Abstract
Background
Cervical cancer is the second largest cause of cancer deaths in women worldwide. It is now evident that persistent infection with high-risk human papillomavirus (HPV) is necessary for the development and maintenance of cervical cancer. Thus, effective vaccination against HPV represents an opportunity to restrain cervical cancer and other important cancers. The FDA recently approved the HPV vaccine Gardasil for the preventive control of HPV, using HPV virus-like particles (VLP) to generate neutralizing antibodies against major capsid protein, L1. However, prophylactic HPV vaccines do not have therapeutic effects against pre-existing HPV infections and HPV-associated lesions. Furthermore, due to the considerable burden of HPV infections worldwide, it would take decades for preventive vaccines to affect the prevalence of cervical cancer. Thus, in order to speed up the control of cervical cancer and treat current infections, the continued development of therapeutic vaccines against HPV is critical. Therapeutic HPV vaccines can potentially eliminate pre-existing lesions and malignant tumors by generating cellular immunity against HPV-infected cells that express early viral proteins such as E6 and E7.
Objective
This review discusses the future directions of therapeutic HPV vaccine approaches for the treatment of established HPV-associated malignancies, with emphasis on current progress of HPV vaccine clinical trials.
Methods
Relevant literature is discussed.
Results/conclusion
Though their development has been challenging, many therapeutic HPV vaccines have been shown to induce HPV-specific antitumor immune responses in preclinical animal models and several promising strategies have been applied in clinical trials. With continued progress in the field of vaccine development, HPV therapeutic vaccines may provide a potentially promising approach for the control of lethal HPV-associated malignancies.
Keywords: clinical trials, HPV, human papillomavirus, therapeutic, vaccines
1. Introduction
Human papillomavirus (HPV) has been identified as an etiological factor for several important cancers, including anogenital cancers [1], and a subset of head and neck cancers (for review see [2]). Among these cancers, cervical cancer has the most significant morbidity, being the second most common cancer in women with an estimated half a million new cases diagnosed and ~ 274,000 deaths per year [3]. Of the > 200 HPV genotypes identified, four ‘high-risk’ types of HPV (16, 18, 31 and 45) are of particular importance because of their high level of association with ~ 80% of all cervical cancers [4]. Of these high-risk types, HPV type 16 (HPV-16) is the most prevalent, being present in more than half of all cervical cancers [5]. Furthermore, high-risk HPV types are associated with high-grade squamous intraepithelial lesions (HSILs), which are precursors of cervical cancer. The identification of HPV as an etiological factor for cervical cancer has created an opportunity to control cervical cancer through vaccination against HPV.
In order to successfully design vaccines against HPV, it is essential to have a clear understanding of HPV biology. The genomic organization of HPV is quite conserved among various types of papillomaviruses. HPVs are non-enveloped, double-stranded, circular DNA viruses of the family Papovaviridae whose genome encodes six ‘early’ proteins (E1, E2, E4, E5, E6 and E7) and two ‘late’ proteins (L1 and L2). The early proteins interact with cellular gene products and facilitate viral DNA replication, while the late proteins comprise the structural components of the viral capsid involved in the packaging of new virions. Though HPV DNA usually replicates in episomal form, it may integrate into host DNA, which often results in the deletion of some viral genes, including several early (E2, E4 and E5) and late (L1 and L2) genes, leaving E6 and E7 as the principal proteins expressed within the infected cell. Because E2 is a transcriptional repressor of E6 and E7, loss of E2 leads to upregulation of the E6 and E7 genes. The E6 and E7 proteins interact with the p53 and retinoblastoma (Rb) proteins respectively, which are important cell cycle regulatory proteins. The uncontrolled expression of E6 and E7 proteins results in the disruption of cell cycle regulation and leads to genomic instability, thereby contributing to the progression of HPV-associated cervical cancer (for a review, see [6]).
Based on our understanding of HPV biology, we realize that for the prevention of HPV infections it is necessary to develop vaccines that are capable of generating HPV-neutralizing antibodies. The newly licensed HPV preventive vaccine, Gardasil represents a triumph for HPV preventive vaccine development. Gardasil is a quadrivalent HPV L1 virus-like particle (VLP) recombinant vaccine produced by Merck that protects against HPV types 6, 11, 16 and 18. Likewise, the other HPV L1 VLP vaccine, Cervarix developed by Glaxo SmithKline that contains HPV types 16 and 18 is also expected to be available in the market soon. In general, these vaccines provide type-restricted protection, that is they protect against cervical disease relating to the HPV types included in the vaccine but not against other HPV types [7,8]. However, partial cross-protection has been observed for closely related HPV types. For example, vaccination with HPV VLPs against HPV types 16 and 18 also induces protection against HPV types 31 and 45 as well [9,10]. Gardasil and Cervarix have demonstrated excellent safety profiles and are highly effective against the included HPV types. Since HPV-16 and 18 account for ~ 70 – 75% of cervical cancers, Gardasil and Cervarix may protect up to 80% of all cervical cancers, including the partial protection against closely related types (HPV types 31 and 45).
However, Gardasil and Cervarix are unlikely to have an effect on the incidence of cervical cancer. Since cervical cancer has a high prevalence in developing countries, vaccines need to be made available in low-resource areas in order to affect the incidence of cervical cancer worldwide. Gardasil, which costs several hundred US dollars per person and Cervarix, which is expected to cost a similar amount, may not be ideal for low-resource areas. Furthermore, the current HPV L1 VLP preventive vaccines require refrigeration for storage, which might be problematic in remote and low-resource areas. Thus, in low-resource settings, the relative benefits of these vaccines may be restricted. In order to have an effect on the incidence of cervical cancer, it is therefore necessary to develop cost-effective, stable and effective preventive vaccines that are capable of inducing broader protection against most HPV types and which are suitable for low-resource areas.
Another important obstacle to the elimination of cervical cancer is the prevalence of established HPV infections and HPV-associated disease. The existing HPV L1 VLP vaccines, Gardasil and Cervarix, do not generate therapeutic effects against pre-existing HPV infection. Since infected basal epithelial cells and cervical cancers cells do not express detectable levels of capsid antigen (L1 and/or L2), preventive HPV vaccines targeting L1 and/or L2 are unlikely to be effective in the elimination of pre-existing infection and HPV-related disease. This is a serious concern since there is currently a considerable burden of HPV infections worldwide. It is estimated that it would take ~ 20 years from the implementation of mass vaccination for highly effective preventive vaccines to affect the cervical cancer rates due to the prevalence of a significant population with existing HPV infections and the slow process of carcinogenesis. Thus, in order to accelerate the control of cervical cancer and treat currently infected patients, it is important to develop therapeutic vaccines against HPV.
2. Therapeutic human papillomavirus vaccines
Therapeutic vaccines can be used to treat established HPV infections and could therefore have an immediate effect on the prevalence of HPV-associated malignancies. Therapeutic vaccine strategies aim to eliminate pre-existing lesions and even malignant tumors by generating cell-mediated immunity against HPV-infected cells. Current approaches include live-vector-based, peptide- and protein-based, nucleic acid-based and cell-based vaccines. Table 1 summarizes the strengths and weaknesses of each strategy and Table 2 summarizes the current strategies to enhance the potency of each of these vaccine approaches. This review discusses the future directions of therapeutic HPV vaccine approaches for the treatment of established HPV-associated malignancies, with emphasis on current progress of HPV vaccine clinical trials. Table 3 lists the various therapeutic HPV vaccine clinical trials.
Table 1.
Characteristics of therapeutic HPV vaccine approaches.
| Vaccine approach | Advantages | Disadvantages |
|---|---|---|
| Viral-vector-based (i.e., vaccinia, adenovirus, alphavirus) | High immunogenicity Different immunological properties of viruses Wide variety of vectors available Can potentially be engineered to express cytokines or other stimulatory molecules |
Risk of toxicity in using live viruses Potential pre-existing immunity Inhibited repeat immunization Immunodominance of viral vector antigens over HPV tumor antigens |
| Bacterial-vector-based (i.e., Listeria, Salmonella, Lactococcus) | High immunogenicity Can deliver either engineered plasmids or HPV tumor proteins to antigen-presenting cells Wide variety of vectors available |
Risk of toxicity in using live bacteria Potential pre-existing immunity Inhibited repeat immunization |
| Peptide based | Stable, easy to produce, safe Can combine multiple epitopes Can enhance peptides for MHC binding |
Low immunogenicity Must determine epitopes Must match patient’s HLA |
| Protein based | Stable, easy to produce, safe Multiple known adjuvants No HLA restriction |
Usually better induction of antibody response than CTL response |
| DNA | Easy to produce, store and transport Versatility in ability to add targeting and/or co-stimulatory genes Capable of multiple immunizations Variety of delivery methods (i.e., direct injection, gene gun, intranasal, biojector) Sustained expression of antigen on MHC-peptide complex (compared with peptide/protein vaccines) |
Intrinsically weak immunogenicity Concern of integration into genome or cellular transformation |
| RNA | Non-infectious, transient; no risk of chromosomal integration or cellular transformation Capable of multiple immunizations RNA replicons replicate within the cell to enhance antigen expression Multiple vectors are available |
Difficult to store and handle Labor-intensive preparation Difficult to prepare large amounts |
| DC based | High immunogenicity; uses the most potent antigen-presenting cells Methods are available to generate large numbers of DCs Multiple methods of antigen loading available Potency can be enhanced by gene transduction or cytokine treatment |
Labor intensive, costly, ex vivo, individualized cell processing Variable quality control and a lack of standard criteria for quality of vaccines Do not necessarily home to draining lymph nodes Possibility of tolerization by immature DCs |
| Tumor cell based | Useful if tumor antigen unknown Potency can be enhanced by gene transduction or cytokine treatment Likely to express relevant tumor antigens |
Safety concerns about injecting tumor cells into patients Labor-intensive procedure Weak antigen presentation by tumor cells Requires availability of tumor cell lines or autologous tumor cells |
DC: Dendritic cell; HPV: Human papillomavirus.
Table 2.
Strategies to enhance therapeutic HPV vaccine potency.
| Vaccine approach | Improvement strategies |
|---|---|
| Viral-vector-based (i.e., vaccinia, adenovirus, alphavirus) | Adjuvant and fusion protein strategies to further augment immunogenicity Circumvent neutralizing antibodies |
| Bacterial-vector-based (i.e., Listeria, Salmonella, Lactococcus) | Adjuvant and fusion protein strategies to further augment immunogenicity Circumvent neutralizing antibodies |
| Peptide-based | Epitope enhancement Lipopeptide formulations Adjuvant and fusion protein strategies to enhance immunogenicity |
| Protein-based | Adjuvant and fusion protein strategies to enhance immunogenicity and CTL response |
| DNA | Increase number of antigen-expressing DCs Enhance antigen processing and presentation in DCs Improve DC interaction with T cells to augment T-cell-mediated immune responses Enhance DC activation |
| RNA | Combine with DNA for use as DNA-launched RNA replicons |
| DC based | Improve efficient loading of antigen to DCs Prolong DC survival |
| Tumor cell based | Address safety concerns Manipulate ex vivo to express cytokines |
CTL: Cytotoxic T lymphocyte; DC: Dendritic cell; HPV: Human papillomavirus.
Table 3.
Therapeutic HPV vaccine clinical trials.
| Type | Vaccine construct |
Target subtype(s) |
Antigen | Sponsor | Study subjects | Ref. |
|---|---|---|---|---|---|---|
| Live vector based | Recombinant Vaccinia Virus (TA-HPV) | HPV-16, HPV-18 | E6 and E7 mutated to inactivate Rb binding | Xenova/Cantab | Phase I/II trial in patients with advanced cervical cancer | [27] |
| Phase I with early-stage cervical cancer patients | [29] | |||||
| Phase II in patients with high-grade VIN or VAIN | [32] | |||||
| Phase II with high grade VIN or VAIN patients | [30] | |||||
| Phase I/II in patients with high grade VIN | [28] | |||||
| Recombinant Vaccinia Virus (MVA-E2) | HPV-16, HPV-18 | E2 | Instituto Mexicano del Seguro Social (IMSS) | Phase I/II trial in patients with CIN1–3 | [33] | |
| Phase II with high grade CIN | [34] | |||||
| Phase I/II in patients with flat con dyloma lesions | [35] | |||||
| Peptide based | Peptide | HPV-16 | E7 epitopes (aa 11 – 22 and 86 – 93) and PADRE | Netherlands National Institute of Public Health and Environmental Protection | Phase I/II trial in patients with cervical cancer | [43] |
| Phase I/II trial in patients with advanced cervical cancer | [44] | |||||
| Peptide | HPV-16 | E7 epitopes (aa 12 – 20 alone or aa 12 – 20 mixed with aa 86 – 93 linked to a T cell epitope peptide) | NCI | Phase I trial in patients with CIN 2/3 or VIN 2/3 | [45] | |
| Lipopeptide | HPV-16 | Lipidated E7 (HLA-A * 0201-restricted epitope, aa 86 – 93 lipopeptide) | NCI | Phase I trial in patients with cervical or vaginal cancer | [46] | |
| Protein based | Fusion protein | HPV-16 | Recombinant E6/E7 protein with ISCOMATRIX adjuvant | CSL Limited | Phase I trial in patients with CIN 1–3 | [57] |
| Fusion protein (PD-E7/D16E7) | HPV-16 | E7 linked to the first 108 aa of Haemophilus influenzae protein D | GlaxoSmithKline | Phase I/II trial in patients with CIN1 or CIN3 | [59] | |
| Fusion protein (TA-GW) | HPV-6 | L2/E7 | Xenova/Cantab | Phase I trial in healthy volunteers | [56] | |
| Phase IIa trial in patients with genital warts | [55] | |||||
| Fusion protein (TA-CIN) | HPV-6 | L2/E6/E7 | Xenova/Cantab | Phase I trial in healthy volunteers | [58] | |
| Fusion protein (SGN-00101 or HSPE7) | HPV-16 | E7 linked to HSP65 from Mycobacterium bovis | StressGen | Phase I/II trial in HIV patients with AIN | [61] | |
| Phase III trial in patients with AIN and Phase II trial in patients with AGW and RRP | [60] | |||||
| DNA based | DNA (ZYC101) | HPV-16 | E7 epitope (aa 83 – 95) | Zycos | Phase I trial in patients with anal HSIL | [91] |
| Phase I trial in patients with CIN2/3 | [90] | |||||
| DNA (ZYC101a) | HPV-16, HPV-18 | E6 and E7 epitopes | Zycos | Phase II trial in patients with CIN2/3 | [92] | |
| DNA | HPV-16 HPV-16 | E7 contained in the pNGVL4a-Sig/E7(detox)/HSP70 plasmid | NCI | Clinical trials have begun for patients with CIN2/3 | * | |
| Clinical trials to begin soon for patients with advanced HNSCC | § | |||||
| DC based | DC | HPV-18 | E7 | NA | Case report of a single patient with cervical cancer | [111] |
| DC | HPV-16, HPV-18 | Autologous DCs pulsed with rHPV16E7 or HPV18E7 | NA | Trial in cervical cancer patients with recurrent disease refractory to standard treatment modalities | [113] | |
| DC | HPV-16, HPV-18 | Autologous DCs pulsed with rHPV16E7 or HPV18E7 | NA | Clinical pilot study in patients with late stage cervical cancer | [112] | |
| Prime boost | Prime with fusion protein (TA-CIN), boost with recombinant vaccinia virus (TA-HPV) | HPV-16, HPV-18 | L2/E6/E7 (TA-CIN) + E6 and E7 mutated to inactivate Rb binding (TA-HPV) | Xenova | Phase II trial in patients with AGIN 3 | [125] |
| Phase II trial in patients with high-grade AGIN | [126] | |||||
| Prime with recombinant vaccinia virus (TA-HPV), boost with fusion protein (TA-CIN) | HPV-16, HPV-18 | E6 and E7 mutated to inactivate Rb binding (TA-HPV) + L2/E6/E7 (TA-CIN) | Xenova | Phase I/II trial in patients with high grade AGIN | [127] |
C Trimble, pers. commun.
M Gillison, pers. commun.
AA: Amino acids; AGIN: Anogenital intraepithelial neoplasia; AGW: Anogenital warts; AIN: Anal intraepithelial neoplasia; CIN: Cervical intraepithelial neoplasia; CRT: Calreticulin; DC: Dendritic cell; HLA: Human leukocyte antigen; HNSCC: Head and neck squamous cell carcinoma; HPV: Human papillomavirus; HSIL: High-grade squamous intraepithelial lesion; HSP: Heat-shock protein; LGIN: Lower genital tract intraepithelial neoplasia; NCI: National Cancer Institute; PADRE: Pan-DR binding T helper peptide; RRP: Recurrent respiratory papillomatosis (warts of the upper airways); VAIN: Vaginal intraepithelial neoplasia; VIN: Vulvar intraepithelial neoplasia.
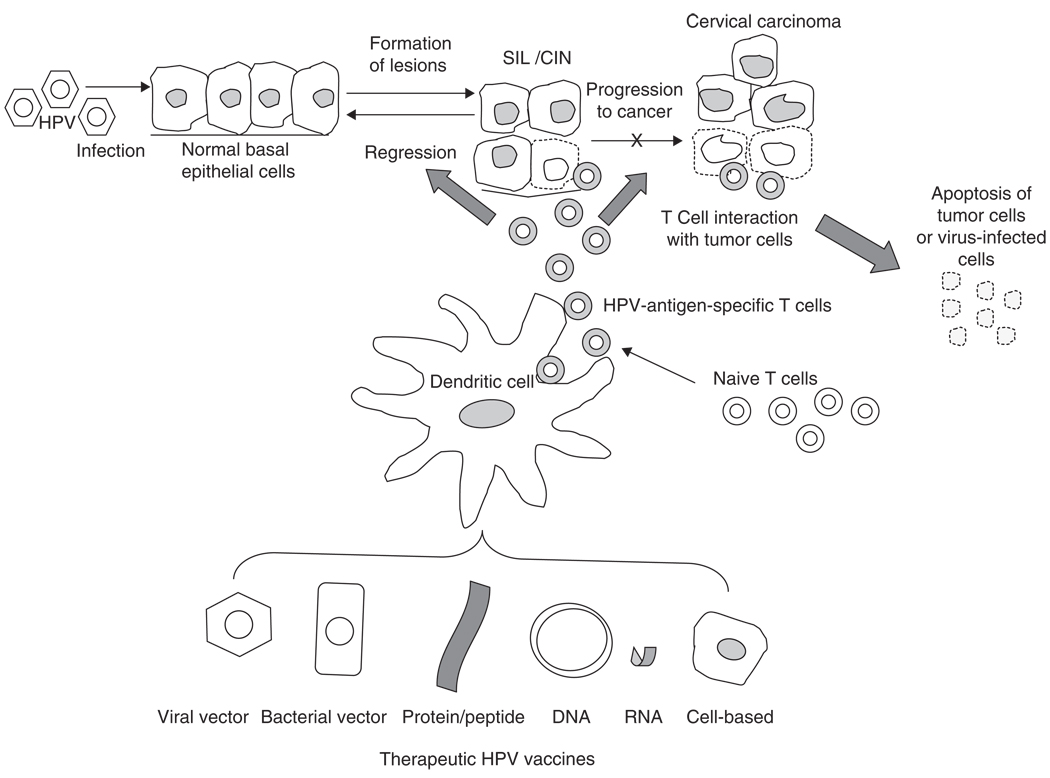
In order to eliminate existing lesions, a therapeutic vaccine should target HPV antigens that are continuously expressed in the infected cells and cancer cells. Thus, the choice of target antigen is extremely important for therapeutic HPV vaccine design. The HPV early proteins are potential target antigens since they are expressed throughout the life cycle and help regulate progression of the disease. In particular, the HPV-encoded proteins E6 and E7 represent ideal targets for the development of therapeutic HPV vaccines. Firstly, E6 and E7 proteins are constitutively expressed by HPV-associated tumors. Secondly, because E6 and E7 are critical for the induction and maintenance of cellular transformation in HPV-infected cells, it is unlikely that the tumor cells can escape immune attack through antigen loss. Thirdly, as E6 and E7 are foreign proteins, immunization against HPV-associated tumors circumvents some common cancer-vaccine-associated problems such as immune tolerance. Thus, many therapeutic HPV vaccine strategies have focused primarily on stimulating the production and activation of T cells by targeting E6 and/or E7 proteins Figure 1 summarizes various therapeutic HPV vaccine strategies and their effect on HPV progress.
Figure 1. Therapeutic HPV vaccines and HPV progression.
Microtrauma is believed to allow HPV to access basal epithelial cells. HPV infection promotes epithelial cell proliferation leading to SIL or CIN, and in some cases, progressing to invasive cervical carcinoma. This diagram provides an overview of the immunologic effects of therapeutic vaccination with live vector-based (viral/bacterial), protein or peptide-based, DNA or RNA-based or cell-based vaccines (DCs or tumor cells). DCs are the most potent professional APCs that prime T cells in vivo. DCs also migrate to secondary lymphoid organs to select and stimulate antigen-specific T cells. Thus, many therapeutic vaccine strategies have focused on targeting antigen to professional APCs, such as DCs, and enhancing antigen processing and presentation in DCs in order to augment T-cell-mediated immune responses. DCs activate the HPV-antigen-specific CTLs. These CTLs mediate immune clearance by apoptosis of virus-infected cells, thus blocking progression to cervical cancer or inducing regression of CIN lesions.
APC: Antigen-presenting cell; CIN: Cervical intraepithelial neoplasia; CTL: Cytotoxic T lymphocyte; DC: Dendritic cell; HPV: Human papillomaviurs; SIL: Squamous intraepithelial lesions.
2.1 Live-vector-based vaccines
Live-vector-based vaccines can be classified into: i) bacterial vectors, such as Salmonella typhimurium and Listeria monocytogenes; and ii) viral vectors, such as adenovirus (AdV) and vaccinia virus. The use of vector-based vaccines for the delivery of antigen to dendritic cells (DC) is an appealing strategy for HPV vaccination. They can be used to express HPV E6 and/or E7 for the treatment of HPV-associated malignancies. Many live-vector vaccines are highly immunogenic because they can replicate within host cells and facilitate intercellular spread of antigen. In addition, the wide variety of available vectors allows for the use of custom vectors that produce a desired effect.
Though live-vector-based vaccines have strong immunogenicity and are able to spread antigen efficiently from cell to cell, there are several potential limitations that must be addressed. The production of neutralizing antibodies in the host during vaccination could reduce the potency of repeat immunizations. In addition, there is the potential risk of toxicity associated with the use of live vectors in human patients. Vaccination with live vectors may also elicit immunosuppressive factors in the host; eliminating these factors may improve the efficacy of these vaccines.
2.1.1 Bacterial vectors
Attenuated bacteria can be used to deliver genes or proteins of interest to DCs. Examples include Listeria monocytogenes, Salmonella, Lactococcus lactis [11,12], Lactobacillus plantarum [13], and bacillus Calmette-Guérin. Bacterial vector-based vaccines have been shown to be capable of eliciting strong E7-specific T-cell-mediated immune responses. In particular, L. monocytogenes has emerged as an exciting prospect as a live-vector-based vaccine due to its ability to generate both CD8+ and CD4+ immune responses and induce regression of established tumors expressing a model antigen. L. monocytogenes is a Gram-positive intracellular bacterium that usually infects macrophages. Unlike other intracellular pathogens, however, it evades phagocytosis by macrophages by secreting a factor, listeriolysin O, to escape into the cytoplasm of the macrophage. Its presence in both the endosomal compartments and the cytoplasm allows it to deliver antigens or carry foreign antigens into both MHC-I and MHC-II pathways, inducing strong cellular immune responses. Recently, it has been shown that Listeria-based vaccines against E7 can induce the regression of solid implanted tumors in transgenic mice with tissue-specific expression of HPV 16 E6 and E7 oncoproteins and overcome central tolerance by expanding low avidity CD8+ T cells specific for E7 [14]. Several strategies employing fusion proteins have been used to enhance the Listeria-based vaccine potency [15,16]. Recently, a Phase I/II clinical trial is currently ongoing using the Listeria-based therapeutic HPV vaccine targeting the HPV E7 antigen in patients with cervical cancer (Y Paterson, pers. commun.).
2.1.2 Viral vectors
The high immunogenecity of viral vectors has also been harnessed in a variety of recombinant DNA and RNA vectors. These live-vector-based vaccines, which include vaccinia virus, adenovirus, adeno-associated virus and alphavirus RNA replicon particles such as Sindbis virus and Venezuelan equine encelphalitis (VEE) virus, have mostly been tested in preclinical models (for a review, see [17]). Among viral vectors, the vaccinia virus is considered to be particularly promising for antigen-specific immunotherapy due to its high efficiency of infection. Vaccinia virus constructs encoding E7 linked to proteins that enhance antigen presentation in DCs have been shown to generate E7-specific immune responses that can cause regression of E7-expressing tumors in mice [18–20].
Preclinical studies have also shown that adenoviruses can serve as effective vectors. In one adenoviral vaccine model testing a papillomavirus protein, E2, a therapeutic effect of reduced papilloma-forming sites has been observed in rabbits [21]. Recent studies employed the CRT/E7 fusion delivered by a replication-deficient adenovirus vector (Ad-CRT/E7) and it was observed that vaccination with Ad-CRT/E7 protected mice against E7-expressing tumor challenge and induced a therapeutic effect against established tumors. Rechallenge with tumor cells showed that these mice generated long-term immunological memory against E7-expressing TC-1 cells [22]. Additionally, several adeno-associated viruses have been engineered to express E7 linked to either Mycobacterium tuberculosis heat-shock protein 70 (hsp70) [23] or the cytokine interleukin (IL)-12 [24], and have been shown to induce E7-specific immune responses in vivo.
Another viral vector of interest is the novel Semliki Forest virus (SFV), which has been shown to be capable of inducing immune responses strong enough to break host tolerance to HPV antigens. This has significant implications in established infections and cancers where the observed lack of clinical response may be due to immune tolerance of HPV-infected cells [25,26].
A recombinant vaccinia vector encoding an HPV-16/18 E6/E7 fusion protein, termed TA-HPV, has been evaluated in Phase I/II clinical trials. The vaccine was well tolerated and induced T-cell-mediated immune responses in patients with cervical intraepithelial neoplasia (CIN) [27–29]. TA-HPV has also been tested with some success in patients with other HPV-associated malignancies including vulvar intraepithelial neoplasia (VIN) [30,31] and vaginal intraepithelial neoplasia (VAIN) [32]. In a clinical study in VIN and VAIN patients vaccinated with TA-HPV, 5 out of 12 patients showed at least a 50% reduction in lesion diameter over a 24-week period, and 1 patient showed complete regression of the lesion [32].
More recently, a recombinant vaccinia vector encoding the E2 viral protein, termed MVA-E2, has been tested in patients with CIN [33,34] and flat condyloma lesions [35]. These studies demonstrated that patients developed antibodies against the MVA-E2 vaccine and generated a specific cytotoxic T lymphocyte (CTL) response against papilloma-transformed cells. However, no definitive conclusion can yet be drawn as to whether the vaccines can generate an E2-specific immune response. One possibility is that the E2-mediated cell death leads to the uptake of the antigen by the immune cells via cross-priming. However, no data are available to support this notion.
2.2 Peptide-/protein-based vaccines
2.2.1 Peptide-based vaccines
Direct administration of peptides derived from HPV antigens provides a means of vaccination against HPV. HPV antigenic proteins are taken up by dendritic cells (DCs) and presented in association with the MHC class I and/or class II pathways on human leukocyte antigen (HLA) molecules, to mount an immune response against the pathogen. In general, peptide-based vaccines are safe, easy to produce, and stable. One limitation, however, is that peptide-based vaccines are MHC-specific. Due to the highly polymorphic nature of these HLA molecules, however, it is necessary to identify specific immunogenic epitopes of HPV antigens before a vaccine can be developed, and it may be difficult to produce a peptide-based vaccine that is effective in a variety of patients with different HLA haplotypes, making it impractical for large scale vaccination treatments. Another drawback is that peptide vaccines tend to be poorly immunogenic. Consequently, most of the research in this area has focused on the use of adjuvants such as chemokines, cytokines and costimulatory molecules to enhance vaccine potency (for a review, see [36]).
In preclinical studies, progress has been achieved in augmenting peptide vaccine potency by employing the intranasal route of administration [37], linking peptides to immunostimulatory molecules to generate protective immunity and specific CTL responses [38] and using DC-activating agents such as 4′-monophosphoryl lipid A (MPL) and GM-CSF to increase and sustain levels of CTL responses [39]. Combining peptides with CpG oligodeoxynucleotide (CpG ODN), which provides a ‘danger signal’ for Toll-like receptor 9 by mimicking bacterial DNA, has also been shown to enhance the immunogenicity of peptide vaccines [40,41]. Other methods for potentiating peptide-based vaccines include the linkage of peptides to lipids and the enhancement of epitopes to prevent peptide degradation. In particular, therapeutic vaccination with E6 and E7 long peptides has been shown to result in the control of both established virus-induced lesions and latently infected sites in a preclinical cottontail rabbit papillomavirus model [42].
There have been several clinical trials employing peptide-based vaccines. In recent Phase I/II human clinical trials, several peptide vaccines have been found to be safe and well tolerated [43,44]. In one study, 18 women with high grade cervical or vulvar intraepithelial neoplasia were vaccinated with lipidated HPV-16 E7 peptides. Of the 18 patients, 3 showed cleared dysplasia and 6 demonstrated measurable lesion regression [45]. In another non-randomized Phase I clinical trial using lipidated E7 peptide, it was found that the vaccine did not elicit regression of tumor burden [46]. A recent study has demonstrated that VIN 3 patients vaccinated with 30–35-mer overlapping peptides of HPV-16 E6 and E7 sequences in Montamide ISA 51 adjuvant generated HPV-16-specific T H 1/T H 2 cells infiltrating both the vaccination site and the VIN lesions in 6 out of 9 patients analyzed [47]. Several such strategies employing synthetic peptide vaccines are currently being developed (for review see [48]).
2.2.2 Protein-based vaccines
Like peptide vaccines, protein-based vaccines are safe and easy to produce. Moreover, protein-based vaccination can circumvent the limited specificity of MHC responses associated with peptide-based vaccines. Protein antigens can be processed and presented on the surface of DCs, and they contain all of the possible human leukocyte antigen (HLA) epitopes of an antigen. Thus, protein-based vaccination circumvents the need to determine the HLA types of prospective patients. However, like peptide-based vaccines, protein-based vaccines also suffer from low immunogenicity, and as a result, adjuvant and fusion protein strategies are often used to enhance vaccine potency. Another limitation of protein-based vaccines is that since proteins used for vaccination are administered exogenously, antigen-presenting cells (APCs) may only occasionally encounter and engulf an injected protein for MHC class I presentation. Additionally, a potential disadvantage of using proteins for therapeutic vaccination is that proteins may elicit better antibody responses than CTL responses.
Preclinical studies have focused on addressing these drawbacks in a number of different ways. To enhance immunogenecity, adjuvant and fusion protein strategies have been used, such as adding the liposome-polycation-DNA (LPD) adjuvant [49] or the saponin-based adjuvant ISCOMATRIX [50]. Because exogenously administered protein vaccines are not usually efficiently engulfed and presented by APCs, there have also been strategies aimed at promoting protein uptake by APCs. For example, fusing the antigen of interest with special proteins, such as Bordetella pertussis adenylyl cyclase (CyaA), a protein that targets APCs through specific interaction with αM β2 integrin [51], or with the translocation domain of bacterial exotoxin Pseudomonas aeruginosa exotoxin A (EXA) [52], has been shown to enhance MHC class I and II presentation. Heat-shock proteins (hsp) have also been shown to help enhance protein-based vaccines by targeting antigen to APCs [53]. The unusually immunogenic nature of hsp derived from bacteria makes them good adjuvant-free carriers for protein-based vaccines. For example, we have shown that a fusion protein comprised of HPV-16 E7 and Mycobacterium bovis bacille Calmette–Guérin hsp65 could induce CTL responses in mice leading to tumor regression [54].
Various protein vaccines have moved to clinical trials. Fusion proteins containing HPV capsid proteins and HPV early proteins can potentially induce prophylactic and therapeutic immune responses. One example of this experimental fusion vaccine is TA-GW, a fusion of HPV-6 L2 and E7 absorbed onto Alhydrogel. It has been well tolerated by patients in two clinical trials and was effective in clearing HPV genital warts in a subset of patients [55,56]. A vaccine containing an HPV-16 E6/E7 fusion protein mixed with the ISCOMATRIX adjuvant has also recently been tested in a Phase I study. Immunization with this protein-based vaccine was shown to be safe and immunogenic and resulted in significantly enhanced CD8+ T cell responses to both E6 and E7 in vaccinated patients compared with those observed in placebo recipients [57]. Another protein vaccine, TA-CIN, a fusion of HPV-16 L2, E6 and E7, induced antibodies in all the women tested and induced T cell immunity in a subset of them, proving to be safe [58]. A vaccine termed PD-E7, comprised of mutated HPV-16 E7 fused with a fragment of Haemophilus influenzae protein D and formulated in the GlaxoSmithKline Biologicals adjuvant AS02B, has been evaluated in Phase I/II clinical trials and was shown to induce significant E7-specific CTL responses in patients with CIN-1 and CIN-3 lesions [59]. Recently, a fusion of HPV-16 E7 and M. bovis hsp65 has been shown to be well tolerated in patients with high-grade anal intraepithelial neoplasia (AIN) [60,61] ; however, further tests are needed to determine the clinical efficacy of the vaccine. A recent trial employing the same vaccine was conducted in women with CIN III lesions. However, there was no significant difference in regression in women infected with HPV 16 compared with those without HPV 16 infection [62].
2.3 Nucleic acid-based vaccines
2.3.1 DNA-based vaccines
DNA vaccines have emerged as an attractive and potentially effective strategy for antigen-specific immunotherapy. Naked DNA is safe, stable, relatively easy to manufacture and can be used to sustain the expression of antigen in cells for longer periods of time than RNA or protein vaccines. Furthermore, unlike live-vector vaccines, DNA vaccines do not elicit neutralizing antibody production in the patient, and thus can be repeatedly administered to the same patient effectively.
Although HPV DNA vaccines are considered to be relatively safe compared with other forms of vaccines such as viral-vector-based vaccines, some concerns have been raised. For example, DNA may potentially integrate into the host genome, causing genomic instability, though there is no evidence that shows that integration of DNA occurs in numerous organs or tissues. Vaccination with E6 and/or E7 DNA also has the potential hazard of cellular trans formation, as E6 and E7 are virus-encoded oncoproteins, which could be addressed through modification of E6 or E7, which abolishes the transformative capacity of these proteins. Generally, however, DNA vaccines are poorly immunogenic because DNA lacks the intrinsic ability to amplify or spread from transfected cells to surrounding cells in vivo. Several strategies have been developed to enhance the potency of DNA vaccines by: i) increasing the number of antigen-expressing DCs; ii) enhancing antigen processing and presentation in DCs; and iii) improving DC interaction with T cells to augment T-cell-mediated immune responses (for reviews, see [63,64]).
2.3.1.1 Strategies for increasing antigen-expressing dendritic cell populations
One approach for boosting antigen-expressing DC populations is to find the most effective routes for delivery of DNA vaccines. Among the different routes of DNA administration, vaccination via gene gun has been to shown to be one of the most potent methods for the delivery of genes of interest into DCs [65]. The gene gun is used to fire DNA-coated gold particles into the epidermis and efficiently transfect intradermal DCs that can mature and migrate to the lymphoid organs for T cell priming. This represents a convenient and effective method for the in vivo introduction of naked DNA into DCs. Another method for increasing the number of antigen-expressing DCs by DNA vaccines is to promote the spread of encoded antigen between DCs by linking antigen with proteins capable of intercellular transport. The authors have investigated the use of DNA encoding HPV-16 E7 fused to herpes simplex virus type 1 VP22 (HSV-1 VP22), a viral protein with intercellular trafficking properties, in a DNA vaccine. In vivo experiments showed that the vaccine dramatically enhanced E7-specific CD8+ T-cell responses and generated greater antitumor effects than DNA vaccines encoding E7 alone [66]. We then generated a vaccine encoding E7 linked to Marek’s disease virus type 1 VP22 (MDV-1 VP22), a protein with some homology to HSV-1 VP22, and observed powerful antitumor immunity as well [67]. Other strategies for improving antigen-expressing DC populations include the linkage of antigen to molecules that can target the antigen to the surface of DCs, such as FMS-like tyrosine kinase 3 (flt3) ligands, which bind with flt3 receptors on DCs, and hsp, which bind with scavenger receptors on DCs such as CD91.
2.3.1.2 Strategies for enhancing antigen expression, processing and presentation in dendritic cells
One method for improving antigen expression of DNA vaccines in DCs is codon optimization. This technique is used to modify antigen gene sequences by replacing rarely used codons with more commonly recognized codons, and can enhance translation of a DNA vaccine in DCs. It has also been shown to be effective in increasing the CTL response induced by DNA vaccines [68–70].
DCs must present antigens through the MHC class I pathway to generate populations of antigen-specific CD8+ T cells. Linkage of antigen to proteins that target the antigen to the endoplasmic reticulum or facilitate proteasome degradation, for example, can lead to enhanced MHC class I antigen presentation and greater ensuing CTL responses. The authors have demonstrated the potent effects against E7-expressing tumors of vaccines encoding E7 linked to various MHC class I-targeting proteins and protein domains, including M. tuberculosis hsp70 [71], γ-tubulin [72], the extracellular domain of Flt3-ligand [73], and the translocation domain of Pseudomonas aeruginosa exotoxin A [74]. Among these strategies, it was found that DNA vaccines containing E7 linked to calreticulin (CRT), a protein shown to significantly enhance MHC class I antigen presentation, elicited the greatest E7-specific CD8+ T-cell responses among all the DNA vaccines tested [75]. These findings suggest that the use of CRT for the generation of potent antigen-specific immune responses may be applicable to other types of immunotherapeutic vaccines.
Another important strategy for circumventing antigen processing and eliciting stable MHC class I presentation of a peptide encoded by a DNA vaccine is the employment of MHC class I single-chain trimer (SCT) technology [76]. This technique involves the linkage of an antigenic peptide to β2-microglobulin and MHC class I heavy chain, producing a single-chain construct encoding an MHC class I molecule fused to the peptide antigen. It has been shown that immunization of mice with a DNA vaccine encoding a SCT composed of an immunodominant CTL epitope of HPV-16 E6, β2-microglobulin and H-2Kb MHC class I heavy chain (termed pIRES-E6- β2m-Kb) could generate increased E6 peptide-specific CD8+ T-cell responses compared with mice vaccinated with DNA encoding wild type E6 [76].
On the other hand, fusion of antigen to MHC class II-targeting molecules can redirect an antigen to the class II pathway and generate greater CD4+ T cell responses that augment CTL responses (for a review, see [77]). We have previously shown that linkage of antigen to a signal peptide for the endoplasmic reticulum (Sig) and the lysosomal targeting domains of lysosome-associated membrane protein 1 (LAMP-1) can enhance MHC class II antigen presentation and generate significant CD4+ T cell responses [78]. This construct, termed Sig/E7/LAMP-1, was tested in the context of a DNA vaccine and produced greater numbers of E7-specific CD4+ T cells and also higher E7-specific CTL activity in mice than vaccines composed of Sig/E7 or wild-type E7 DNA alone [79].
The MHC class II invariant chain has recently been employed in the context of a DNA vaccine to effectively enhance class II presentation of antigens. In the endoplasmic reticulum (ER), Ii binds to MHC class II molecules and the class II-associated invariant -chain peptide (CLIP) region of the invariant chain occupies the class II peptide-binding groove, preventing premature binding of antigenic peptides into the groove. In the lysosomal compartment, CLIP is replaced by an antigenic peptide and the MHC class II/peptide complex is presented on the surface of the DC. By substituting the CLIP region of the invariant chain with a desired T helper (TH) epitope such as pan-DR helper T-lymphocyte epitope (PADRE) (Ii-PADRE), the epitope can be efficiently presented via the class II pathway. Mice vaccinated with a DNA vaccine encoding Ii-PADRE generated significant PADRE-specific CD4+ T cell immune responses. Furthermore, coadministration of DNA encoding E7 and DNA encoding Ii-PADRE in mice was shown to elicit potent CD8+ T-cell responses compared with coadministration of DNA encoding E7 and DNA encoding unmodified invariant chain [80].
2.3.1.3 Strategies for enhancing dendritic cell and T-cell interaction
Once an antigen has been processed and presented, the interactions between DCs and T cells become critical for T-cell activation. After priming, DCs can become targets of activated armed effector T cells. To prevent T cell-mediated apoptosis in DCs, DNA encoding antiapoptotic proteins can be used to prolong DCs survival and enhance the long-term ability of DCs to prime T cells. In our studies, co-delivery of DNA encoding E7 with DNA encoding inhibitors of apoptosis such as B-cell leukemia/lymphoma × (BCL-xL), B-cell lymphoma protein 2 (BCL-2), X-linked inhibitor of apoptosis protein (XIAP) and dominant-negative caspases was shown to enhance E7-specific CD8+ T cell responses in mice [81]. Approaches involving the linkage of the E7 gene with Escherichia coli β-glucuronidase have also been employed to enhance vaccine potency [82]. In addition, DNA vaccines employing strategies to enhance antigen presentation with strategies to prolong DC life were shown to further improve antigen-specific CTL responses [83–85]. The introduction of DNA-encoding antiapoptotic proteins into cells, however, raises concerns of oncogenicity. Inhibition of proapoptotic proteins using RNA interference (RNAi) may potentially alleviate these problems. We have recently demonstrated that coadministration of DNA vaccines encoding E7 with short interfering RNA (siRNA) targeting the key proapoptotic proteins BCL-2 homologous antagonist/killer (Bak) and BCL2-associated X protein (Bax) was able to effectively improve DC resistance to apoptotic cell death and enhance antitumor CD8+ T cell responses in mice [86].
The activation of naive antigen-specific T cells is dependent on signals delivered by DCs to T cells, such as co-stimulatory factors and cytokines. Numerous studies using this strategy have shown enhanced antigen-specific immune responses. Examples include coadministration of HPV DNA vaccines with DNA encoding GM-CSF [87], IL-2 [88] or IL-12 [89].
Several DNA vaccines have translated into clinical trials. A microencapsulated DNA vaccine encoding multiple HLA-A2-restricted E7-derived epitopes, termed ZYC-101 (ZYCOS, Inc., now acquired by MGI Pharma), has been tested in patients with CIN-2/3 lesions [90] and in patients with high-grade anal intra-epithelial lesions [91]. The vaccine was well tolerated in both trials. Out of the 12 individuals with anal displasia, 10 had increased immune responses to the peptide epitopes encoded within the DNA vaccine. Out of 15 women with CIN-2/3 5 had complete histological responses and 11 induced HPV-specific T-cell responses. A new version of the vaccine, termed ZYC-101a, encodes HPV-16 and HPV-18 E6- and E7-derived epitopes and was shown to resolve CIN-2/3 lesions in a subset of young women enrolled in the trial [92]. At the Johns Hopkins Hospital, a Phase I trial using a DNA vaccine encoding modified HPV-16 E7 DNA (which abolished the Rb binding site) linked to M. tuberculosis hsp70 (pNGVL4a-Sig/E7(detox)/hsp70) was performed in patients with CIN-2/3 lesions. The vaccine was well tolerated by all patients, and some of the patients who received the maximum dose of DNA vaccine (4 mg/vaccination) showed detectable E7-specific CD8+ T-cell immune responses (C Trimble, pers. commun.). The same DNA vaccine has also been tested in a subset of HPV-16-positive patients with head and neck squamous cell carcinoma. The same group of investigators have also planned to initiate a Phase I trial with a DNA vaccine encoding modified HPV-16 E7 DNA linked to CRT in patients with stage 1B1 cervical cancer using a good manufacturing practice-grade gene gun device (C Trimble, pers. commun.).
2.3.2 RNA replicon vaccines
The use of RNA replicons is a relatively new and potentially interesting strategy for HPV vaccination. RNA replicons are naked RNA molecules that replicate within transfected cells. They may be derived from alphaviruses, such as Sindbis virus [93,94], Semliki Forest virus [95,96], and VEE [97]. These subgenomic alphavirus RNA vaccines are self-replicating and self-limiting, and may be administered as either RNA or DNA, which is then transcribed into RNA replicons. RNA replicon-based vectors have several potential advantages for cancer vaccine development. For example, RNA replicons can replicate in a wide range of cell types and can be used to produce sustained levels of antigen expression in cells, making them more immunogenic than conventional DNA vaccines. Many replicon vectors do not contain viral structural genes, and thus no infectious particles are produced and the host immune response to these vectors is likely to be limited. Thus, RNA-replicon-based vaccines can be used in patients repeatedly. In addition, RNA replicons alleviate the risk of potential chromosomal integration and cellular transformation associated with naked DNA vaccines. This is particularly important in the development of vaccines targeting potentially oncogenic proteins such as E6 and E7. However, RNA replicons are less stable than DNA. One alternative has been to combine the benefits of RNA replicon and DNA vaccines into a DNA-launched RNA replicon, termed ‘suicidal’ DNA.
DNA-launched RNA replicons have been used for HPV vaccine development in preclinical models [98,99]. This ‘suicidal DNA’ is transcribed into RNA within the transfected cell and provides a stable and efficient way to express tumor antigen. However, transfected cells eventually undergo apoptosis. When DNA-launched RNA replicons are delivered to DCs via gene gun, this apoptotic change mediated by suicidal DNA may lead to diminished immune responses. To prevent this, suicidal DNA encoding the E7 antigen linked to antiapoptotic proteins such as BCL-xL was administered to mice and was shown to delay cell death in DCs and generate significantly higher E7-specific CD8+ T-cell immune responses and better antitumor effects than DNA encoding wild type E7 alone [99]. Another alternative is to generate replication-defective, self-limiting replicon particles using a safe packaging cell line. These replicon particles offer advantages over naked nucleic acid vaccines such as efficient gene delivery and large-scale production, and may prove useful in the development of effective RNA-based HPV vaccines.
The potency of HPV RNA replicon vaccines can also be enhanced by employment of the intracellular targeting and intercellular spreading strategies used in DNA-based immunization [100–102]. Another replicon system uses a flavivirus termed Kunjin (KUN) to deliver replicons. Vaccination of mice with KUN replicons expressing an HPV-16 E7 epitope induced specific T-cell responses and protected mice from tumor challenge [103]. A new generation of KUN replicon vectors has been developed which allows for the synthesis of replicon RNA from plasmid DNA. The primary advantage of this DNA-based KUN replicon system is that the replicon does not induce cellular apoptosis, and thus antigen presentation by DCs to T cells is prolonged, enhancing the elicited immune response [104]. However, despite the general success of RNA replicons in preclinical models, RNA replicon-based vaccines have had limited clinical testing.
2.4 Whole cell vaccines
2.4.1 Dendritic cell-based vaccines
The use of DC-based vaccines represents a potentially plausible strategy for therapeutic HPV vaccines against HPV-associated malignancies. To create a dendritic cell-based HPV vaccine, it is necessary to load DCs with viral antigens and deliver them into patients. DCs can be prepared ex vivo by the physical loading of MHC class I and class II molecules, and antigen loading can be accomplished by pulsing the DCs with antigenic peptides or proteins or by transfecting DCs with DNA or RNA encoding HPV antigen. Significant advances in our knowledge of DC differentiation, migration and maturation, as well as antigen processing and presentation have provided a rationale for the usage of DCs as natural adjuvants in antigen-specific cancer immunotherapy (for a review, see [105]).
However, while effective DC-based vaccines can be produced using autologous DCs, individualized vaccines are cumbersome to generate and therefore large-scale production is quite challenging. Additionally, the culturing technique used to prepare DCs may affect the quality of the vaccine, leading to heterogeneity in vaccine quality and a lack of standard criteria for evaluating the vaccines. Furthermore because it is critical for DCs to home to the lymphoid organs where the majority of T cells are located, the route of administration is an important issue for DC-based vaccines for maximizing the effects of the vaccine.
Preclinical models have tried to address these concerns. For effective loading of tumor antigen into DCs, one strategy is to deliver genes to DCs using adenoviral vectors targeted to CD40 via bispecific antibodies. Recombinant adenoviral vectors carrying HPV E7 have been shown to elicit strong antitumor immunity in mice after challenge with E7-expressing tumors [106]. Once DCs are loaded with tumor antigen, the vaccine can be administered via intramuscular, subcutaneous, or intravenous delivery. Among these, it was shown that intramuscular delivery is the most effective method for generating large numbers of E7-specific CD8+ T cell precursors [107]. It is also possible to infect DCs with tumor antigen-expressing vaccinia virus [108] or fuse DCs with tumor cells.
Several methods have been used to enhance the potency of DC-based HPV vaccines. As mentioned previously, prolongation of DC survival improves T cell priming by DCs. It has been shown that transfecting E7-loaded DCs with small interfering RNA (siRNA) targeting the Bak and Bax proapoptotic proteins leads to downregulation of Bak and Bax protein expression in the DCs, which resulted in increased resistance of the DCs to T-cell-mediated apoptosis. Vaccination of mice with E7-loaded DCs transfected with siRNA targeting Bak and Bax led to enhanced E7-specific immune responses and antitumor effects [109]. Furthermore, strategies to prolong DC survival can be used in conjunction with a strategy to improve MHC class I/II presentation of antigen to further enhance DC-based vaccine potency [110]. Thus, some of the strategies used to modify of the properties of DCs described in previous sections may potentially be used to enhance DC-based vaccines.
Several clinical trials of DC-based HPV vaccines in cancer patients have been reported. Although most of the studies were Phase I/II trials focused on assessing feasibility and safety of DC-based vaccines, successful induction of antigen-specific immune responses have been observed. In a case report, subcutaneous injection of HPV-18 E7-pulsed DCs in a patient with metastatic cervical cancer led to inhibition of tumor progression. Although the vaccine did not cause complete remission in this patient, the individual’s health status improved and no significant side effects were observed [111]. In a clinical pilot study, autologous DCs pulsed with E7 protein were shown to induce T-cell responses in some late-stage cervical cancer patients [112]. In more recent trials, administration of DCs pulsed with recombinant HPV-16 or HPV-18 E7 was shown to induce E7-specific CD4+ T-cell immune responses in two out of four patients and E7-specific CD8+ T-cell responses in all four patients with cervical cancers refractory to standard treatments [113]. However, no objective clinical responses were observed.
2.4.2 Tumor-cell-based vaccines
The use of tumor cells in HPV vaccines represents a second approach to whole-cell immunization. Tumor cells can be manipulated ex vivo to express immunomodulatory proteins, such as cytokines, in order to enhance their immunogenicity. Cytokine genes used in HPV-transformed tumor vaccines include IL-2 [114], IL-12 [115,116], and GM-CSF [116,117]. One advantage of tumor-cell-based vaccines is the convenience that tumor antigens need not be clearly identified, though cervical cancer has a clear viral etiology, as opposed to other types of cancers without well-defined tumor-specific antigens.
Tumor-cell-based HPV vaccines have been tested in preclinical models. For example, vaccination of mice with GM-CSF-expressing E7-positive tumor cells led to an E7-specific CTL response and potent antitumor effects against E7-expressing tumors in vivo [117]. Although tumor-cell-based vaccines, such as GM-CSF-transduced autologous or allogeneic tumor cells have been used in clinical trials for other cancers such as pancreatic cancer, renal cell carcinoma, melanoma and prostate carcinoma, tumor-cell-based vaccines have not been tested against HPV-associated malignancies in the clinical arena. However, using tumor-cell-based vaccines carries the risk of introducing new cancers, which is not acceptable for relatively healthy HPV carriers or patients with mild cervical neoplasia. In addition, although effective tumor vaccines can be derived from autologous tumor cells, individualized vaccine production is cumbersome and large-scale vaccine production is impractical. Furthermore, inconsistencies in the potency and purity of these vaccines restrict their efficacy. Thus, tumor cell-based vaccines have limited scope for HPV vaccine development.
3. Combined approaches: the future of human papillomavirus vaccination
Prime-boost regimens are perhaps the most effective treatment strategy for vaccination against HPV. Because nucleic acid vaccines often generate relatively weak CTL responses, combinatorial vaccination approaches are used to circumvent this limitation. Priming with a DNA or RNA vaccine and then boosting with a viral-vector vaccine has been shown to result in enhanced immune responses relative to single modality vaccinations. For example, in preclinical models, priming the immune system with a DNA vaccine and then boosting with either a live-viral-vector vaccine [118,119] or a tumor vaccine [120] has been shown to elicit stronger antitumor effects than either vaccine alone. In another study, mice first primed with a Sindbis virus RNA replicon containing E7 linked to M. tuberculosis hsp70 (E7/hsp70) and then boosted with a vaccinia vector encoding E7/hsp70 showed E7-specific CTL responses as well as strong antitumor effects [121]. The efficacy of other treatment strategies, such as protein-based vaccination, can also be enhanced by prime-boost approaches [122]. On the other hand, combining therapeutic vaccines with antiviral or anticancer agents may also be effective for the treatment of HPV-associated disease [123,124].
Some prime-boost regimens have proven effective in clinical trials. In prime-boost vaccine trials using heterologous HPV vaccines (TA-CIN followed by TA-HPV) in the management of anogenital intraepithelial neoplasia, patients experienced HPV-16-specific T cell responses and showed symptomatic improvement without serious adverse effects [125,126]. In another study, patients were initially primed with TA-HPV and subsequently vaccinated with a booster containing HPV-16 L2/E6/E7. Nine out of ten patients developed HPV 16-specific immune and/or serological responses, and three showed a reduction in lesion size or experienced symptomatic relief [127]. A clinical trial using pNGVL4a/Sig/E7(detox)/Hsp70 DNA prime followed by TA-HPV vaccinia boost is currently being planned at Johns Hopkins University in patients with CIN2/3 lesions (C Trimble, pers. commun.). In general, clinical outcomes have suggested that prime-boost regimens may be more effective than single-modality vaccinations, and thus this strategy merits further study as one of the most promising approaches for the treatment of HPV-associated malignancies.
Combination approaches including chemotherapy, radiation or other biotherapeutic agents combined with HPV therapeutic vaccination may also serve to enhance the therapeutic HPV vaccine potency. For example, a recent study has shown that the chemotherapeutic agent epigallocatechin-3-Gallate (EGCG), a chemical derived from green tea, could induce tumor cellular apoptosis and enhance the tumor-antigen-specific T-cell immune responses elicited by DNA vaccination [31]. In addition, peptide/protein-based vaccines have been combined with chemotherapy and/or radiation to improve therapeutic antitumor effects [128,129]. A particular study has combined the employment of recombinant E7 protein-based subunit vaccines in the presence of CpG oligonucleotide adjuvant with chemotherapy using cisplatin and demonstrated therapeutic antitumor synergy against established E7-expressing tumors [128]. These successful results have led to the planning of a Phase I clinical trial at Johns Hopkins involving the combination of oral EGCG administration with intradermal administration of CRT/E7 DNA vaccination via gene gun in patients with advanced HPV-associated head and neck squamous cell carcinomas (HPV-HNSCC) (S Pai, pers. commun.).
The therapeutic effects of HPV vaccines may be further enhanced by combination with blocking of the factors that inhibit T-cell activation, such as cytotoxic T lymphocyte antigen-4 (CTLA-4) and programmed cell death protein 1 (PD-1). These molecules are negative coregulators in the T cell costimulatory pathway. Thus, antibody-mediated blockade of CTLA-4 and PD-1 can potentially be used to prolong antitumoral T cell responses (for review, see [130,131]). The combination of HPV therapeutic vaccines with agents that influence the tumor microenvironment may also potentially be used to generate enhanced therapeutic effects against HPV-associated malignancies. It is now clear that several factors present in the tumor microenvironment may potentially hinder immunotherapy. These factors include the expression of B7 homolog-1 B7-H1 [132], signal transducer and activator of transcription 3 (STAT3) [133] and MHC class I polypeptide-related sequence (MIC)-A and B [134], indoleamine 2,3-dioxygenase (IDO) enzyme [135], and galectin-1 [136] on tumor cells, immunosuppressive cytokines such as IL-10 [137] and TGF-β[138], T regulatory cells [139], myeloid-derived suppressor cells [140]. It is conceivable that agents capable of blocking these molecules may potentially be used to enhance the therapeutic effects of the HPV vaccines.
4. Expert opinion
The identification and characterization of high-risk human papillomavirus as a necessary causal agent for cervical cancer provides a promising possibility for the eradication of HPV-related malignancies. In the development of therapeutic HPV vaccines, we have focused on identifying and targeting the most relevant antigens associated with cervical cancer, the E6 and E7 HPV oncoproteins, which represent tumor-specific antigens and potentially ideal targets for therapeutic HPV vaccines. Based on our studies and those of others in the field, we conclude that the various current approaches, including peptide- and protein-based, live-vector-based, nucleic acid-based, and cell-based immunization, are each associated with strengths and weaknesses (Table 1). It is important to consider using strategies such as prime-boost regimens and/or combinations strategies using molecules that are capable of blocking the negative regulators on T cells to further enhance the T cell immune responses. Furthermore, increasing understanding of the molecular mechanisms that hinder immune attack in the tumor microenvironment will lead to the identification of novel molecular targets that can be blocked in order to enhance the therapeutic effect of HPV vaccines. It is conceivable that effective therapy against HPV-associated malignancies will probably require a combination of therapeutic HPV vaccines with the employment of innovative agents that are capable of eliminating the suppressive factors present in the tumor microenvironment. With continued endeavor in the development of HPV therapeutic vaccines, we can foresee that HPV therapeutic vaccines will become an important approach that can be combined with existing forms of therapy such as chemotherapy and radiation therapy to generate better control of HPV-associated malignancies.
Acknowledgements
This review is not intended to be an encyclopedic one, and the authors apologize to those not cited. The authors gratefully acknowledge R Roden for his critical review of the manuscript.
Declaration of interest
The work is supported by the National Cancer Institute (NCI) specialized program of research excellence (SPORE) in Cervical Cancer P50 CA098252 and NCI 1RO1 CA114425-01.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1. Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. • This paper reports that, worldwide, more than 99% of cervical carcinomas are associated with HPV, suggesting that HPV maybe a cause of cervical cancer
- 2.Devaraj K, Gillison ML, Wu TC. Development of HPV vaccines for HPV-associated head and neck squamous cell carcinoma. Crit Rev Oral Biol Med. 2003;14:345–362. doi: 10.1177/154411130301400505. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 4.Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 5.Bosch FX, Manos MM, Munoz N, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 6.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 7.Harper DM, Franco EL, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364:1757–1765. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 8.Villa LL, Costa RL, Petta CA, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II effi cacy trial. Lancet Oncol. 2005;6:271–278. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 9.Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367:1247–1255. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 10.Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 11.Bermudez-Humaran LG, Langella P, Miyoshi A, et al. Production of human papillomavirus type 16 E7 protein in Lactococcus lactis. Appl Environ Microbiol. 2002;68:917–922. doi: 10.1128/AEM.68.2.917-922.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bermudez-Humaran LG, Cortes-Perez NG, Le Loir Y, et al. An inducible surface presentation system improves cellular immunity against human papillomavirus type 16 E7 antigen in mice after nasal administration with recombinant lactococci. J Med Microbiol. 2004;53:427–433. doi: 10.1099/jmm.0.05472-0. [DOI] [PubMed] [Google Scholar]
- 13.Cortes-Perez NG, Azevedo V, Alcocer-Gonzalez JM, et al. Cell-surface display of E7 antigen from human papillomavirus type-16 in Lactococcus lactis and in Lactobacillus plantarum using a new cell-wall anchor from lactobacilli. J Drug Target. 2005;13:89–98. doi: 10.1080/10611860400024219. [DOI] [PubMed] [Google Scholar]
- 14.Souders NC, Sewell DA, Pan ZK, et al. Listeria-based vaccines can overcome tolerance by expanding low avidity CD8+ T cells capable of eradicating a solid tumor in a transgenic mouse model of cancer. Cancer Immun. 2007;7:2. Available from: www.cancerimmunity.org/v7p2/070102.htm. [PMC free article] [PubMed] [Google Scholar]
- 15.Sewell DA, Douven D, Pan ZK, et al. Regression of HPV-positive tumors treated with a new Listeria monocytogenes vaccine. Arch Otolaryngol Head Neck Surg. 2004;130:92–97. doi: 10.1001/archotol.130.1.92. [DOI] [PubMed] [Google Scholar]
- 16.Sewell DA, Shahabi V, Gunn GR, 3rd, et al. Recombinant Listeria vaccines containing PEST sequences are potent immune adjuvants for the tumor-associated antigen human papillomavirus-16 E7. Cancer Res. 2004;64:8821–8825. doi: 10.1158/0008-5472.CAN-04-1958. [DOI] [PubMed] [Google Scholar]
- 17.Monahan SJ, Salgaller ML. Viral vectors for gene transfer into antigen presenting cells. Curr Opin Mol Ther. 1999;1:558–564. [PubMed] [Google Scholar]
- 18.Hsieh CJ, Kim TW, Hung CF, et al. Enhancement of vaccinia vaccine potency by linkage of tumor antigen gene to gene encoding calreticulin. Vaccine. 2004;22:3993–4001. doi: 10.1016/j.vaccine.2004.03.057. [DOI] [PubMed] [Google Scholar]
- 19.Lamikanra A, Pan ZK, Isaacs SN, et al. Regression of established human papillomavirus type 16 (HPV-16) immortalized tumors in vivo by vaccinia viruses expressing different forms of HPV-16 E7 correlates with enhanced CD8(+) T-cell responses that home to the tumor site. J Virol. 2001;75:9654–9664. doi: 10.1128/JVI.75.20.9654-9664.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin KY, Guarnieri FG, Staveley-O’Carroll KF, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–26. [PubMed] [Google Scholar]
- 21.Brandsma JL, Shlyankevich M, Zhang L, et al. Vaccination of rabbits with an adenovirus vector expressing the papillomavirus E2 protein leads to clearance of papillomas and infection. J Virol. 2004;78:116–123. doi: 10.1128/JVI.78.1.116-123.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez-Gutierrez JG, Elpek KG, Montes de Oca-Luna R, et al. Vaccination with an adenoviral vector expressing calreticulin-human papillomavirus 16 E7 fusion protein eradicates E7 expressing established tumors in mice. Cancer Immunol Immunother. 2007;56:997–1007. doi: 10.1007/s00262-006-0247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu DW, Tsao YP, Kung JT, et al. Recombinant adeno-associated virus expressing human papillomavirus type 16 E7 peptide DNA fused with heat shock protein DNA as a potential vaccine for cervical cancer. J Virol. 2000;74:2888–2894. doi: 10.1128/jvi.74.6.2888-2894.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin HS, Park EK, Lee JM, et al. Immunization with adenoviral vectors carrying recombinant IL-12 and E7 enhanced the antitumor immunity to human papillomavirus 16-associated tumor. Gynecol Oncol. 2005;97:559–567. doi: 10.1016/j.ygyno.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 25.Riezebos-Brilman A, Regts J, Freyschmidt EJ, et al. Induction of human papilloma virus E6/E7-specific cytotoxic T-lymphocyte activity in immune-tolerant, E6/E7-transgenic mice. Gene Ther. 2005;12:1410–1414. doi: 10.1038/sj.gt.3302536. [DOI] [PubMed] [Google Scholar]
- 26.Daemen T, Riezebos-Brilman A, Regts J, et al. Superior therapeutic efficacy of alphavirus-mediated immunization against human papilloma virus type 16 antigens in a murine tumour model: effects of the route of immunization. Antivir Ther. 2004;9:733–742. [PubMed] [Google Scholar]
- 27.Borysiewicz LK, Fiander A, Nimako M, et al. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet. 1996;347:1523–1527. doi: 10.1016/s0140-6736(96)90674-1. [DOI] [PubMed] [Google Scholar]
- 28.Adams M, Borysiewicz L, Fiander A, et al. Clinical studies of human papilloma vaccines in cervical cancer. Adv Exp Med Biol. 2001;495:419–427. doi: 10.1007/978-1-4615-0685-0_61. [DOI] [PubMed] [Google Scholar]
- 29.Kaufmann AM, Stern PL, Rankin EM, et al. Safety and immunogenicity of TA-HPV, a recombinant vaccinia virus expressing modified human papillomavirus (HPV)-16 and HPV-18 E6 and E7 genes, in women with progressive cervical cancer. Clin Cancer Res. 2002;8:3676–3685. [PubMed] [Google Scholar]
- 30.Davidson EJ, Boswell CM, Sehr P, et al. Immunological and clinical responses in women with vulval intraepithelial neoplasia vaccinated with a vaccinia virus encoding human papillomavirus 16/18 oncoproteins. Cancer Res. 2003;63:6032–6041. [PubMed] [Google Scholar]
- 31.Kang TH, Lee JH, Song CK, et al. Epigallocatechin-3-gallate enhances CD8+ T cell-mediated antitumor immunity induced by DNA vaccination. Cancer Res. 2007;67:802–811. doi: 10.1158/0008-5472.CAN-06-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baldwin PJ, van der Burg SH, Boswell CM, et al. Vaccinia-expressed human papillomavirus 16 and 18 e6 and e7 as a therapeutic vaccination for vulval and vaginal intraepithelial neoplasia. Clin Cancer Res. 2003;9:5205–5213. [PubMed] [Google Scholar]
- 33.Corona Gutierrez CM, Tinoco A, Navarro T, et al. Therapeutic vaccination with MVA E2 can eliminate precancerous lesions (CIN 1, CIN 2, and CIN 3) associated with infection by oncogenic human papillomavirus. Hum Gene Ther. 2004;15:421–431. doi: 10.1089/10430340460745757. [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Hernandez E, Gonzalez-Sanchez JL, Andrade-Manzano A, et al. Regression of papilloma high-grade lesions (CIN 2 and CIN 3) is stimulated by therapeutic vaccination with MVA E2 recombinant vaccine. Cancer Gene Ther. 2006;13:592–597. doi: 10.1038/sj.cgt.7700937. [DOI] [PubMed] [Google Scholar]
- 35.Albarran YCA, de la Garza A, Cruz Quiroz BJ, et al. MVA E2 recombinant vaccine in the treatment of human papillomavirus infection in men presenting intraurethral flat condyloma: a phase I/II study. Biodrugs. 2007;21:47–59. doi: 10.2165/00063030-200721010-00006. [DOI] [PubMed] [Google Scholar]
- 36.Roden RB, Monie A, Wu TC. Opportunities to improve the prevention and treatment of cervical cancer. Curr Mol Med. 2007;7:490–503. doi: 10.2174/156652407781387127. [DOI] [PubMed] [Google Scholar]
- 37.Manuri PR, Nehete B, Nehete PN, et al. Intranasal immunization with synthetic peptides corresponding to the E6 and E7 oncoproteins of human papillomavirus type 16 induces systemic and mucosal cellular immune responses and tumor protection. Vaccine. 2007;25:3302–3310. doi: 10.1016/j.vaccine.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qin Y, Wang XH, Cui HL, et al. Human papillomavirus type 16 E7 peptide(38–61) linked with an immunoglobulin G fragment provides protective immunity in mice. Gynecol Oncol. 2005;96:475–483. doi: 10.1016/j.ygyno.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 39.Zwaveling S, Ferreira Mota SC, Nouta J, et al. Established human papillomavirus type 16-expressing tumors are effectively eradicated following vaccination with long peptides. J Immunol. 2002;169:350–358. doi: 10.4049/jimmunol.169.1.350. [DOI] [PubMed] [Google Scholar]
- 40.Chen YF, Lin CW, Tsao YP, Chen SL. Cytotoxic-T-lymphocyte human papillomavirus type 16 E5 peptide with CpG-oligodeoxynucleotide can eliminate tumor growth in C57BL/6 mice. J Virol. 2004;78:1333–1343. doi: 10.1128/JVI.78.3.1333-1343.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daftarian P, Mansour M, Benoit AC, et al. Eradication of established HPV 16-expressing tumors by a single administration of a vaccine composed of a liposome-encapsulated CTL-T helper fusion peptide in a water-in-oil emulsion. Vaccine. 2006;24:5235–5244. doi: 10.1016/j.vaccine.2006.03.079. [DOI] [PubMed] [Google Scholar]
- 42.Vambutas A, DeVoti J, Nouri M, et al. Therapeutic vaccination with papillomavirus E6 and E7 long peptides results in the control of both established virus-induced lesions and latently infected sites in a pre-clinical cottontail rabbit papillomavirus model. Vaccine. 2005;23:5271–5280. doi: 10.1016/j.vaccine.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 43.Ressing ME, van Driel WJ, Brandt RM, et al. Detection of T helper responses, but not of human papillomavirus-specific cytotoxic T lymphocyte responses, after peptide vaccination of patients with cervical carcinoma. J Immunother. 2000;23:255–266. doi: 10.1097/00002371-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 44.van Driel WJ, Ressing ME, Kenter GG, et al. Vaccination with HPV16 peptides of patients with advanced cervical carcinoma: clinical evaluation of a phase I–II trial. Eur J Cancer. 1999;35:946–952. doi: 10.1016/s0959-8049(99)00048-9. [DOI] [PubMed] [Google Scholar]
- 45.Muderspach L, Wilczynski S, Roman L, et al. A phase I trial of a human papillomavirus (HPV) peptide vaccine for women with high-grade cervical and vulvar intraepithelial neoplasia who are HPV 16 positive. Clin Cancer Res. 2000;6:3406–3416. [PubMed] [Google Scholar]
- 46.Steller MA, Gurski KJ, Murakami M, et al. Cell-mediated immunological responses in cervical and vaginal cancer patients immunized with a lipidated epitope of human papillomavirus type 16 E7. Clin Cancer Res. 1998;4:2103–2109. [PubMed] [Google Scholar]
- 47.Melief CJ, Welters MJP, Kenter GG, et al. Long peptide vaccine-induced migration of HPV16 specific type 1 and 2T-cells into the lesions of VIN 3 patients. Cancer Immun. 2007;7:20. [Google Scholar]
- 48.Bijker MS, Melief CJ, Offringa R, van der Burg SH. Design and development of synthetic peptide vaccines: past, present and future. Expert Rev Vaccines. 2007;6:591–603. doi: 10.1586/14760584.6.4.591. [DOI] [PubMed] [Google Scholar]
- 49.Cui Z, Huang L. Liposome-polycation-DNA (LPD) particle as a carrier and adjuvant for protein-based vaccines: Therapeutic effect against cervical cancer. Cancer Immunol Immunother. 2005;54:1180–1190. doi: 10.1007/s00262-005-0685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stewart TJ, Drane D, Malliaros J, et al. ISCOMATRIX adjuvant: an adjuvant suitable for use in anticancer vaccines. Vaccine. 2004;22:3738–3743. doi: 10.1016/j.vaccine.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 51.Preville X, Ladant D, Timmerman B, Leclerc C. Eradication of established tumors by vaccination with recombinant Bordetella pertussis adenylate cyclase carrying the human papillomavirus 16 E7 oncoprotein. Cancer Res. 2005;65:641–649. [PubMed] [Google Scholar]
- 52.Liao CW, Chen CA, Lee CN, et al. Fusion protein vaccine by domains of bacterial exotoxin linked with a tumor antigen generates potent immunologic responses and antitumor effects. Cancer Res. 2005;65:9089–9098. doi: 10.1158/0008-5472.CAN-05-0958. [DOI] [PubMed] [Google Scholar]
- 53.Qian X, Lu Y, Liu Q, et al. Prophylactic, therapeutic and anti-metastatic effects of an HPV-16mE6Δ/mE7/TBhsp70Δ fusion protein vaccine in an animal model. Immunol Lett. 2006;102:191–201. doi: 10.1016/j.imlet.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 54.Chu NR, Wu HB, Wu T, et al. Immunotherapy of a human papillomavirus (HPV) type 16 E7-expressing tumour by administration of fusion protein comprising Mycobacterium bovis bacille Calmette-Guerin (BCG) hsp65 and HPV16 E7. Clin Exp Immunol. 2000;121:216–225. doi: 10.1046/j.1365-2249.2000.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lacey CJ, Thompson HS, Monteiro EF, et al. Phase IIa safety and immunogenicity of a therapeutic vaccine, TA-GW, in persons with genital warts. J Infect Dis. 1999;179:612–618. doi: 10.1086/314616. [DOI] [PubMed] [Google Scholar]
- 56.Thompson HS, Davies ML, Holding FP, et al. Phase I safety and antigenicity of TA-GW: a recombinant HPV6 L2E7 vaccine for the treatment of genital warts. Vaccine. 1999;17:40–49. doi: 10.1016/s0264-410x(98)00146-7. [DOI] [PubMed] [Google Scholar]
- 57.Frazer IH, Quinn M, Nicklin JL, et al. Phase 1 study of HPV16-specific immunotherapy with E6E7 fusion protein and ISCOMATRIX adjuvant in women with cervical intraepithelial neoplasia. Vaccine. 2004;23:172–181. doi: 10.1016/j.vaccine.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 58.de Jong A, O’Neill T, Khan AY, et al. Enhancement of human papillomavirus (HPV) type 16 E6 and E7-specifi c T-cell immunity in healthy volunteers through vaccination with TA-CIN, an HPV16 L2E7E6 fusion protein vaccine. Vaccine. 2002;20:3456–3464. doi: 10.1016/s0264-410x(02)00350-x. [DOI] [PubMed] [Google Scholar]
- 59.Hallez S, Simon P, Maudoux F, et al. Phase I/II trial of immunogenicity of a human papillomavirus (HPV) type 16 E7 protein-based vaccine in women with oncogenic HPV-positive cervical intraepithelial neoplasia. Cancer Immunol Immunother. 2004;53:642–650. doi: 10.1007/s00262-004-0501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chu NR. Therapeutic vaccination for the treatment of mucosotropic human papillomavirus-associated disease. Expert Opin Biol Ther. 2003;3:477–486. doi: 10.1517/14712598.3.3.477. [DOI] [PubMed] [Google Scholar]
- 61.Palefsky JM, Berry JM, Jay N, et al. A trial of SGN-00101 (HspE7) to treat high-grade anal intraepithelial neoplasia in HIV-positive individuals. AIDS (London, England) 2006;20:1151–1155. doi: 10.1097/01.aids.0000226955.02719.26. [DOI] [PubMed] [Google Scholar]
- 62.Einstein MH, Kadish AS, Burk RD, et al. Heat shock fusion protein-based immunotherapy for treatment of cervical intraepithelial neoplasia III. Gynecol Oncol. 2007;106:453–460. doi: 10.1016/j.ygyno.2007.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hung CF, Wu TC. Improving DNA vaccine potency via modification of professional antigen presenting cells. Curr Opin Mol Ther. 2003;5:20–24. [PubMed] [Google Scholar]
- 64.Tsen SW, Paik AH, Hung CF, Wu TC. Enhancing DNA vaccine potency by modifying the properties of antigen-presenting cells. Expert Rev Vaccines. 2007;6:227–239. doi: 10.1586/14760584.6.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trimble C, Lin CT, Hung CF, et al. Comparison of the CD8+ T cell responses and antitumor effects generated by DNA vaccine administered through gene gun, biojector, and syringe. Vaccine. 2003;21:4036–4042. doi: 10.1016/s0264-410x(03)00275-5. [DOI] [PubMed] [Google Scholar]
- 66.Hung CF, Cheng WF, Chai CY, et al. Improving vaccine potency through intercellular spreading and enhanced MHC class I presentation of antigen. J Immunol. 2001;166:5733–5740. doi: 10.4049/jimmunol.166.9.5733. [DOI] [PubMed] [Google Scholar]
- 67.Hung CF, He L, Juang J, et al. Improving DNA vaccine potency by linking Marek’s disease virus type 1 VP22 to an antigen. J Virol. 2002;76:2676–2682. doi: 10.1128/JVI.76.6.2676-2682.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheung YK, Cheng SC, Sin FW, Xie Y. Plasmid encoding papillomavirus type 16 (HPV16) DNA constructed with codon optimization improved the immunogenicity against HPV infection. Vaccine. 2004;23:629–638. doi: 10.1016/j.vaccine.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 69.Liu WJ, Gao F, Zhao KN, et al. Codon modified human papillomavirus type 16 E7 DNA vaccine enhances cytotoxic T-lymphocyte induction and anti-tumour activity. Virology. 2002;301:43–52. doi: 10.1006/viro.2002.1584. [DOI] [PubMed] [Google Scholar]
- 70.Lin CT, Tsai YC, He L, et al. A DNA vaccine encoding a codon-optimized human papillomavirus type 16 E6 gene enhances CTL response and anti-tumor activity. J Biomed Sci. 2006;13:481–488. doi: 10.1007/s11373-006-9086-6. [DOI] [PubMed] [Google Scholar]
- 71.Chen C-H, Wang T-L, Hung C-F, et al. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res. 2000;60:1035–1042. [PubMed] [Google Scholar]
- 72.Hung CF, Cheng WF, He L, et al. Enhancing major histocompatibility complex class I antigen presentation by targeting antigen to centrosomes. Cancer Res. 2003;63:2393–2398. [PubMed] [Google Scholar]
- 73.Hung C-F, Hsu K-F, Cheng W-F, et al. Enhancement of DNA vaccine potency by linkage of antigen gene to a gene encoding the extracellular domain of Flt3-ligand. Cancer Res. 2001;61:1080–1088. [PubMed] [Google Scholar]
- 74.Hung C-F, Cheng W-F, Hsu K-F, et al. Cancer immunotherapy using a DNA vaccine encoding the translocation domain of a bacterial toxin linked to a tumor antigen. Cancer Res. 2001;61:3698–3703. [PubMed] [Google Scholar]
- 75.Kim JW, Hung CF, Juang J, et al. Comparison of HPV DNA vaccines employing intracellular targeting strategies. Gene Ther. 2004;11:1011–1018. doi: 10.1038/sj.gt.3302252. [DOI] [PubMed] [Google Scholar]
- 76. Huang CH, Peng S, He L, et al. Cancer immunotherapy using a DNA vaccine encoding a single-chain trimer of MHC class I linked to an HPV-16 E6 immunodominant CTL epitope. Gene Ther. 2005;12:1180–1186. doi: 10.1038/sj.gt.3302519. • This paper describes an innovative strategy for bypassing antigen processing as a method for generating stable antigen presentation in DCs, thus enhancing DNA vaccine potency.
- 77.Moniz M, Ling M, Hung CF, Wu TC. HPV DNA vaccines. Front Biosci. 2003;8:d55–d68. doi: 10.2741/936. [DOI] [PubMed] [Google Scholar]
- 78.Wu TC, Guarnieri FG, Staveley-O’Carroll KF, et al. Engineering an intracellular pathway for major histocompatibility complex class II presentation of antigens. Proc Natl Acad Sci USA. 1995;92:11671–11675. doi: 10.1073/pnas.92.25.11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ji H, Wang TL, Chen CH, et al. Targeting human papillomavirus type 16 E7 to the endosomal/lysosomal compartment enhances the antitumor immunity of DNA vaccines against murine human papillomavirus type 16 E7-expressing tumors. Hum Gene Ther. 1999;10:2727–2740. doi: 10.1089/10430349950016474. [DOI] [PubMed] [Google Scholar]
- 80. Hung CF, Tsai YC, He L, Wu TC. DNA vaccines encoding Ii-PADRE generates potent PADRE-specific CD4(+) T-cell immune responses and enhances vaccine potency. Mol Ther. 2007;15:1211–1219. doi: 10.1038/sj.mt.6300121. • This paper describes a novel strategy to enhance DNA vaccine potency by inducing a strong CD4+ T helper cell immune response.
- 81.Kim TW, Hung CF, Ling M, et al. Enhancing DNA vaccine potency by coadministration of DNA encoding antiapoptotic proteins. J Clin Invest. 2003;112:109–117. doi: 10.1172/JCI17293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smahel M, Pokorna D, Mackova J, Vlasak J. Enhancement of immunogenicity of HPV16 E7 oncogene by fusion with E. coli beta-glucuronidase. J Gene Med. 2004;6:1092–1101. doi: 10.1002/jgm.596. [DOI] [PubMed] [Google Scholar]
- 83.Kim TW, Hung CF, Boyd D, et al. Enhancing DNA vaccine potency by combining a strategy to prolong dendritic cell life with intracellular targeting strategies. J Immunol. 2003;171:2970–2976. doi: 10.4049/jimmunol.171.6.2970. [DOI] [PubMed] [Google Scholar]
- 84.Kim TW, Hung CF, Boyd DA, et al. Enhancement of DNA vaccine potency by coadministration of a tumor antigen gene and DNA encoding serine protease inhibitor-6. Cancer Res. 2004;64:400–405. doi: 10.1158/0008-5472.can-03-1475. [DOI] [PubMed] [Google Scholar]
- 85.Kim TW, Hung CF, Zheng M, et al. A DNA vaccine co-expressing antigen and an anti-apoptotic molecule further enhances the antigen-specific CD8+ T-cell immune response. J Biomed Sci. 2004;11:493–499. doi: 10.1007/BF02256098. [DOI] [PubMed] [Google Scholar]
- 86. Kim TW, Lee JH, He L, et al. Modification of professional antigen-presenting cells with small interfering RNA in vivo to enhance cancer vaccine potency. Cancer Res. 2005;65:309–316. • This study presents an innovative strategy to enhance DNA vaccine potency by prolonging DC survival to enhance T cell interaction.
- 87.Leachman SA, Tigelaar RE, Shlyankevich M, et al. Granulocyte-macrophage colony-stimulating factor priming plus papillomavirus E6 DNA vaccination: effects on papilloma formation and regression in the cottontail rabbit papillomavirus-rabbit model. J Virol. 2000;74:8700–8708. doi: 10.1128/jvi.74.18.8700-8708.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen CH, Wu TC. Experimental vaccine strategies for cancer immunotherapy. J Biomed Sci. 1998;5:231–252. doi: 10.1007/BF02255855. [DOI] [PubMed] [Google Scholar]
- 89.Kim MS, Sin JI. Both antigen optimization and lysosomal targeting are required for enhanced anti-tumour protective immunity in a human papillomavirus E7-expressing animal tumour model. Immunology. 2005;116:255–266. doi: 10.1111/j.1365-2567.2005.02219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sheets EE, Urban RG, Crum CP, et al. Immunotherapy of human cervical high-grade cervical intraepithelial neoplasia with microparticle-delivered human papillomavirus 16 E7 plasmid DNA. Am J Obstet Gynecol. 2003;188:916–926. doi: 10.1067/mob.2003.256. [DOI] [PubMed] [Google Scholar]
- 91. Klencke B, Matijevic M, Urban RG, et al. Encapsulated plasmid DNA treatment for human papillomavirus 16-associated anal dysplasia: a Phase I study of ZYC101. Clin Cancer Res. 2002;8:1028–1037. • This study represents the first therapeutic HPV DNA vaccine clinical trial in patients with anal dysplasia.
- 92.Garcia F, Petry KU, Muderspach L, et al. ZYC101a for treatment of high-grade cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2004;103:317–326. doi: 10.1097/01.AOG.0000110246.93627.17. [DOI] [PubMed] [Google Scholar]
- 93.Hariharan MJ, Driver DA, Townsend K, et al. DNA immunization against herpes simplex virus: enhanced efficacy using a Sindbis virus-based vector. J Virol. 1998;72:950–958. doi: 10.1128/jvi.72.2.950-958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brandsma JL, Shylankevich M, Su Y, et al. Vesicular stomatitis virus-based therapeutic vaccination targeted to the E1, E2, E6, and E7 proteins of cottontail rabbit papillomavirus. J Virol. 2007;81:5749–5758. doi: 10.1128/JVI.02835-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Berglund P, Quesada-Rolander M, Putkonen P, et al. Outcome of immunization of cynomolgus monkeys with recombinant Semliki Forest virus encoding human immunodeficiency virus type 1 envelope protein and challenge with a high dose of SHIV-4 virus. AIDS Res Hum Retroviruses. 1997;13:1487–1495. doi: 10.1089/aid.1997.13.1487. [DOI] [PubMed] [Google Scholar]
- 96.Berglund P, Smerdou C, Fleeton MN, et al. Enhancing immune responses using suicidal DNA vaccines. Nat Biotechnol. 1998;16:562–565. doi: 10.1038/nbt0698-562. [DOI] [PubMed] [Google Scholar]
- 97.Pushko P, Parker M, Ludwig GV, et al. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology. 1997;239:389–401. doi: 10.1006/viro.1997.8878. [DOI] [PubMed] [Google Scholar]
- 98.Hsu KF, Hung CF, Cheng WF, et al. Enhancement of suicidal DNA vaccine potency by linking Mycobacterium tuberculosis heat shock protein 70 to an antigen. Gene Ther. 2001;8:376–383. doi: 10.1038/sj.gt.3301408. [DOI] [PubMed] [Google Scholar]
- 99.Kim TW, Hung CF, Juang J, et al. Enhancement of suicidal DNA vaccine potency by delaying suicidal DNA-induced cell death. Gene Ther. 2004;11:336–342. doi: 10.1038/sj.gt.3302164. [DOI] [PubMed] [Google Scholar]
- 100.Cheng WF, Hung CF, Hsu KF, et al. Enhancement of sindbis virus self-replicating RNA vaccine potency by targeting antigen to endosomal/lysosomal compartments. Hum Gene Ther. 2001;12:235–252. doi: 10.1089/10430340150218387. [DOI] [PubMed] [Google Scholar]
- 101.Cheng W-F, Hung C-F, Chai C-Y, et al. Enhancement of Sindbis virus self-replicating RNA vaccine potency by linkage of herpes simplex virus type 1 VP22 protein to antigen. J Virol. 2001;75:2368–2376. doi: 10.1128/JVI.75.5.2368-2376.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cheng WF, Hung CF, Hsu KF, et al. Cancer immunotherapy using Sindbis virus replicon particles encoding a VP22-antigen fusion. Hum Gene Ther. 2002;13:553–568. doi: 10.1089/10430340252809847. [DOI] [PubMed] [Google Scholar]
- 103.Herd KA, Harvey T, Khromykh AA, Tindle RW. Recombinant Kunjin virus replicon vaccines induce protective T-cell immunity against human papillomavirus 16 E7-expressing tumour. Virology. 2004;319:237–248. doi: 10.1016/j.virol.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 104.Varnavski AN, Young PR, Khromykh AA. Stable high-level expression of heterologous genes in vitro and in vivo by noncytopathic DNA-based Kunjin virus replicon vectors. J Virol. 2000;74:4394–4403. doi: 10.1128/jvi.74.9.4394-4403.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Santin AD, Bellone S, Roman JJ, et al. Therapeutic vaccines for cervical cancer: dendritic cell-based immunotherapy. Curr Pharm Des. 2005;11:3485–3500. doi: 10.2174/138161205774414565. [DOI] [PubMed] [Google Scholar]
- 106.Tillman BW, Hayes TL, DeGruijl TD, et al. Adenoviral vectors targeted to CD40 enhance the efficacy of dendritic cell-based vaccination against human papillomavirus 16-induced tumor cells in a murine model. Cancer Res. 2000;60:5456–5463. [PubMed] [Google Scholar]
- 107.Wang T-L, Ling M, Shih I-M, et al. Intramuscular administration of E7-transfected dendritic cells generates the most potent E7-specific anti-tumor immunity. Gene Ther. 2000;7:726–733. doi: 10.1038/sj.gt.3301160. [DOI] [PubMed] [Google Scholar]
- 108.Mackova J, Kutinova L, Hainz P, et al. Adjuvant effect of dendritic cells transduced with recombinant vaccinia virus expressing HPV16-E7 is inhibited by co-expression of IL12. Int J Oncol. 2004;24:1581–1588. [PubMed] [Google Scholar]
- 109.Peng S, Kim TW, Lee JH, et al. Vaccination with dendritic cells transfected with BAK and BAX siRNA enhances antigen-specific immune responses by prolonging dendritic cell life. Hum Gene Ther. 2005;16:584–593. doi: 10.1089/hum.2005.16.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim TG, Kim CH, Won EH, et al. CpG-ODN-stimulated dendritic cells act as a potent adjuvant for E7 protein delivery to induce antigen-specific antitumour immunity in a HPV 16 E7-associated animal tumour model. Immunology. 2004;112:117–125. doi: 10.1111/j.1365-2567.2004.01851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Santin AD, Bellone S, Gokden M, et al. Vaccination with HPV-18 E7-pulsed dendritic cells in a patient with metastatic cervical cancer. N Engl J Med. 2002;346:1752–1753. doi: 10.1056/NEJM200205303462219. [DOI] [PubMed] [Google Scholar]
- 112.Ferrara A, Nonn M, Sehr P, et al. Dendritic cell-based tumor vaccine for cervical cancer II: results of a clinical pilot study in 15 individual patients. J Cancer Res Clin Oncol. 2003;129:521–530. doi: 10.1007/s00432-003-0463-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Santin AD, Bellone S, Palmieri M, et al. HPV16/18 E7-pulsed dendritic cell vaccination in cervical cancer patients with recurrent disease refractory to standard treatment modalities. Gynecol Oncol. 2006;100:469–478. doi: 10.1016/j.ygyno.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 114.Bubenik J, Simova J, Hajkova R, et al. Interleukin 2 gene therapy of residual disease in mice carrying tumours induced by HPV 16. Int J Oncol. 1999;14:593–597. doi: 10.3892/ijo.14.3.593. [DOI] [PubMed] [Google Scholar]
- 115.Hallez S, Detremmerie O, Giannouli C, et al. Interleukin-12-secreting human papillomavirus type 16-transformed cells provide a potent cancer vaccine that generates E7-directed immunity. Int J Cancer. 1999;81:428–437. doi: 10.1002/(sici)1097-0215(19990505)81:3<428::aid-ijc17>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 116.Mikyskova R, Indrova M, Simova J, et al. Treatment of minimal residual disease after surgery or chemotherapy in mice carrying HPV16-associated tumours: Cytokine and gene therapy with IL-2 and GM-CSF. Int J Oncol. 2004;24:161–167. [PubMed] [Google Scholar]
- 117.Chang EY, Chen CH, Ji H, et al. Antigen-specific cancer immunotherapy using a GM-CSF secreting allogeneic tumor cell-based vaccine. Int J Cancer. 2000;86:725–730. doi: 10.1002/(sici)1097-0215(20000601)86:5<725::aid-ijc19>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 118.Wlazlo AP, Deng H, Giles-Davis W, Ertl HC. DNA vaccines against the human papillomavirus type 16 E6 or E7 oncoproteins. Cancer Gene Ther. 2004;11:457–464. doi: 10.1038/sj.cgt.7700723. [DOI] [PubMed] [Google Scholar]
- 119.Chen CH, Wang TL, Hung CF, et al. Boosting with recombinant vaccinia increases HPV-16 E7-specific T cell precursor frequencies of HPV-16 E7-expressing DNA vaccines. Vaccine. 2000;18:2015–2022. doi: 10.1016/s0264-410x(99)00528-9. [DOI] [PubMed] [Google Scholar]
- 120.Rittich S, Duskova M, Mackova J, et al. Combined immunization with DNA and transduced tumor cells expressing mouse GM-CSF or IL-2. Oncol Rep. 2005;13:311–317. [PubMed] [Google Scholar]
- 121.Lin CT, Hung CF, Juang J, et al. Boosting with recombinant vaccinia increases HPV-16 E7-Specific T cell precursor frequencies and antitumor effects of HPV-16 E7-expressing Sindbis virus replicon particles. Mol Ther. 2003;8:559–566. doi: 10.1016/s1525-0016(03)00238-7. [DOI] [PubMed] [Google Scholar]
- 122.Mackova J, Stasikova J, Kutinova L, et al. Prime/boost immunotherapy of HPV16-induced tumors with E7 protein delivered by Bordetella adenylate cyclase and modified vaccinia virus Ankara. Cancer Immunol Immunother. 2006;55:39–46. doi: 10.1007/s00262-005-0700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Christensen ND, Han R, Cladel NM, Pickel MD. Combination treatment with intralesional cidofovir and viral-DNA vaccination cures large cottontail rabbit papillomavirus-induced papillomas and reduces recurrences. Antimicrob Agents Chemother. 2001;45:1201–1209. doi: 10.1128/AAC.45.4.1201-1209.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sobotkova E, Duskova M, Smahel M, et al. Chemotherapy and immunotherapy of tumours induced by gene-modified HPV16-transformed cells. Oncol Rep. 2004;12:877–883. [PubMed] [Google Scholar]
- 125.Fiander AN, Tristram AJ, Davidson EJ, et al. Prime-boost vaccination strategy in women with high-grade, noncervical anogenital intraepithelial neoplasia: clinical results from a multicenter phase II trial. Int J Gynecol Cancer. 2006;16:1075–1081. doi: 10.1111/j.1525-1438.2006.00598.x. [DOI] [PubMed] [Google Scholar]
- 126.Smyth LJ, Van Poelgeest MI, Davidson EJ, et al. Immunological responses in women with human papillomavirus type 16 (HPV-16)-associated anogenital intraepithelial neoplasia induced by heterologous prime-boost HPV-16 oncogene vaccination. Clin Cancer Res. 2004;10:2954–2961. doi: 10.1158/1078-0432.ccr-03-0703. [DOI] [PubMed] [Google Scholar]
- 127.Davidson EJ, Faulkner RL, Sehr P, et al. Effect of TA-CIN (HPV 16 L2E6E7) booster immunisation in vulval intraepithelial neoplasia patients previously vaccinated with TA-HPV (vaccinia virus encoding HPV 16/18 E6E7) Vaccine. 2004;22:2722–2729. doi: 10.1016/j.vaccine.2004.01.049. [DOI] [PubMed] [Google Scholar]
- 128.Bae SH, Park YJ, Park JB, et al. Therapeutic synergy of human papillomavirus E7 subunit vaccines plus cisplatin in an animal tumor model: causal involvement of increased sensitivity of cisplatin-treated tumors to CTL-mediated killing in therapeutic synergy. Clin Cancer Res. 2007;13:341–349. doi: 10.1158/1078-0432.CCR-06-1838. [DOI] [PubMed] [Google Scholar]
- 129.Ye GW, Park JB, Park YJ, et al. Increased sensitivity of radiated murine cervical cancer tumors to E7 subunit vaccine-driven CTL-mediated killing induces synergistic anti-tumor activity. Mol Ther. 2007;15:1564–1570. doi: 10.1038/sj.mt.6300149. [DOI] [PubMed] [Google Scholar]
- 130.Peggs KS, Quezada SA, Korman AJ, Allison JP. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol. 2006;18:206–213. doi: 10.1016/j.coi.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 131.Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother. 2007;56:739–745. doi: 10.1007/s00262-006-0272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Goldberg MV, Maris CH, Hipkiss EL, et al. Role of PD-1 and its ligand, B7-H1, in early fate decisions of CD8 T cells. Blood. 2007;110:186–192. doi: 10.1182/blood-2006-12-062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 134.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 135.Munn DH, Mellor AL. IDO and tolerance to tumors. Trends Mol Med. 2004;10:15–18. doi: 10.1016/j.molmed.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 136.Rubinstein N, Alvarez M, Zwirner NW, et al. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; A potential mechanism of tumor-immune privilege. Cancer Cell. 2004;5:241–251. doi: 10.1016/s1535-6108(04)00024-8. [DOI] [PubMed] [Google Scholar]
- 137.Yue FY, Dummer R, Geertsen R, et al. Interleukin-10 is a growth factor for human melanoma cells and down-regulates HLA class-I, HLA class-II and ICAM-1 molecules. Int J Cancer. 1997;71:630–637. doi: 10.1002/(sici)1097-0215(19970516)71:4<630::aid-ijc20>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 138.Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-β signaling in T cells. Nat Med. 2001;7:1118–1122. doi: 10.1038/nm1001-1118. [DOI] [PubMed] [Google Scholar]
- 139.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 140.Nagaraj S, Gupta K, Pisarev V, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]