Abstract
The incidence of melanoma is continuing to increase worldwide. UV exposure is a known risk factor for melanoma. Geographic location is known to influence UV exposure and the distribution of the incidence of melanoma. Furthermore, epidemiologic data suggest that gender and genetics may influence the distribution of melanoma on the body surface and histopathologic characteristics of the lesion. This article describes what is known about the impact of gender, ethnicity and geography on the progression of melanoma. Advanced-stage cutaneous melanoma has a median survival time of less than 1 year. Surgical removal, radiotherapy, chemotherapy, targeted therapies and a variety of immunotherapies have been utilized in the treatment of melanoma. Current treatment strategies and the results of recent clinical trials are also discussed in this article.
Keywords: anatomic distribution, epidemiology, ethnicity, gender, geography, melanoma
Once a rare cancer, the incidence of malignant melanoma skin cancer in most developed countries has risen faster than any other cancer type since the mid-1950s [1–12]. Cutaneous malignant melanoma (CMM) is the sixth most commonly diagnosed cancer in the USA in both genders [10]. An important feature of melanoma is that the incidence rate is highest in lighter skinned patients and is much rarer in darker skinned individuals. Furthermore, melanoma is one of the most deadly skin cancers; one person dies every hour from melanoma in the USA. Unfortunately, melanoma strikes individuals in the prime of their lives (median age: 52 years), almost a decade before most solid tumors arise (e.g., breast, colon, lung or prostate). Detection and surgical treatment of early-stage disease seems to prevent progression in most cases. However, patients with deep primary tumors or tumors that metastasize to regional lymph nodes frequently develop distant metastases. Median survival after the onset of distant metastases is only 6–9 months, and the 5-year survival rate is less than 5% [13].
There are two likely etiologic pathways to the development of melanoma. The most often-noted pathway is considered to be development from a common nevus (mole) with slow progression to a ‘melanoma in situ’, although multiple common nevi may only be a ‘marker’ of risk and not on the actual pathway to development. This radial growth can then develop into a vertical growth phase with a high capacity for metastases. The other pathway has melanomas arising rapidly without nevus involvement into aggressive lesions [13]. Solar UV radiation is an established risk factor for melanoma, with the magnitude of the risk depending on patterns of sun exposure (intermittent or cumulative UV exposure), frequency of excessive sun exposure and inherited susceptibility to its effects [14–18].
Epidemiology of melanoma
It is estimated that the annual increase in the incidence rate of melanoma has been approximately 3–7% per year worldwide for Caucasians [19]. This increased incidence may be attributable to better and earlier detection of melanomas and enhanced public awareness [11–13,20]. However, another significant part of this rise in incidence may be related to a change in sun-seeking behavior, as more people are exposed to natural and/ or artificial UV radiation [14,15]. An analysis of melanoma trends stratified by thickness, based on the Surveillance, Epidemiology and End Results (SEER) registry data from 1988 to 2006, showed that all four thickness categories (≤1, 1.01–2, 2.01–4 and >4 mm) increased in incidence over the 19-year study period [21]. Furthermore, Jemal et al. reported that this increase was different by gender; females had a greater increase in thin lesions (4.1% per year) than men, where the increase was greatest in thick melanomas (6.1% per year) [4]. Another analysis of nine SEER registry databases (1975–2006) showed that age-specific melanoma incidence rates were greater among women than men prior to age 40 years at diagnosis. In addition, melanomas on the trunk were more frequently diagnosed in US women than men [22,23]. Lipsker and colleagues hypothesized that, based on European data, the changes in trends reveal three different types of melanoma with different etiology and different risk factors [23,24]. This idea has been supported by other studies in which different sun exposure patterns (chronic vs intermittent) were related to the appearance of melanoma at different anatomic sites, suggesting divergent causal pathways for the disease [25,26].
Ultraviolet exposure is a known risk factor for melanoma. However, much less is known about the relationship between recent and life-time UV exposure and the interaction between UV radiation and genetic factors. UV-induced DNA damage (i.e., UV footprints) is sometimes but not always detected in melanoma tumors [27–30].
Indoor tanning is an artificial source of intermittent UV radiation that became popular in the early 1980s worldwide. Its popularity took advantage of media-influenced cultural images of tanned skin and its association with fitness. In a 2009 report of 116 US cities, public health researchers reported that the average number of tanning salons were much higher than any coffee shop or fast food chain restaurants; indoor tanning has developed into a business that gains US$5 billion in profits each year [31].
Tanning devices emit both UVA and UVB wavelengths. UVB was once considered the putative factor for skin carcinogenesis, but UVA has also been found to be absorbed by melanocytes. Furthermore, indoor tanning devices have changed over time; early devices emitted higher amounts of UVB rather than solar UV spectrum, which contributes to a higher frequency of sunburns; a risk factor for the development of melanoma. Later devices have been designed to emit more UVA to address concerns about burning. In the 1990s, high-speed or high-intensity devices were introduced to achieve a deep tan. In addition, high-pressure devices emitting mostly UVA became popular.
Several studies have been conducted to elucidate the interaction(s) between the tanning device, UV dose and risk of developing melanoma. A recent, large, population-based epidemiological study (n = 2268) conducted at the University of Minnesota (MN, USA) found an elevated odds ratio 1.74 of cutaneous malignant melanoma among indoor tanners when compared with those who never used indoor tanning [32]. They also observed a strong dose–response relationship between malignant melanoma risk and total hours, sessions or years engaged in indoor tanning activity. The authors concluded that, based on their analyses, no device can be considered ‘safe’ for use, as any device used, regardless of the UVB–UVA ratio they emitted, was found to contribute to an increased risk of melanoma among indoor tanners.
In addition, burns from indoor tanning were fairly common among users and conferred a similar risk of malignant melanoma to that seen from sunburns. Young age at tanning initiation was not found to increase age-group-specific susceptibility among adolescents as previously thought; rather they found that indoor tanning contributed to increased cumulative UV exposure among participants [32]. Similar results were also detected in a different population, using nested case–control study design, in the Norwegian Women and Cancer study [33].
In 2009, the International Agency of Research on Cancer (IARC) classified indoor tanning devices as carcinogenic to humans; they based this classification on ‘sufficient evidence’ from animal models [34]. Effective 1 July 2010, indoor tanning services experienced a 10% increase in taxation rates in the USA. This tax increase was included in the framework of the recent US healthcare system reform. Furthermore, a stronger sense of awareness of the health risks of indoor tanning can be seen worldwide. Prompted by key points from the IARC’s report – such as an increase in basal cell carcinomas, squamous cell carcinomas and ocular melanomas among tanning bed users, and a 75% increase in cutaneous melanoma risk among those who started tanning before age 30 years – nations across Northern Europe, Australia, New Zealand, North America and even Brazil have sought legislation to restrict tanning bed use, especially among young people.
Understanding the relationship between UV exposure and the progression of melanoma is important in the development of appropriate prevention strategies. Inherent differences in histopathologic characteristics of melanoma lesions, such as tumor thickness, are thought to be indicative of UV exposure patterns.
Thick versus thin melanoma
Breslow thickness is the depth of a melanoma lesion measured from the basement membrane of the epidermis to the deepest identified melanoma tumor cells. Thickness is the most important prognostic factor for melanoma, as deeper melanomas metastasize more easily via the bloodstream and the lymphatic system. There are other melanomas, usually with a thick Breslow index (>1.0 mm), that appear to have developed in the absence of nevus involvement, but their etiology is largely unknown. New epidemiologic data support the hypothesis that these melanomas are very different from thinner melanomas and their initiation through UV exposure is questionable. There are sparse scientific data on how human immunological factors influence these thick, possibly more genetically induced melanomas [35,36].
Anatomic location
Using epidemiologic data as a basis, Green proposed the theory of site-dependent susceptibility of melanocytes to malignant transformation [37]. This can be explained by people with a low propensity for melanocyte proliferation who require continual sun exposure to initiate melanocytes to clonally expand; thus, this occurs on areas of the body that are continually exposed to sunlight, while people with a high propensity for melanocyte proliferation, seen as a high number of nevi, develop melanomas on areas of the body that are intermittently exposed to the sun such as the trunk of the body [38]. Thus, based on this meta-analysis covering several continents and varied geographical locations, different etiologic pathways of melanoma development by anatomical site seem to exist.
It is important to emphasize that there is no positive association between melanoma thickness and time to diagnosis on a population basis. Results from a large, population-based cancer registry (n = 3772) and treatment unit data sets (n = 1300) in Australia independently showed that the time from detection of a change in the skin (first noticing a suspicious spot) to diagnosis was not longer in those who had thick lesions compared with those who had thin melanomas [36,37]. These findings support the idea that the different melanoma subtypes represent different biological characteristics and etiologic pathways. However, these findings should not be interpreted to mean that the time of melanoma diagnosis is not important. On the contrary, seeking medical care and attention for an uncertain skin lesion is highly recommended and needs to be stressed as an effective public health measure for improved survival in melanoma.
It has long been debated whether the site of a melanoma lesion is an indicator of the differential pathway in disease development. Epidemiologic findings regarding this hypothesis are inconclusive. The site of melanoma at diagnosis is not necessarily directly linked to localized UV exposures; several studies support this observation [39–43]. In addition, a Swedish occupational cohort study of melanoma cases diagnosed in men between 1970 and 1989 reported high risk ratios for melanoma on the trunk with localized occupational UV exposure to other body sites, suggesting that the effect of UV radiation is not restricted to exposed sites [43]. These findings led to the idea that melanomas develop through more than one biologic pathway.
The ‘divergent pathway’ hypothesis is based upon epidemiologic and experimental observations, which predict that individuals predisposed to few nevi will tend to develop melanomas on habitually sun-exposed body sites; conversely, nevus-prone individuals will develop melanomas on body sites with large numbers of nevi, such as the back [44]. This model was supported by a pooled analysis of ten case–control studies of melanoma in women in which it was observed that higher nevus counts on the arm were strongly associated with an increased risk of melanoma of the trunk and limbs but less so of the head and neck [44]. In addition, Caini et al. performed a meta-analysis using 24 observational studies to determine if melanoma risk factors differ depending on body site and histological subtype [38]. They found high nevus counts to be associated with melanoma on sites not usually exposed to the sun.
A pooled analysis performed by Chang et al. of 5700 melanoma cases and 7216 controls from seven studies from Europe, five studies from North America, one study from Hawaii and two studies from Australia revealed that melanoma risk at different body sites is associated with different amounts and patterns of sun exposure [45]. Specifically, intermittent, recreational sun exposure and sunburns were strong predictors of melanoma on less frequently sun-exposed body sites such as the trunk, at all latitudes. Continuous sun exposure (occupational) was associated with risk of head and neck melanoma, and total sun exposure was associated with an increased risk of melanoma on the limbs (both measured at low latitudes) [45]. Chang and colleagues concluded that these analyses were consistent with the ‘divergent pathway’ hypothesis. Further support for the divergent pathway hypothesis comes from evidence of differences in genome-wide alterations in DNA copy number and mutational status of BRAF and N-RAS genes in melanomas arising from different sites and with different levels of sun exposure. This is discussed in more detail later.
Similarly, analysis of patients from the Queensland (Australia) cancer registry revealed that head and neck melanoma patients were more likely than patients with trunk melanomas to have higher levels of occupational sun exposure but lower levels of recreational sun exposure [46]. Thus, head and neck melanomas were associated with chronic patterns of sun exposure, while truncal melanomas were associated with intermittent patterns of sun exposure. Whiteman and others have hypothesized that the anatomic site at which a melanoma develops is reflective of the susceptibility of host melanocytes to proliferate in response to sunlight [45,46]. Bulliard et al. conducted a seminal study that was the largest of its kind at the time, in which they compared the site distribution of melanoma in 41,331 incident cases from two phenotypically comparable populations from New Zealand and Canada [47]. The authors chose these two populations of patients because they share a primarily European ancestry. However, the incidence rates and UV exposures of the two countries are vastly different. The trunk and face were the predominant areas for lesions in the Canadian patients and the lower limbs were the most common sites in the New Zealand patients. Bulliard and colleagues were able to determine that the risk of developing melanoma varied in a site-specific manner and that environmental and lifestyle factors influence the site distribution in these populations [47].
Role of gender
Several groups throughout the world have identified differences in the anatomic distribution of melanomas in males when compared with females. Interestingly, melanomas on the trunk of the body present frequent mutations in BRAF, which is strongly associated with MC1R polymorphisms. MC1R expression is modulated by endocrine sex hormones; melanocyte regulation in the trunk may have hormone-related features since distribution is different between males and females [48].
A higher rate for CMM among women, predominantly in geographic regions with low sunlight indices, has been observed in the international literature. Countries with a high incidence of CMM, such as Australia, tend to have a slight predominance of females over males or a balance between genders [49]. These findings suggest the existence of a gender difference with changes in latitude. Clark et al. compared the distribution of melanomas seen at their institution (University of Pennsylvania, PA, USA) in the 1970s and 2004 to determine whether anatomic distribution changed over time. Males in the 1970s had higher odds of developing melanoma on the trunk and females on the extremities [50]. In 2004 women had increased odds of developing melanoma on the chest and men on their lower legs when compared with the 1970s. The authors attribute changes in distribution to changes in clothing and lifestyle. Pruthi et al. also suggest that these changes in body site distribution of melanoma over time could be caused by marked changes in the influence of risk factors for the disease, increased awareness, increased sun-protective practices and differences in sun-exposure behaviors [51].
Gender differences in body site distribution of melanoma lesions have been thought to be a result of inherent differences between males and females. Differences in clothing, hair style, occupation, sun-seeking behavior, and preventive measures and seeking medical care have all been considered as potential reasons for the higher incidence of lesions on the lower extremities in women and lesions of the head, neck and truncal areas of the body in men [47]. However, the reason(s) for the observed gender differences may go beyond societal differences among males and females. Speculations of a relationship between steroid hormones and melanoma arose when population studies revealed that females have a survival advantage over males, which was evident between 1973 and 1997 when males had a rate of death from melanoma that was twice as high as that of females [52]. Furthermore, the natural history of malignant melanoma in women has been reported to be rare before puberty, rise steeply through child-bearing years and decrease during menopausal years, thus implicating the involvement of estrogen. This phenomenon has led to the suggestion that hormones play an important role in melanoma [52]. Nonetheless, initial studies were unable to identify the presence of estrogen receptor (ER) α in melanocytic tumors. In addition, the unresponsiveness of melanomas to anti-estrogen therapies led to the assumption that estrogen was not involved in malignant melanoma [53]. ERβ has more recently been characterized and ERβ protein levels have been found to be associated with lesion thickness, with lower ERβ protein levels found in thicker tumors. Investigators have speculated that ERβ may be associated with the metastatic process in melanoma by regulating its invasive capacity [54]. Yet, epidemiologic studies evaluating the risk of melanoma in relation to hormonal and reproductive factors such as oral contraceptive use, hormone-replacement therapy, pregnancy and menopause are conflicting; some studies have failed to demonstrate a causal relationship, while others have found a dose-dependent increased risk of melanoma with estrogen use [55–57]. Thus, the existence of a relationship between hormones and melanoma remains uncertain.
Ethnicity & geography
The incidence of melanoma has been steadily increasing in Caucasian populations worldwide. Although gender appears to influence anatomic site distribution and mortality from melanoma, the increase in incidence appears to include both men and women. Trends in melanoma appear to be consistent throughout the world and vary only when comparisons are drawn with respect to latitude. Observed differences in epidemiologic studies of melanoma are most often attributed to variations in UV exposure from intermittent to chronic.
Chang et al. conducted a pooled analysis of seven studies from Europe, North America, Hawaii and Australia to investigate the complex relationship between sun exposure and melanoma risk at different latitudes [45]. The authors found recreational sun exposure and sunburn to be strong predictors of melanoma at all latitudes; measures of occupational and total sun exposure predicted melanoma predominately at low latitudes.
Africa
Melanoma is extremely rare among black populations in Africa and most cases of melanoma tend to be of the acral lentiginous subtype [58,59]. Immunosuppression as a result of HIV infection (Kaposi sarcoma), chronic ulceration, inflammation, albinism and UV exposure are identified risk factors for skin cancer in Africans. A retrospective study in Port Harcourt, Nigeria, identified 15 cases of melanoma at the University Hospital over an 11-year period [60]. In this study, the lower limbs and feet were the most common anatomic site; these melanomas were diagnosed at stage III or higher, and the majority of the cases were female. Africans have greater levels of melanin than Caucasians which provide protection from the carcinogenic effects of solar radiation. The acral lentiginous melanomas seen in Africans and African–Americans develop on areas of the body such as the nail beds, soles of the feet and palms of the hands. Interestingly, a retrospective analysis of melanoma in South Africans of mixed ancestry revealed histological type and anatomic distribution that is characteristic of black populations, yet the 5-year survival rate in these patients was similar to that seen in white populations [61]. These findings suggest the influence of genetics in the development and progression of melanoma.
Asia
In a recent study conducted in Japan, Uehara et al. identified prognostic factors for survival in cutaneous melanoma [62]. In this study population, thinner tumors, female gender and location on the extremity were found to be favorable prognostic features in malignant melanoma. Similar to findings in the USA and Europe, thickness was the most important prognostic factor in predicting survival in the melanoma patient in this Japanese study, with the best cut-off point being 4 or 5 mm.
Australia
Australia has among the highest incidence rates of melanoma in the world. Incidence rates trend toward a south–north gradient within the eastern states of Australia. In Queensland over a 20-year time period (1982–2002), incidence rates were highest for the trunk followed by the upper limbs in males, and the lower limbs followed by the upper limbs and trunk for females [49].
Canada
In a study initiated to examine historical changes in the incidence and anatomic site presentation of cutaneous melanoma during a 50-year period in Canada, it was observed that the trunk was the predominant site for melanoma in male patients younger than 60 years of age and the lower extremities were the most common site among female patients under 60 years of age [51]. Incidence rates appear to have slowed for young individuals; however, among those aged 60 years and older, the rates continue to rise.
Europe
In Norway, a country at high latitude that stretches over a long distance north–south, Cicarma et al. observed 2–2.5-times greater incidence in the south than the north during 1966–1986 and 1997–2007 [63]. Truncal melanomas predominated as the most prevalent site of lesions for both the north and south, and this did not change over the years studied. In this study, the high CMM incidence in a country with a low UV index could be related to the fact that most Scandinavian populations are of a high-risk skin type: fair skin, hair and eye color. Similarly, analysis of incident melanoma cases diagnosed between 1978 and 2004 revealed a significant increase over time in age-standardized rates over the 27-year time period [64]. In France, a retrospective study of melanomas diagnosed between 1980 and 2004 found a steady increase in thin melanomas predominantly on the trunk in men [65].
Regional cancer registries throughout Italy, particularly in the northern region of the country, collect epidemiologic figures and clinical characteristics of malignant melanoma. Similar to other countries in Europe and throughout the world, the trunk and lower limbs appear to be the most frequent sites of melanomas [66]. In a case–control study examining individual characteristics in patients diagnosed between 1992 and 1994, CMM tended to be located on the trunk in men and on the lower limbs in women [67]. Data on incident cases of malignant melanoma from the Schleswig–Holstein cancer registry in Germany diagnosed between 1998 and 2001 were analyzed [68]. Similar to other countries, in this population melanomas were predominantly located on the trunk in men and on the lower limbs in women.
Latin America
Studies from Argentina and Uruguay have also observed the predominant location of melanomas in women to be the lower extremities and the trunk for men. In Chile the anatomic distribution in women was the same as for Argentina and Uruguay; however, men tended to have a higher number of lesions on the ears, backs of the hands and soles of the feet [69]. Zemelman et al. conducted a study of malignant melanoma in Chile using recorded cases from five state hospitals [69]. They found significant differences between men and women. Women showed less aggressive histological types than men and presented more superficial tumors. There was predominance for melanomas on the limbs in women; conversely, a higher number of melanomas were found on the backs of the hands, soles of the feet and ears in men. The authors attribute the majority of this observed difference to genetic composition, as well as a contribution from sun exposure patterns [69].
Clinical and histopathological data on patients from a Dermatologic Clinic in São Paulo (Brazil) with cutaneous melanoma diagnosed over a 13-year period was analyzed to determine if correlations between the variables existed [70]. The trunk and feet were the most predominant sites for lesions in both males and females. In this cohort, patients presented with thick ulcerated tumors and there was a greater prevalence of female patients, nonwhite patients and acral lentiginous melanomas.
Middle East
The incidence of melanoma was evaluated over a 15-year period in Yazd, a central region of Iran [71]. In this study, males were 1.5-times more likely to have melanoma than females. The gender difference was speculated to be due to clothing and body covering customs, as well as lower levels of employment outside the home for women.
Incidence rates of melanoma among Arab immigrants residing in the USA have been found to be significantly higher than those residing in the Middle East, with women having a fivefold increase in the incidence of melanoma. The authors attribute this difference to better disease surveillance in the USA, acculturation of immigrants, and the immigrant population not being a true representation of the general population of the home countries [72].
USA
Woodall et al. used state of residence at the time of diagnosis (north vs south) in melanoma patients to compare clinicopathologic factors to determine if a geographical difference exists [73]. They found a shift in clinicopathologic factors based on sun exposure without a significant impact on outcome.
Other groups suggest that genetic predisposition may be more important than geography in the incidence of melanoma [74]. In fact, the incidence of melanoma may be influenced by gene–environment interactions that are not completely understood.
Using 11 US cancer registries, Eide and Weinstock examined the association between UV index, latitude and melanoma incidence [75]. They found that higher UV indices were significantly associated with an increase in melanoma incidence and lower latitude had significant correlation with incidence of melanoma in non-Hispanic whites only. Melanoma incidence was not significantly associated with index in black, Hispanic, Asian/pacific islander and Native American populations.
Incidence rates for melanoma are lower among Hispanic (4.5/100,000) and black populations (1.0/100,000) when compared with non-Hispanic white populations (21.6/100,000) in the USA. However, these minority groups are more likely to have melanomas that metastasize and have poorer outcomes [76]. More importantly, a study of ethnic differences among melanoma patients revealed a 1.96–3.01-fold greater risk of disease-specific mortality in minorities compared with white populations [77]. A lack of public education efforts tailored toward minorities, socioeconomic status, access to care and lack of early diagnosis are thought to contribute to the disparity in survival that exists. Furthermore, low incidence rates make it difficult for the initiation of large-scale epidemiologic studies to investigate the etiology of melanoma and the identification of risk factors associated with this disease in Hispanic and African–American populations. It has been hypothesized that physiologic differences in these ethnic groups could contribute to the increased mortality seen. The concept of admixture within ancestral genes has not been explored as a potential cause of genetic susceptibility for the development of melanoma in ethnic minorities. Ethnic differences in the skin phenotype and aggregation of mutations in genes associated with risk for developing melanoma are influenced by the geographic location of an individual.
Agredano et al. analyzed the correlation between increasing accessibility to air travel and melanoma incidence [78]. Using declining leisure-specific airfares as a proxy for increased access to sunny locations, they found that increasing rates of melanoma corresponded strongly with declining airfares in the USA. Similar results were seen in Norway when increasing air passenger mileage corresponded strongly with increasing melanoma incidence [79].
Occupational exposure has been reported among flight attendants and pilots. It is suspected that a higher proportion of UVB exposure can occur at a higher altitude while the proportion of UVA increases only minimally. Furthermore, the major aircraft flight routes are frequently directed above the dew point corresponding to higher UVB exposure during flights. Season of the year, time of day, cloudiness and amount of atmospheric aerosol also influence UVB exposure among pilots and flight attendants, contributing to higher occupational UV exposures [79]. In addition, these findings also suggest changes in recreational patterns that may be contributing to the public health problem of melanoma. As individual sun exposure patterns become more complex as a result of more frequent air travel, and potentially increased intermittent sun exposure, the contribution of these exposures to the development and progression of melanoma then becomes more difficult to assess.
Treatment options in malignant melanoma
Geographic differences in the treatment of melanoma are generally dictated by the availability of resources and/or infrastructure. Furthermore, melanoma is considered a disease of industrialized nations and in other areas of the world, scarce resources are usually not directed towards a rare disease as is the case for melanoma in countries such as Africa. However, although the incidence of melanoma is low in these countries, mortality remains high. The remaining section of this article describes recent strategies and advances in the treatment of melanoma. It should be noted that the treatments described here are those developed in countries with resources in which the great majority of melanomas occur in light-skinned individuals.
Aside from the availability of resources, therapeutic options for malignant melanoma are dependent upon the stage at diagnosis. Standard treatments for less invasive early-stage tumors are usually surgical excision without any additional therapies. Stage IV cutaneous malignant melanoma is considered incurable as the disease has disseminated to distant sites and organs throughout the body. The median survival time for patients with metastatic disease is 6–9 months and the 5-year survival rate is less than 5% [80]. To date, Phase III randomized controlled trials have failed to improve overall survival in patients with metastatic melanoma [81]. The reader is referred to Agarwala’s 2009 clinical review for a more detailed discussion of current systemic therapeutic strategies for metastatic melanoma [80].
Surgical excision
Surgical excision is the most common, well-accepted treatment for melanoma. Surgical margins used as part of the course of treatment for melanoma are based on multiple clinical trials of primary melanomas and are defined as follows:
5-mm surgical resection for in situ lesions;
1-cm margin for lesions with less than a 2-mm Breslow thickness;
2-cm surgical margin for lesions with 2-mm or greater Breslow thickness [82].
Breslow thickness is often used as a proxy for invasiveness and prognosis of malignant melanoma. Based on several follow-up studies [83–85], the effectiveness of thinner margins seemed to be validated as there was no statistical difference in overall survival rates and local tumor recurrence even in the thick Breslow lesion group.
Radiotherapy
Cutaneous malignant melanoma was long considered a radio-therapy-resistant tumor. This idea was based on the fact that melanocytes are known to easily repair DNA damage induced by low-dose radiation. However, clinical evidence suggests that certain types of melanoma, in particular, mucosal melanomas of the head and neck region, should be treated with radiation to prevent recurrence. Lesions of the sinonasal, nasopharyngeal and oral cavities appear to respond to radiotherapy [86,87]. In addition, lentigo maligna melanoma (LMM) relapses frequently when only surgical removal is employed [88]. Unresectable CMM lesions can also be treated with thermal neutron irradiation [86,89]. Recently, Henderson and colleagues demonstrated the utility of radiotherapy in melanoma patients using a multicenter randomized study of 250 patients with isolated regional recurrence. Their study was designed to assess the effects of adjuvant radio-therapy on regional recurrence, survival, morbidity and quality of life [90]. In this population of patients, those randomized to the radiotherapy group showed statistically significant improvement in lymph node field control compared with patients in the observation group.
Immunotherapy
Melanoma has been demonstrated to be a particularly immuno-genic tumor; thus, immunotherapy of melanoma has developed as a dynamic field for clinical research. To date, immunotherapies have been developed for malignant melanoma patients with distant metastases (stage IV) and also for stage II–III patients with micrometastatic disease among the fraction of patients having microscopic spread of tumor detected in lymph nodes. Melanoma vaccines and stimulation of immune responses by interrupting immune-inhibitory mechanisms are among the strategies that have been utilized with varied success [91].
Interferon-based approaches
Since 1986, the US FDA has approved the use of IFN-α as a cancer immunotherapy agent. Randomized clinical trials revealed that high-dose IFN-α, when administered in combination with chemotherapy, did not prove to be more effective than chemotherapy alone [92]. Yet the most updated quantitative evaluation of 14 randomized controlled clinical trials has revealed that IFN-α adjuvant therapy improves disease-free survival. The hazard ratio for disease recurrence was 0.82 (95% CI: 0.77–0.87; p < 0.001) and the improved overall survival observed in four of the 14 trials had a hazard ratio for death of 0.89 (95% CI: 0.83–0.96; p = 0.002) in advanced-stage cutaneous melanoma patients [93].
Cytokines
Cytokines are frequently administered to stage IV patients in an attempt to boost immune system tumor surveillance and melanocyte apoptosis. IL-2 (Aldesleukin, Proleukin®, Prometheus Inc., CA, USA), a cytokine produced by T cells, is involved in immunostimulatory effects on the immune system, such as the stimulation of natural killer cells [80]. High-dose IL-2 intravenous treatment was demonstrated to be effective in the treatment of melanoma and was approved for this use by the FDA in 1998. Specifically, IL-2 has been shown to increase T-cell function and increase memory T-cell production; a response rate of 16% has been observed in melanoma patients with advanced metastasis [94]. However, the use of high-dose IL-2 is limited by the high toxicities caused by this treatment [95].
New cytokines have been included recently in a search for new tools in immunotherapy in melanoma. IL-15 and IL-21 are similar in structure to IL-2, both of which stimulate T-cell-specific immune responses. Co-treatment with both cytokines may improve CD8+ and CD4+ T-cell responses [96]. IL-21 seems to be a promising agent as it does not enhance the production of regulatory/suppressor T cells, which is a common problem with IL-2 treatment [97,98].
Adoptive immunotherapy
Adoptive immunotherapy is a method utilizing ex vivo activation and expansion of tumor-reactive lymphocytes from the patient, which are then administered back to the same individual. Tumor-infiltrating lymphocytes are T cells with specific tumor reactivity and they offer a promising treatment strategy for tumor regression and targeted tumor eradication techniques in which response rates as high as 72% have been seen [99]. Other cell types such as dendritic cells and macrophages have also been employed in Phase I trials [100].
Vaccines
Vaccines are specialized immunostimulants designed and produced to initiate host immune responses, build long-term memory function and ultimately achieve tumor regression in melanoma [101]. Vaccines are used among patients with intermediate- to high-risk stage IV melanomas with very low response rates. Cancer vaccine trials in 440 melanoma patients, conducted at the National Cancer Institute Surgery Branch (MA, USA), had an overall objective response rate of only 2.6%. That result was comparable to the 4.0% response rate reported in 40 studies that involved 756 melanoma patients [101,102].
The principle behind melanoma vaccine development is to administer a single antigen or a mixture of antigens that are specific to the cancerous melanocyte growth and induce lymphocyte activation through increased recognition of these antigen(s). The ultimate goal is to enhance the anti-tumor immune response and tumor surveillance to the point that total tumor regression is achieved [103]. Previously, various single antigens were chosen among discovered melanocyte-derived proteins. Proteins such as melanoma antigen-encoding gene (MAGE)-1 and −3 are shared and commonly expressed among different solid tumors including melanomas. Other protein compounds specific to certain melanoma lineages such as Melan-A/melanoma antigen recognized by T cells (MART)-1, tyrosinase or gp100 and proteins that are overexpressed antigens in melanomas have been utilized. These antigens can be used explicitly as melanoma vaccine antigens. The Cancer Immunity website contains a list of potential tumor antigens in vaccine development [201].
Immune checkpoint inhibitors
Immune checkpoints are thought to be exploited by tumors as they inhibit T-cell functions in normal physiologic settings [104]. The cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) is an immune checkpoint molecule that downregulates T-cell activation pathways [104]. Recently, ipilimumab (MDX-010), a human monoclonal antibody, has been utilized to inhibit CTLA-4 and thus promote anti-tumor immunity. Ipilimumab has been successful when used as a monotherapy in Phase II studies, as well as when combined with other agents including cancer vaccines [105]. Specifically, ipilimumab has frequently been used to induce tumor regression and block immunosuppression among advanced melanoma patients [105]. A combination of autologous engineered tumor cells producing granulocyte colony-stimulating factors with periodic infusions of CTLA-4-blocking antibodies produced meaningful changes in infiltrating lymphocytes and tumor necrosis. This work expands the therapeutic options for the treatment of melanoma. The therapy had a longer time frame (at least 12 weeks) to achieve an objective response of approximately 15% for patients. Several trials administering fully human anti-CTLA-4 monoclonal antibodies to melanoma patients recorded some autoimmune disease-initiating side effects. As several immune-related adverse events (IRAEs) were reported previously during treatment with ipilimumab, more recent clinical trials have tried to improve positive and negative effects of CTLA-4-blocking antibodies [106].
Glycoprotein 100 peptide, derived from melanosomal protein, has been found to induce immune responses and potentially improve the efficacy of high-dose IL-2 treatment in patients with metastatic melanoma. However, it appears to have limited anti-tumor activity when used as a single treatment. Hodi and colleagues conducted a Phase III study using gp100 as an active control to determine whether ipilimumab, with or without gp100, improved overall survival among patients with previously treated metastatic melanoma. In this study population, ipilimumab administration, with and without gp100 peptide vaccine, improved overall survival in patients with previously treated metastatic melanoma [81].
Another treatment strategy utilizing immune check points involves blocking the programmed death-1 (PD-1) receptor with antibody dosing. PD-1 is an inhibitory receptor expressed on antigen-activated T cells and is thought to suppress anti-tumor immunity [107]. Furthermore, a known ligand for this receptor has been found to be highly expressed in murine and human tumors, and its expression is associated with poor prognosis for patients with certain epithelial cancers. Brahmer and colleagues conducted a Phase I trial to evaluate the anti-tumor activity, safety and tolerability of PD-1 receptor inhibition in patients with advanced treatment-refractory solid tumors [108]. In this trial, PD-1 antibody dosing appeared to have anti-tumor activity and was generally well tolerated by the patients. Anti-PD-1 has been demonstrated to be highly synergistic when combined with tumor vaccines in murine models. Brahmer and colleagues suggest that more effective utility of this antibody will involve combinatorial therapies with vaccines, targeted therapies or other immunomodulators [108].
Chemotherapy
Cytotoxic chemotherapy is often used in melanoma treatment. Anti-tumor effects have been demonstrated with dacarbazine, platinum analogs and nitrosoureas [108]. The effectiveness of dacarbazine has been validated for several years; however, a response rate of only approximately 7.5% has been confirmed in patients from the two largest and most recent randomized trials [80]. A recent analysis found that survival of longer than 18 months was detected only in a fraction of patients [109]. Perez and colleagues conducted a Phase II trial of carboplatin, weekly paclitaxel and biweekly bevacizumab in stage IV melanoma patients [110]. Bevacizumab is a VEGF monoclonal antibody that provides antiangiogenic effects. In melanoma, chemotherapy-induced overproduction of VEGF has been identified as a causative factor in the development of a more tumorigenic and metastatic phenotype that can ultimately lead to chemotherapy resistance [111]. Combined therapy was utilized in this trial as chemotherapy and bevacizumab combinations have been demonstrated to work synergistically in other cancers. In this trial, this combination was moderately to well tolerated and demonstrated some clinical activity. Further studies are needed to determine the relative value of adding bevacizumab to carboplatin–paclitaxel (CP) treatment.
A similar trial was not as successful in combining chemotherapeutics and biologic agents; sorafenib, a multikinase inhibitor that prevents tumor cell proliferation and angiogenesis, was trialled in combination with CP. In this Phase III, randomized placebo-controlled study with unresectable stage III or IV melanoma patients, the addition of sorafenib to CP did not improve any of the end points measured over placebo plus CP [111].
Targeted therapies
Targeted therapies refer to treatments designed to inhibit biochemical pathways activated by mutations in tumors [105]. Success of these therapies is dependent upon the identification of a ‘target’ or genetic abnormality in a cancer. In melanoma, two targets have been clinically validated in protein kinase-signaling pathways; BRAF and cKIT mutations. Inhibition of BRAF using the general BRAF inhibitor, sorafenib, decreases cell proliferation and invasion, and induces cell death in melanoma cell lines, yet it has demonstrated limited utility in clinical trials (reviewed in [112]). The mutant-specific BRAF inhibitor, PLX4032, has achieved up to a 70% clinical response rate in a Phase I trial in metastatic melanoma patients [113]. The success of this targeted therapy appears to be transient as the melanomas from many of the patients who initially responded to PLX4032 later became resistant. Targeting other areas of this pathway in combination with BRAF inhibition has been suggested as a potential mechanism to overcome resistance (reviewed in [95]).
Point mutations in cKIT, a tyrosine growth factor receptor, result in activation of proliferative and prosurvival signaling pathways [95]. Initial case reports of patients treated with imatinib, a small-molecule cKIT inhibitor, have provided promising results, with patients experiencing dramatic to durable responses to treatment [114]. Additional studies are needed to determine whether resistance mechanisms exist and if KIT inhibition leads to activation of other signaling pathways.
Targeted therapies in melanoma have had varied success in clinical trials in part because they rely on a simplistic approach to melanoma; inhibition/activation of a single target. The complexity of melanoma is clearly observed in melanomas that lack a mutation of interest yet respond to targeted therapies.
Curtin and colleagues identified the clinical importance of understanding to what extent the heterogeneity of the site, degree of UV exposure and histologic characteristics of melanoma is caused by distinct biologic pathways, and suggest that elucidation of these biologic mechanisms will result in separate targeted therapeutic approaches and prevention strategies [115]. The importance of this type of approach to melanoma was clearly demonstrated in their findings of markedly different patterns of genetic alterations in cutaneous, acral and mucosal melanomas at different sites and with different levels of sun exposure.
Expert commentary
Recently, several genetic-susceptibility studies have been conducted to identify potential genes linked to the development of melanoma. Remarkable results to date include findings from genome-wide association studies, which have identified chromosomal regions that had previously been suspected to be associated with familial melanoma. There is a great need for cohort studies to follow healthy people to determine if and when they develop melanoma. Genetic-susceptibility factors directly and indirectly influence the phenotype of an individual – proven to be one of the most important indicators of risk for melanoma. The question remains whether this relationship is direct or indirect, and which genes are involved. It has been established that skin type, number of nevi, type of sun exposure and family history are linked to risk. In addition, certain individual behaviors, such as sun seeking, use of sun protection and different exposure patterns (intermittent vs chronic), may influence the etiology of the disease – all of which must be taken into account in these large-scale epidemiologic studies.
Geographic areas with higher risk and available resources draw more cutting-edge research that explores the interrelationship between exposure, genetics and phenotype. However, research in other populations with different incidence patterns and disease etiologies, such as in ethnic groups, is needed, especially since these individuals tend to have more aggressive forms of melanoma at diagnosis and experience a higher mortality from melanoma.
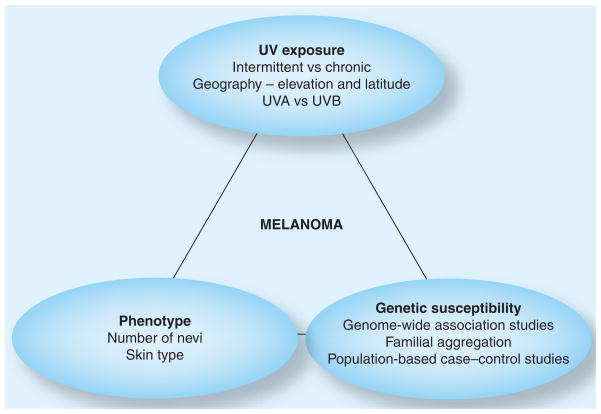
Although data to support a gene–environment interaction for the etiology of melanoma does not exist, it is a good model for studying melanoma. However, this is complicated by the vast differences in exposures and genetic backgrounds of individuals affected (Figure 1).
Figure 1.
The complex interaction of issues that currently represent the field.
Five-year view
The issue of place of residence during the lifetime of an individual needs to be factored into future studies to elucidate the impact of geography. More accurate measures of lifetime and current UV exposure patterns need to be developed and would allow for the identification of the amount and pattern of UV exposure necessary to initiate disease and to identify the different pathways leading to melanoma.
In order to successfully design preventive measures, it will be crucial to understand how protective factors are related to sun exposure, and how vitamin D production is involved in prevention and progression. As advances are made in the understanding of genetic susceptibility for melanoma, prevention and treatment strategies will need to be adapted; this will probably continue to evolve over the next 5 years.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
The project described was supported by Award Number K12GM088021 from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
- 1.Hall H, Miller D, Rogers J, Bewerse B. Update on the incidence and mortality from melanoma in the United States. J Am Acad Dermatol. 1999;40:35–42. doi: 10.1016/s0190-9622(99)70562-1. [DOI] [PubMed] [Google Scholar]
- 2.Marks R. Epidemiology of melanoma. Clin Exp Dermatol. 2000;25:459–463. doi: 10.1046/j.1365-2230.2000.00693.x. [DOI] [PubMed] [Google Scholar]
- 3.Garbe C, Roderick G, McLeod C, Buettner PG. Time trends of cutaneous melanoma in Queensland, Australia and Central Europe. Cancer. 2000;89:1269–1278. doi: 10.1002/1097-0142(20000915)89:6<1269::aid-cncr11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Devesa SS, Hartge P, Tucker MA. Recent trends in melanoma incidence among whites in the United States. J Natl Cancer Inst. 2001;93:678–683. doi: 10.1093/jnci/93.9.678. [DOI] [PubMed] [Google Scholar]
- 5.Marrett LD, Nguyen HL, Armstrong BK. Trends in the incidence of cutaneous malignant melanoma in New South Wales, 1983–1996. Int J Cancer. 2001;92:457–462. doi: 10.1002/ijc.1203. [DOI] [PubMed] [Google Scholar]
- 6.Diepgen TL, Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002;146:1–6. doi: 10.1046/j.1365-2133.146.s61.2.x. [DOI] [PubMed] [Google Scholar]
- 7.Bevona C, Sober AJ. Melanoma incidence trends. Dermatol Clin. 2002;20:589–595. doi: 10.1016/s0733-8635(02)00037-2. [DOI] [PubMed] [Google Scholar]
- 8.Veierod MB, Weiderpass E, Thorn M, et al. A prospective study of pigmentation, sun exposure, and risk of cutaneous malignant melanoma in women. J Natl Cancer Inst. 2003;95:1530–1538. doi: 10.1093/jnci/djg075. [DOI] [PubMed] [Google Scholar]
- 9.Thompson JF, Scolyer RA, Kefford RF. Cutaneous melanoma. Lancet. 2005;365:687–701. doi: 10.1016/S0140-6736(05)17951-3. [DOI] [PubMed] [Google Scholar]
- 10.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56(2):106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 11.de Vries E, Bray FI, Coebergh JW, Parkin DM. Changing epidemiology of malignant cutaneous melanoma in Europe 1953–1997: rising trends in incidence and mortality. Br J Dermatol. 2006;154:539–541. doi: 10.1002/ijc.11360. [DOI] [PubMed] [Google Scholar]
- 12.Geller AC, Swetter SM, Brooks K, Demierre MF, Yaroch AL. Screening, early detection, and trends for melanoma: current status (2000–2006) and future directions. J Am Acad Dermatol. 2007;57(4):555–572. doi: 10.1016/j.jaad.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 13.Houghton AN, Polsky D. Focus on melanoma. Cancer Cell. 2002;2(4):275–278. doi: 10.1016/s1535-6108(02)00161-7. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong B, Kricker A. How much melanoma is caused by sun exposure? Melanoma Res. 1993;3:395–401. doi: 10.1097/00008390-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Elwood JM, Jopson J. Melanoma and sun exposure: an overview of published studies. Int J Cancer. 1997;73:198–203. doi: 10.1002/(sici)1097-0215(19971009)73:2<198::aid-ijc6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 16.Solomon CC, White E, Kristal AR, Vaughan T. Melanoma and lifetime UV radiation. Cancer Causes Control. 2004;15:893–902. doi: 10.1007/s10552-004-1142-9. [DOI] [PubMed] [Google Scholar]
- 17•.Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma II. Sun exposure. Eur J Cancer. 2005;41:45–60. doi: 10.1016/j.ejca.2004.10.016. This seminal epidemiological meta analysis summarizes 57 studies examining the association between sun exposure and the risk of developing cutaneous melanoma. [DOI] [PubMed] [Google Scholar]
- 18.CDC. Sunburn prevalence among adults – United States, 1999, 2003, and 2004. MMWR Morb Mortal Wkly Rep. 2007;56(21):524–528. [PubMed] [Google Scholar]
- 19.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94(2):153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 20.Lens MB, Dawes M. Global perspectives of contemporary epidemiological trends of cutaneous malignant melanoma. Br J Dermatol. 2004;150:179–185. doi: 10.1111/j.1365-2133.2004.05708.x. [DOI] [PubMed] [Google Scholar]
- 21.Criscione VD, Weinstock MA. Melanoma thickness trends in the United States, 1988–2006. J Invest Dermatol. 2010;130(3):793–797. doi: 10.1038/jid.2009.328. [DOI] [PubMed] [Google Scholar]
- 22.Bradford PT, Anderson WF, Purdue MP, et al. Rising melanoma incidence rates of the trunk among younger women in the United States. Cancer Epidemiol Biomarkers Prev. 2010;19(9):2401–2406. doi: 10.1158/1055-9965.EPI-10-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipsker D, Engel F, Cribier B, Velten M, Hedelin G. Trends in melanoma epidemiology suggest three different types of melanoma. Br J Dermatol. 2007;157:338–343. doi: 10.1111/j.1365-2133.2007.08029.x. [DOI] [PubMed] [Google Scholar]
- 24.Lipsker DM, Hedelin G, Heid E, Grosshans EM, Cribier BJ. Striking increase of thin melanomas contrasts with stable incidence of thick melanomas. Arch Dermatol. 1999;135:1451–1456. doi: 10.1001/archderm.135.12.1451. [DOI] [PubMed] [Google Scholar]
- 25.Whiteman DC, Stickley M, Watt P, et al. Anatomic site, sun exposure, and risk of cutaneous melanoma. J Clin Oncol. 2006;24(19):3172–3177. doi: 10.1200/JCO.2006.06.1325. [DOI] [PubMed] [Google Scholar]
- 26.Dal H, Boldemann C, Lindelöf B. Does relative melanoma distribution by body site 1960–2004 reflect changes in intermittent exposure and intentional tanning in the Swedish population? Eur J Dermatol. 2007;17(5):428–434. doi: 10.1684/ejd.2007.0242. [DOI] [PubMed] [Google Scholar]
- 27.Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16(5):991–997. doi: 10.1158/1055-9965.EPI-06-1038. [DOI] [PubMed] [Google Scholar]
- 28.Thomas NE, Berwick M, Cordiero-Stone M. Could BRAF mutations in melanocytic lesions arise from DNA damage induced by ultraviolet radiation? J Invest Dermatol. 2006;126(8):1693–1696. doi: 10.1038/sj.jid.5700458. [DOI] [PubMed] [Google Scholar]
- 29.Poynter JN, Elder JT, Fullen DR, et al. BRAF and NRAS mutations in melanoma and melanocytic nevi. Melanoma Res. 2006;16(4):267–273. doi: 10.1097/01.cmr.0000222600.73179.f3. [DOI] [PubMed] [Google Scholar]
- 30.Thomas NE. BRAF somatic mutations in malignant melanoma and melanocytic naevi. Melanoma Res. 2006;16(2):97–103. doi: 10.1097/01.cmr.0000215035.38436.87. [DOI] [PubMed] [Google Scholar]
- 31.Hoerster KD, Garrow RL, Mayer JA, et al. Density of indoor tanning facilities in 116 large U.S. cities. Am J Prev Med. 2009;36:243–246. doi: 10.1016/j.amepre.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lazovich D, Vogel RI, Berwick M, et al. Indoor tanning and risk of melanoma: a case–control study in a highly exposed population. Cancer Epidemiol Biomarkers Prev. 2010;19(6):1557–1568. doi: 10.1158/1055-9965.EPI-09-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veierød MB, Parr CL, Lund E, Hjartåker A. Reproducibility of self-reported melanoma risk factors in a large cohort study of Norwegian women. Melanoma Res. 2008;18(1):1–9. doi: 10.1097/CMR.0b013e3282f120d2. [DOI] [PubMed] [Google Scholar]
- 34•.El Ghissassi F, Baan R, Straif K, et al. Special report: policy. A review of human carcinogens – part D: radiation. Lancet Oncol. 2009;10:751–752. doi: 10.1016/s1470-2045(09)70213-x. One of the most important International Agency for Research on Cancer reports published recently presenting scientific evidence of human and animal studies on increased risk of developing basal cell carcinomas, squamous cell carcinomas, and cutaneous and ocular melanomas among tanning bed users. [DOI] [PubMed] [Google Scholar]
- 35.Baade PD, English DR, Youl PH, et al. The relationship between melanoma thickness and time to diagnosis in a large population-based study. Arch Dermatol. 2006;142:1422–1427. doi: 10.1001/archderm.142.11.1422. [DOI] [PubMed] [Google Scholar]
- 36.Hersey P, Silalr RW, Howe CG, et al. Factors related to the presentation of patients with thick primary melanomas. Med J Aust. 1991;154(9):583–587. doi: 10.5694/j.1326-5377.1991.tb121217.x. [DOI] [PubMed] [Google Scholar]
- 37.Green A. A theory of the site distribution of melanomas: Queensland, Australia. Cancer Causes Control. 1992;3(6):513–516. doi: 10.1007/BF00052747. [DOI] [PubMed] [Google Scholar]
- 38.Caini S, Gandini S, Sera F, et al. Meta-analysis of risk factors for cutaneous melanoma according to anatomical site and clinic–pathological variant. Eur J Cancer. 2009;45:3054–3063. doi: 10.1016/j.ejca.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Franceschi S, Levi F, Randimbison L, La Vecchia C. Site distribution of different types of skin cancer: new aetiological clues. Int J Cancer. 1996;67(1):24–28. doi: 10.1002/(SICI)1097-0215(19960703)67:1<24::AID-IJC6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 40.Bulliard JL, Cox B, Semenciw R. Trends by anatomic site in the incidence of cutaneous malignant melanoma in Canada, 1969–1993. Cancer Causes Control. 1999;10(5):407–416. doi: 10.1023/a:1008964621225. [DOI] [PubMed] [Google Scholar]
- 41.Bulliard JL. Site-specific risk of cutaneous malignant melanoma and pattern of sun exposure in New Zealand. Int J Cancer. 2000;85(5):627–632. doi: 10.1002/(sici)1097-0215(20000301)85:5<627::aid-ijc5>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 42.Leiter U, Garbe C. Epidemiology of melanoma and nonmelanoma skin cancer – the role of sunlight. Adv Exp Med Biol. 2008;624:89–103. doi: 10.1007/978-0-387-77574-6_8. [DOI] [PubMed] [Google Scholar]
- 43.Perez-Gomez B, Pollán M, Gustavsson P, et al. Cutaneous melanoma: hints from occupational risks by anatomic site in Swedish men. Occup Environ Med. 2004;61(2):117–126. doi: 10.1136/oem.2002.006320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olsen CM, Zens MS, Stukel TA, et al. Nevus density and melanoma risk in women: a pooled analysis to test the divergent pathway hypothesis. Int J Cancer. 2009;124(4):937–944. doi: 10.1002/ijc.24011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang YM, Barrett JH, Bishop DT, et al. Sun exposure and melanoma risk at different latitudes: a pooled analysis of 5700 cases and 7216 controls. Int J Epidemiol. 2009;38:814–830. doi: 10.1093/ije/dyp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whiteman DC, Stickley M, Watt P, Hughes MC, Davis MB, Green AC. Anatomic site, sun exposure, and risk of cutaneous melanoma. J Clin Oncol. 2006;24(19):3172–3177. doi: 10.1200/JCO.2006.06.1325. [DOI] [PubMed] [Google Scholar]
- 47.Bulliard JL, Cox B, Elwood JM. Comparison of the site distribution of melanoma in New Zealand and Canada. Int J Cancer. 1997;72:231–235. doi: 10.1002/(sici)1097-0215(19970717)72:2<231::aid-ijc5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 48.Perez-Gomez B, Aragones N, Pollan M. Divergent cancer pathways for early onset and late onset cutaneous malignant melanoma. A role for sex–site interaction. Cancer. 2010;115:2499. doi: 10.1002/cncr.24985. [DOI] [PubMed] [Google Scholar]
- 49.Buettner PG, MacLennan R. Geographical variation of incidence of cutaneous melanoma in Queensland. Aust J Rural Health. 2008;16:269–277. doi: 10.1111/j.1440-1584.2008.00987.x. [DOI] [PubMed] [Google Scholar]
- 50.Clark LN, Shin DB, Troxel AB, Khan S. Association between the anatomic distribution of melanoma and sex. J Am Acad Dermatol. 2007;56:768–773. doi: 10.1016/j.jaad.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 51.Pruthi DK, Guilfoyle R, Nugent Z, Wiseman MC, Demers AA. Incidence and anatomic presentation of cutaneous malignant melanoma in central Canada during a 50-year period: 1956 to 2005. J Am Acad Dermatol. 2009;61(1):44–50. doi: 10.1016/j.jaad.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt A, Nanney LB, Boyd AS, King LE, Ellis DL. Oestrogen receptor-β expression in melanocytic lesions. Exp Dermatol. 2006;15:971–980. doi: 10.1111/j.1600-0625.2006.00502.x. [DOI] [PubMed] [Google Scholar]
- 53.Driscoll MS, Grant-Kels JM. Nevi and melanoma in pregnancy. Dermatol Clin. 2006;24:199–204. doi: 10.1016/j.det.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 54.de Giorgi V, Mavilla C, Massi D, et al. Estrogen receptor expression in cutaneous melanoma. Arch Dermatol. 2009;145:30–36. doi: 10.1001/archdermatol.2008.537. [DOI] [PubMed] [Google Scholar]
- 55.Holly EA, Cress RD, Ahn DK. Cutaneous melanoma in women: III. Reproductive factors and oral contraceptive use. Am J Epidemiol. 1995;141:943–950. doi: 10.1093/oxfordjournals.aje.a117361. [DOI] [PubMed] [Google Scholar]
- 56.Smith MA, Fine JA, Barnhill RL, Berwick M. Hormonal and reproductive influences and risk of melanoma in women. Int J Epidemiol. 1998;27:751–757. doi: 10.1093/ije/27.5.751. [DOI] [PubMed] [Google Scholar]
- 57.Koomen ER, Joosse A, Herings, et al. Estrogens, oral contraceptives and hormonal replacement therapy increase the incidence of cutaneous melanoma: a population-based case–control study. Ann Oncol. 2009;20(2):358–364. doi: 10.1093/annonc/mdn589. [DOI] [PubMed] [Google Scholar]
- 58.Adegbidi H, Yedomon H, Atadokpede F, Balley-Pognon MC, do Ango-Padonou F. Skin cancers at the National University Hospital of Cotonou from 1985 to 2004. Int J Dermatol. 2007;46(Suppl 1):26–29. doi: 10.1111/j.1365-4632.2007.03459.x. [DOI] [PubMed] [Google Scholar]
- 59.Asuquo ME, Ebughe G. Cutaneous cancers in Calabar, Southern Nigeria. Dermatol Online J. 2009;15(4):11. [PubMed] [Google Scholar]
- 60.Seleye-Fubara D, Etebu EN. Histological review of melanocarcinoma in Port Harcourt. Niger J Clin Pract. 2005;8(2):110–113. [PubMed] [Google Scholar]
- 61.Swan MC, Hudson DA. Malignant melanoma in South Africans of mixed ancestry: a retrospective analysis. Melanoma Res. 2003;13(4):415–419. doi: 10.1097/00008390-200308000-00012. [DOI] [PubMed] [Google Scholar]
- 62.Uehara S, Kamo R, Harada T, Ishii M. Survival analysis of malignant melanoma in Japan – multivariate analysis of prognostic factors. Osaka City Med J. 2009;55(1):35–52. [PubMed] [Google Scholar]
- 63.Cicarma E, Juzeniene A, Porojnicu A, Bruland O, Moan J. Latitude gradient for melanoma incidence by anatomic site and gender in Norway 1966–2007. J Photochem Photobiol B Biol. 2010;101(2):174–178. doi: 10.1016/j.jphotobiol.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 64.Doherty VR, Brewster DH, Jensen S, Gorman D. Trends in skin cancer incidence by socioeconomic position in Scotland, 1978–2004. Br J Cancer. 2010;102:1661–1664. doi: 10.1038/sj.bjc.6605678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lipsker D, Engel F, Cribier B, Velten M, Hedelin G. Trends in melanoma epidemiology suggest three different types of melanoma. Br J Dermatol. 2007;157:338–343. doi: 10.1111/j.1365-2133.2007.08029.x. [DOI] [PubMed] [Google Scholar]
- 66.Amerio P, Manzoli L, Auriemma M, Carbone A, Proietto G, Angelucci D. Epidemiology and clinical and pathologic characteristics of cutaneous malignant melanoma in Abruzzo (Italy) Int J Dermatol. 2009;48:718–722. doi: 10.1111/j.1365-4632.2009.03974.x. [DOI] [PubMed] [Google Scholar]
- 67.Naldi L, Altieri A, Imberti GL, Gallus S, Bosetti C, La Vecchia C. Sun exposure, phenotypic characteristics, and cutaneous malignant melanoma. An analysis according to different clinico-pathological variants and anatomical locations (Italy) Cancer Causes Control. 2005;16:893–899. doi: 10.1007/s10552-005-2300-4. [DOI] [PubMed] [Google Scholar]
- 68.Katalinic A, Kunze U, Schäfer T. Epidemiology of cutaneous melanoma and non-melanoma skin cancer in Schleswig–Holstein, Germany: incidence, clinical subtypes, tumour stages and localization (epidemiology of skin cancer) Br J Dermatol. 2003;149(6):1200–1206. doi: 10.1111/j.1365-2133.2003.05554.x. [DOI] [PubMed] [Google Scholar]
- 69.Zemelman V, Roa J, Ruiz T, Valenzuela CY. Malignant melanoma in Chile: an unusual distribution of primary sites in men from low socioeconomic strata. Clin Exp Dermatol. 2006;31:335–338. doi: 10.1111/j.1365-2230.2005.02038.x. [DOI] [PubMed] [Google Scholar]
- 70.Ferrari Júnior NM, Muller H, Ribeiro M, Maia M, Sanches Júnior JA. Cutaneous melanoma: descriptive epidemiological study. Sao Paulo Med J. 2008;126(1):41–47. doi: 10.1590/S1516-31802008000100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Noorbala MT, Kafaie P. Analysis of 15 years of skin cancer in central Iran (Yazd) Dermatol Online J. 2007;13(4):1. [PubMed] [Google Scholar]
- 72.Nasseri K, Mills PK, Allan M. Cancer incidence in the Middle Eastern population of California, 1988–2004. Asian Pac J Cancer Prev. 2007;8(3):405–411. [PMC free article] [PubMed] [Google Scholar]
- 73.Woodall CE, Martin RCG, Stromberg AJ, et al. Do melanoma patients from southern climates have a worse outcome than those from northern climates? Am Surg. 2009;75:687–692. [PubMed] [Google Scholar]
- 74.Cockburn M, Hamilton A, Mack T. The simultaneous assessment of constitutional behavioral, and environmental factors in the development of large nevi. Cancer Epidemiol Biomarkers Prev. 2007;16(2):200–206. doi: 10.1158/1055-9965.EPI-06-0273. [DOI] [PubMed] [Google Scholar]
- 75.Eide MJ, Weinstock MA. Association of UV index, latitude, and melanoma incidence in nonwhite populations – US Surveillance, Epidemiology and End Results (SEER) Program, 1992–2001. Arch Dermatol. 2005;141(4):477–481. doi: 10.1001/archderm.141.4.477. [DOI] [PubMed] [Google Scholar]
- 76.Rouhani P, Hu S, Kirsner RS. Melanoma in Hispanic and black Americans. Cancer Control. 2008;15:248–253. doi: 10.1177/107327480801500308. [DOI] [PubMed] [Google Scholar]
- 77.Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907–1914. doi: 10.1001/archinte.166.17.1907. [DOI] [PubMed] [Google Scholar]
- 78.Agredano YZ, Chan JL, Kimball RC, Kimball AB. Accessibility to air travel correlates strongly with increasing melanoma incidence. Melanoma Res. 2006;16(1):77–81. doi: 10.1097/01.cmr.0000195696.50390.23. [DOI] [PubMed] [Google Scholar]
- 79.Haldorsen T, Reitan J, Tveten U. Cancer incidence among Norwegian airline cabin attendants. Int J Epidemiol. 2001;30:825–830. doi: 10.1093/ije/30.4.825. [DOI] [PubMed] [Google Scholar]
- 80•.Agarwala SS. Current systemic therapy for metastatic melanoma. Expert Rev Anticancer Ther. 2009;9(5):587–595. doi: 10.1586/era.09.25. Clinical report that reviews current therapies for metastatic melanoma and provides the status of recently completed and ongoing trials. [DOI] [PubMed] [Google Scholar]
- 81•.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. Summarizes recent advancement of treatment options, and combination use of ipilimumab and vaccines against advanced melanomas, which result in fewer side effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haigh P, DiFronzo L, McCready D. Optimal excision margins for primary cutaneous melanoma: a systematic review and meta-analysis. Can J Surg. 2003;46:419–426. [PMC free article] [PubMed] [Google Scholar]
- 83.Veronesi U, Cascinelli N, Adamus J, et al. Thin stage I primary cutaneous malignant melanoma. Comparison of excision with margins of 1 and 3 cm. N Engl J Med. 1988;318:1159–1162. doi: 10.1056/NEJM198805053181804. [DOI] [PubMed] [Google Scholar]
- 84.Veronesi U, Cascinelli N. Narrow excision (1-cm margin) a safe procedure for thin cutaneous melanoma. Arch Surg. 1991;126:438–441. doi: 10.1001/archsurg.1991.01410280036004. [DOI] [PubMed] [Google Scholar]
- 85.Balch CM, Soong SJ, Smith T, et al. Long-term results of a prospective surgical trial comparing 2-cm v. 4-cm excision margins for 470 patients with 1 to 4-mm melanomas. Ann Surg Oncol. 2001;8:101–108. doi: 10.1007/s10434-001-0101-x. [DOI] [PubMed] [Google Scholar]
- 86.Fukuda H, Hiratsuka J, Kobayashi T, et al. Boron neutron capture therapy (BNCT) for malignant melanoma with special reference to absorbed doses to the normal skin and tumor. Aust Phys Eng Sci Med. 2003;26(3):97–103. doi: 10.1007/BF03178777. [DOI] [PubMed] [Google Scholar]
- 87.McLean N, Tighiouart M, Muller S. Primary mucosal melanoma of the head and neck. Comparison of clinical presentation and histopathologic features of oral and sinonasal melanoma. Oral Oncol. 2008;44(11):1039–1046. doi: 10.1016/j.oraloncology.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 88.Testori A, Rutkowski P, Marsden J, et al. Surgery and radiotherapy in the treatment of cutaneous melanoma. Ann Oncol. 2009;20(Suppl 6):vi22–vi29. doi: 10.1093/annonc/mdp257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Santa Cruz GA, Bertotti J, Marín J, et al. Dynamic infrared imaging of cutaneous melanoma and normal skin in patients treated with BNCT. Appl Radiat Isot. 2009;67(Suppl 7–8):S54–S58. doi: 10.1016/j.apradiso.2009.03.093. [DOI] [PubMed] [Google Scholar]
- 90.Henderson MA, Burmeister B, Thompson JF, et al. Adjuvant radiotherapy and regional lymph node field control in melanoma patients after lymphadenectomy: results of an intergroup randomized trial (ANZMTG 01.02/TROG 02.01) J Clin Oncol. 2009;27(Suppl 18) (Abstract LBA9084) [Google Scholar]
- 91.Hersey P. Immunotherapy for melanoma. Asia Pac J Clin Oncol. 2010;6(Suppl 1):S2–S8. doi: 10.1111/j.1743-7563.2010.01269.x. [DOI] [PubMed] [Google Scholar]
- 92.Vuoristo MS, Hahka-Kemppinen M, Parvinen LM, et al. Randomized trial of dacarbazine versus bleomycin, vincristine, lomustine and dacarbazine (BOLD) chemotherapy combined with natural or recombinant interferon-α in patients with advanced melanoma. Melanoma Res. 2005;15:291–296. doi: 10.1097/00008390-200508000-00010. [DOI] [PubMed] [Google Scholar]
- 93.Mocellin S, Pasquali S, Rossi CR, Nitti D. Interferon α adjuvant therapy in patients with high-risk melanoma: a systematic review and meta-analysis. J Natl Cancer Inst. 2010;102(7):493–501. doi: 10.1093/jnci/djq009. [DOI] [PubMed] [Google Scholar]
- 94.Atkins MB, Kunkel L, Sznol M, et al. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000;6(Suppl 1):S11–S14. [PubMed] [Google Scholar]
- 95.Davies MA, Samuels Y. Analysis of the genome to personalize therapy for melanoma. Oncogene. 2010;29(41):5545–5555. doi: 10.1038/onc.2010.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kirkwood JM, Ibrahim JG, Sondak VK, et al. High dose and low-dose interferon α-2b in high risk melanoma: first analysis of Intergroup trial E1690/ S9111/C9190. J Clin Oncol. 2000;18(12):2444–2458. doi: 10.1200/JCO.2000.18.12.2444. [DOI] [PubMed] [Google Scholar]
- 97.Davis ID, Skak K, Smyth MJ, et al. Interleukin-21 signaling: functions in cancer and autoimmunity. Clin Cancer Res. 2007;13:6926–6932. doi: 10.1158/1078-0432.CCR-07-1238. [DOI] [PubMed] [Google Scholar]
- 98.Thompson JA, Curti BD, Redman BG, et al. Phase I study of recombinant interleukin-21 in patients with metastatic melanoma and renal cell carcinoma. J Clin Oncol. 2008;26:2034–2039. doi: 10.1200/JCO.2007.14.5193. [DOI] [PubMed] [Google Scholar]
- 99.Prieto PA, Durflinger KH, Wunderlich JR, Rosenberg SA, Dudley ME. Enrichment of CD8+ cells from melanoma tumor-infiltrating lymphocyte cultures reveals tumor reactivity for use in adoptive cell therapy. J Immunother. 2010;33(5):547–556. doi: 10.1097/CJI.0b013e3181d367bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ellyard JI, Quah BJ, Simson L, Parish CR. Alternatively activated macrophage possess antitumor cytotoxicity that is induced by IL-4 and mediated by arginase-1. J Immunother. 2010;33(5):443–452. doi: 10.1097/CJI.0b013e3181cd8746. [DOI] [PubMed] [Google Scholar]
- 101.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eggermont AM, Gore M. Randomised adjuvant trials in melanoma: surgical and systemic. Semin Oncol. 2007;34:509–515. doi: 10.1053/j.seminoncol.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 103.Kirkwood JM, Tarhini AA, Panelli MC, et al. Next generation of immunotherapy for melanoma. J Clin Oncol. 2008;26(20):3445–3454. doi: 10.1200/JCO.2007.14.6423. [DOI] [PubMed] [Google Scholar]
- 104.Topalian SL, Chen K, Taube J, et al. Immunology. In: Balch C, Houghton AN, Sober AJ, et al., editors. Cutaneous Melanoma. 5. Quality Medical Publishing; MO, USA: 2009. pp. 865–882. [Google Scholar]
- 105.Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer. 2007;7:95–106. doi: 10.1038/nrc2051. [DOI] [PubMed] [Google Scholar]
- 106.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100(14):8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tarhini AA, Iqbal F. CTLA-4 blockade: therapeutic potential in cancer treatments. Onco Targets Ther. 2010;3:15–25. doi: 10.2147/ott.s4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brahmer JR, Drake CG, Wllner I, et al. Phase I study of a single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Atkins MB. The treatment of metastatic melanoma chemotherapy and biologics. Curr Opin Oncol. 1997;9(2):205–213. doi: 10.1097/00001622-199703000-00016. [DOI] [PubMed] [Google Scholar]
- 110.Perez DG, Suman VJ, Fitch TR, et al. Phase 2 trial of carboplatin, weekly paclitaxel, and biweekly bevacizumab in patients with unresectable stage IV melanoma. Cancer. 2009;115:119–127. doi: 10.1002/cncr.23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim C, Lee CW, Kovacic L, Shah A, Klasa R, Savage KJ. Long-term survival in patients with metastatic melanoma treated with DTIC or temozolomide. Oncologist. 2010;10:16. doi: 10.1634/theoncologist.2009-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hauschild A, Agarwala SS, Trefzer U, et al. Results of a Phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009;21(17):2823–2830. doi: 10.1200/JCO.2007.15.7636. [DOI] [PubMed] [Google Scholar]
- 113.Fisher DE, Barnhill R, Hodi FS, et al. Melanoma from bench to bedside: meeting report from the 6th international melanoma congress. Pigment Cell Melanoma Res. 2010;23:14–26. doi: 10.1111/j.1755-148X.2009.00655.x. [DOI] [PubMed] [Google Scholar]
- 114.Flaherty KT, Hodi FS, Bastian BC. Mutation-driven drug development in melanoma. Curr Opin Oncol. 2010;22:178–182. doi: 10.1097/cco.0b013e32833888ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115•.Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–2147. doi: 10.1056/NEJMoa050092. Seminal paper providing evidence of distinct genetic pathways involved in the development of melanomas through variations in genetic alterations in melanomas arising from different sites and with different levels of sun exposure. [DOI] [PubMed] [Google Scholar]
Website
- 201. [Accessed 11 June 2010];Cancer Immunity: Peptide database: shared tumor-specific antigens. www.cancerimmunity.org/peptidedatabase/tumorspecific.htm.