, Webb RC. Penile erection requires association of soluble guanylyl cyclase with endothelial caveolin-1 in rat corpus cavernosum. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1302–R1308. doi: 10.1152/ajpregu.00601.2005. [DOI] [PubMed] [Google Scholar]