Abstract
Background
Helicobacter pylori is a gram-negative bacterium incriminated in gastroduodenal ulcers, and mucosa-associated lymphoid tissue lymphoma imposing a major burden on health care systems worldwide. Honeys have been shown to have in vitro activity against microaorganisms and suitable for use in ulcers, infected wounds and burns.
Objective
The study was aimed at evaluating the antimicrobial potential of honeys (Manuka−, Capillano®, Eco- and Mountain) at different concentrations (10%v/v, 20%v/v, 50%v/v and 75%v/v) against clinical isolates of H. pylori.
Methods
H. pylori was isolated from gastric biopsies of patients with gastroduodenal pathologies following standard microbiological procedures. Antimicrobial susceptibility of the isolates to different honey varieties was determined by the disk diffusion assay. Also, the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the most potent honey was determined by the agar dilution method. Data were analysed using the Fisher exact test and statistical significance considered at p<0.05.
Results
All the four honey varieties exhibited antibacterial activity. The strongest inhibitory activity (82.22%) was demonstrated by Mountain honey at 75%v/v, followed by Capillano® and Manuka− honeys (75.56%), and Eco-honey (73.36%) at the same concentration. However, no statistically significant difference (p>0.05) was noted between the honeys at different concentrations. The MIC and MBC concentrations of Mountain honey were in the range 0.117 – 0.938ì/mL and 0.366 – 2.965ìg/mL respectively. The antimicrobial potential of these honeys at different concentrations were highly comparable to clarithromycin, the positive control.
Conclusion
These honeys may contain compounds with therapeutic potential against our local isolates of H. pylori.
Keywords: H. pylori, gastric biopsies, honey, antimicrobial activity, Cameroon
Introduction
Helicobacter pylori is a gram-negative, spiral or curved rod, microaerophilic organism that is responsible for a significant cause of morbidity and mortality imposing a major burden on health care systems worldwide. While the majority of infections are asymptomatic, the association of H. pylori colonization of the stomach with chronic gastritis, peptic ulcer disease, and gastric malignancies is now well documented in both adults and children1–3 Eradication of infection is the first therapeutic approach that constitutes a reliable and long-term prophylaxis of peptic ulcer relapse4. The triple therapy regimen of treatment with a proton-pump inhibitor or bismuth salt plus two antibiotics is recommended in all patients positively diagnosed5. Also, with an increase in the prevalence of antibiotic resistant H. pylori, the noncompliance of patients to treatment regimen due to numerous side effects and the cost of the drugs6, especially for people in the developing world with low socio-economic status and a high prevalence of H. pylori, it is imperative to search for an alternatively cheap and safe remedy to address the problem.
More recently, there has been growing interest in the use of honey as a “natural” remedy for the control of microbial infections7. It has been shown to be active against a diverse range of micro-organisms and reports of its inhibitory effect on specific micro-organisms have been extensively documented7–10. Honey is well known for its positive actions within a wound environment. It maintains a moist wound environment that promotes healing, and its high viscosity helps to provide a protective barrier to prevent infection11. In Cameroon, honey is commonly used by many people with the belief that it has antibacterial properties, in addition to being cheap and easily available. It is used as an antiseptic for wounds and burns, and for the treatment of cough and gastrointestinal complications.
The observation that honey in New Zealand and Saudi Arabia at concentrations approximating 20% v/v can inhibit the growth of H. pylori in vitro and the finding that Medihoney and Manuka honeys have been shown to have in vivo activity and suitable for use in ulcers, infected wounds and burns are important findings7,9,11, which could be exploited clinically. Although these and several other studies have documented the antimicrobial activity of honey against several pathogens including H. pylori, we are not aware of any study in Cameroon that has investigated the antibacterial activity of honey on H. pylori isolates, especially so when we have documented a high prevalence of the organism in our environment3, as well as an increasing trend in antibiotic resistant bacteria12. Against this background, the present study was initiated to evaluate different honey varieties including a local variety for their antimicrobial effect on clinical isolates of H. pylori circulating in Cameroon.
Materials and methods
Bacterial isolates
H. pylori was cultured from biopsies of patients presenting with gastroduodenal pathologies at the Douala General Hospital after Informed consent was obtained from them and ethical approval (Protocol number HGD/LN158/LHN/SE/DMT/10/05) from the hospital's management board . Briefly, biopsies were inoculated on Columbia agar base plates (Conda Pronadisa, Spain) supplemented with 7% sterile defibrinated sheep blood, amphotericin B (250mg) and Campylobacter supplements; trimethoprim (5mg/L), vancomycin (10mg/L) and polymyxin B (2500 units/L). All plates were incubated at 37°C for 3 to 5 days under microaerophilic conditions (5–6% O2, 10% CO2, 80–85% N2) (Anaerocult© Darmstadt, Germany). Isolates were identified following previously reported schemes5,13. A total of 304 isolates were obtained and characterized. Fifteen of the isolates were subjected to antimicrobial assays.
Preparation of concentrations of honey
Four honey varieties were used. They included: Manuka− (Ma, New Zealand), Capillano® (Cp, Australia), Eco (Ec, Kenya) and Mountain (Mn, Cameroon) honey varieties. They were purchased from the supermarket. Different concentrations of each honey constituting, 10% v/v, 20% v/v, 50% v/v and 75% v/v were made in sterile distilled water. This was done by dissolving the respective volumes: 0.1mL, 0.2mL, 0.5mL, 0.75mL of each honey into corresponding volumes of sterile distilled water to give a 1mL preparation. Filter paper disks of 6mm diameter were prepared by the method of Cheesbrough14. The disks were impregnated with the different concentrations of each honey.
Susceptibility testing of honey
The disk diffusion (Kirby-Bauer) technique was employed as previously described12. Brain heart infusion agar (BHI) constituted with yeast extract agar (YEA) (Biotec Laboratory Ltd, UK) was prepared according to the manufacturer's instructions. Disks impregnated with the different concentrations of each honey were employed in the study. A 0.5 McFarland standard was prepared by the method of Koneman et al.15 and 5mL put into a sterile test tube. An inoculum of each clinical isolate was prepared from subculture of bacterial suspension. Briefly, it was prepared as follows: 4–5 colonies of the isolates were emulsified in sterile distilled water and the turbidity adjusted to 1.5 × 108 CFU/mL (corresponding to 0.5 McFarland standards). A sterile cotton swab was dipped into the standardized bacterial suspension and used to evenly inoculate the BHI agar plates. The plates were allowed to dry for 3–5 minutes. Thereafter, all disks were placed on the plates and pressed gently to ensure complete contact with agar. A distance of at least 15mm was maintained from the edges of the plates to prevent overlapping of inhibition zones. A clarithromycin disk (15ì g) was used as the positive control. Fifteen minutes following placement of the disks, the plates were incubated at 37°C for 2–5 days. They were then examined and the diameter of the zone of inhibition measured. The experiment was repeated 3x for each strain. H. pylori control strain NCTC 11638 was included in all the experiments.
Determination of minimum inhibitory concentration (MIC)
The MIC was determined by the agar dilution method according to the scheme of Nariman et al.16. Brain heart infusion broth medium (Biotec Laboratories Limited, UK) was prepared following the manufacturer's instructions. Briefly, the H. pylori isolates were each inoculated into 2mL of BHI broth in sterile test tubes. Tubes were incubated in a microaerophilic environment at 37°C for 48 hours. The inoculum was then serially diluted 10-fold. The dilution containing 1.5 × 108 CFU/ mL was used as the required inoculum size. As a result of the potent antibacterial activity demonstrated by Mountain honey on the isolates, it was used to determine the minimum inhibitory concentration. Seventy-five percent of honey was prepared in sterile distilled water. It was serially diluted 2-fold with BHI broth medium. The 1st tube was the control and contained just 1mL of honey. One milliliter of 75% v/v of the honey was transferred into the second tube and serially diluted to the 12th tube. Twenty microliter of inoculum was dispensed into the 12 tubes. Tubes were incubated under micro-aerophilic conditions at 37 °C for 2–5 days and visually examined. MIC was recorded as the lowest concentration of the honey that inhibited bacterial growth (no visible growth or turbidity).
Determination of minimum bactericidal concentration (MBC)
MBC was determined by the method of Ngemenya et al.17. A volume of 0.2mL of the contents of each MIC tube was diluted 10 fold in sterile distilled water. A loopful was plated on to freshly prepared Columbia blood agar plates and incubated under microaerophilic conditions at 37°C for 48 hours. Plates were examined and MBC was recorded as the lowest concentration of honey at which no colony of H. pylori was formed on the plate.
Statistical analysis
The Fisher exact test was employed at 5% significance level to determine if there was any statistically significant difference in the mean zone diameters of the four honey varieties at different concentrations; mean zone diameters of the control antibiotic to the different honeys at different concentrations; the lowest and highest MIC and MBC of mountain honey and the control antibiotic.
Results
Susceptibility testing of honey
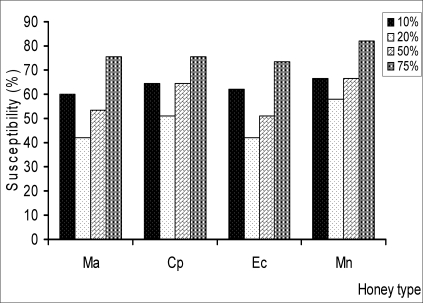
All the different concentrations of honey showed antibacterial activity against the isolates. The zones of inhibition ranged from 0–50mm. The susceptibilities of the isolates to the honeys at the various concentrations are shown in fig. 1. The greatest inhibitory activity was demonstrated by Mountain honey (37/45, 82.22%) at 75%v/v concentration while the least was observed with Manuka” and Eco- honeys (19/45, 42.22%) respectively at 20%v/v concentrations. However, the mean inhibition diameters of the different concentrations (10%v/v, 20%v/v, 50%v/v and 75%v/v) of the various honeys did not reach statistical significance (p>0.05).
Fig.1.
Susceptibility of H. pylori to different varieties of commercial honey at 10%v/v, 20%v/v, 50%v/v, 75%v/v against H. pylori isolates. Zone diameter of sensitive isolates was = 15mm. Ma, Manuka; Cp, Capillano; Ec,Eco; Mn, Mountain
Minimum inhibitory and bactericidal concentration determination
The most active honey was further assayed to determine its minimum inhibitory concentration and minimum bactericidal concentration against 15 H. pylori isolates. Consequently, Mountain honey at 75%v/v was used. The MIC ranged from 0.117–0.938ì g/mL while the MBC was 0.366 - 2.965ì g/mL (Table 1).
Table 1.
Minimum inhibitory and bactericidal concentrations of Mountain honey against H. pylori isolates.
| H. pylori isolates | MIC (ì g/mL) |
MBC (ì g/mL) |
| 1 | 0.938 | 1.465 |
| 2 | 0.938 | 2.930 |
| 3 | 0.469 | 0.733 |
| 4 | 0.469 | 1.465 |
| 5 | 0.469 | 2.931 |
| 6 | 0.187 | 2.964 |
| 7 | 0.469 | 1.466 |
| 8 | 0.234 | 0.366 |
| 9 | 0.234 | 0.731 |
| 10 | 0.469 | 2.931 |
| 11 | 0.117 | 1.463 |
| 12 | 0.187 | 2.965 |
| 13 | 0.938 | 2.931 |
| 14 | 0.469 | 1.466 |
| 15 | 0.234 | 1.425 |
MIC: Minimum inhibitory concentration; MBC: Minimum bactericidal concentration
Discussion
In Australia, two honeys, Medihoney® (Capillano) and Manuka™ (various brands) are sold as therapeutic honey suitable for use in ulcers, infected wounds and burns7. Against this background, we opted to investigate the anti-H. pylori activity of different honeys on our local clinical isolates. We observed that the honeys inhibited the growth of H. pylori isolates at concentrations = 10%. They produced zones of inhibition at the different concentrations which depended on the type of honey. This is in accordance with other studies which have equally documented that honey has significant inhibitory activity against a number of bacterial and various enteropathogens, moulds, and yeasts with unique properties that render it bacteriostatic and bactericidal18. However, the mean (± standard deviation) inhibition diameters of the honeys at different concentrations did not reach statistical significance (p>0.05). This may suggest that Mountain and Eco-honeys possess antibacterial activity similar to those of Manuka− and Capillano® honeys which are commercially available antibacterial honeys7.
The observed differences in the activity of the different honeys at the different concentrations, may also suggest that there could be regional differences as well as the nature of honey production influencing the inhibitory activity as previously suggested18. We are constrained to support this hypothesis because the honeys used in our study which included; Manuka− (New Zealand), Capillano® (Australia), Eco-honey (Kenya), and Mountain honey (Cameroon), reflects geographical distribution. In addition, the variations could be attributed to the presence of additional components derived from the nature of honey production, as bees are able to take nectar from whatever source that is available to them at the time7. Flavonoids, proteinacious compounds and their high sugar content have also been reported to play a role in their antimicrobial activity19. Other factors, though not investigated in our study could also be responsible for the variations. These could be in the level of hydrogen peroxide that arises in honey, or non-peroxide factors which have been documented as the major antibacterial factors in honey20,21. Hydrogen peroxide is produced by enzymatic activity of glucoseoxidase in dilute honey. This may therefore hold for our study since our honeys were diluted, especially so when we consider that at 10% v/v concentration (the most dilute of all the concentrations used), a greater antimicrobial activity was exhibited than the 20% v/v concentration for Ma and Eco honeys. This is however, the subject of our future investigations.
Our results revealed Mountain honey to be the most potent of the honeys at all concentrations, followed by Manuka− and Capillano® at 75% v/v concentration. This result compares well with that of Lusby et al.11 in which the antibacterial activity of rewa rewa, a locally produced honey demonstrated significant antibacterial activity when compared to Manuka−, and Capillano® honeys.. Our results further showed that at the highest concentration (75% v/v), there was an increase inhibition of H. pylori probably due to a greater concentration of honey, with the honeys demonstrating percentage susceptibilities in the range 73% – 82% (fig. 1). This observation is unexpected as susceptibility is supposed to increase at lower dilutions. We speculate that this may be because the concentration of honey in the agar might have decreased by diffusion with time. The MIC and MBC of Mountain honey were also determined since it presented the most potent activity. The MIC ranged from 0.117 – 0.938ì g/mL and the MBC from 0.366 – 2.965 ì g/mL. The lowest and highest MIC and MBC values of mountain honey were statistically compared to determine any variation in their efficacy against the isolates. No statistically significant difference (p> 0.05) was observed between the lowest and highest MIC and MBC values. This is in accordance with the study of Agbaje et al.21 who also documented low MIC and MBC values of local Nigerian honeys against their isolates of Staphylococcus albus.
Comparing the mean (± standard deviation) inhibition diameters of the control antibiotic to the honeys at different concentrations, we observed there was no statistically significant difference in the values (p>0.05) between clarithromycin and all the honeys at concentrations = 50% v/v. Also, the MIC of clarithromycin (0.025µg/ml to 1 µg/ml) did not have a significant difference (p> 0.05) in activity to mountain honey. This may suggest that the honeys at these concentrations are likely to contain substances whose antibacterial activities are comparable to that of clarithromycin.
Conclusion
Mountain, Manuka−, Capillano®, and Eco- honeys exhibited inhibitory activity against H. pylori isolates at concentrations = 10% v/v. These honeys may contain compounds with therapeutic potential against our local isolates of H. pylori. We also demonstrated that our locally produced honey had a very good antibacterial activity comparable to the commercial honeys.. It may therefore be necessary to study other locally produced but yet untested honeys for their antimicrobial activity. Further research is however required to ascertain the healing property of Mountain honey in- vivo as well as its mechanism of action.
Acknowledgements
This study received part funding from the International Foundation for Science and the Organisation of the Islamic Conference Standing Committee on Scientific and Technological Cooperation through a grant to Dr. RN NDIP. We are also grateful to the Department of Child Health, University of Glasgow for providing the growth supplement used in the study.
References
- 1.Sherif M, Mohran Z, Fathy H, Rockabrand DM, Rozmajzl PJ, Frenck RW. Universal high-level primary metronidazole resistance in Helicobacter pylori isolated from children in Egypt. J Clin Microbiol. 2004;42(10):4832–4834. doi: 10.1128/JCM.42.10.4832-4834.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asrat D, Nilson I, Mengistu Y, Ashenafi S, Ayenew K, Al-Soud WA, Wadstrom T, Kassa E. Prevalence of Helicobacter pylori infection among adult dyspeptic patients in Ethiopia. Annals Trop Med Parasitol. 2004;98(2):181–189. doi: 10.1179/000349804225003190. [DOI] [PubMed] [Google Scholar]
- 3.Ndip RN, Malange AE, Akoachere JFT, Mackay WG, Titanji VPK, Weaver LT. Helicobacter pylori antigens in the faeces of asymptomatic children in the Buea and Limbe health districts of Cameroon: a pilot study. Trop Med Int Health. 2004;9(9):1036–1040. doi: 10.1111/j.1365-3156.2004.01299.x. [DOI] [PubMed] [Google Scholar]
- 4.Yuen B, Zbinden R, Fried M, Bauerfeind P, Bernardi M. Cultural recovery and determination of antimicrobial susceptibility in Heliocobacter pylori by using commercial transport and isolation media. Infection. 2005;33:77–81. doi: 10.1007/s15010-005-4071-y. [DOI] [PubMed] [Google Scholar]
- 5.McNulty C the PHLS Helicobacter Working group, author. Helicobacter pylori susceptibility testing by disc diffusion. J Antimicrob Chemother. 2002;49:601–609. doi: 10.1093/jac/49.4.601. (Owen, R., Tompkins, R., Hawtin, P., McColl, K., Price, A., Smith, G., Teare, L) [DOI] [PubMed] [Google Scholar]
- 6.Moayyedi P, Axon ATR. Is there a rationale for eradication of Helicobacter pylori cost-benefit: the case for. Br Med Bulletin. 1998;54(1):229–241. doi: 10.1093/oxfordjournals.bmb.a011674. [DOI] [PubMed] [Google Scholar]
- 7.Davis C. The use of Australian honey in moist wound management. Rural industries research and development corporation report. 2005:1–18. [Google Scholar]
- 8.Adebolu TT. Effect of natural honey on local isolates of diarrhea- causing bacteria in South Western Nigeria. Afri J Biotech. 2005;4(10):1172–1174. [Google Scholar]
- 9.Al-Waili NS, Akmal M, Al-Waili FS, Saloom KY, Ali A. The antimicrobial potential of honey from United Arab Emirates on some microbial isolates. Med Sci Monitor. 2005;11(12):BR 433–438. [PubMed] [Google Scholar]
- 10.Alnaqdy A, Al-Jabri A, Al Mahrooqi Z, Nzeako B, Nsanze H. Inhibitory effect of honey on the adherence of Salmonella to intestinal epithelial cells in vitro. Int J Food Microbiol. 2005;103:347–351. doi: 10.1016/j.ijfoodmicro.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 11.Lusby PE, Coombes AL, Wilkinson JM. Bactericidal activity of different honeys against pathogenic bacteria. Arch Med Res. 2005;36:464–467. doi: 10.1016/j.arcmed.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 12.Ndip RN, Dilonga HM, Ndip LM, Akoachere JFT, Nkuo-Akenji T. Pseudomonas aeruginosa isolates recovered from clinical and environmental samples in Buea, Cameroon: current status on biotyping and antibiogram. Trop Med Int Health. 2005;10(1):74–81. doi: 10.1111/j.1365-3156.2004.01353.x. [DOI] [PubMed] [Google Scholar]
- 13.Ndip RN, Mackay WG, Farthing MJG, Weaver LT. Culturing Helicobacter pylori from clinical specimens: review of microbiological methods. J Pediatr Gastroenterol Nutr. 2003;36:616–622. doi: 10.1097/00005176-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Cheesbrough M. district laboratory practice in tropical countries. Part II. Low price edition. The Edinburgh Building Cambridge: Cambridge University Press; 2000. Biochemical test to identify bacteria; antimicrobial susceptibility testing; pp. 1933–1934. 63–70; 132–143. [Google Scholar]
- 15.Koneman WE, Allen DS, Janda MW, Scherchenberger CP, Winn WC., Jr. color atlas and text book of diagnostic microbiology. 4th edition. JB Lippincott company; 1992. Antimicrobial susceptibility testing; p. 624. 629, 637. [Google Scholar]
- 16.Nariman F, Eftekhar F, Habibi Z, Falsafi T. Anti-Helicobacter pylori activities of six Iranian plants. Helicobacter. 2004;9(2):140–151. doi: 10.1111/j.1083-4389.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- 17.Ngemenya MN, Mbah JA, Tane P, Titanji VK. Antibacterial effects of some Cameroonian medicinal plants against common pathogenic bacteria. Afri J Trad Compl Alternative med. 2006;3(2):84–93. [Google Scholar]
- 18.Osato MS, Reddy SG, Graham DY. Osmotic effect of honey on growth and viability of Helicobacter pylori. Dig Dis Sci. 1999;44(3):462. doi: 10.1023/a:1026676517213. [DOI] [PubMed] [Google Scholar]
- 19.Mundo MA, Padilla-Zakour OI, Worobo RW. Growth inhibition of foodborne pathogens and food spoilage organisms by select raw honey. Int J Food Microbiol. 2004;97(1):1–8. doi: 10.1016/j.ijfoodmicro.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 20.Iurlina MO, Fritz R. Characterization of micro-organisms in Argentinean honeys from different sources. Int J Food Microbiol. 2005;105:297–304. doi: 10.1016/j.ijfoodmicro.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Agbaje EO, Ogunsanya T, Aiwerioba OIR. Conventional use of honey as antibacterial agent. Annals Afri Med. 2006;5(2):78–81. [Google Scholar]