Abstract
Schwann cell myelination is tightly regulated by timely expression of key transcriptional regulators that respond to specific environmental cues, yet molecular mechanisms underlying such a process are poorly understood. Here, we report that HDAC1/2-regulated acetylation state of NF-κB is critical in orchestrating the myelination program. Mice lacking HDAC1/2 exhibit severe dysmyelination with Schwann cell development arrested at the immature stage. We find that NF-κB p65 becomes heavily acetylated in HDAC1/2 mutants, inhibiting the expression of positive regulators of myelination, while inducing the expression of differentiation inhibitors. We observe that NF-κB protein complex switches its association with p300 to that with HDAC1/2 as Schwann cells differentiate. NF-κB and HDAC1/2 act coordinately to regulate the transcriptionally-linked chromatin state for Schwann cell myelination. Thus, our results reveal an HDAC-mediated developmental switch for controlling myelination in the peripheral nervous system.
Keywords: Schwann cell differentiation and myelination, chromatin remodeling, epigenetic regulation, HDAC, HAT, transcription factors, NF-κB
Myelination of axons by Schwann cells in the peripheral nervous system (PNS) is essential for axonal insulation and saltatory conduction of action potentials. Peripheral myelin defects underlie various inherited or acquired demyelinating neuropathies in the PNS, leading to motor and sensory disabilities 1. Schwann cell myelination is a well-characterized developmental process, with a different set of regulators sequentially induced to play distinct roles at discrete stages, until mature myelin is formed 2. Recent studies revealed a transcriptional cascade that positively and negatively controls Schwann cell differentiation in a spatiotemporally specific manner 2,3. Since expression of these factors is regulated by different environmental cues, it is conceivable that epigenetic mechanisms play important roles to instruct the myelination program. A well-studied chromatin remodeling is mediated by histone methylation and acetylation, the latter being dynamically controlled by histone acetyltransferases (HATs) and histone deacetylases (HDACs) 4. Recently, HDAC1 and HDAC2 were shown to be essential for CNS myelination 5,6, however their function and underlying mechanisms in Schwann cell differentiation and myelination remain elusive.
To define the role of HDAC1 and HDAC2 in Schwann cell development, we generated HDAC single (HDAC1cKO or HDAC2cKO) and double HDAC1/2 mutants (dCKO, HDAC1fl/fl;HDAC2fl/fl;DhhCre/+) mice, in which HDAC1/2 were deleted in the Schwann cell lineage directed by Dhh-Cre 7. HDAC single mutants appeared normal as compared to heterozygous control littermates, while dCKO mice developed severe tremors, hindlimb paralysis and died around postnatal week 2 (Supplementary Fig. S1).
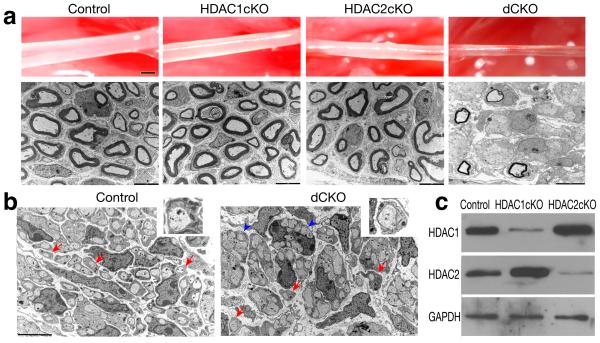
The sciatic nerves isolated from dCKO mice at P7 appeared much thinner and translucent compared to the control and HDAC1 or 2 single mutants (Fig. 1a). Electron microscopy ultrastructural analysis revealed that there was indeed severe myelin deficit in dCKO, and majority of Schwann cells appeared to associate with multiple axon bundles, but without forming myelin sheaths around axons (Fig. 1a). Similarly, at P0, the majority of Schwann cells failed to establish a 1:1 relationship with individual axons although a few Schwann cells were able to sort the axons (Fig. 1b). The absence of a discernable myelination defect in HDAC1 or HDAC2 single mutants suggests that these HDACs function redundantly during Schwann cell differentiation. Consistently, we observed upregulation of HDAC2 or HDAC1 in HDAC1cKO or HDAC2cKO sciatic nerves, respectively (Fig. 1c), suggesting a compensatory effect in each other's absence. Since HDAC1 or 2 single mutant phenotypes were indistinguishable from heterozygous control, we focused on the control (HDAC1fl/+;HDAC2fl/+;DhhCre/+) and dCKO for subsequent analyses. In line with the electron microscopy analysis, in dCKO sciatic nerves, expression of mature myelin components such as Mbp, Mag and Mpz was significantly downregulated (Fig. 2), indicating a severe defect in Schwann cell differentiation. In contrast, the number of immature Schwann cells expressing S100β and p75 was comparable to the control at P4 (Fig. 2a), and their proliferation was unaffected based on Ki67 expression and BrdU incorporation (Supplementary Fig. 2a,b). There was a slight increase in the percentage of apoptotic cells in the nerves (Supplementary Fig. 2c), however it did not alter the overall number of immature Schwann cells. These results suggest that Schwann cell development becomes arrested at the immature stage in the absence of HDAC1/2.
Figure 1. Ablation of HDAC1/2 in the Schwann cell lineage results in severe myelination defects in sciatic nerves.
(a) Appearance (upper panel) and electron microscopy analysis (lower panel, cross-section) of sciatic nerves from control and HDAC1cKO, HDAC2cKO and dCKO mutants at P7. (b) Electron microscopy analysis of cross sections of control and dCKO sciatic nerves at P0. Inserts are shown for an individual sorted axon (arrows). Arrowheads indicate unsorted axons. (c) Western blot analysis of HDAC1 and HDAC2 expression using sciatic nerves from control, HDAC1cKO and HDAC2cKO at P4. GAPDH as a loading control. Full-length blots/gels are presented in Supplementary Fig. 6. Scale bars in a, 1 mm (up panels) and 5 μm (low panels); b, 5 μm.
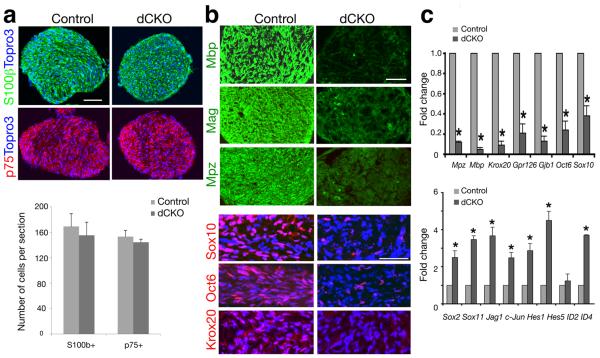
Figure 2. Effects of HDAC1/2 deletion on Schwann cell precursor formation and differentiation.
(a) Cross sections of sciatic nerves of control and dCKO mice at P4 were immunostained with antibodies to S100β and p75. Cell nuclei were counterstained with Topro3. Lower panel, quantification of S100β+ or p75+ cells per cross-section. (b) Sciatic nerves of control and dCKO mice at P5 were immunostained with antibodies with myelin components (cross-sections) and transcriptional regulators (longitudinal sections) as indicated. (c) qRT-PCR analysis of myelin-associated genes, promyelinating transcriptional regulators (upper panels) and negative regulators (lower panels) in sciatic nerves of control and dCKO mice at P4 (*P < 0.01). Scale bars in a, 60 μm; b, 40 μm.
Since Schwann cell development is controlled by a series of positive and negative regulatory factors 2,3, we next measured mRNA levels of these regulators. In dCKO sciatic nerves, we observed not only a significant reduction in expression of positive regulators including Oct6/SCIP/Pou3f1, Sox10, Krox20/Egr2 and GPR126, but also a concomitant increase in negative regulators of Schwann cell differentiation such as Sox2, Sox11, Jagged1, c-Jun, Hes1, Hes5, ID2 and ID4 (Fig. 2c). Protein levels of key Schwann cell differentiation regulators such as Sox10, Oct6 and Krox20 were correlated with their mRNA levels assayed by qRT-PCR (Fig. 2b,c). These results suggest that HDAC1/2-dependent epigenetic modifications control the overall transcriptional program to orchestrate proper Schwann cell differentiation.
Dysmyelination in dCKO sciatic nerves suggests that HDAC1/2 are likely to target critical transcription factors for Schwann cell differentiation. As a candidate molecule, we focused on NF-κB since it is crucial for Schwann cell differentiation by regulating expression of key differentiation regulators such as Oct6 8, and in addition, its p65 subunit was known to undergo a reversible acetylation/deacetylation by HATs (e.g. p300) and HDACs 9. Western-blot analysis revealed that the amount of acetylated p65 dramatically increased in dCKO sciatic nerves, while the total amount of p65 did not differ between control and dCKO at P4 (Fig. 3a). Consistently, we observed a robust increase in the expression intensity of acetylated p65 in dCKO sciatic nerves via immunohistochemistry (Fig. 3b). Acetylated tubulin levels did not increase in dCKO (Fig. 3a), suggesting that the effect of HDAC1/2 deletion on p65 acetylation was specific. Similarly to the lack of myelination defect, p65 acetylation levels were not altered in HDAC1 or HDAC2 single mutants (Supplementary Fig. 3).
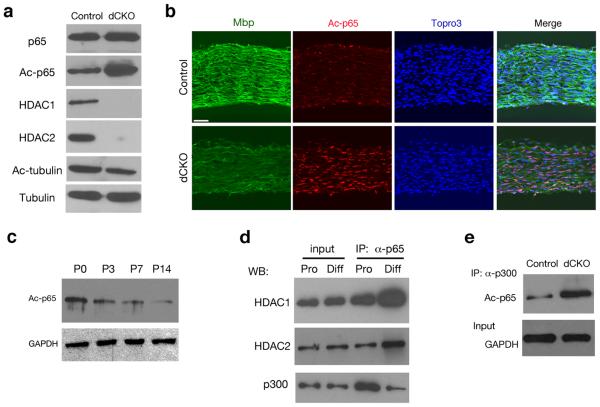
Figure 3. p65 subunit of NF-κB is a key substrate of HDAC1/2 for Schwann cell differentiation.
(a) Lysates of control and dCKO sciatic nerves at P4 were subject to immunoblotting analysis with antibodies to p65, acetyl-p65 K310 (Ac-p65), HDAC1, HDAC2, acetyl-tubulin and tubulin as indicated. (b) Sciatic nerves of control and dCKO mice at P4 were immunostained with antibodies to Mbp and acetyl p65. Cell nuclei were counterstained with Topro3. (c) Western blot analysis of p65/RelA acetylation in wildtype sciatic nerves at P0, P3, P7 and P14 with antibodies to acetyl p65 and GAPDH as indicated. (d) Lysates from primary Schwann cells under the proliferation (Pro) and differentiation (Diff) condition for 4 days were co-immunoprecipitated (IP) with anti-p65 antibody and blotted with anti-HDAC1, HDAC2, p300/CBP and GAPDH antibody, respectively. (e) Lysates of control and dCKO sciatic nerves at P4 were immunoprecipitated with anti-p300 and blotted with anti-acetyl p65. GAPDH as an input control. Full-length blots/gels are presented in Supplementary Fig. 6. Scale bars in b, 50 μm.
To determine the extent of p65 acetylation changes during the course of Schwann cell development, we detected that the amount of acetylated p65 decreased gradually from P0 to P14 (Fig. 3c). In addition, under the differentiation condition that promoted expression of myelination-associated genes (Supplementary Fig. 4), the amount of p65 that interacted with HDAC1 and 2 increased significantly in differentiating Schwann cells compared to proliferating Schwann cells (Fig. 3d). On the other hand, the association of p65 with p300/CBP, the major HAT for p65 acetylation 9, declined dramatically in differentiating Schwann cells (Fig. 3d). Conversely, we observed an enhancement of acetyl p65/p300 interaction by immunoprecipitation in myelin-deficient dCKO sciatic nerves (Fig. 3e). Together, these results suggest that as Schwann cells differentiate, NF-κB p65 complex switches its association with p300 to that with HDAC1/2, thereby undergoing deacetylation process. Thus, these results identify the p65 subunit of NF-κB as a key substrate of HDAC1/2 in regulating Schwann cell differentiation.
NF-κB p65 subunit is known to be acetylated at K310, K314, and K315, while K310 acetylation was reported to be critical for its transcriptional activity in vitro 10,11. To determine whether the acetylation state of p65 regulates myelin gene transcription, we introduced wildtype p65 and p65 mutants carrying mutations in the acetylation sites to primary rat Schwann cells, along with reporters for differentiation activators or inhibitors. Transfection of wildtype p65 substantially transactivated reporter activities of Sox10 or the myelin gene Mpz (Fig. 4a), while inhibiting the ID4 or Hes5 promoter activity (Fig. 4b). Overexpression of p65 acetylation mutants exhibited an enhanced ability to activate reporter activities of Sox10 and Mpz promoters (Fig. 4a), and similarly caused a further decrease in ID4 and Hes5 reporter activities (Fig. 4b). These results suggest that the acetylation state of NF-κB p65 K310, K314 and K315 sites play a role in transcriptional control of the Schwann cell myelination program.
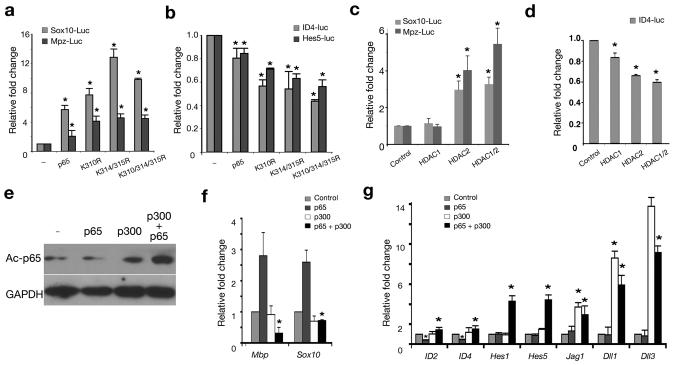
Figure 4. The acetylation state of NF-κB regulated by HDAC1/2 is critical for the Schwann cell differentiation program.
(a-b) Primary rat Schwann cells were transfected with expressing vectors for p65, mutant p65 carrying acetylation site mutation(s) and control vector pCMV (−), together with luciferase reporters of Sox10- and Mpz-luc (a) and ID4- and Hes5-luc reporters (b), respectively. (c-d) Primary rat Schwann cells were transfected with control and expressing vectors for HDAC1, 2 or both together with luciferase reporters Sox10-luc and Mpz-luc (c) or ID4-luc (d), respectively. (e) The lysates of primary Schwann cells transfected with expression vectors for p65 and/or p300 were immunoblotted by anti-acetyl p65 and GAPDH. Full-length blots/gels are presented in Supplementary Fig. 6. (f-g) Primary Schwann cells were transfected with expressing vectors p65, p300 or both p65 and p300. qRT-PCRs were performed to detect myelin associated genes Mbp and Sox10 (f), as well as differentiation inhibitors ID2, ID4, Hes1, Hes5, Jagged1 (Jag1), Delta 1 (Dll1) and Delta 3 (Dll3) (g). Fold changes over controls were measured from three independent experiments in a-d and f-g (n=3, *P < 0.01). Error bars shown are the mean ± s.d.
We next tested whether introducing HDAC1/2 could mimic the effect of the p65 acetylation mutations in primary Schwann cells. Indeed, combination of HDAC1/2 resulted in a 3-5-fold increase in Sox10 and Mpz promoter activities (Fig. 4c), while it caused a 40% reduction in ID4 promoter activity (Fig. 4d). Overexpression of p300 alone increased endogenous p65 acetylation, which was further enhanced when introduced along with p65 (Fig. 4e). Strikingly, introduction of p300 substantially blocked endogenous expression of Mbp and Sox10 with and without p65 in primary rat Schwann cells (Fig. 4f). In contrast to the positive regulators of myelination, overexpression of p300 upregulated the expression of differentiation inhibitors such as ID2, ID4, Notch effectors (Hes1, Hes5) as well as Notch signaling ligands (Jagged1, Delta1 and Delta3) 2,3 (Fig. 4g). Together, these results indicate that HDAC-regulated deacetylation of NF-κB selectively activates expression of positive regulators while repressing differentiation inhibitors to promote Schwann cell myelination.
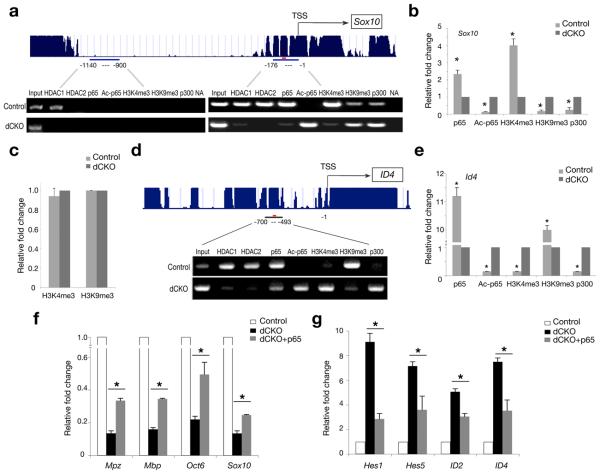
Since HDAC1/2 are essential for gene transcription during Schwann cell myelination, we hypothesized that the acetylation state of p65 regulated by HDACs may signal transcription-dependent changes in chromatin structure. To address the hypothesis, we performed chromatin immunoprecipitation assays with activating and repressive trimethyl histone marks, H3K4me3 and H3K9me3 12,13, respectively, in addition to p65 and acetylated p65. As for the target genes, we chose Schwann cell regulatory genes Sox10 and ID43 since both contained a highly conserved NF-κB binding site in their promoter regions. In particular, the Sox10 promoter carrying the NF-κB site was shown to direct reporter gene expression in Schwann cells both in vivo and in vitro 14.
In control sciatic nerves, HDAC1, HDAC2 and p65 were recruited to the Sox10 promoter region carrying the NF-κB binding site but not to the region that lack the NF-κB consensus site (Fig. 5a). In contrast, in dCKO sciatic nerves, p65 recruitment was hardly detectable while acetyl-p65 recruitment increased dramatically (Fig. 5a), which coincided with the increase of p300 recruitment (Fig. 5a,b). Of particular interest is that robust recruitment of an activating histone mark H3K4me3 to the promoter region observed in control was no longer detected in dCKO. Instead, the recruitment of a repressive histone mark H3K9me3 increased significantly in dCKO (Fig. 5a,b). As a control, the recruitment level of H3K4me3 and H3K9me3 on a housekeeping gene GAPDH promoter was comparable between control and dCKO sciatic nerves (Fig. 5c).
Figure 5. HDAC1/2 and NF-κB cooperate to regulate epigenetic marks on the critical genes for Schwann cell differentiation.
(a) Upper panel, the conservation map of the Sox10 promoter among various vertebrates. Lower panel, semi-quantitative ChIP-PCR assays for indicated protein/mark recruitment on the Sox10 promoter from control and dCKO sciatic nerves at P5. NA, no antibody control. (b) Quantification of enrichment of indicated protein/mark association with the Sox10 proximal promoter region by qRT-PCR. (n = 3; *P < 0.01). (c) qRT-PCR analysis of relative enrichment of H3K4me3 and H3K9me3 marks on an active promoter of GAPDH in control and dCKO SNs at P5. (d) Upper panel, the conservation map of the ID4 promoter among various vertebrates. Lower panel, ChIP-PCR assays for protein recruitment on the promoter of ID4 in control and dCKO SNs at P5. (e) qRT-PCR for enrichment of protein/marks on the Sox10 proximal promoter region in control and dCKO sciatic nerves. Relative fold enrichment in control versus dCKO sciatic nerves (b,c,e) was shown from three independent experiments (n = 3; *P < 0.01). Blue lines in a, d; PCR amplification of promoter elements with or without a NF-κB binding site (red dot) as indicted. (f-g) Primary Schwann cells isolated from dCKO sciatic nerves at P1 were transfected with control and p65 expression vectors and harvested 48 hrs after transfection. qRT-PCR was performed to detect myelination associated genes Mpz, Mbp, Oct6 and Sox10 (f), as well as differentiation inhibitory genes ID2, ID4, Hes1 and Hes5 (g) from three independent experiments (n = 3; *P < 0.01).
Within the ID4 promoter, on the other hand, the repressive mark H3K9me3 was robustly recruited in control sciatic nerves, and H3K4me3 recruitment was barely detectable (Fig. 5d). Conversely, in the absence of HDAC1/2, H3K4me3 was strongly recruited along with acetyl-p65 and p300, while H3K9me3 occupancy was significantly reduced (Fig. 5d,e). Since trimethylation on histone H3-K4 and H3-K9 is the histone modification that is often associated with gene activation and repression 12,13, respectively, our observations suggest that HDAC1/2 channel through the acetylation state of NF-κB on the promoter region of Schwann cell regulatory genes to induce a change of chromatin configuration in coordination with transcriptional outcome. As a further support, introducing p65 into Schwann cells isolated from dCKO sciatic nerves resulted in a significant increase in expression of myelin genes and positive regulators of Schwann cell differentiation (Fig. 5f), but a reduction of differentiation inhibitors (Fig. 5g). Thus, p65 overexpression could, at least in part, rescue the defects of the Schwann cell differentiation program caused by the loss of HDAC1/2, suggesting that p65 is a critical target of HDAC1/2 for Schwann cell differentiation.
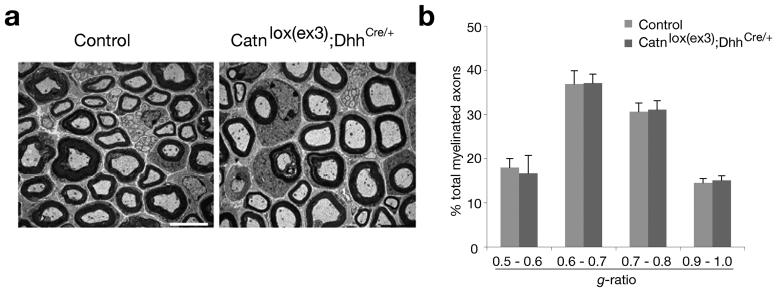
Requirement of HDACs for the formation of both myelinating oligodendrocytes and Schwann cells suggests that HDAC1/2 have a conserved function in CNS and PNS myelination by regulating common differentiation activators (e.g. Sox10 and YY1) and inhibitors (e.g. ID4, Sox2 and Hes5) 6,15. However, HDACs may have divergent effects on distinct signaling pathways and molecular targets in the CNS and PNS. We and others have shown previously that HDAC1/2 regulate Wnt signaling 5, whose activation inhibits myelination and remyelination in the CNS 5,16. Intriguingly, activation of canonical Wnt signaling by β-catenin stabilization in the Schwann cell lineage did not affect Schwann cell myelination in β-catenin activating mice (Catnlox(ex3)/+;DhhCre/+) (Fig. 6). There is a potential discrepancy regarding Wnt/β-catenin activities in Schwann cell development between our study and a companion study 17, in which HDAC1 was found to control Schwann cell survival by regulating Wnt/β-catenin signaling 17. Although the reasons for the discrepancy is not clear, it might be due to potential differences in the cellular context and timing.
Figure 6. Activation of canonical Wnt/β-catenin signaling does not inhibit Schwann cell myelination.
(a) Electron microscopy of sciatic nerve cross-sections from control (Catnlox(ex3)) and Wnt/β-catenin activating mice in the Schwann cell lineage (Catnlox(ex3)/+;DhhCre/+) at P15. (b) The g-ratio (ratio of axon diameter/ myelinated fiber diameter) of the myelinated axons was measured in sciatic nerves of control and β-catenin activating mice. Histogram presented the percentage of counts for myelinated axons at the different range of g-ratio as mean ± s.d. Scale bar, 5 μm.
In the PNS, NF-κB signaling is required for Schwann cell myelinogenesis 8. We show here that HDAC1/2 control Schwann cell differentiation, at least in part, by modifying NF-κB acetylation state and cooperating with NF-κB. At present, the role of NF-κB signaling in CNS myelination is yet to be elucidated. Our data reveal a developmental switch of NF-κB p65 complex with HATs e.g. p300 over to that with HDAC1/2 on target promoters during Schwann cell differentiation (Supplementary Fig. 5). In addition, recruitment of chromatin modifiers e.g. histone methyltransferases by HDACs and their effectors such as NF-κB may also contribute to the specificity of target gene expression (Supplementary Fig. 5). Our present study suggests that HDAC1/2 and NF-κB act coordinately to refine the epigenetic landscape for gene transcription that is required for Schwann cell myelination. Enhancing their activities may have therapeutic benefits for promoting myelin repair.
Methods
Generation of mice with HDAC1/2 mutant mice in the Schwann cell lineage
Mice carrying the HDAC1 and HDAC2 floxed alleles were bred with Schwann cell lineage expressing Dhh-cre line 7, to generate HDAC1fl/fl;DhhCre/+(HDAC1CKO), HDAC2fl/fl;DhhCre/+(HDAC2CKO), or HDAC1fl/fl;HDAC2fl/fl;DhhCre/+ (dCKO), and control heterozygous (HDAC1fl/+;HDAC2fl/+;DhhCre/+) offspring. The control mice developed and behaved the same as age-matched wild-type mice. Since HDAC1 or 2 single mutant phenotypes were indistinguishable from heterozygous control, we mainly focused on the control (HDAC1fl/+;HDAC2fl/+;DhhCre/+) and dCKO for subsequent analyses. Conditional β-catenin activation (Catnlox(ex3)) 5,18 mice were generated by breeding with the Dhh-Cre line to generate control (Catnlox(ex3)/+) and β-catenin activating mice (Catnlox(ex3)/+;DhhCre/+), in which β-catenin is stabilized in the Schwann cell lineage. Dhh-Cre-mediated excision of the exon 3 of the β-catenin allele produced a shortened, stable form of β-catenin that lacks its N-terminal phosphorylation and ubiquitination sites, resulting in constitutive activation of canonical Wnt signaling in the Schwann cell lineage. Animal use and studies were approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center at Dallas.
Immunohistochemistry, electron microscopy and Western blot analysis
Sciatic nerves were fixed in 4% paraformaldehyde for 10 min, and then were embedded in OCT, cut into 10-um as cross or longitudinal sections. For BrdU pulse labeling, animals at P4 were injected intraperitoneally with 100 mg BrdU/kg body weight 2 hrs prior to sciatic nerve collection. Primary antibodies were used: mouse anti-Mbp, anti-Mpz, anti-Mag (Covance), rabbit anti-Krox20/Egr2 (Covance), goat anti-Oct6 (Santa Cruz), goat anti-Sox10 (Abcam), mouse anti-BrdU (BD Biosciences), rabbit anti-Ki67 (Thermo Scientific), rabbit anti-caspase 3 (Millipore), anti-p300/CBP (Santa Cruz), anti-NF-κB p65/RelA (Santa Cruz) and anti-acetylated NF-κB p65 (acetyl K310) (Abcam). The acetyl-p65 antibody detected NF-κB p65 acetylation at K310, the major acetylation site in p65. Currently, the antibodies for NF-κB acetylated at K314 and K315 are not commercially available. Topro3 (Molecular probe) was used for cell nuclei stain. All images were acquired using Zeiss LSM 510 confocal microscope. For electron microscopy, sciatic nerves were dissected and fixed in fixative solution containing 2% paraformaldehyde and 2% glutaraldehyde and 0.1M cacodylic acid (pH7.2) and processed for electron microscopy. Western blot analysis was performed according to the method described previously 19. GAPDH or tubulin was used as an input control.
Quantitative RT-PCR
qRT-PCR was carried out using the ABI Prism 7700 Sequence Detector System (Perkin-Elmer Applied Biosystems) using Gapdh (TaqMan kit, Applied Biosystems) as an internal as previously described 5. qPCR primers for mouse species: Mpz F: ctggtccagtgaatggtct, R: gtcccttggcatagtggaaa; Mbp F: tcacagaagagaccctcaca, R: gccgtagtgggtagttcttg; OCT6 F:gttctcgcagaccaccatct, R: gtctcctccagccacttgtt; SOX10 F: agcccaggtgaagacagaga, R: gtcaaactggggtcgtgag; Hes1 F: tctggaaatgactgtgaaca, R: gtcacctcgttcatgcactc; Hes5 F: agctacctgaaacacagcaaagcc, R: taaagcagcttcatctgcgtgtcg; ID2 F: tgaacgactgctactccaagctca, R: gtgctgcaggatttccatcttggt; ID4 F: gcagtgcgatatgaacgactgcta, R: taacgtgctgcaggatctccactt; GPR126 F: ttatgtgagctgtgccgggtactt, R: attcctcccacagatctgcaccat; Gjb1 F: ttcagaatcatggtgctggtggtg, R: accaagataagctgcagggaccat; Krox20 F: caggagtgacgaaaggaagc, R: accagaggctgaagactgg; SOX2 F: cacaactcggagatcagcaa, R: ctccgggaagcgtgtactta; SOX11 F: gctggaagatgctgaaggac, R: gtcgggataatcagccatgt; C-Jun F: acccccactcagttcttgtg, R: agttgctgaggttggcgtag; Jag-1 F: caaatgagtgcgaggccaaacctt, R: agccaggaaggcaatcacagtagt qPCR primers for rat species: Mbp F: ttgactccatcgggcgcttcttta, R: gctgtgccacatgtacaaggatca; SOX10 F: gctatccaggctcactacaag R: actgcagctctgtctttgg; ID2 F: atggaaatcctgcagcacgtcatc, R: acgtttggttctgtccaggtctct; ID4 F: actgtgcctgcagtgcgatatgaa, R: tgcaggatctccactttgctgact; Hes1 F: agaaaaattcctcgtccccg, R: tttcatttattcttgcccggc; Hes5 F: accagcccaactccaaac, R: agtaaccctcgctgtagtcc; Jag1 F: gactacgagggcaagaactg, R: gttggaagagatataccgcacc; Dll-1 F: ttctctggcttcaactgtgag, R: tcatccacattgtcctcgc; Dll-3 F: attctatgggcctcgatgtgaggt, R: aggatcttcaccgccaacacacaa
Primary Schwann cell culture and transfection
Rat Schwann cells were prepared from sciatic nerves of newborn rats (1–2 d old) as described previously20. For routine culture, rat Schwann cells were grown under the proliferation condition [DMEM (Gibco) with 10% fetal bovine serum (FBS) (Hyclone) supplemented with 10 ng/ml neuregulin (R&D) and 5 μM forskolin (Sigma-Aldrich)]. Cells between passages 2 and 4 were used in all experiments. To promote differentiation, Schwann cells were cultured in a differentiation medium [DMEM with N2 (Gibco) supplemented with 5 μM forskolin], resulting in a substantial increase of expression of differentiation markers including Mpz and Krox20. Primary mouse Schwann cells were prepared from sciatic nerves of newborn mice (P1) as described by Yeisr et al.21 and cultured on poly-D-Lysine (Sigma) coated plate in DMEM (Gibco) with 10% fetal bovine serum (FBS) (Hyclone) supplemented with 50ng/ml beta heregulin (EGF domain) (R&D system). The primary Schwann cells were greater than 95% pure and used for transfection study after isolation. For reporter assays, rat Schwann cells were transfected with luciferase reporters and expression vectors using lipofectamine 2000. Transfected cells were cultured for 48 hr before harvesting to measure the luciferase activity using luciferase assay kit (Promega). The pRSV-renilla luciferase plasmid was included to control for variable transfection efficiencies between different experiments (n ≥ 3 times).
Chromatin Immunoprecipitation (ChIP) assays
ChIP assay was performed as described 19. Briefly, sciatic nerves were dissected from control and HDAC mutant mice and immediately fixed in 1% formaldehyde for 25 min at room temperature. Nerves were washed in PBS, homogenized in 150mM NaCl, 10% glycerol, 0.3% Triton X-100 and 50mM Tris-HCl (pH 8.0) containing protease inhibitor cocktail (Roche) as described by Jang et al. 22. Lysates were then sonicated with a Bioruptor sonicator (Diagenode) to ~500 bp. Sheared chromatin (~30 μg) was incubated with 2 μg of anti-HDAC1, anti-HDAC2, anti-p65, anti-acetylated p65 K310, anti-trimethyl-histone3 K4 (Abcam), anti-trimethyl-histone3 K9 (Abcam) and anti-p300 (Abcam), respectively, for each ChIP assay. Real-time PCR was carried out using quantitative SYBR green PCR mix (Applied Biosystems, Inc.). The relative fold-enrichments were determined by the 2−ΔCT methods as previously described 23. Samples were normalized to input chromatin. Primers used for ChIP-PCR analysis: Sox10 proximal promoter (−1/−176): Forward AGGCAGC AGTGCGGGTCACA; Reverse: TGACTGAGCCGCTGCAGACG; Sox10 5′UTR(−900/−1140): Forward AATCATTGAGGCCTTGATTC; Reverse CTCTACAGCCTAGTTAG TGT. ID4 promoter (−700/−493): Forward ATTCCAGCCAGCA CATTCC; Reverse GGAGTGACCAGCCAATCAG; GAPDH proximal promoter: Forward TTTGAAA TGTGCACGCACCAAGCG; Reverse TGAGTCCTATCCTGGGAACCATCA.
Statistic analysis
Quantifications were performed from at least three experimental groups. Data are presented as mean ± s.d. in the graphs. P values are from Student's two-tailed t test to compare two sets of data. P value < 0.05 is considered to be statistically significant.
Supplementary Material
ACKNOWLEDGEMENTS
Authors would like to thank D. Meijer for Dhh-Cre mice, B. Carter and J. Chan for critical comments, and P. Casaccia for initial discussion. We thank Q. Weng and Z. Ma for technical support, O. Nakagawa and J. Chen for p300/CBP and p65/RelA expression vectors, and Dr. R. Kageyama for the Hes5 luciferase reporter. This study was funded in part by grants from the US National Institutes of Health (NS072427) and the National Multiple Sclerosis Society (RG3978) to QRL.
Footnotes
AUTHOR CONTRIBUTIONS
Y.C. conducted the majority of the experiments and analyzed the data. H.W. and X.X. contributed to HDAC mutant generation, phenotype analysis and biochemical assays. S.O.K, J.S. and H.A.S. provide reagents and inputs. M.H. provided p65 mutant expressing vectors. K.A.N. provided CNP-Cre mice. E.N.O provided floxed HDAC1/2 mice and inputs. Q.R.L. supervised the project, analyzed the data and wrote the manuscript.
FINANCNCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Suter U, Scherer SS. Disease mechanisms in inherited neuropathies. Nat Rev Neurosci. 2003;4:714–726. doi: 10.1038/nrn1196. [DOI] [PubMed] [Google Scholar]
- 2.Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- 3.Svaren J, Meijer D. The molecular machinery of myelin gene transcription in Schwann cells. Glia. 2008;56:1541–1551. doi: 10.1002/glia.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- 5.Ye F, et al. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat Neurosci. 2009;12:829–838. doi: 10.1038/nn.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen S, et al. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat Neurosci. 2008 doi: 10.1038/nn.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaegle M, et al. The POU proteins Brn-2 and Oct-6 share important functions in Schwann cell development. Genes Dev. 2003;17:1380–1391. doi: 10.1101/gad.258203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nickols JC, Valentine W, Kanwal S, Carter BD. Activation of the transcription factor NF-kappaB in Schwann cells is required for peripheral myelin formation. Nat Neurosci. 2003;6:161–167. doi: 10.1038/nn995. [DOI] [PubMed] [Google Scholar]
- 9.Calao M, Burny A, Quivy V, Dekoninck A, Van Lint C. A pervasive role of histone acetyltransferases and deacetylases in an NF-kappaB-signaling code. Trends Biochem Sci. 2008;33:339–349. doi: 10.1016/j.tibs.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. EMBO J. 2002;21:6539–6548. doi: 10.1093/emboj/cdf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothgiesser KM, Fey M, Hottiger MO. Acetylation of p65 at lysine 314 is important for late NF-kappaB-dependent gene expression. BMC Genomics. 2010;11:22. doi: 10.1186/1471-2164-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin C, Zhang Y. The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol. 2005;6:838–849. doi: 10.1038/nrm1761. [DOI] [PubMed] [Google Scholar]
- 13.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 14.Antonellis A, et al. Identification of neural crest and glial enhancers at the mouse Sox10 locus through transgenesis in zebrafish. PLoS Genet. 2008;4:e1000174. doi: 10.1371/journal.pgen.1000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He Y, et al. Yy1 as a molecular link between neuregulin and transcriptional modulation of peripheral myelination. Nat Neurosci. 2010;13:1472–1480. doi: 10.1038/nn.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fancy SP, et al. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 2009;23:1571–1585. doi: 10.1101/gad.1806309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacob C, et al. HDAC1 and HDAC2 control the transcriptional program of myelination and the survival of Schwann cells. Nat Neurosci. 2011 doi: 10.1038/nn.2762. in press. [DOI] [PubMed] [Google Scholar]
- 18.Harada N, et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. Embo J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, et al. The oligodendrocyte-specific G protein-coupled receptor GPR17 is a cell-intrinsic timer of myelination. Nat Neurosci. 2009;12:1398–1406. doi: 10.1038/nn.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brockes JP, Fields KL, Raff MC. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979;165:105–118. doi: 10.1016/0006-8993(79)90048-9. [DOI] [PubMed] [Google Scholar]
- 21.Yeiser EC, Rutkoski NJ, Naito A, Inoue J, Carter BD. Neurotrophin signaling through the p75 receptor is deficient in traf6-/- mice. J Neurosci. 2004;24:10521–10529. doi: 10.1523/JNEUROSCI.1390-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang SW, LeBlanc SE, Roopra A, Wrabetz L, Svaren J. In vivo detection of Egr2 binding to target genes during peripheral nerve myelination. J Neurochem. 2006;98:1678–1687. doi: 10.1111/j.1471-4159.2006.04069.x. [DOI] [PubMed] [Google Scholar]
- 23.Bernstein BE, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.