Abstract
An expanding spectrum of acute and chronic inflammatory diseases are considered “autoinflammatory” diseases. This review considers autoinflammatory diseases as being distinct from “autoimmune” diseases. Autoimmune diseases are associated with dysfunctional T-cells and treated with “biologicals” including anti-TNFα, CTLA-Ig, anti-IL-12/23, anti-CD20, anti-IL-17 and anti-IL-6 receptor. In contrast, autoinflammatory diseases are uniquely due to a dysfunctional monocyte caspase-1 activity and secretion of IL-1β; indeed, blocking IL-1β results in a rapid and sustained reduction in the severity of most autoinflammatory diseases. Flares of gout, Type-2 diabetes, heart failure and smoldering multiple myeloma are examples of seemingly unrelated diseases, which are uniquely responsive to IL-1β neutralization.
Keywords: cytokines, inflammation, diabetes, heart failure
Introduction
This review describes the ever-increasing areas within medicine for which there is a role for IL-1. Although it has been 26 years since the cDNA’s for IL-1β [1] and IL-1α [2] were first reported, IL-1-like “activities” had been studied earlier in models of inflammation such as fever [3], augmentation of lymphocyte activation [4] and induction of hepatic acute-phase protein synthesis [5]. With the availability of recombinant IL-1 in 1985, these and other activities confirmed the multiple properties of IL-1. For the fields of biology, immunology and medicine, that a single small protein could evoke nearly the entire host response to inflection or injury was validated [6]. Studies also revealed new and unexpected functions for IL-1; for example, new paradigms of therapeutic relevance emerged. Of these, blocking the activity of IL-1 became goal for clinical medicine. Later this concept was applied to other cytokines such as tumor necrosis factor (TNFα) [7, 8]. Much later to Toll-like Receptors (TLR) [9] joined the IL-1 family of receptors [10]. In fact, the biological activities of IL-1 and TLR are indistinguishable because the IL-1 Toll Receptor (TIR) domain is the functional domain for both IL-1 and TLR signaling [11, 12]. Without IL-1, concepts such as “innate immunity” would have remained “non-specific host defense” and studies on the function of the Toll protein would have remained in the field of fruit fly embryology.
Blocking IL-1 in Rheumatoid Arthritis
In clinical medicine, blocking the activity IL-1 has made a considerable impact on reducing disease severity. The IL-1 receptor antagonist (IL-1Ra) is approved for treating rheumatoid arthritis including reducing the destructive joint process. IL-1Ra binds to the IL-1 Receptor Type I (IL-1RI) with a high affinity without triggering a response; thus, IL-1Ra is a perfect receptor antagonist without any agonist activity. Natural IL-1Ra is structurally related to IL-1β and is a glycosylated protein. Anakinra (KinertR) is a recombinant form of natural IL-1Ra but is non-glycosylated. Because anakinra is injected each day and because the injection is often painful, anakinra is not popular with patients or rheumatologists. By comparison, there is widespread use of anti-TNFα agents in treating rheumatoid arthritis, which may be due to both a reduction in joint inflammation and a rapid (within a few days) reduction in the depressive effects of TNFα on the central nervous system. Other agents are also used to treat rheumatoid arthritis such as CTLA-4 Ig, anti IL-6 receptor monoclonal antibodies and depleting antibodies to CD20 expressed on B-lymphocytes. However, after one full year of treatment, the reduction in disease severity in patients with rheumatoid arthritis treated with anakinra is comparable to other treatment [13, 14]. IL-1 is potent inhibitor of proteoglycan synthesis in cartilage [15] and joint space narrowing and erosions in patients with rheumatoid arthritis treated with anakinra are clearly improved [13, 16, 17]. Moreover, unlike TNFα blocking therapies, there have been no reports of opportunistic infections particularly in reactivation of Mycobacterium tuberculosis in patients treated with IL-1β blocking agents.
Is there a role for anti-IL-1β in treating osteoarthritis?
In the joint, IL-1β is the mediator of reduced chondrocyte proteoglycan synthesis, increased synthesis of matrix metallo-proteinases and the release of nitric oxide [18]. Mice deficient in IL-1β are protected from inflammation-induced loss of cartilage [15]. Mice deficient in TNFα are not. The role of IL-1β in the destructive processes of osteoarthritis has also been studied in rabbits, pigs, dogs and horses [19]. There has been a placebo-controlled trial of intraarticular anakinra. Although there was a clear dose-dependent (50 mg versus 150 mg) reduction in pain and stiffness scores, the benefit did not extend beyond one month [20]. The modest reduction may be due to the heterogeneity of the osteoarthritis population in general but also to the short duration of IL-1RI blockade by anakinra. There is an ongoing study of anti-IL-1β monoclonal antibodies in osteoarthritis using direct intraarticular injection.
Classic Autoinflammatory Diseases
The initial descriptions of autoinflammatory diseases
As shown in Table 1, an increasing number of chronic inflammatory diseases are termed “autoinflammatory”. This term was originally coined for a group of rare, periodic febrile diseases such as Familial Mediterranean Fever (FMF) [21], Familial Cold Autoinflammatory Syndrome (FCAS) [22] and TNF Receptor Associated Periodic Syndrome (TRAPS) [23]. These diseases are characterized by recurrent fevers, leukocytosis, elevated acute phase proteins, myalgias and generalized fatigue. These autoinflammatory diseases have an identifiable genetic cause. In the case of Familial Cold Autoinflammatory Syndrome (FCAS) [22], the single amino acid mutation occurs in a gene coding for a protein termed “cryopyrin”. Since that seminal discovery, there has been an unprecedented level of investigation into how this gene product affects inflammation. The name is derived from the clinical description of the disease. Upon exposure to cold, such as cold air or cold water, the affect subjects develop fevers, leukocytosis and generalized “flu-like symptoms”’, hence the conjunction of “cryo” for cold and “pyrin” for hot or fever. Another systemic autoinflammatory disease is Muckle-Wells Syndrome (MWS), which is also due to a mutation in cryopyrin [24]. A more severe disease associated with a mutation in cryopyrin is Neonatal Onset Neonatal Onset Multisystem Inflammatory Disease (NOMID) [25]. Together, FCAS, MWS and NOMID are now called Cryopyrin Associated Periodic Syndromes (CAPS). Patients with CAPS often develop hearing loss. CAPS patients treated with either anakinra [25–27], a soluble IL-1 receptor (rilonacept) [28]or a monoclonal anti-human IL-1β (canakinumab) [29] experience a rapid, sustained and near complete resolution of the disease.
Table 1.
The Spectrum of Auto-inflammatory Diseases
| Classic Auto-inflammatory Diseases |
| Familial Mediterranean Fever |
| Cryopyrin Associated Periodic Syndromes (CAPS) |
| Hyper IgD Syndrome |
| Adult and Juvenile Still’s Disease |
| Behçet’s Disease |
| Schnitzler’s Syndrome |
| TNF Receptor-Associated Periodic Syndrome |
| PAPA Syndrome; Blau’s Syndrome; Sweet’s Syndrome |
| Probable Auto-inflammatory Diseases |
| Urticarial Vasculitis |
| Anti-synthetase Syndrome |
| Recurrent Idiopathic Pericarditis |
| Relapsing Perichondritis |
| Common Diseases Treatable by Blocking IL-1β |
| Urate Crystal Arthritis (gout) |
| Type-2 Diabetes |
| Smoldering Multiple Myeloma |
| Post-myocardial Infarction Heart Failure |
| Osteoarthritis |
CAPS is a grouping of Familial Cold Auto-inflammatory Syndrome, Muckle-Wells Syndrome and Neonatal Onset Multi-inflammatory Disease
However, there are many patients with near identical disease manifestations without these mutations. They have similar responses to IL-1β blockade, as do patients with the mutations [25, 30]. Initially, the concept of “autoinflammation” considered responses to signals such “cold” as triggers. Today, to use the term “autoinflammatory” for a particular disease has an additional meaning. For the most part, the use of “autoinflammation” for these are chronic inflammatory diseases is due to the dramatic, rapid and sustained improvement following a reduction in IL-1β activity by blockade of the IL-1 receptor with anakinra or neutralizing IL-1β with a soluble IL-1 receptor (rilonacept) or anti-IL-1β monoclonal antibodies (canakinumab, Xoma 052). As shown in Figure 1, the “auto” in autoinflammation also considers the fact that IL-1 induces itself [31] and may explain why a single administration of an anti-IL-1β monoclonal antibody results in prolonged resolution of disease activity after the antibody is cleared from the circulation [29]. Another characteristic of patients with autoinflammatory diseases is the response to reducing IL-1β activity is observed in patients who are refractory to corticosteroids, cyclosporine, azathiaprine or colchicine. Whereas anti-TNFα is often used to treat such refractory patients, the response to TNFα blockade is modest at best but often not sustained. It is also possible that even a modest response to anti-TNFα in these patients is due to a reduction in IL-1 activity since TNFα induces IL-1 [32]
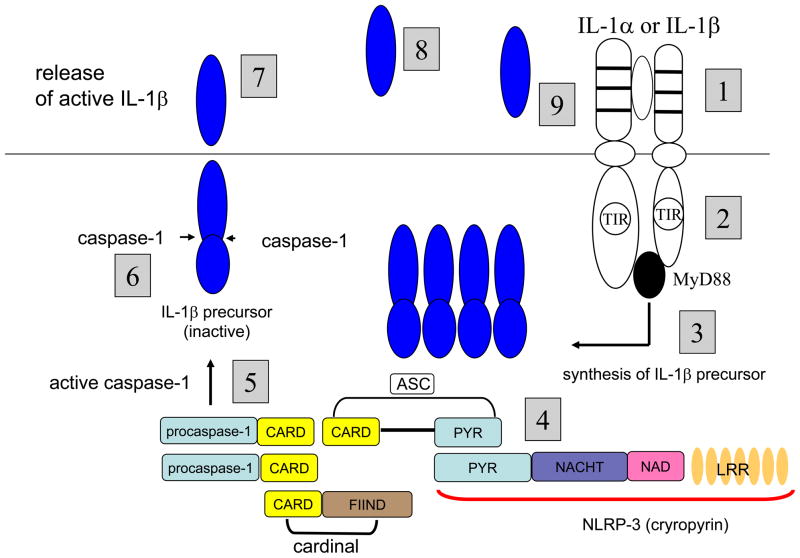
Figure 1. IL-1-mediated Autoinflammation.
1. Myeloid lineage cells expressing IL-1RI bind either IL-1α or IL-1β. 2. The Toll-IL-1-Receptor (TIR) domains of the IL-1RI-IL-1R Accessory Protein complex recruit MyD88 and initiate signal transduction. 3. Following NFκB or AP-1 translocation to the nucleus, the IL-1β precursor is synthesized. 4. Oligomerization of components of the NLRP3 inflammasome with procaspase-1 [36]. 5. Activation of caspase-1. 6. Cleavage of the IL-1β precursor by caspase-1. 7. Release of active IL-1β into the extracellular space [30, 93–95]. 8. Once released, IL-1β can bind to IL-1RI on non-myeloid cells such as endothelial and mesenchymal cells and stimulate the production of other cytokines and chemokines as well as mediators of inflammation, for example, COX-2, inducible nitric oxide and phospholipase A2. In addition, increases adhesion molecules, facilitating the emigration of inflammatory cells into injured or infected tissues. 9. On myeloid cells expressing IL-1RI, IL-1β activates the receptor and generates a signal resulting in more IL-1β [31]. Therapeutically, the cycle of IL-1-induced IL-1 is specifically arrested by reducing the activity of IL-1β via IL-1RI blockade (anakinra), binding of IL-1 to soluble IL-1 receptors (rilonacept) or neutralization by monoclonal anti-IL-1β antibodies (canakinumab, Xoma 052). Naturally, the cycle of IL-1-induced IL-1 can be controlled by endogenous IL-1Ra or endogenous IL-1RI type II.
The concept of autoinflammation as the pathogenic basis for a disease process that is highly responsive to IL-1β blockade is that the “auto” is IL-1 itself, either IL-1α or IL-1β. IL-1 induces its own gene expression, processing and secretion [31] and therefore chronic IL-1 triggering its own receptor can be interpreted as an autoinflammatory process. Nearly all epithelial tissues and keratinocytes contain the IL-1α precursor, which unlike the IL-1β precursor, binds to the IL-1 receptor and is active. Upon death of cells, the IL-1α precursor is released and induces myeloid cell-mediated inflammation in vivo [33, 34]. IL-1α is also expressed as an integral membrane protein, which is highly active in inducing chemokines from mesenchymal cells [35].
The distinction between autoinflammatory and autoimmune diseases
Autoinflammatory diseases lack associations with Class II MHC haplotypes, whereas autoimmune diseases exhibit distinct MHC associated haplotype susceptibilities. There are no auto-reactive T-cells driving disease activity in patients with autoinflammatory diseases. Autoimmune diseases are responsive to anti-TNFα, CTLA4-Ig, anti-IL-6 receptor, depletion of CD20 B-cells, anti-IL-12/IL-23 antibodies, anti-IL-17, anti-α-3 intergrin and anti-LFA. Each of these agents targets T- and B-cell functions. However, these latter therapeutic agents have no sustained effects in treating autoinflammatory diseases. In contrast, autoinflammatory diseases are uniquely responsive to IL-1β blockade. The monocyte-macrophage rather than the T-cell is the culprit in patients with autoinflammatory diseases and the defect is increased secretion of active IL-1β [25, 36, 37].
Role of caspase-1 in the pathogenesis of autoinflammatory diseases
Caspase-1 is the intracellular cysteine protease that cleaves the N-terminal 116 amino acids of the IL-1β precursor thus converting the inactive precursor to the active “mature” form. Caspase-1 exists in cells of myeloid origin such as tissue macrophages and dendritic cells as an inactive zymogen and requires conversion to an active enzyme by autocatalysis. However, in circulating human blood monocytes, caspase-1 is present in an active state [38]; when the monocyte is stimulated to synthesize the IL-1β precursor, cleavage of the precursor takes place and mature IL-1β is secreted over several hours. Caspase-1 is also constitutively active in highly metastatic human melanoma cells [39]. In general, the release of active IL-1β from blood monocytes is tightly controlled with less than 20% of the total synthetic IL-1β precursor being processed and released. Although the release of active IL-1β from blood monocytes of healthy subjects takes place over several hours [38], the process can be accelerated by the exogenous addition of ATP [40], which triggers the P2X7 purinergic receptor [41]. ATP activation of the P2X7 receptor opens the potassium channel, and simultaneously as intracellular potassium levels fall, caspase-1 is activated, the IL-1β precursor is cleaved and secretion take place [41].
The fall in intracellular potassium is thought to bring about the oligomerization of a highly specialize group of intracellular proteins, which bind to and convert procaspase-1 to an active enzyme. The increase in the secretion of active IL-1β can be due to a mutation in cryopyrin [22]. Cryopyrin is now termed nucleotide-binding domain and leucine-rich repeat containing protein 3 (NLRP3). In 2004, cryopyrin (NLRP3) was found to associate with procaspase-1 and other intracellular proteins and the complex was termed the “inflammasome” [36]. The inflammasome activates procaspase-1, resulting in active caspase-1 followed by the processing and secretion of active IL-1
Treating Rare Autoinflammatory Diseases with IL-1β Blockade
As shown in Table 1, rare autoinflammatory diseases are specifically responsive to IL-1β blockade. Although it is possible to demonstrate increased secretion of active IL-1β secretion in some of the diseases shown in Table 1, it is not necessary. Also, patients with active autoinflammatory diseases (fever, leukocytosis, elevated CRP) often do not have statistically significant elevated circulating IL-1β. In fact, increased secretion of IL-1β from blood monocytes of patients with autoinflammatory diseases are approximately 5–10-fold greater than that of healthy subjects [25, 37] and circulating IL-1β in patients with CAPS is also only 5-fold greater than levels in healthy subjects [42]. As such, demonstrated of elevated serum IL-1β is not a requirement for classification of autoinflammation. Therefore, injecting anakinra or other IL-1 blocker into patients serves as both a diagnostic as well as therapeutic exercise in defining an autoinflammatory disease.
Type-2 Diabetes, a Chronic, Low-Grade Autoinflammatory Disease
IL-1β and Type-2 Diabetes
Increasing evidence indicates that Type-2 diabetes is a chronic inflammatory disease [43, 44]. The selective cytotoxic effects of IL-1β for the insulin-producing pancreatic beta cell and not the alpha cells has been studied since 1986 [45]. Initially, IL-1β was considered to play a pathogenic role in Type-1 diabetes. Although this is still the case, in recent years, it was shown that high concentrations of glucose stimulated IL-1β production from the beta-cell itself [46] implicating a role for IL-1β in Type-2 diabetes. Increasing evidence points to an inflammatory process underlying the failure of the beta-cell to secrete sufficient amount of insulin in patients with Type-2 diabetes [44]. The insulitis is due to a pathological activation of the innate inflammatory system by metabolic stress, which appears to be mediated by IL-1 signaling. This raises the question of the cellular sources of IL-1β as well as the mechanisms of IL-1β production in Type-2 diabetes.
Over nutrition is the main cause of Type-2 diabetes. Accordingly, exposure of human islets to glucose or to free fatty acids (FFA) induces the production and release of IL-1β [47, 48] [49]. Furthermore, the adipokine leptin can also induce IL-1β production [50]. In support of those studies, gene expression for IL-1β was over one hundred-fold higher in beta cells isolated from the islets of Type-2 diabetic patients compared to non-diabetic patients [48]. In addition to high glucose concentrations, FFA also stimulates the production of IL-1β from the beta-cell [47]. As shown in Figure 2, the combination of high glucose concentrations plus FFA act together in inducing IL-1β. The source of FFA is the adipocyte, which is also a source of IL-1β [51]. In fact, the adipocyte produces IL-1β, which is under the control of caspase-1. Once released, IL-1β can amplify its signals by self-activation via engagement of the IL-1 receptor I leading to a vicious inflammatory cycle of IL-1-induced IL-1 [31, 48, 52]. Hence, Type-2 diabetes falls into the group of auto-inflammatory diseases and blocking IL-1β activity breaks the cycle.
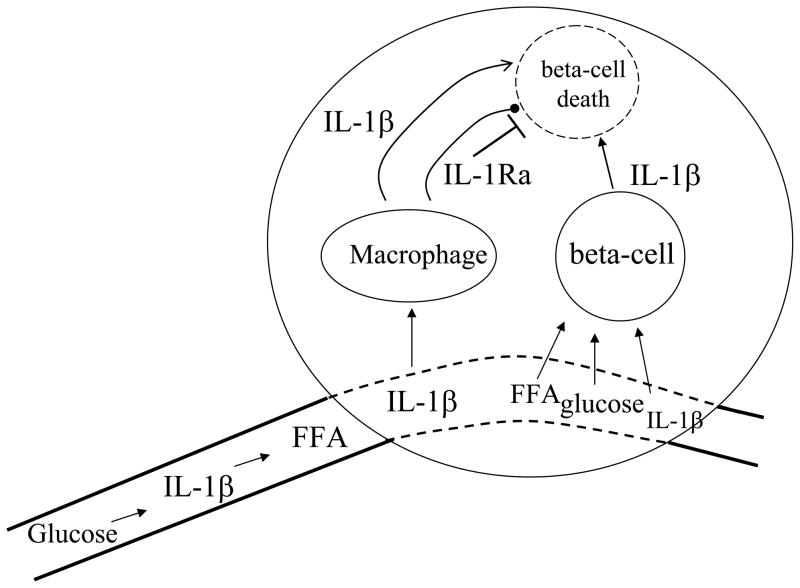
Figure 2. IL-1β-mediated loss of the insulin-producing beta-cell in Type-2 diabetes.
The islet receives its blood supply from a highly fenestrated vascular bed. Glucose, FFA and IL-1β itself as well as other cytokines enter the islet. In Type-2 diabetes, the islet contains increased numbers of macrophages, which produce chemokines and IL-1β itself as well as IL-1Ra. Macrophage IL-1β and macrophage IL-1Ra compete for occupancy of the IL-1RI on the beta-cell. In Type-2 diabetes, a low production of endogeneous IL-1Ra may contribute to beta-cell death. IL-1β, glucose and FFA enter the islet and directly stimulate beta-cell IL-1β production, which is toxic for the beta-cells. With time, beta-cell mass decreases and exogenous insulin is required. Arresting the cycle of IL-1β-induced IL-1β may restore dysfunctional beta-cells as well as arrest the loss of beta-cells.
Adipocyte Differentiation and the Role of Caspase-1
Caspase-1 influences the differentiation of adipocyte and insulin sensitivity via IL-1β-mediated mechanisms. High fat diet or obese ob/ob mice have increased caspase-1 and elevated levels of IL-1β. In contrast, adipose tissue from caspase-1 deficient mice is histologically distinct compared to fat from wild type mice; furthermore, caspase-1 deficient mice have decreased body fat and improved insulin sensitivity [53]. Adipocytes from caspase-1 deficient mice or mice deficient in NLRP3 are more metabolically active with higher insulin sensitivity and increased production of adiponectin compared to adipocytes from wild-type mice. Gene expression for PPARγ and GLUT4 were also increased in fat from caspase-1 or NLRP3 deficient mice. In the ob/ob obese mouse or in wild type mice fed a high fat diet, fat tissue reveals higher caspase-1 activity with elevated production of active IL-1β. Fat cells from wild-type mice treated with a specific caspase-1 inhibitor primarily affect IL-1β secretion.
Clinical Trials Blocking IL-1β in Type-2 Diabetes
The first clinical proof of a role for IL-1 in the pathogenesis of Type-2 diabetes is a randomized, placebo controlled study of anakinra for 13 weeks. In that study, improved insulin production and glycemic control was observed in anakinra-treated patients [54]. The fall in glycated hemoglobin was statistically significant and nearly 0.5% lower than that in placebo treated patients. There were no episodes of hypoglycemia during the trial. In addition to improved glycemic control, C-peptide levels increased and the ratio of proinsulin to insulin decreased, both indicators of improved beta-cell function. Not unexpectedly, serum IL-6 and CRP levels decreased significantly. However, insulin resistance, insulin-regulated gene expression in skeletal muscle and the body-mass index were unaffected by anakinra [54] in either study groups.
In the 39 weeks following the 13-week course of anakinra, patients who responded to anakinra used 66% less insulin to obtain the same glycemic control compared to baseline requirements [55]. The proinsulin to insulin ratio also improved. Patients with low circulating levels of endogenous IL-1Ra before the trial responded to anakinra and in the 39 weeks following the 13-week treatment period, these responders maintained the improvement in stimulated C-peptide [55]. These observations suggest that blocking IL-1β even for a short period of time restores the function of the beta cells or possibly allows for partial regeneration of beta cells.
The observation of anakinra trial in Type-2 diabetes has been confirmed using a specific neutralizing monoclonal antibody to IL-1β [56] and also provides more evidence that short-term blockade of IL-1β restores the function of the beta cells and possibly regeneration. Similar to the anakinra trial, the effect of a single administration of the monoclonal antibody to IL-1β resulted decreased glycated hemoglobin A1C, increased C-peptide levels, greater insulin production following a glucose challenge and decreased IL-6 and CRP levels [57]. The reduction in IL-1β-mediated inflammation is not limited to the islet but is rather systemic. Therefore, it is likely that improved glycemic control reflects not only less toxicity on the beta-cell in the islet but also reduced inflammation in the adipose tissue.
In considering the ability of a short reduction in IL-1β-mediated inflammation to improve the function of the insulin-producing beta-cell, one must consider the balance of IL-1β to endogenous levels of IL-1IL-1Ra (see Figure 2). Indeed, in parallel to increased IL-1β [46], IL-1Ra expression is decreased in islets of patients with Type-2. During the anakinra trial, it was noticed that clinical responses to anakinra correlated with baseline serum IL-1Ra levels [55], in that levels were significantly lower in patients responding to anakinra than in non-responders. This difference was similar 39 weeks after withdrawal of anakinra therapy, suggesting a stable phenotypic trait. Serum IL-1Ra levels correlated with variations in the 5’-promotor of the IL-1Ra gene. The C allele of the single nucleotide polymorphism (SNP) rs4251961 in the IL-1Ra gene that is associated with low serum IL-1Ra was significantly overrepresented in responders. Therefore, therapy with anakinra or anti-IL-1β neutralizing antibodies could be considered in the more than 40% Type-2 diabetic carriers of this variation.
Insulin Resistance
In blocking IL-1β in patients with Type-2 diabetes, there has been data suggesting that insulin resistance has changed. In general, unlike healthy subjects, patients with Type-2 diabetes with high steady state circulating insulin levels do not respond a glucose challenge by increasing the secretion of insulin. This failure has been interpreted as an indication of a non-responsive beta-cell and a hall-mark of the disease. With IL-1β blockade, there is a restoration of beta cell function in that insulin levels rise appropriately following a glucose challenge [54, 57]. It is possible that with prolonged IL-1β blockade in Type 2 diabetes, the reduction in systemic inflammation may reduce insulin resistance at the level of liver and skeletal muscle responses.
Failure of TNFα blockade to alter insulin resistance in humans
Initially, a great deal of attention was generated by the observation that TNFα expression in the obese Zucker rat correlated with the development of insulin resistance and the metabolic syndrome [58]. Although an adenoviral construct designed to inhibit TNFα activity did not reverse insulin resistance in the Zucker rat [59], in the obese ob/ob mouse, anti-TNFα treatment restored insulin receptors in wounded skin [60] and insulin resistance in the liver [61]. Other studies suggested that blocking TNFα in rodents corrects insulin sensitivity in models of the metabolic syndrome. Therefore, studies in humans with obesity, Type-2 diabetes as well as frank metabolic syndrome have been carried out. There are at least six published studies using neutralization of TNFα with monoclonal antibodies (infliximab or adalimumab) or soluble TNFα receptors (etanercept or a construct of the p55 TNFα receptor fused to the Fc of IgG) [62–67]. The doses of anti-TNFα agents used in each of these studies are the same dose, which ate highly effective in treating patients with rheumatoid arthritis, psoriasis or Crohn’s Disease.
In nearly all studies, a decrease in parameters of inflammation was observed. For example, decreases in CRP were consistently reported [62–64] and in some studies, circulating IL-6 was also decreased. In some studies the anti-inflammatory adipokine adiponectin increased [62, 66, 67]. Even fibrinogen, a risk for patients with the metabolic syndrome, decreased [62]. However, in each study, there was no change in insulin sensitivity. Insulin levels following an oral glucose tolerance test were unaffected after 4 weeks of etanercept (25 mg twice a week) [63]. C-peptide and glucose clearance was also unaffected by anti-TNFα antibodies [65]. Steady-state glucose levels were unchanged. In one study, muscle adiposity increased [67]. The decrease in circulating fatty acids reported in patients rheumatoid arthritis patients treated with anti-TNFα is likely due to a reduction of the ability of TNFα to inhibit lipoprotein lipase activity [67]. The ability of TNFα to inhibit lipoprotein lipase activity was first described by Cerami in 1981 [7]. Thus the increase in muscle adiposity may be due to the deposition of fat as a result of decreased lipolysis by blocking TNFα. One can conclude that a causative or contributing role for TNFα in insulin resistance in Type 2 diabetes in humans lacks support.
The case for IL-1β in and the loss of beta-cell mass in diabetes
From a historical viewpoint, the evidence that IL-1β was toxic for the insulin-producing beta-cell starts in 1985 using an immunoaffinity column containing anti-human IL-1β polyclonal antibodies [45]. This was a milestone report that opened the field of “soluble factors” from mononuclear phagocytes playing a pivotal role in the pathogenesis of diabetes. At the time, a role for immunocompetent cells in Type-2 diabetes was not appreciated. Thereafter, recombinant human IL-1β was shown to account for the death of the beta-cell while sparing the alpha-cell [68]. The topic has been reviewed by Mandrup-Poulsen who carried out the original studies [44]. The direct loss of beta-cell mass due to IL-1β is likely the result of more than one mechanism of action. In the rodent, IL-1β induces nitric oxide (inducible), a known toxic mediator for the beta-cell. Another mechanism is the induction of FasL by IL-1β. [69]. Regardless of the mechanism of action, clinical proof of the importance of IL-1β in the loss of beta cell mass has been obtained in patients with Type-2 diabetes.
Long-term Effects of Blocking IL-1β in Type-2 Diabetes
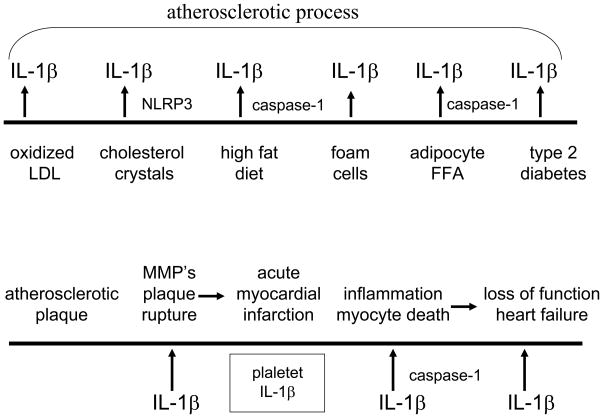
As shown in Figure 3, there are several places that IL-1β contributes to the time-line of atherosclerosis leading to myocardial infarction and heart failure. The increase in cardiovascular events associated with Type-2 diabetes also contributes to an increase in the atherosclerotic process. In Type-1 diabetes, the vascular complications of the disease are thought to be due to the chronic hyperglycemia. As such, if blocking IL-1β-mediated loss of beta-cell function results in improved control of glycemia, then one would anticipate reduced atherogenesis. Patients with Type-2 diabetes that have survived a myocardial infarction are at higher risk for a second infarction. With prolonged reduction in IL-1β activity in Type-2 diabetes, there is also a direct and beneficial effect on the heart. Similar to the ability of IL-1β to induce cell death in the beta-cell, IL-1β also is toxic for the cardic myocyte [70, 71]. In a placebo-controlled trial of patients with ST elevation myocardial infarction, daily anakinra was added to standard of therapy the day after angioplasty for 14 days. Serial imaging and echocardiographic studies after 14 weeks revealed that left ventricular remodeling was significantly reduced in patients receiving anakinra compared to patients receiving 14 days of placebo [71]. These findings are consistent with myocardial infarction models in mice in that blocking IL-1 results is a similar reduction in remodelling [72]. Therefore, reducing IL-1β-mediated inflammation in the islet may also benefit IL-1β-induced inflammation in coronary arteries, peripheral arteries and the myocardium itself.
Figure 3. Time-line for a role of IL-1β in heart failure.
Top. Left to right. The pathogenesis of atherosclerosis is affected by IL-1β production by oxidized LDL and cholesterol crystals as demonstrated in human monocytes. In animal studies, high fat diets, lipid laden foam cells and FFA from adipocytes have demonstrated a role for IL-1β. In animal models as well as in humans, IL-1β contributes to the loss of insulin-production beta-cells resulting in hyperglycemia and endothelial cell damage. Bottom. Left to right. The atherosclerotic plaque is at risk for rupture via IL-1β-induced matrix metalloproteases. Once the integrity of the plaque surface in lost, platelets initiate clot formation. IL-1β is released from activated platelets and contributes to the inflammation of the plaque via increased chemokines and inflammatory cells[96]. During the ischemia of the infracted myocardium, IL-1β induces myocyte cell death via a caspase-1 mechanism. With loss of myocytes, there is loss of function clinically termed heart failure. In patients surviving ST-elevated myocardial infarction (STEMI), administration of anakinra for 14 days improved myocardial function compared to placebo-treated patients [71]
Recurrent Attacks of Gout
Induction of IL-1β by Monosodium Urate Crystals
Recent clinical trials with IL-1β blockade have revealed an impressive and sustained reduction in patients with recurrent attacks of gouty arthritis [73–76]. Even with the use of allopurinol to reduce the systemic levels of uric acid and the anti-inflammatory properties of colchicine, there is no dearth of patients with recurrent episodes of painful gouty arthritis poorly controlled with these regimens. These patients often require intermittent courses of glucocorticoids. Thus, the success of IL-1β blocking therapies is a welcome addition for treating refractory gouty arthritis in these patients. Despite the availability of several widely used TNFα blocking therapies for rheumatoid arthritis and other autoimmune diseases, there is a paucity of reports that blocking TNFα provides an effective reduction in gout severity. One explanation for the lack of clinical trials of TNFα blockade in gout attacks is that efficacy of TNFα blockade in refractory gout is less than expected. One study reports a weak response with rather high doses of infliximab [77]. There are also few publications on monosodium urate (MSU) crystals inducing TNFα from human and mouse cells unless co-stimulated with endotoxins. Therefore, IL-1β blockade may be used for inducing long-term remissions in refractory patients and replace glucocorticoids. If IL-1β blockade becomes the standard of care in refractory gout, it would be consistent with the unique role of IL-1β in the pathogenesis of autoinflammatory diseases.
What precipitates the gouty attack?
Despite several reports that the addition of MSU crystals to mononuclear phagocytes induces IL-1β secretion [78, 79], MSU as a sole activator of active IL-1β is inconsistent with the clinical reality of gouty attacks. For example, why do less than 10% of persons with hyperuricemia and deposits MSU crystals in joints develop the disease and the most patients do not? Why do acute flares of gout often occur in the middle of the night? Why do patients receiving anti-tumor therapies have high uric acid levels but no clinical signs of gout? Why do persons with hyperuricemia have attacks of gout when they diet and lose weight? Although the associations of food and alcohol consumption with attacks of gout have been known for many centuries, how such life-style events relate to IL-1β activity has not been explored. Thus published studies that show MSU crystals alone induce active IL-1β requires a careful re-evaluation to be consistent with the clinical associations of gout attacks. A study by Joosten and co-workers [80], goes a long way in providing the basis for the production of IL-1β by MSU and concludes that one needs more than MSU to trigger an attack of painful gouty arthritis.
Pure MSU crystals per se do not induce IL-1β from primary peripheral blood mononuclear cells (PBMC) isolated from healthy donors or mouse peritoneal macrophages [80, 81]. In order to relate the association with dietary intake of fatty foods, free fatty acids (FFA) of increasing lengths were also added to human or mouse macrophages and similar to MSU alone, did not result in the secretion of IL-1β. However, the combination of MSU plus FFA induced the release of high levels of IL-1β into the supernatants of either PBMC or mouse macrophages. The study identified the eighteen carbon fatty acid (stearic acid) as the most effective lipid for stimulating IL-1β in combination with MSU crystals.
The evaluation of studies adding exogenous stimuli to human PBMC for cytokine production must assess the role of TLR ligands, particularly endotoxin (lipopolysaccahride, LPS), as these are unusually potent inducers of IL-1β in the picomolar range. Although some have reported that MSU crystals alone induce IL-1β from macrophages [78, 79], others have not been able to demonstrate this response unless a small amount of bacterial endotoxin is present as a “priming agent” [78]. In fact, there is a specific synergism of MSU crystals with LPS for the production of IL-1β [81]. Moreover, the concept that MSU crystals per se induce IL-1β is inconsistent with that fact that there is no inflammation where there is ample presence of urate crystal (tophi). Moreover, reports of MSU crystals inducing IL-1β do not explain why the attacks are precipitated with intake of large amounts of food. MSU crystals needs a second signal in inducing active IL-1β. However, from a clinical perspective, a second signal from LPS is hardly relevant to gout. There is usually no infection that triggers the flare of gout in susceptible persons. On the other hand, free fatty acids are relevant to gout. Indeed, one signal is provided by the high levels of MSU crystals in susceptible patients and a second signal comes from a rise in FFA following and episode of overnutrition. Neither MSU crystals nor FFA alone induce the release of biologically active IL-1β; both are needed.
Two signals are needed for IL-1β induction
It is highly likely that the synovial macrophage is already stimulated with MSU crystals secondary to the hyperuricemia. Then a rise in serum lipids provides the trigger for IL-1β synthesis, processing and release, thus precipitating the inflammatory flare of the attacks. The two-signal concept for the secretion of IL-1β is not a new concept and has a firm foundation in understanding the molecular mechanisms for synthesis and release of the active cytokine. Unlike IL-1α, the IL-1β precursor is not constitutively present in blood monocytes or tissue macrophages but requires a stimulus to be induced. Although many cytokines are translated soon after transcription, this is not the case with IL-1β. For example, freshly obtained human blood monocytes adhering to glass or plastic transcribe large amounts of IL-1β mRNA without any significant translation [82]. Naturally generated or recombinant complement C5a also results in transcription without translation [83]. The first signal is sometimes termed “priming” and drives gene expression for the IL-1β precursor. Unless a second signal is provided, the mRNA is degraded or the poly-adenylated mRNA falls off the ribosome soon after initiation of translation [84]. The signal for completion of ribosomal translation can come from various endogenous sources. Low picomolar concentrations of IL-1 itself provide a second signal for rapid translation of the IL-1β precursor [82–84]. It is now clear that saturated FFA also provide a signal for translation. In the case of IL-1β production in gout, MSU crystals likely provide the transcriptional priming signal and FFA the second signal. The role of TLR in this scenario comes from the ability of TLR2 to recognize FFA [80].
MSU crystals injected into the joint
As of this writing, there appears to be one report on the effect of MSU crystals injected into a joint of experimental pigs [85]. Most investigators study MSU crystal responses in primary as well as cell line lines. In vivo, MSU crystals are commonly injected into the peritoneal cavity or a skin pouch, both methods being rather different from the responses of the synovium to inflammatory agents. Similar to the observations in human PBMC and mouse macrophages, MSU crystals alone was not inflammatory as there was no joint swelling or induction of neutrophil infiltration following even high doses of MSU in the joints [80]. The C18 FFA alone was also without effect but the combination of C18 plus MSU crystals induced joint swelling, neutrophil infiltration and cytokines and chemokines. Both the IL-1β precursor and the processed form were elevated. Mice deficient in three molecular pathways for IL-1β processing and secretion were evaluated. Not unexpectedly, mice deficient in caspase-1 or ASC exhibited markedly reduced synovial inflammation in response to the MSU-C18 combination, and in mice deficient in ASC, histological examination of the joints revealed near complete protection. However, mice deficient in NLRP3 responded with same inflammatory response as did wild-type mice. Other studies have also observed an NLRP3- independent role of IL-1β processing [86]. The role of NLR3 may require TLR4 for activation of caspase-1.
Role of the inflammasome
In freshly obtained human PBMC, MSU crystals clearly synergizes with LPS via the TLR4 for the production and processing of the IL-1β [81], demonstrating that MSU crystals requires a second signal via a TLR. Since saturated FFA are inflammatory, how does FFA provide the TLR signal for MSU-induced inflammation? FFA appears to signal via TLR2 and not TLR4. These findings are consistent with those of others in that TLR2 recognizes FFA’s as endogenous ligands [87]. The role of FFA’s has also been studied in the context of Type-2 diabetes. FFA induces IL-1β, IL-6 and IL-8 in insulin-producing human islets and IL-1β in mouse islets [47]. Moreover, high glucose concentrations enhanced IL-1β-inducing properties of FFA-induced in human islets. Blocking the IL-1RI with IL-1Ra strongly inhibited FFA-mediated expression of IL-1β and chemokines in human and mouse islets. Similar to inflammation by MSU injected into the peritoneal cavity [78], the FFA-induced IL-1β and chemokine production was dependent on MyD88 [47].
In freshly isolated human blood monocytes, the rate limiting step in IL-1β processing and secretion is at the level of transcription/translation and not at the level of caspase-1. Caspase-1 is constitutively active in blood monocytes, even before the cells are cultured [38]. As such once the IL-1β precursor is synthesized, processing and secretion takes place. On the other hand, in macrophages derived from monocytes, caspase-1 is not active and requires a signal from ATP. Thus, in tissue macrophages three signals are required, once for transcription and one for translation and one for activation of caspase-1, the latter being the role of the inflammasome. Endotoxins provides the first two signals in the blood monocyte as well as in the macrophage. It is not uncommon that a low concentration of LPS is used to “prime” cells before another stimulant (such as MSU) is added to induce IL-1β. But in the case of gout, LPS as a priming agent is clinically irrelevant. One can conclude that MSU is the priming agent by inducing gene expression for IL-1β and fatty acids provides a uniquely clinically relevant translational signal. A similar mechanism likely takes place in the production of IL-1β in Type-2 diabetes.
Unexpectedly, the production of joint inflammation by the combination of MSU crystals plus C18 was not observed in NALP3 deficient mice. This finding is different from that reported by others and raises the issue of the presence of LPS in MSU preparations. Nevertheless, in mice deficient in ASC, there was an impressive reduction in MSU/FFA inflammation. In fact, the lack of inflammation in the ASC deficient mice was observed at the level of gene expression for IL-1β. One interpretation is that ASC is needed for an upstream effect on IL-1β gene expression and not just as the required co-factor for NALP3 activation of caspase-1. In mice deficient in caspase-1, a reduction in joint inflammation was reduced, although not to the extent as was observed in mice deficient in ASC [80]. Thus the reduction in IL-1β gene expression in ASC deficient mice would explain the lack of inflammation more than a reduced activation of caspase-1.
Role of the neutrophil
Since neutrophils dominate the inflammation of gouty arthritis in humans, the role of the neutrophil needs to be considered. Cell death of neutrophils provides a wealth of possibilities for inflammation. For the synovial macrophage, dead neutrophils provide a source of ATP and other small molecules for activating caspase-1. Neutrophils also provide a source proteinase-3, which can process the IL-1β precursor into an active cytokine [88]. The gouty attack is likely triggered by over nutrition with FFA providing the second signal in MSU primed cells, followed by the secretion of active IL-1β, which in turn, induces IL-8 and the infiltration of neutrophils. Large numbers of neutrophils augment the inflammation by providing enzymes and ATP, which induces more active IL-1β.
Is Smoldering Myeloma an Autoinflammatory Disease?
Targeting IL-1β in smoldering/indolent myeloma
Smoldering myeloma presents a challenge to medicine as the population ages [89]. Decades of research have focused on the role of IL-1β and IL-6 in the pathogenesis of multiple myeloma [90, 91]. Similar to mature B-cells, the myeloma plasma cell produces IL-1. In the microenvironment of the bone marrow, stromal cells respond to low concentrations IL-1 and release large amounts of IL-6, which in turn promotes the survival and expansion of the myeloma cells. Although IL-6 is a primary growth and survival cytokine for myeloma cells, antibodies to IL-6 have not been effective in treating the diseases. Lust, Donovan and co-workers reasoned that in the indolent stages of multiple myeloma, blocking IL-1β would provide better control of IL-6 activity. Bone marrow cells from patients with smoldering myeloma were co-cultured with a myeloma cell line actively secreting IL-1β. Although the addition of dexamethasone reduced stromal cell IL-6 production, the amount of IL-6 remained sufficiently high enough to protect the plasma cell against dexamethasone -mediated apoptosis. However, anakinra added to these co-cultures significantly reduced IL-6 by nearly 90% and the combination of anakinra plus dexamethasone induced myeloma cell death [92].
The Clinical Trial
Patients with smoldering or indolent myeloma were selected with the clinical objective of slowing or preventing progression to active disease. Based on in vitro data, 47 patients with smoldering/indolent myeloma at high risk for progression to full-blown multiple myeloma were treated with daily anakinra for six months. During the 6 months, there was a decrease in CRP in most but not all patients, which paralleled a decrease in the plasma cell labeling index, a measure of myeloma cell proliferation in unfractionated bone marrow cells. After 6 months of anakinra, a low dose of dexamethasone (20 mg per week) was added. Of the 47 patients that received anakinra (25 with dexamethasone), progression-free survival was over three years and in 8 patients over 4 years [92]. Compared to historical experience, the findings indicate a significant failure to progress to active disease and a modest fall in CRP predicted responders who continued with stable disease. Patients with a decrease in serum CRP of 15% or greater after 6 months of anakinra monotherapy resulted in progression-free survival greater than 3 years compared to 6 months in patients with less than a 15% fall during anakinra therapy (p<0.002). Thus, an effective reduction in IL-1β activity using CRP as the marker for IL-1β-induced IL-6 halts progression to active myeloma. With the use of anakinra in over 100,000 patients with rheumatoid arthritis or auto-inflammatory diseases, no opportunistic infections, including Mycobacterium tuberculosis reactivation, have been reported.
Acknowledgments
Supported by NIH Grants AI-15614, CA-04 6934 and JDRF 26-2008-893. The author thanks Mihai Netea, Leo Joosten and Jos van der Meer for many helpful suggestions in the preparation of this MS.
Footnotes
Conflict of interest statement
I do hold a patent in antibodies to human IL-1β. I receive royalities less than 5,000 USD each year for the sale of these antibodies as reagents. I receive no royalties on the use of anti-human IL-1β for clinical use.
References
- 1.Auron PE, Webb AC, Rosenwasser LJ, Mucci SF, Rich A, Wolff SM, Dinarello CA. Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci USA. 1984;81:7907–7911. [Google Scholar]
- 2.Lomedico PT, Gubler R, Hellmann CP, et al. Cloning and expression of murine interleukin-1 cDNA in Escherichia coli. Nature. 1984;312:458–462. doi: 10.1038/312458a0. [DOI] [PubMed] [Google Scholar]
- 3.Dinarello CA, Renfer L, Wolff SM. Human leukocytic pyrogen: purification and development of a radioimmunoassay. Proc Natl Acad Sci USA. 1977;74:4624–4627. doi: 10.1073/pnas.74.10.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenwasser LJ, Dinarello CA, Rosenthal AS. Adherent cell function in murine T-lymphocyte antigen recognition. IV. Enhancement of murine T-cell antigen recognition by human leukocytic pyrogen. J Exp Med. 1979;150:709–714. doi: 10.1084/jem.150.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McAdam KPWJ, Dinarello CA. In: Induction of serum amyloid A synthesis by human leukocytic pyrogen, in Bacterial Endotoxins and Host Response. Agarwal MK, editor. Amsterdam: Elsevier/North-Holland and Biomedical Press; 1980. pp. 167–178. [Google Scholar]
- 6.Dinarello CA. Interleukin-1 and the pathogenesis of the acute-phase response. New England Journal Of Medicine. 1984;311:1413–1418. doi: 10.1056/NEJM198411293112205. [DOI] [PubMed] [Google Scholar]
- 7.Kawakami M, Cerami A. Studies of endotoxin-induced decrease in lipoprotein lipase activity. J Exp Med. 1981;154:631–639. doi: 10.1084/jem.154.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dayer JM, Beutler B, Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985;162:2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gay NJ, Keith FJ. Drosophila Toll and IL-1 receptor. Nature. 1991;351:355–356. doi: 10.1038/351355b0. [DOI] [PubMed] [Google Scholar]
- 10.Sims JE, March CJ, Cosman D, et al. cDNA expression cloning of the IL-1 receptor, a member of the immunoglobulin superfamily. Science. 1988;241:585–589. doi: 10.1126/science.2969618. [DOI] [PubMed] [Google Scholar]
- 11.Heguy A, Baldari C, Bush K, et al. Internalization and nuclear localization of interleukin 1 are not sufficient for function. Cell Growth Differ. 1991;2:311–315. [PubMed] [Google Scholar]
- 12.Heguy A, Baldari CT, Macchia G, Telford JL, Melli M. Amino acids conserved in interleukin-1 receptors (IL-1Rs) and the Drosophila toll protein are essential for IL-1R signal transduction. J Biol Chem. 1992;267:2605–2609. [PubMed] [Google Scholar]
- 13.Bresnihan B, Newmark RD, Robbins S, McCabe DP, Genant HK. Anakinra reduces the rate of joint destruction after 1 year of treatment in a randomized controlled cohort of patients with rheumatoid arthritis. Arthrit Rheumat. 2000;43 (suppl 9):S289. [Google Scholar]
- 14.Genant HK, Bresnihan B, Ng E, Robbins S, Newmark RD, McCabe D. Treatment with anakinra reduces the rate of joint destruction and shows accelerated benefit in the secon 6 months of treatment for patients with rheumatoid arthritis. Ann Rheumat Dis. 2001;40(suppl 1):169. (abs) [Google Scholar]
- 15.Joosten LA, Helsen MM, Saxne T, van De Loo FA, Heinegard D, van Den Berg WB. IL-1 alpha beta blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF-alpha blockade only ameliorates joint inflammation. J Immunol. 1999;163:5049–5055. [PubMed] [Google Scholar]
- 16.Abramson SB, Amin A. Blocking the effects of IL-1 in rheumatoid arthritis protects bone and cartilage. Rheumatology (Oxford) 2002;41:972–980. doi: 10.1093/rheumatology/41.9.972. [DOI] [PubMed] [Google Scholar]
- 17.Bresnihan B, Cobby M. Clinical and radiological effects of anakinra in patients with rheumatoid arthritis. Rheumatology (Oxford) 2003;42(Suppl 2):ii22–28. doi: 10.1093/rheumatology/keg329. [DOI] [PubMed] [Google Scholar]
- 18.Joosten LA, Smeets RL, Koenders MI, et al. Interleukin-18 promotes joint inflammation and induces interleukin-1-driven cartilage destruction. Am J Pathol. 2004;165:959–967. doi: 10.1016/S0002-9440(10)63357-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39:237–246. [PubMed] [Google Scholar]
- 20.Chevalier X, Goupille P, Beaulieu AD, et al. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2009;61:344–352. doi: 10.1002/art.24096. [DOI] [PubMed] [Google Scholar]
- 21.Masters SL, Simon A, Aksentijevich I, Kastner DL. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease. Annu Rev Immunol. 2009;27:621–668. doi: 10.1146/annurev.immunol.25.022106.141627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDermott MF, Aksentijevich I, Galon J, et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell. 1999;97:133–144. doi: 10.1016/s0092-8674(00)80721-7. [DOI] [PubMed] [Google Scholar]
- 24.Hawkins PN, Lachmann HJ, Aganna E, McDermott MF. Spectrum of clinical features in Muckle-Wells syndrome and response to anakinra. Arthr Rheumat. 2004;50:607–612. doi: 10.1002/art.20033. [DOI] [PubMed] [Google Scholar]
- 25.Goldbach-Mansky R, Dailey NJ, Canna SW, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. New England Journal Of Medicine. 2006;355:581–592. doi: 10.1056/NEJMoa055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffman HM, Rosengren S, Boyle DL, et al. Prevention of cold-associated acute inflammation in familail cold autoinflammatory syndrome by interleukin-1 receptor antagonist prevents. Lancet. 2004;364:1779–1785. doi: 10.1016/S0140-6736(04)17401-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hawkins PN, Lachmann HJ, McDermott MF. Interleukin-1 receptor antagonist in the Muckle-Wells syndrome. New England Journal Of Medicine. 2003;348:2583–2584. doi: 10.1056/NEJM200306193482523. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman HM, Throne ML, Amar NJ, et al. Efficacy and safety of rilonacept (interleukin-1 trap) in patients with cryopyrin-associated periodic syndromes: Results from two sequential placebo-controlled studies. Arthritis Rheum. 2008;58:2443–2452. doi: 10.1002/art.23687. [DOI] [PubMed] [Google Scholar]
- 29.Lachmann HJ, Kone-Paut I, Kuemmerle-Deschner JB, et al. Use of canakinumab in the cryopyrin-associated periodic syndrome. New England Journal Of Medicine. 2009;360:2416–2425. doi: 10.1056/NEJMoa0810787. [DOI] [PubMed] [Google Scholar]
- 30.Gattorno M, Piccini A, Lasiglie D, et al. The pattern of response to anti-interleukin-1 treatment distinguishes two subsets of patients with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2008;58:1505–1515. doi: 10.1002/art.23437. [DOI] [PubMed] [Google Scholar]
- 31.Dinarello CA, Ikejima T, Warner SJ, Orencole SF, Lonnemann G, Cannon JG, Libby P. Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J Immunol. 1987;139:1902–1910. [PubMed] [Google Scholar]
- 32.Dinarello CA, Cannon JG, Wolff SM, et al. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986;163:1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 34.Cohen I, Rider P, Carmi Y, et al. Differential release of chromatin-bound IL-1alpha discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. Proc Natl Acad Sci U S A. 2010;107:2574–2579. doi: 10.1073/pnas.0915018107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaplanski G, Farnarier C, Kaplanski S, Porat R, Shapiro L, Bongrand P, Dinarello CA. Interleukin-1 induces interleukin-8 from endothelial cells by a juxacrine mechanism. Blood. 1994;84:4242–4248. [PubMed] [Google Scholar]
- 36.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1β processing inflammasome with increased activity in Muckle-Wells auto-inflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 37.Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med. 2005;201:1479–1486. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Netea MG, Nold-Petry CA, Nold MF, et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood. 2009;113:2324–2335. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okamoto M, Liu W, Luo Y, et al. Constitutively active inflammasome in human melanoma cells mediating autoinflammation via caspase-1 processing and secretion of interleukin-1beta. J Biol Chem. 2009;285:6477–6488. doi: 10.1074/jbc.M109.064907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gattorno M, Tassi S, Carta S, et al. Pattern of interleukin-1beta secretion in response to lipopolysaccharide and ATP before and after interleukin-1 blockade in patients with CIAS1 mutations. Arthritis Rheum. 2007;56:3138–3148. doi: 10.1002/art.22842. [DOI] [PubMed] [Google Scholar]
- 41.Perregaux DG, McNiff P, Laliberte R, Conklyn M, Gabel CA. ATP acts as an agonist to promote stimulus-induced secretion of IL-1 beta and IL-18 in human blood. J Immunol. 2000;165:4615–4623. doi: 10.4049/jimmunol.165.8.4615. [DOI] [PubMed] [Google Scholar]
- 42.Lachmann HJ, Lowe P, Felix SD, et al. In vivo regulation of interleukin 1beta in patients with cryopyrin-associated periodic syndromes. J Exp Med. 2009;206:1029–1036. doi: 10.1084/jem.20082481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1b in type 2 diabetes. Curr Opin Endocrinology. 2010 doi: 10.1097/MED.0b013e32833bf6dc. in press. [DOI] [PubMed] [Google Scholar]
- 44.Donath MY, Storling J, Berchtold LA, Billestrup N, Mandrup-Poulsen T. Cytokines and beta-cell biology: from concept to clinical translation. Endocr Rev. 2008;29:334–350. doi: 10.1210/er.2007-0033. [DOI] [PubMed] [Google Scholar]
- 45.Mandrup-Poulsen T, Bendtzen K, Nerup J, Dinarello CA, Svenson M, Nielsen JH. Affinity-purified human interleukin I is cytotoxic to isolated islets of Langerhans. Diabetologia. 1986;29:63–67. doi: 10.1007/BF02427283. [DOI] [PubMed] [Google Scholar]
- 46.Maedler K, Sergeev P, Ris F, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110:851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boni-Schnetzler M, Boller S, Debray S, et al. Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin-1 receptor I. Endocrinology. 2009;150:5218–5229. doi: 10.1210/en.2009-0543. [DOI] [PubMed] [Google Scholar]
- 48.Boni-Schnetzler M, Thorne J, Parnaud G, et al. Increased interleukin (IL)-1beta messenger ribonucleic acid expression in beta -cells of individuals with type 2 diabetes and regulation of IL-1beta in human islets by glucose and autostimulation. J Clin Endocrinol Metab. 2008;93:4065–4074. doi: 10.1210/jc.2008-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maedler K, Sergeev P, Ehses JA, et al. Leptin modulates beta cell expression of IL-1 receptor antagonist and release of IL-1beta in human islets. Proc Natl Acad Sci U S A. 2004;101:8138–8143. doi: 10.1073/pnas.0305683101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maedler K, Storling J, Sturis J, et al. Glucose- and interleukin-1beta-induced beta-cell apoptosis requires Ca2+ influx and extracellular signal-regulated kinase (ERK) 1/2 activation and is prevented by a sulfonylurea receptor 1/inwardly rectifying K+ channel 6.2 (SUR/Kir6. Diabetes. 2004;53:1706–1713. doi: 10.2337/diabetes.53.7.1706. [DOI] [PubMed] [Google Scholar]
- 51.Stienstra R, Joosten LA, Koenen T, van der Meer JWM, Tack CJ, Kanneganti T, Netea MG. The inflammasome-mediated caspase-1 activation controls apipocyte differentiation and insulin sensitivity. Cytokine. 2009;48:134. doi: 10.1016/j.cmet.2010.11.011. (abs) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toda Y, Tsukada J, Misago M, Kominato Y, Auron PE, Tanaka Y. Autocrine induction of the human pro-IL-1beta gene promoter by IL-1beta in monocytes. J Immunol. 2002;168:1984–1991. doi: 10.4049/jimmunol.168.4.1984. [DOI] [PubMed] [Google Scholar]
- 53.van Asseldonk EJP, Stienstra R, Koenen TB, et al. The effect of the interleukin-1 cytokine family members IL-1F6 and IL-1F8 on adipocyte differentiation. Obesity. 2010 doi: 10.1038/oby.2010.55. in press. [DOI] [PubMed] [Google Scholar]
- 54.Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. New England Journal Of Medicine. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 55.Larsen CM, Faulenbach M, Vaag A, Ehses JA, Donath MY, Mandrup-Poulsen T. Sustained effects of interleukin-1 receptor antagonist treatment in type 2 diabetes. Diabetes Care. 2009;32:1663–1668. doi: 10.2337/dc09-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donath MY, Weder C, Brunner A, et al. XOMA 052, a potential disease modifying anti-1L-1beta antibody, shows sustained HbA1c reductions 3 Months after a single injection with no increases in safety parameters in subjects with Type 2 diabetes. Diabetes. 2009;58:A30. [Google Scholar]
- 57.Donath MY, Boni-Schnetzler M, Ellingsgaard H, Ehses JA. Islet inflammation impairs the pancreatic beta-cell in type 2 diabetes. Physiology (Bethesda) 2009;24:325–331. doi: 10.1152/physiol.00032.2009. [DOI] [PubMed] [Google Scholar]
- 58.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 59.Cheung AT, Ree D, Kolls JK, Fuselier J, Coy DH, Bryer-Ash M. An in vivo model for elucidation of the mechanism of tumor necrosis factor-alpha (TNF-alpha)-induced insulin resistance: evidence for differential regulation of insulin signaling by TNF-alpha. Endocrinology. 1998;139:4928–4935. doi: 10.1210/endo.139.12.6336. [DOI] [PubMed] [Google Scholar]
- 60.Goren I, Muller E, Pfeilschifter J, Frank S. Severely impaired insulin signaling in chronic wounds of diabetic ob/ob mice: a potential role of tumor necrosis factor-alpha. Am J Pathol. 2006;168:765–777. doi: 10.2353/ajpath.2006.050293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Z, Yang S, Lin H, et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343–350. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 62.Bernstein LE, Berry J, Kim S, Canavan B, Grinspoon SK. Effects of etanercept in patients with the metabolic syndrome. Arch Intern Med. 2006;166:902–908. doi: 10.1001/archinte.166.8.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dominguez H, Storgaard H, Rask-Madsen C, et al. Metabolic and vascular effects of tumor necrosis factor-alpha blockade with etanercept in obese patients with type 2 diabetes. J Vasc Res. 2005;42:517–525. doi: 10.1159/000088261. [DOI] [PubMed] [Google Scholar]
- 64.Rosenvinge A, Krogh-Madsen R, Baslund B, Pedersen BK. Insulin resistance in patients with rheumatoid arthritis: effect of anti-TNFalpha therapy. Scand J Rheumatol. 2007;36:91–96. doi: 10.1080/03009740601179605. [DOI] [PubMed] [Google Scholar]
- 65.Ofei F, Hurel S, Newkirk J, Sopwith M, Taylor R. Effects of an engineered human anti-TNF-alpha antibody (CDP571) on insulin sensitivity and glycemic control in patients with NIDDM. Diabetes. 1996;45:881–885. doi: 10.2337/diab.45.7.881. [DOI] [PubMed] [Google Scholar]
- 66.Paquot N, Castillo MJ, Lefebvre PJ, Scheen AJ. No increased insulin sensitivity after a single intravenous administration of a recombinant human tumor necrosis factor receptor: Fc fusion protein in obese insulin-resistant patients. J Clin Endocrinol Metab. 2000;85:1316–1319. doi: 10.1210/jcem.85.3.6417. [DOI] [PubMed] [Google Scholar]
- 67.Lo J, Bernstein LE, Canavan B, Torriani M, Jackson MB, Ahima RS, Grinspoon SK. Effects of TNF-alpha neutralization on adipocytokines and skeletal muscle adiposity in the metabolic syndrome. Am J Physiol Endocrinol Metab. 2007;293:E102–109. doi: 10.1152/ajpendo.00089.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bendtzen K, Mandrup-Poulsen T, Nerup J, Nielsen JH, Dinarello CA, Svenson M. Cytotoxicity of human pI 7 interleukin-1 for pancreatic islets of Langerhans. Science. 1986;232:1545–1547. doi: 10.1126/science.3086977. [DOI] [PubMed] [Google Scholar]
- 69.Maedler K, Donath MY. Beta-cells in type 2 diabetes: a loss of function and mass. Horm Res. 2004;62 (Suppl 3):67–73. doi: 10.1159/000080503. [DOI] [PubMed] [Google Scholar]
- 70.Abbate A, Van Tassell BW, Seropian IM, et al. Interleukin-1beta modulation using a genetically engineered antibody prevents adverse cardiac remodelling following acute myocardial infarction in the mouse. Eur J Heart Fail. 2010;12:319–322. doi: 10.1093/eurjhf/hfq017. [DOI] [PubMed] [Google Scholar]
- 71.Abbate A, Kontos MC, Grizzard JD, et al. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction. Am J Cardiol. 2010;105:1371–1377. doi: 10.1016/j.amjcard.2009.12.059. [DOI] [PubMed] [Google Scholar]
- 72.Abbate A, Salloum FN, Vecile E, et al. Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation. 2008;117:2670–2683. doi: 10.1161/CIRCULATIONAHA.107.740233. [DOI] [PubMed] [Google Scholar]
- 73.Singh D, Huston KK. IL-1 inhibition with anakinra in a patient with refractory gout. J Clin Rheumatol. 2009;15:366. doi: 10.1097/RHU.0b013e3181be2423. [DOI] [PubMed] [Google Scholar]
- 74.So A, De Smedt T, Revaz S, Tschopp J. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther. 2007;9:R28. doi: 10.1186/ar2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Terkeltaub R. Update on gout: new therapeutic strategies and options. Nat Rev Rheumatol. 2010;6:30–38. doi: 10.1038/nrrheum.2009.236. [DOI] [PubMed] [Google Scholar]
- 76.Terkeltaub R, Sundy JS, Schumacher HR, et al. The interleukin 1 inhibitor rilonacept in treatment of chronic gouty arthritis: results of a placebo-controlled, monosequence crossover, non-randomised, single-blind pilot study. Ann Rheum Dis. 2009;68:1613–1617. doi: 10.1136/ard.2009.108936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fiehn C, Zeier M. Successful treatment of chronic tophaceous gout with infliximab (Remicade) Rheumatol Int. 2006;26:274–276. doi: 10.1007/s00296-005-0617-7. [DOI] [PubMed] [Google Scholar]
- 78.Chen CJ, Shi Y, Hearn A, et al. MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J Clin Invest. 2006;116:2262–2271. doi: 10.1172/JCI28075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 80.Joosten LA, Netea MG, Myloni E, et al. Fatty acids engagement with TLR2 drive IL-1beta producion via inflammasome acitvation by urate crystals in gouty arthritis. Arthr Rheumat. 2010 in press. [Google Scholar]
- 81.Giamarellos-Bourboulis EJ, Mouktaroudi M, Bodar E, van der Ven J, Kullberg BJ, Netea MG, van der Meer JW. Crystals of monosodium urate monohydrate enhance lipopolysaccharide-induced release of interleukin 1 beta by mononuclear cells through a caspase 1-mediated process. Ann Rheum Dis. 2009;68:273–278. doi: 10.1136/ard.2007.082222. [DOI] [PubMed] [Google Scholar]
- 82.Schindler R, Clark BD, Dinarello CA. Dissociation between interleukin-1β mRNA and protein synthesis in human peripheral blood mononuclear cells. J Biol Chem. 1990;265:10232–10237. [PubMed] [Google Scholar]
- 83.Schindler R, Gelfand JA, Dinarello CA. Recombinant C5a stimulates transcription rather than translation of IL-1 and TNF; cytokine synthesis induced by LPS, IL-1 or PMA. Blood. 1990;76:1631–1638. [PubMed] [Google Scholar]
- 84.Kaspar RL, Gehrke L. Peripheral blood mononuclear cells stimulated with C5a or lipopolysaccharide to synthesize equivalent levels of IL-1β mRNA show unequal IL-1β protein accumulation but similar polyribosome profiles. J Immunol. 1994;153:277–286. [PubMed] [Google Scholar]
- 85.Chapman PT, Yarwood H, Harrison AA, et al. Endothelial activation in monosodium urate monohydrate crystal-induced inflammation: in vitro and in vivo studies on the roles of tumor necrosis factor alpha and interleukin-1. Arthritis Rheum. 1997;40:955–965. doi: 10.1002/art.1780400525. [DOI] [PubMed] [Google Scholar]
- 86.Kolly L, Karababa M, Joosten LA. Inflammatory role of ASC. 2009 doi: 10.4049/jimmunol.0802173. [DOI] [PubMed] [Google Scholar]
- 87.Nguyen MT, Favelyukis S, Nguyen AK, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 88.Joosten LA, Netea MG, Fantuzzi G, et al. Inflammatory arthritis in caspase 1 gene-deficient mice: Contribution of proteinase 3 to caspase 1-independent production of bioactive interleukin-1beta. Arthritis Rheum. 2009;60:3651–3662. doi: 10.1002/art.25006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kyle RA, Remstein ED, Therneau TM, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. New England Journal Of Medicine. 2007;356:2582–2590. doi: 10.1056/NEJMoa070389. [DOI] [PubMed] [Google Scholar]
- 90.Torcia M, Lucibello M, Vannier E, et al. Modulation of osteoclast-activating factor activity of multiple myeloma bone marrow cells by different interleukin-1 inhibitors. Exp Hematol. 1996;24:868–874. [PubMed] [Google Scholar]
- 91.Lust JA, Donovan KA. The role of interleukin-1 beta in the pathogenesis of multiple myeloma. Hematol Oncol Clin North Am. 1999;13:1117–1125. doi: 10.1016/s0889-8588(05)70115-5. [DOI] [PubMed] [Google Scholar]
- 92.Lust JA, Lacy MQ, Zeldenrust SR, et al. Induction of a chronic disease state in patients with smoldering or indolent multiple myeloma by targeting interleukin 1{beta}-induced interleukin 6 production and the myeloma proliferative component. Mayo Clin Proc. 2009;84:114–122. doi: 10.4065/84.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Andrei C, Dazzi C, Lotti L, Torrisi MR, Chimini G, Rubartelli A. The secretory route of the leaderless protein interleukin 1beta involves exocytosis of endolysosome-related vesicles. Mol Biol Cell. 1999;10:1463–1475. doi: 10.1091/mbc.10.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Andrei C, Margiocco P, Poggi A, Lotti LV, Torrisi MR, Rubartelli A. Phospholipases C and A2 control lysosome-mediated IL-1 beta secretion: Implications for inflammatory processes. Proc Natl Acad Sci U S A. 2004;101:9745–9750. doi: 10.1073/pnas.0308558101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tassi S, Carta S, Vene R, Delfino L, Ciriolo MR, Rubartelli A. Pathogen-induced interleukin-1beta processing and secretion is regulated by a biphasic redox response. J Immunol. 2009;183:1456–1462. doi: 10.4049/jimmunol.0900578. [DOI] [PubMed] [Google Scholar]
- 96.Kaplanski G, Porat R, Aiura K, Erban JK, Gelfand JA, Dinarello CA. Activated platelets induce endothelial secretion of interleukin-8 in vitro via an interleukin-1-mediated event. Blood. 1993;81:2492–2495. [PubMed] [Google Scholar]