Abstract
Voltage-sensitive dye activity within the thin, unfoliated turtle cerebellar cortex (Cb) was recorded in vitro during eighth cranial nerve (nVIII) stimulation. Short latency responses were localized to the middle of the lateral edges of both ipsilateral and contralateral Cb [vestibulocerebellum (vCb)]. Even with a severed contralateral Cb peduncle, stimulation of the nVIII ipsilateral to the intact peduncle evoked contralateral vCb responses with a mean latency of only 0.25 ms after the ipsilateral responses, even though the distance between them was ∼5 mm. We investigated whether a rapidly conducting commissure exists between each vCb by stimulating one of them directly. Responses in both vCb spread sagittally, but, surprisingly, there was no sequential activation along a transverse Cb beam between them. In contrast, stimulation medial to either vCb evoked transverse beams that required ∼20 ms to cross the Cb. Therefore, the rapid commissural connection between each vCb is not mediated by slowly conducting parallel fibers. Also, the vCb was not strongly activated by climbing fiber stimulation, suggesting that inputs to vCb involve distinct cerebellar circuits. Responses between the two vCb remained following knife cuts through the rostral and caudal Cb along the midline, through both peduncles, and even shallow midline cuts to the middle Cb through its white matter and granule cell layer. Commissural responses were still observed only with a narrow transverse bridge between each vCb or in thick transverse Cb slices. Horseradish peroxidase transport from one vCb labeled transverse axons traveling within the Purkinje cell layer that were larger than parallel fibers and lacked varicosities. In sagittal sections, cross-section profiles of myelinated axons were observed around Purkinje cells midway between the rostral and caudal Cb. This novel pathway for transverse communication between lateral edges of turtle Cb suggests that afferents may directly conduct vestibular information rapidly across the Cb to coordinate vestibulomotor reflex behaviors.
Keywords: optical recordings, parallel fiber beam, Purkinje cells, voltage-sensitive dye, imaging
the microstructure of the cerebellar cortex (Cb), which is highly conserved across most vertebrate species, functions to coordinate body movements (Larsell 1967; Llinas 1969; Voogd and Glickstein 1998; Voogd and Wylie 2004). The Cb is particularly known for its control of postural balance and oculomotor reflexes (Precht and Llinas 1969), where vestibular afferents provide direct input to Cb beginning early in evolution. This role for Cb was first described for reptiles more than a century ago when Rolando in 1809 removed their cerebellum and reported that they had disturbed movements (as described by Larsell 1967). More recently, hypotheses have focused on Cb's role in controlling the timing of these behaviors (Kistler et al. 2000; Kitazawa and Wolpert 2005; Xu et al. 2006; Yarom and Cohen 2002).
Whereas the microstructure of the circuit is highly conserved, gross brain structure exhibits more variability, putting the turtle Cb in a distinct position in the evolutionary tree (Larsell 1967). Unlike most fish and amphibians, the turtle Cb lacks two distinct cerebellar components: a medial corpus cerebelli and two separate, more lateral auricles (Larsell 1967). Like mammalian Cb, the turtle has a single continuous three-layered Cb, but it lacks gross regional specializations. We studied the turtle Cb to understand if its anatomically uniform, mammalian-like cortical structure uses two distinct circuits to mediate sensorimotor processing (Devor 2000): afferents from the inferior olive (IO) and other afferents supplied directly from sensory nerve of the vestibular system (Bangma and ten Donkelaar 1982; Schwarz and Schwarz 1983) within the structure called the vestibulocerebellum (vCb; Larsell 1967).
To study the topography and timing of the entire cerebellar cortical surface, we developed an optical recording technique using an in vitro turtle preparation (Brown and Ariel 2009). The turtle Cb is a thin unfolded neural sheet, ∼1-mm thick that narrows substantially within 0.5 mm of its most lateral edge. It rests over the fourth ventricle and connects to each side of the brainstem by a single cerebellar peduncle. The turtle's resistance to hypoxia permits neurons in an in vitro, intact brainstem to remain responsive to natural sensory stimuli: visual patterns imaged on retina (Ariel and Fan 1993; Fan et al. 1993) and head rotation (Fan et al. 1997). Voltage-sensitive dye recording techniques developed by Saltzberg and Grinvald (Baker et al. 2005; see modern reviews by Ebner and Chen 1995; Saltzberg et al. 1977; Wu et al. 1998) were applied to this in vitro preparation to measure neuronal activity as absorbance changes of transilluminated light (Brown and Ariel 2009; Konnerth et al. 1987). This technique permits the measurement of physiological activity with submillisecond resolution from a two-dimensional sheet of cortical tissue during electrical stimulation within the Cb and to the eighth cranial nerve (nVIII).
A previous study (Ariel and Fan 1993) recording through a single micropipette showed that extracellular field potentials to nVIII stimulation were evoked across much of the turtle Cb. Their amplitudes were largest in the rostrolateral Cb. Here, optical recordings show that nVIII stimulation activates a more limited region but causes a rapid bilateral response. The timing and topography of this response are distinct from a parallel fiber beam of activation, suggesting that an independent vestibulocerebellar path connects the two vestibulocerebellar cortices. An anatomical commissure of thick myelinated fibers in the Purkinje cell layer (PCL) was identified that might mediate this rapid connection. These findings are discussed in the context of an evolutionary perspective of two modes of Cb sensorimotor processing, both using the same standard Cb circuitry to either process direct sensory inputs bilaterally or modify sensory inputs using parallel and climbing fibers.
METHODS
Common red-ear pond turtles, Trachemys scripta elegans, were housed at room temperature in an aquarium on a 16:8-h light-dark cycle, where they swam and basked at will in the heat of an incandescent bulb. Optical recordings were performed on cerebella that were ∼5-mm wide, corresponding to turtles whose carapace lengths were 15–18 cm. Anatomical studies used 8–14 cm turtles. Turtles were handled and housed in accordance with National Institutes of Health Guidelines. The protocols of animal care and surgical procedures were approved by the Saint Louis University Animal Care Committee.
Turtle brainstem preparation.
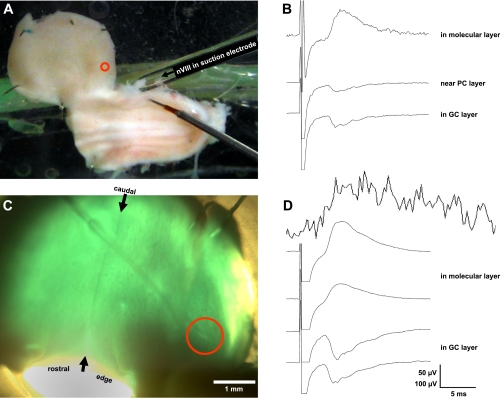
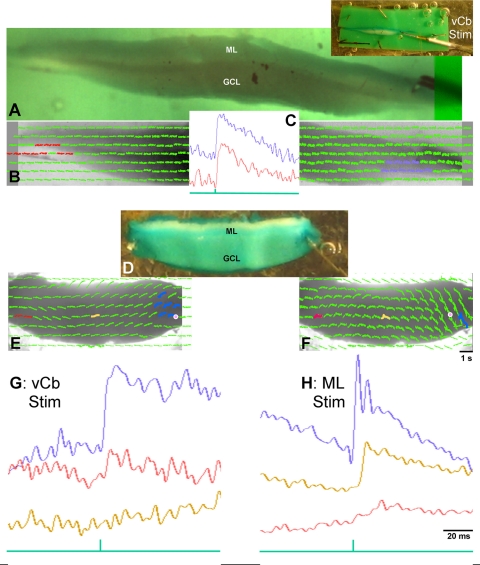
The details of this preparation and the optical recording methods have been presented in detail elsewhere (Brown and Ariel 2009; Fan et al. 1993; Rosenberg and Ariel 1990; Ariel and Brown 2010). Briefly, the brain was removed from the anesthetized animal, its telencephalon was quickly ablated, and the brain stem was placed in a Petri dish of oxygenated physiological media. One cerebellar peduncle was cut, and the dura and choroid plexus were then carefully removed around the cerebellum to facilitate unimpeded staining by immersion for up to an hour in a voltage-sensitive oxonol dye RH482 (NK3630; Nippon Kankoh; 1 mg /2.5 ml of oxygenated physiological media). In some experiments, the intact tissue was then sliced on a tissue chopper (Stoelting, Chicago, IL) in the transverse plane for thick slice recordings. Otherwise, the intact stained tissue was transferred to a recording chamber and a suction electrode was secured to the nVIII ipsilateral to the intact peduncle (Fig. 1A). The Cb was deflected ventricular-surface-up onto the recording chamber floor. Its coverglass floor is covered with a 1-mm layer of Sylgard to allow the Cb to be stabilized (Fig. 1A, see 5 pins around the Cb perimeter but avoiding the lateral edge). The Cb was imaged through a fixed stage microscope (BX-51; Olympus, Tokyo Japan). For the accurate registration of Cb topography with the photodiode-array signals, photomicrographs of the transilluminated tissue were captured by a digital camera attached to the trinocular port of the microscope (Fig. 1C).
Fig. 1.
Turtle brainstem-cerebellum preparation maintained in vitro. A: photograph of an unstained preparation in the recording chamber viewed with ambient room illumination. Cerebellar cortex (Cb) is rotated away from the brainstem by cutting its left peduncle and pinning it ventricular side up to the Sylgard floor of the top shallow compartment of the chamber. Brainstem was pinned dorsal side up in the bottom, deeper compartment that also contains the ground wire. Thus the image shows the cerebellum's rostral edge down, yet the cut rostral edge of the brainstem is to the left. A pipette with a polished tip serves as a suction electrode to record from the right eighth cranial nerve (nVIII). Beneath the suction electrode is a tungsten bipolar stimulating electrode directed towards the vestibular nucleus (data not shown). Entering the cerebellum at the center of the red circle is a ∼100 KΩ glass micropipette that is not visible (but see C). B: traces of cerebellar field potentials recorded through a ∼100 KΩ glass micropipette (not visible) at three depths as it was advanced from the ventricular surface. Average voltage traces are shown vertically offset, in response to twenty 100-μs, 100-μA pulses, at a shallow, intermediate, and deep position, roughly corresponding to the granule cell (GC) layer, near the Purkinje cell (PC) layer and the molecular layer (ML). C: cerebellar cortical preparation (different from A) was stained with the voltage-sensitive dye RH482, transilluminated, and photographed through the ×2 objective of the microscope. Midline raphe is observed connecting the rostral and caudal midpoints (see arrows). The shank of the glass micropipette can be seen entering the cerebellum yet its tip is no longer visible within the red circle. D: like B, traces of field potentials are shown for 4 Cb depths as it was advanced from the ventricular side of the cerebellum. Note the clear stimulus artifact. Above those traces is a trace without a stimulus artifact measured by optical diodes imaging the Cb within the red circle. Unlike the averaged, filtered voltage trace (3 dB below 1 KHz), the optical response is not filtered. Its response shows no artifact and presumably represents absorbance changes from the entire tissue depth. Although this optical recording is quite noisy, its onset and peak timing are similar to the voltage traces recorded by the pipette (voltage calibration: 50 μV for B and 100 μV for D).
Optical recordings.
Light through a standard microscope condenser passed through a 715 ± 35 nm interference filter (Chroma Technology, Brattleboro, VT) to transilluminate the tissue so that an increase in absorbance by the voltage-sensitive dye RH482 is equivalent to a membrane depolarization. The image was recorded optically by a 464-photodiode array (WuTech H-469IV PDA; Wutech, Gaithersburg, MD) using one of two microscope objectives (×2 PlanApo N NA 0.08 or ×5 UMplanFL NA 0.15; Olympus). At those magnifications, each diode detects 0.06 and 0.024 mm2 of Cb, respectively.
The fractional change in light absorption due to stimulation, relative to light intensity at rest, was recorded for each of the 464 diodes by the NeuroPlex software (RedShirt Imaging, Fairfield, CT). Some of the diode traces had a baseline drift because the WuTech H-469IV PDA exhibits a very long time constant (∼20 s). To generate pseudocolor images, it was often necessary to remove any artifactual drift by applying a minimal digital filter to each diode's trace. Timing data were always measured without this high-pass filtering.
For nVIII stimulation, care was taken to extract a long stump of the right nVIII from the temporal bone during the dissection so that the suction electrode would seal on the nerve and not on the brainstem surface (Fig. 1A). This electrode was a fire-polished thin-wall glass capillary tube (outer diameter/inner diameter: 1.5/1.12 mm) attached to a standard electrophysiological pipette holder. The cut peduncle (Fig. 1C, bottom left) and the adjacent disconnected Cb hemisphere are seen on the left of the midline raphe (Fig. 1C, between rostral and caudal arrows). For intracerebellar microstimulation, tungsten bipolar microelectrodes (125-μM tip separation), the tips of which had a right angle bend, were advanced perpendicular to the left Cb surface. These electrodes delivered 100-μs biphasic 25–200 μA current pulses (AM-Systems 2100, Carlsborg, WA). In some experiments, extracellular field potentials were recorded for comparison with the optical data (Brown and Ariel 2009). A glass recording micropipette (25-μm tip diameter, filled with 3 M K-acetate, DC resistance <1 MΩ) was positioned in the vCb to record potentials with respect to a chlorided silver ground wire in the recording chamber.
Anatomical processing.
For all histochemical experiments, perfusion of oxygenated physiological media bilaterally through the neck's internal jugular veins was performed to rinse blood from the head immediately after decapitation. For tracing experiments, horseradish peroxidase (HRP; 10% in Tris buffer) was iontophoretically ejected (7-s on/off cycle, 10–30 μA, 20–40 min) from a 20- to 30-diameter tip of a glass pipette through the ventricular Cb surface into the PCL. Eight to ten hours later, the Cb was immersed in phosphate-buffered 4% paraformaldehyde (pH 7.4) for 24 h and then cyroprotected in 30% sucrose/4% fixative solution for an additional 2 h. Then, 50-μm thick transverse sections were cut with a freezing sliding microtome, mounted onto gelatin-subbed slides, and reacted with diaminobenzidine-H2O2. Following this reaction, the sections were dehydrated, cleared, and coverslipped. Some sections were later counterstained with cresyl violet.
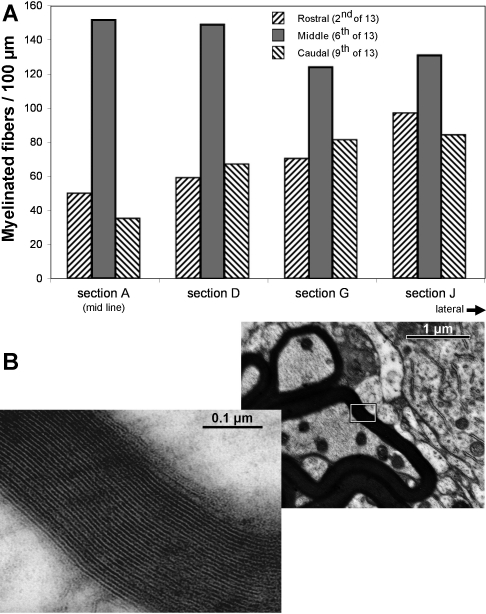
For histological analysis of myelinated axons, two turtles (carapace length of 8 and 14 cm) were studied with similar results. The brains were immersed in freshly made fixative consisting of 2.5% glutaraldehyde and 2% formaldehyde in 0.1 M cacodylate buffer (pH 7.4) and stored at room temperature before overnight shipment to J. Rosenbluth. Each cerebellum was excised, cut into left and right halves, rinsed overnight in three-fourths strength mammalian Ringer solution, and then postfixed for 2–3 h with 2% osmium tetroxide and 1.5% potassium ferricyanide in 0.1 M sodium cacodylate buffer (pH 7.4). Half cerebella were rinsed and dehydrated in an ascending series of methanol solutions (30–100%), soaked overnight in propylene oxide, and infiltrated and embedded in Araldite. One-micrometer sections were then cut on a Sorvall MT2 microtome with a diamond knife beginning at the midsagittal plane and progressing laterally. After they were mounted on glass slides and stained with alkaline toluidine blue, the sections were examined with a Nikon optical microscope and photographed through a ×4 objective and then at 13 rostrocaudal positions through a ×40 objective lens using a Nikon Coolpix 990 digital camera. Sixteen sections were labeled alphabetically, four of which were quantified: sections A, D, G, and J (equivalent to 0, 20, 40, and 60% of the ∼2 mm distance between the midline and the lateral edge of the cerebellum, respectively).
Myelinated profiles were quantified for the cerebellum of the 8-cm turtle. Purkinje cell bodies were identified by their very large size within a monolayer between smaller granule cells and the neuropil of the molecular layer (ML). The transversely sectioned myelinated fiber profiles surrounding Purkinje cell bodies were marked in a high-power frame of ∼300 × 400 μms using ImageJ (National Institutes of Health). Three rostrocaudal levels (2, 6, and 9) were quantified in the midsagittal section (level A), and the corresponding positions were quantified in three more lateral sections (levels D, G, and J). Within a known length of PCL, Purkinje cells and myelinated fiber profiles were counted in each image. Density data are expressed as myelinated fibers per 100 μm. A sample of axon diameter within the myelin profiles was measured in the midline section and compared with a sample of diameters of HRP-labeled axons from the tracing experiments.
Data analysis.
Quantifications of the responsive photodiodes included their response latencies, their peak amplitudes, and their distance from the stimulating point within the Cb. Latencies were detected in these traces at the peak risetime using wavelet analysis (maximal slope after response onset). The response latency pattern was viewed as a grayscale latency array for which the diodes were displayed as gray boxes, the darkness of which was scaled as a function of their response latency. Diodes with the longest response latencies were displayed as darkest, and those with the shortest latencies were lightest. Latency profiles that showed a regular change in the darkening of the boxes across the array indicated that the latency changed along that vector as the response slowly propagated.
Response fields were displayed in dot-amplitude arrays as follows. Response traces were strongly filtered to allow measurement of their average peak amplitudes. The largest response amplitude in each array was displayed as a dot filling the space occupied by its diode. The dot sizes of the remaining diodes were scaled smaller based on their amplitudes. Response fields were also displayed as pseudocolor images generated by NeuroPlex software using minimal high pass filter to remove nonphysiological baseline drifts for display purposes only. In addition, a standard color threshold of 75% (as noted by the black line on the color scale) was established to remove noise so that the response field can be viewed as an overlay on the Cb image. The NeuroPlex system was also used to convert these 464 traces into pseudocolor movies as Supplemental Data (Supplemental Material for this article is available online at the J Neurophysiol website). Each of the 464 diodes was sampled 1,600 times per second while the optical responses would really only last tens of milliseconds. The resulting video files, using the standard mpeg video format of 30 frames per second, appear ∼53 (1,600/30) times slower than the real events.
RESULTS
A previous study (see Figs. 2 and 3 of Ariel and Fan 1993) of turtle Cb responses to nVIII stimulation using a single recording micropipette showed that extracellular field potentials varied in amplitude and polarity depending on recording depth. This study began by repeating that finding in unstained tissue (Fig. 1B) and in stained tissue (Fig. 1D) in which the voltage-sensitive dye signal was measured simultaneously at the same location but across the entire depth of the Cb. The optical signal always resulted in a short latency depolarization, apparently equivalent to electrophysiological signal recorded in the ML. The optical signal was limited to the lateral edge of the Cb, a region thought to represent the turtle vCb (Ariel and Fan 1993). In this and all figures showing the intact Cb, its ventricular surface is viewed with its caudal edge at the top of the image, so that the sagittal midline is roughly oriented vertically. With that orientation, the peduncle to the right remained connected to the brainstem and the peduncle to the left was severed.
Fig. 2.
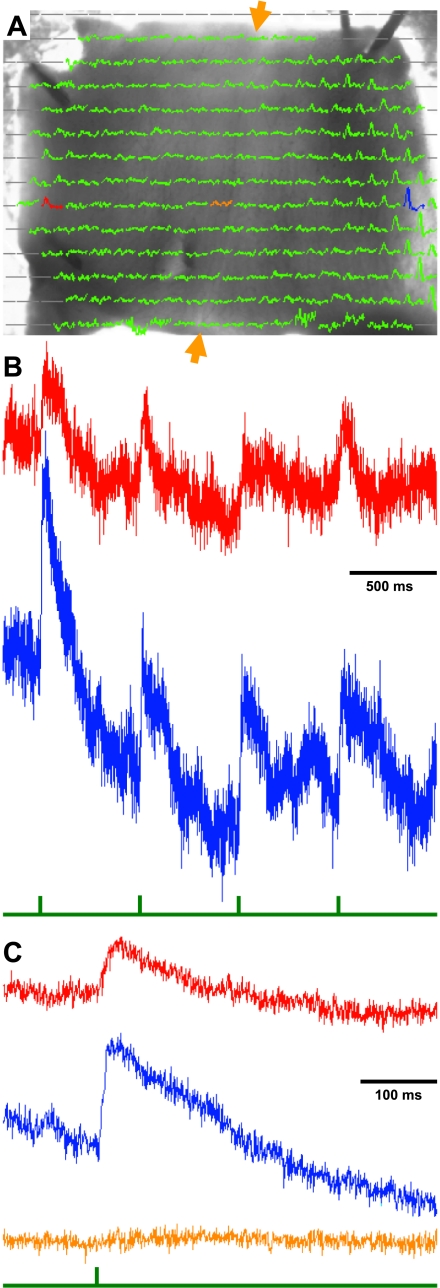
Analysis of cerebellar responses to single nVIII current pulses. A: traces recorded by the photodiode array, superimposed on the monochrome image of the ventricular surface of a transilluminated turtle Cb in the recording chamber (as in Fig. 1C). Gray lines indicate diodes that did not image the transilluminated tissue. The remaining 245-ms traces represent responses to a single 150-μA current pulse to the right nVIII. Red, blue, and tan traces show responses from diodes on the contralateral Cb edge, on the ipsilateral Cb edge, and at the Cb midline, respectively. B: raw traces from diodes in the 2 Cb edges. Without any filtering, these traces occasionally show slow drifts of their baseline, upon which responses occur to the standard stimuli of 4 pulses (interval of 575 ms, sampled at 1.6 KHz). Below the traces is a green stimulus pulse that indicates the current pulse. Note that responses follow each stimulus with a short latency and are larger than the noise even without averaging. C: averages of responses from the four pulses indicate that responses on both sides of the Cb exhibit a similar latency. There is no response (tan trace) from the diode between the 2 responsive edges along the midline (between the tan arrows).
Fig. 3.
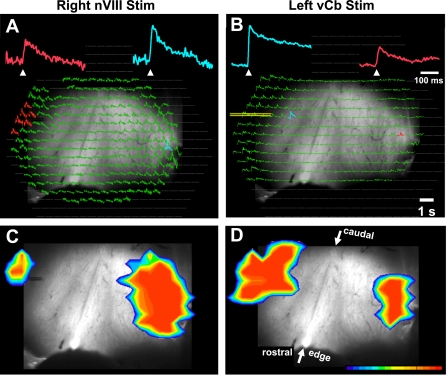
Comparison of bilateral responses to right nVIII (A and C) and left lateral edge stimulation (B and D). A and B: top row shows arrays of average absorbance traces superimposed on the monochrome image of the Cb (as in Fig. 1C). During nVIII stimulation (A) and lateral edge microstimulation (B), diodes along the lateral Cb edges exhibited strong responses. Note the position of the bipolar electrode in left Cb of B. Magnified traces above each array have a color correspond to the diode(s) of the same color in the array. Blue traces are ipsilateral, and red traces are contralateral to the side of stimulation. Note that the red traces are smaller than blue traces, yet have similar latency following the stimulation (arrowhead). C and D: bottom row shows pseudocolor images of the spatial extent of the Cb responses, showing responses near their peak amplitude at the same latency following stimulation. Caudal Cb is up and arrows indicate the rostral and caudal extent of the midline. Cb and nVIII were both stimulated with a 500-Hz train of 5 100-μA pulses. Responses contralateral to the stimulated nVIII are averaged from 6 adjacent diodes stimulated with 5 trains.
Bilateral responses to nVIII stimulation.
By recording responses optically with a 464-photodiode-array, simultaneous responses with the Cb can be measured (Fig. 2A). Those responses are displayed as 245-ms traces superimposed on a monochrome image of the Cb oriented ventricular side up in the recording chamber. The right peduncle is still attached to the brainstem so that stimulation of nVIII through a suction electrode will activate the Cb. Current pulses to the nVIII activated both the lateral cerebellar edges, its presumptive vCb. From the locations of the red and blue diodes highlighted in Fig. 2A, the unfiltered time course of local absorbance changes for a single trial can be viewed in Fig. 2B (top and bottom traces, respectively). Each trace exhibits clear transient depolarizations for each of the four stimulus pulses, as indicated by a green stimulus trace. (These 150-μA, 100-μs current pulse square pulses, here and in subsequent figures, were expanded graphically to the right to improve their visibility.) Averages of those four responses indicate that the contralateral response (red) is smaller than the ipsilateral (blue) responses (Fig. 2C).
When viewing the responses from individual diodes in the array in Fig. 2A, a clear boundary between the vCb and the nonresponsive medial Cb was not obvious. Moreover, the sizes of the responsive region in individual Cb preparations were different, and larger stimulus currents evoked larger responsive regions. In spite of the variability, diodes with the largest amplitude responses were consistently found near the lateral edge at the rostrocaudal midpoint of the Cb (Fig. 2A; see also dot-amplitude array in the Supplemental Data and Fig. 5F). Note that midline diodes exhibited traces that were flat or had minimal responses (Fig. 2C, tan).
Fig. 5.
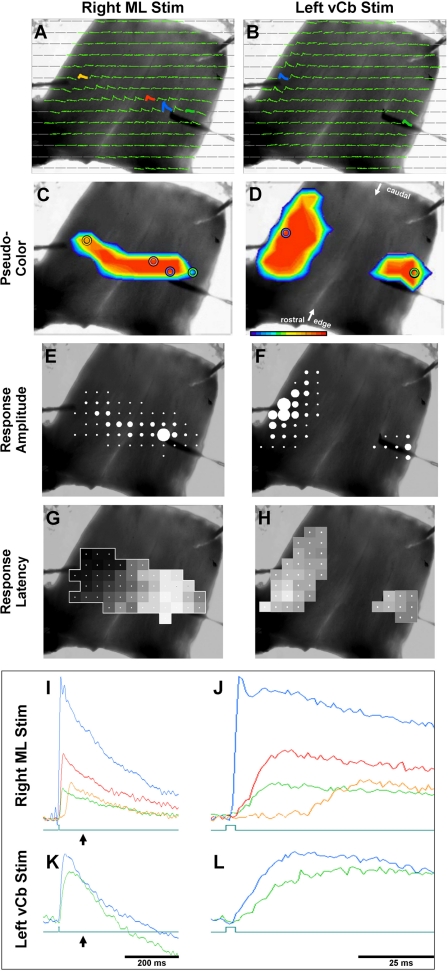
Comparison of transverse conduction timing differences of responses to 100-μA pulses into medial ML (A, C, E, and G) and into lateral edge (vCb; B, D, F, and H). A bipolar microelectrode was place either in the ML of the medial Cb (blue trace in A, I, and J) or in the vCb (blue trace in B, K, and L). Colored circles in C and D correspond to colored traces in A and B. ML microstimulation evoked a depolarization along a transverse parallel fiber beam. I and J: traces of 4 diodes in A are superimposed in I and expanded in time in J. Blue trace responses began immediately following the 100-μs stimulus pulse. Red and green responses, the diode locations of which straddle that of the stimulus, responded with approximately the same onset latency (∼3.8 ms). Orange trace response, the diode of which was located near the end of the transverse beam, responded much later (27 ms). Note that the ML response is bilateral, yet its response along the Cb lateral edge is limited. C–H: responses superimposed on monochrome plots. C and D: pseudocolor frames. E and F: relative response amplitude of each diode (dot-amplitude array, see methods). G and H: relative response latency of each diode (latency-distance plot: white is zero latency and black is maximal latency; see methods). B, D, F, and H: vCb microstimulation evoked an ipsilateral response that spread sagittally and a contralateral response that was more localized. Traces of a diode in each response field are superimposed in K and expanded in time in L. Blue and green responses, the diode locations of which in B were more distant than that of the green and orange diodes of A, had very similar latencies. Green pulses below the traces indicate the onset of the 100-μs stimulus. Arrows below the traces in I and K indicate the point in time shown in the pseudocolor frames C and D.
Bilateral responses to vCb microstimulation in the intact Cb.
Although two direct paths might exist from the nVIII to each vCb, the presence of bilateral responses from unilateral nVIII stimulation suggests that a commissure connects the two vCbs. Several paths for this activation are possible. First, the nVIII afferents could cross soon after entering the penduncle, along the rostral edge of the Cb. A second alternative is that these afferents could run along the lateral edge of the Cb, sending off processes to the ipsilateral vCb as they proceed around the caudal edge to terminate on the contralateral vCb. Third, the nVIII afferents could project toward the ipsilateral vCb and bifurcate there to synapse on the ipsilateral vCb and also form a commissure that continues transversely across the midline to the contralateral vCb. Finally, these afferents could simply terminate on the ipsilateral vCb, some of whose neurons in turn form a commissure that projects transversely across the midline to the contralateral vCb.
These options were first tested to verify the presence a commissure connecting the two vCbs. First, responses to nVIII stimuli were recorded (Fig. 3A) and then a bipolar stimulating electrode was placed in the contralateral response field at a depth approximately halfway between the ventricular surface and the pia. During nVIII stimuli, Fig. 3A shows that many diodes were responsive on the lateral edge ipsilateral to the stimulated nVIII but a much smaller field of diodes responded on the contralateral side (like responses in Fig. 2A). The response amplitude was also larger on the ipsilateral side (see Fig. 3A, blue vs. red trace). Figure 3C shows a pseudocolor image of this response, representing the activated response fields (red) that surpass a threshold. This image is a frame of a pseudocolor movie, using a point in time when the response amplitude was near its peak. The entire response sequence can be viewed as a video in the Supplemental Data.
Having located the contralateral vCb based on nVIII stimulation, a bipolar electrode was positioned in that contralateral vCb for local microstimulation (Fig. 3, B and D, yellow lines overlie the bipolar electrode for better visibility). On that side, ipsilateral to this microstimulation, more diodes were responsive and their response amplitudes were larger, compared with the contralateral side (Fig. 3, B and D, and video files in the Supplement Data). For both stimulation of the nVIII (right) and microstimulation of the vCb (left), their contralateral response fields appear shifted caudally, relative to the stimulated side. However, the underlying monochrome image shows that the midline raphe is rotated and that the centers of both response fields are therefore at the same rostrocaudal level relative to the diagonally oriented midline raphe.
Mean timing and topography.
These findings were analyzed for entire sample of bilateral responses produced by nVIII or vCb stimulation (Table 1). nVIII stimulation evoked ipsilateral responses in 29 preparations, yet only 5 experiments had sufficient contralateral responses for accurate quantification of the bilateral response latency. The factors that limited the contralateral responsiveness to nVIII stimulation are unknown. On the other hand, most of the experiments that stimulated the intact Cb lateral edge at its rostrocaudal midpoint resulted in contralateral responses.
Table 1.
Responses to single current pulses
| Max Slope Time, ms |
Response Peak Time, ms |
|||||
|---|---|---|---|---|---|---|
| To ipsi vCb | Increase contra vCb | To ipsi vCb | Increase contra vCb | Velocity, m/s | Response Strength, ratio contra/ipsi | |
| nVIII stimulation (n = 5) | 8.13 ± 5.15* | 0.25 ± 0.56* | 19.58 ± 8.17 | 7.19 ± 9.22 | 8.13 ± 1.08* | 0.67 ± 0.39 |
| vCb stimulation (n = 22) | 3.15 ± 1.18* | 3.30 ± 1.19* | 13.27 ± 3.5 | 7.50 ± 5.31 | 1.86 ± 1.79* | 0.53 ± 0.88 |
Values are means ± SD. Latency differences following stimulation of eighth cranial nerve (nVIII) and vestibulocerebellum (vCb) groups were subjected to an independent variable t-test for a 95% confidence level, assuming unequal variances. Max slope data were unfiltered. Response peak data were low-pass filtered by a moving boxcar average. Velocity and response strength were measured at max slope. Ipsi, ipsilateral; contra, contralateral.
P < 0.05.
Data in Table 1 indicate that the ipsilateral vCb risetime latency for nVIII stimulation was longer than that of vCb stimulation (8.13 vs. 3.15 ms; P < 0.05), consistent with its longer conduction distance. However, surprisingly, the difference between the ipsilateral and contralateral stimulus response times after nVIII stimulation was shorter that that observed for vCb stimulation (0.25 vs. 3.30 ms; P < 0.05). This second result suggests that the path from the brainstem to the contralateral vCb is shorter and/or has a higher velocity than the path across the Cb. Measurements of mean peak response latencies of these two paths are consistent with the findings using risetime latencies, although peak response times are inherently more variable and were not statistically significant. Table 1 also quantifies another feature observed in Figs. 2 and 3; that the response strength is much smaller on the contralateral side. The mean ratio of contralateral to ipsilateral amplitude is near 0.6 for both the nVIII and vCb stimulation.
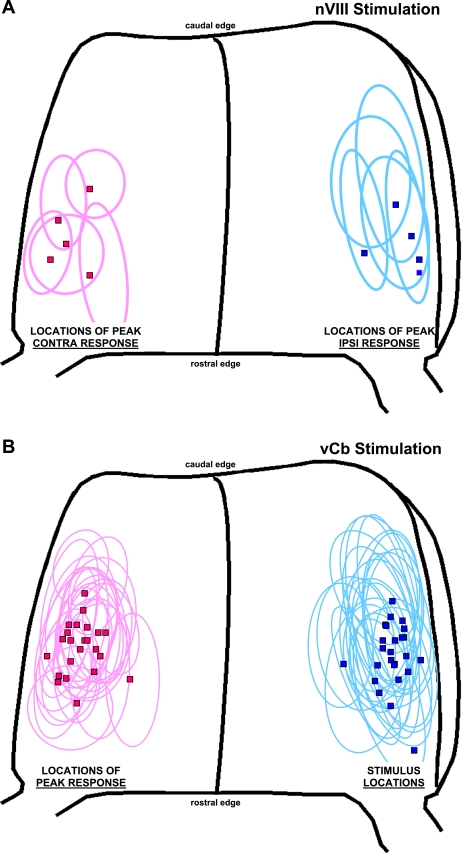
The same data set used for Table 1 was analyzed for the location of the diode with the peak response amplitude and its adjacent responsive field (Fig. 4). The locations for both the nVIII and vCb stimulation appear similar when mapped on the dimensions of a typical Cb (4.89 wide × 3.81 long; Brown and Ariel 2009). Although responses were observed immediately adjacent to the lateral edge, the diodes with the strongest responses were often more medial and not in the most lateral region, 0.5 mm from the edge where the turtle Cb becomes very thin. Because the rostrocaudal levels of these responses are variable, an attempt was made to determine if the rostrocaudal position of the bipolar stimulating electrodes along the lateral edge would be equally effective to evoke a contralateral response. However, we found that stimulation close to the rostral Cb evoked a broad activation of the hemisphere, due to the proximity of the Cb peduncle. Similarly, the caudal Cb could not be effectively tested because slowly propagating parallel fiber beams were evoked from there, without a rapid contralateral edge response (not shown).
Fig. 4.
Graphic summary of the response location within the Cb drawn on the standardized drawing of a turtle Cb. Each marker (square) represents the location of the diode that exhibited the largest peak amplitude in its response field (ellipse). Blue data are ipsilateral (ipsi), and red data are contralateral (contra) to the side of stimulation. A: nVIII stimulation. B: vCb stimulation, for which the blue data include responses to local current stimulation. Mean current for single pulses were 87.7 ± 31.5 μA.
Comparison of response to nVIII stimulation and ML microstimulation.
A likely path to mediate the connection between the lateral edges of the cerebellum would be the parallel fibers. Single parallel fibers have been observed to transverse nearly the full extent of the turtle Cb (Tolbert et al. 2004). Therefore, an experiment was performed to test parallel fiber involvement in vCb → vCb communication. In a simple sense, if these paths used the same axons, action potentials traveling along this path in the opposite direction should collide and not conduct beyond the collision point. This possibility was tested by presenting simultaneous current pulses into the ML of the right Cb and into the left vCb. One might expect spike collision at or near the Cb midline so that the left parallel fiber beam and/or the right vCb would no longer be responsive.
First, control responses were measured to each stimulus independently (Fig. 5 and Fig. S5 video files in the Supplemental Data). Microstimulation to the left vCb evoked responses in the right vCb that were smaller (Fig. 5, B, D, and F, blue vs. green) and slightly delayed in time (Fig. 5, K and L, blue vs. green) (see Figs. 3–4 and 7–9 for similar results). At the stimulation sites, responses to the vCb and to the ML were both strong and had very short latencies (Fig. 5, K vs. L, blue). However, recording sites that were ∼0.6 mm on either side of the ML stimulation site had clear delayed latencies (Fig. 5J, blue vs. red and green). Furthermore, the farthest responsive diode in the contralateral hemisphere (Fig. 5J, tan) had a risetime latency of ∼25 ms following ML stimulation, compared with almost no latency to vCb stimulation (Fig. 5L, green). The long latency to ML stimulation is equivalent to a conduction velocity of 0.18 m/s along the parallel fibers.
Having identified how these responses cross the midline, the possibility of spike collision was tested during simultaneous stimulation of the ML in the right Cb hemisphere and the left vCb (Fig. 6K and video file in the Supplemental Data). From the results shown in Fig. 6, H and K, it is clear that spikes still cross the midline without collision. There were however interesting combinations of response latencies observed to dual stimulation (Fig. 6, J, L, and N). Simultaneous stimulation-evoked traces in the left Cb with a distinctive two-stage excitation (Fig. 6G) of short and long latencies. The tan response from the most lateral diode exhibited a predominant short response, followed by a smaller longer latency response. In contrast, the green response from a more medial position shows a weak short response, followed by a large longer latency response. The blue diode was between the tan and green, both in position and in the extent of short and long latency responses. By comparing Fig. 6G with Fig. 6D, it is clear that stimulation of the left vCb contributes the short-latency depolarizing component (Fig. 6D) during the simultaneous stimulation. Likewise, stimulation of the right ML evokes the long latency component (Fig. 6A) during the simultaneous stimulation. These profiles of sequential depolarizations are indicative of two independent circuits that contribute independently to the responses of the left Cb; namely, a local vCb response followed by a delayed ML response due to slow parallel fiber conduction. Clearly though, simultaneous stimulation did not cause a spike collision between the stimulation sites, or there would have been gaps in the response field, not a full transverse beam (compare Fig. 6K with Fig. 5, C and D).
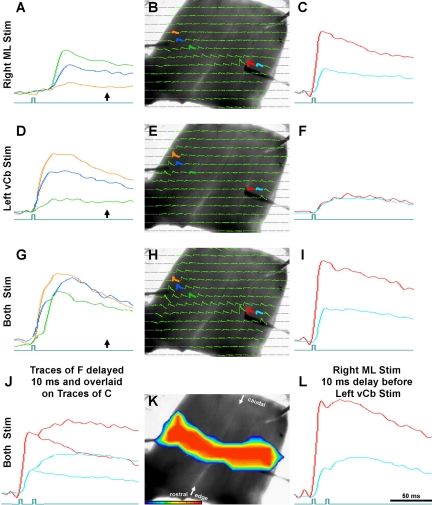
Fig. 6.
Conjoint Cb microstimulation into both medial ML (A–C) and lateral edge (vCb; D–F) using the same Cb shown in Fig. 5. Third and fourth rows show responses to simultaneous stimulation of both (G–L). A shows superposed traces of 3 diodes in the left Cb hemisphere (marked in B), far from the ML stimulation site. Onset times of all these traces are much delayed and the orange trace barely responds as it is in the contralateral vCb. C: superimposed traces of 2 diodes in the right Cb hemisphere, close to the ML stimulation site. Onset of the blue trace is slightly delayed as it is to the right of the stimulation. D and F: responses of vCb microstimulation, using the same diodes as A and C, respectively. Onset times of these traces are very similar. During both stimulation of the ML and vCb, the traces in G have 2 components, a short latency response (due to vCb stimulation) and a delayed response (due to the distant ML stimulation). Unlike I, which shows responses to simultaneous stimulation, L shows responses when the vCb stimulation was delayed by 10 ms relative to the ML stimulation. Both the red and blue traces of L exhibit an early and late response component. These 2 components compare well to the shapes of the overlaid traces of C and F shown in J. See description in text. Arrows below the traces in B, F, and J show the time point to which pseudocolor frames of Fig. 5, C and D, and Fig. 6K correspond, respectively.
The presence of independent components within the response waveform of individual diodes was confirmed by inserting a 10-ms time interval between ML and vCb stimulation (Fig. 6L and video file in the Supplemental Data). Responses of the right Cb to simultaneous stimulation did not show any distinctive two-stage excitation (Fig. 6I). Presumably, two components were not visible because the short distance from the ML stimulus site did not delay the parallel fiber-mediated response, so it overlapped with the rapid vCb response. However, traces evoked by the sequential stimuli (ML then vCb) created two clear depolarizations: a large and early (ML) response followed by a smaller delayed (vCb) response. Identification of the sources for those two depolarizations was supported by a graphic simulation. To the waveforms generated by ML stimuli alone (Fig. 6C), we added the waveforms generated by vCb alone (Fig. 6F) but delayed those latter traces by 10 ms graphically. The resulting simulated waveform (Fig. 6J) closely matches the recorded traces evoked by the actual sequential stimuli (Fig. 6L).
Based on the timing and shapes of these traces, it appears that conduction across the Cb midline uses two distinct paths. Communication between the two vCbs and transmission by parallel fibers each use independent and functionally distinct mechanisms. This conclusion is supported by a fast mean velocity measured between the two vCb responses (Table 1; nVIII: 8.13 m/s; vCb: 1.86 m/s). Those conduction velocities are much faster than that of the parallel fiber beam (0.24 m/s for turtles; Brown and Ariel 2009) (0.18–0.27 m/s for mammals; Wyatt et al. 2005). The distinctive nature of the bilateral vCb circuit is supported by the separate topography of optical signals during vCb and ML stimulation. Although there may be some overlap, the response fields for ML stimuli seldom or only weakly extend laterally to the very edge of the Cb where the vCb is located (Fig. 5, C vs. D).
Comparison of response to nVIII stimulation and IO microstimulation.
Some aspects of responses of the turtle vCb to nVIII stimulation are similar to those reported to IO stimulation (Ariel 2005; Brown and Ariel 2009). Both responses are rapid and are oriented sagittally (Fig. 5 and see Brown and Ariel 2009). Therefore, we reexamined these two responses in the same experiment to evaluate their topographic and temporal similarities and differences (Fig. 7, A–C and D–F). As reported previously, responses to current pulses into the contralateral IO are strongest within a sagittal band centered ∼0.7 mm from the midline (Fig. 7F). Like responses to ML stimulation, responses to IO stimulation seldom extend laterally to the edge of the Cb where the vCb is located (Fig. 7C vs. Fig. 7F and video files in the Supplemental Data). However, IO and ML pathways share common regions of the medial turtle Cb, suggesting that this region is served by functional circuitry that is distinct from that targeted by the vCb pathways (see discussion).
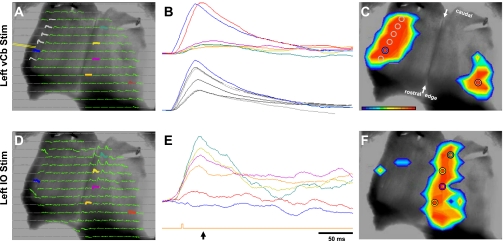
Fig. 7.
Comparison of responses to microstimulation of the left lateral edge (A–C) and left inferior olive (IO; D–F). Left column: arrays of absorbance traces recorded during direct VCb stimulation (A, see position of the bipolar electrode tips) and during microstimulation of IO (not shown), contralateral to the intact right Cb peduncle (D). Pseudocolor images in C and F show the spatial extent of the responses near their peak as measured in A and D. Those images are superimposed on the monochrome image of the ventricular surface of a transilluminated Cb. B and E: magnified filtered traces that correspond to traces in the arrays in A and D and the colored circles in C and F. Arrow below the green stimulus trace under E indicates the point in time that corresponds to the pseudocolor images of C and F. Note that responses to IO microstimulation were in a nearly synchronized sagittal band ipsilateral to the midline. In contrast, the VCb response contralateral to the stimulated lateral edge has a slightly later latency. Below E, the green stimulus pulse indicates the 100-μs current pulse onset yet appears displaced to the right of the response onset because those responses were slightly filtered. Current pulses to vCb and IO were 80 and 50 μA, respectively.
The response risetimes for vCb and IO microstimulation were also examined. As reported previously, responses to IO stimuli are nearly synchronous, independent of their rostrocaudal position (Fig. 7E and see Brown and Ariel 2009). Similarly, responses with the vCb also appear to be nearly synchronous, independent of their rostrocaudal position (Fig. 7B, bottom, grey and blue traces). This synchrony was not observed between the two vCb (Fig. 7B, top, red vs. blue traces). On average, the contralateral response risetime is delayed 3.3 ms relative to the ipsilateral risetime (range 0.62–5.6 ms; Table 1).
Bilateral responses to microstimulation in nonintact Cb.
The rapid conduction between the two vCb suggests that its path is not via parallel fibers, which conduct relatively slowly (Brown and Ariel 2009; Eccles et al. 1966) due to their thin unmyelinated axons (Wyatt et al. 2005). These rapidly conducting axons could be traveling in the transverse plane among the parallel fibers within the ML, or other layers of the Cb, or they could take a circuitous route along either the rostral or the caudal rim of the Cb. To investigate the path through which there is rapid communication between the two vCbs, two more physiological experiments were performed. First, the bilateral responses were measured following a series of knife cuts into the Cb (Fig. 8 and video files in the Supplemental Data). A role of the brainstem in mediating bilateral responses was removed by severing the remaining attached peduncle (Fig. 8B). The bilateral responses also remained after first a rostral midline cut (Fig. 8C), then a caudal midline cut (Fig. 8D), and finally a shallow cut from the ventricular surface along the midline (Fig. 8E). The response contralateral to the stimulus only disappeared after a sagittal knife cut between the two vCb reached the PCL or the ML beneath it (Fig. 8F). The commissure is thereby localized at or near the PCL in a transverse path between the vCb.
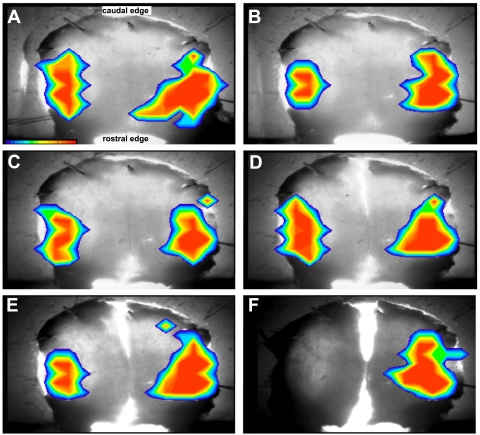
Fig. 8.
Optical responses to right vCb microstimulation, before and after a series of knife cuts of the Cb/brainstem preparation. Control pseudocolor responses (A) are bilateral, shown overlaying an image of the intact Cb with a cut left peduncle, ventricular surface up, caudal end on top. B: response of intact Cb after right peduncle was severed from brainstem. C: response of Cb after the rostral part of mid-line raphe was cut. D: response of Cb after additional cut through caudal part of mid-line raphe. E: response of Cb after an approximate half-millimeter shallow sagittal cut along mid-line raphe into the ventricular surface. F: response of Cb after a deeper sagittal cut into the ML. vCb current pulses were 75 μA.
A second approach to localize the path between the two vCb was to study Cb slices. A previous report (Ariel and Brown 2010) showed optical recordings of a slow beam of activation entirely within the ML from thick transverse slices. Although those findings were quite consistent, there was only limited success in this series of experiments in measuring a fast connection between the lateral edges of the Cb. Two examples are shown in Fig. 9 using Cb slices. As mentioned earlier, the turtle Cb tapers within 0.5 mm of its most lateral edge. With the use of a 1-mm slice (Fig. 9A), bipolar microstimulation just medial to this tapering within the PCL of the right Cb was able to evoke a contralateral response in the left Cb (Fig. 9B and video file in the Supplemental Data). Like the intact tissue, there was only a 4.4-ms difference in risetime latency when comparing the ipsilateral and contralateral responses (Fig. 9C, see average of the blue diodes to the red diodes, respectively). In this and subsequent figures, Cb slices are oriented with its pia (dorsal) side on top. Here, the response is strongest in the PCL and granule cell layer.
Fig. 9.
Bilateral responses observed in 1- to 2-mm transverse slices of Cb. A: 1-mm slice cut from the middle of the Cb, placed ML up, was secured in the chamber by adjacent strips of colored Sylgard to prevent stray light along the edges of the tissue. Inset: low-power photograph shows placement of the bipolar electrode in the lateral edge. B: diode arrays of optical signals in the contralateral and ipsilateral vCb using the ×5 microscope objective. C: plot centered over B shows the averaged responses from 10 ipsilateral (blue) diodes and 10 contralateral (red) diodes to the same stimulus paradigm (single 75-μA pulses). D: 2-mm slice cut from the middle of the Cb with a straight bipolar stimulating electrode placed just below the PC layer of vCb. E and G: responses to 100-μA pulses were observed in ipsilateral (blue) diodes and contralateral (red) diodes. Note the trace from a midline (tan) diode was not responsive. Then, the electrode was advanced mediodorsally into the ML using 50-μA pulses, where different responses were observed (F and H). Response amplitude of the midline (tan) diode is now large and its latency is longer than the ipsilateral (blue) diode. Note the fast response of the contralateral (red) diode is absent (or strongly reduced) while a much delayed response may be present. GCL, granule cell layer.
Figure 9, D–H, shows another example of a thicker slice with a bipolar stimulating electrode placed in the vCb and then advanced medially into the ML. Like the intact tissue show in Fig. 5, this slice exhibits a rapid conduction to the contralateral (left) vCb following lateral edge stimulation of the right Cb (Fig. 9, E and G). Then, more medial ML stimulation evoked a slow parallel fiber beam across the Cb (Fig. 9, F and H). Relative to the risetime at the stimulation site (blue trace), the contralateral vCb (red trace) responded in 1.3 ms (a distance of 5.8 mm, equivalent to 4.66 m/s). In contrast, the midline trace of ML stimulation at a distance of 3.1 mm responded after 10.6 ms (0.29 m/s).
Anatomical evidence of rapid transverse path between the lateral edges of Cb.
The physiological evidence suggests that a rapidly conducting commissure exists roughly within the transverse plane of the Cb. Furthermore, responses following knife cuts and in slices suggest that this path may run in or beneath the PCL and cross in the middle to rostral part of the Cb. Based on their conduction velocities, axons that mediate this response are likely to be thick and well myelinated. The remaining anatomical findings suggest that such myelinated axons exist and do indeed course transversely through the PCL.
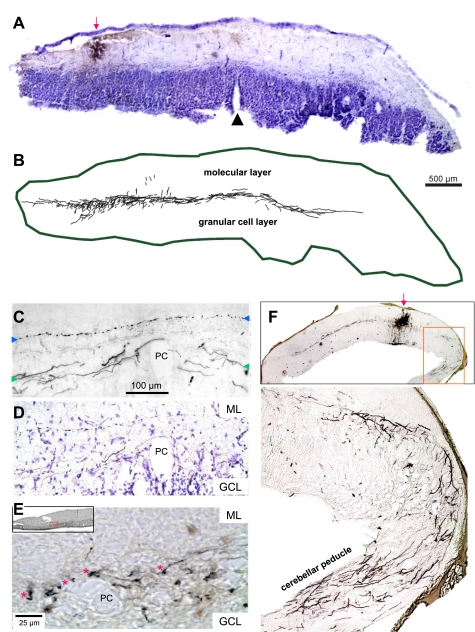
In tracing experiments, HRP injections were made iontophoretically into the PCL of the rostral half of a fresh intact (unstained) Cb in vitro. After 8–10 h, the tissue was immersed in fixative for subsequent slicing of transverse sections and histological processing (see methods). Extending from the injection sites (Fig. 10, B, C, and F) were transversely oriented axons. These travel in the PCL towards both ends of the Cb (Fig. 10C, between the green arrows). These were not typical parallel fibers in the ML (Fig. 10C, between the green arrows), Purkinje cell axons, or afferent Cb inputs that travel sagittally or obliquely in the granule cell layer or in the thin layer of white matter to and from the peduncle (Tolbert et al. 2004). Beneath the injection site, such afferent and efferent axons are also labeled on the ventricular surface (see Fig. 10F, inset). Also at the injection site, cell bodies were locally labeled yet no cell bodies were backfilled elsewhere in the Cb.
Fig. 10.
Examples of distinct axons in the PC layer in transverse 50-μm Cb sections labeled by transport from horseradish peroxidase injections. A: low-power micrograph counter-stained with cresyl violet shows an injection site into left vCb. B: labeled processes were traced using Neurolucida, revealing transverse-oriented axons in the PC layer extending far across the midline. C: high-power micrograph without counterstaining showing labeled transverse-oriented axons adjacent to PCs, 1-mm lateral to the midline, contralateral to injection site. Green arrowheads on each side mark the level of these labeled axons. In contrast to these large smooth axons, note the small beaded parallel fiber labeled in the ML above labeled with blue arrowheads. D: same region as C after cresyl-violet counterstaining shows that the large, smooth, transverse-oriented axons travel in the PC layer, while the parallel fiber is within the ML. E: terminal boutons (adjacent to red asterisks) are observed at even higher magnification near PCs more laterally in Cb (see box in inset). F: low-power image of injection site into rostral Cb, from which transverse-oriented labeled axons emanate, travel long distances in the PC layer. Boxed region is magnified below to show very lateral axons turning ventrally to enter the peduncle.
The transversely oriented labeled axons reported here are also distinct from parallel fibers that travel in the ML, exhibiting thin axons (0.6 μm) with numerous regular varicosities (Tolbert et al. 2004) (Fig. 10, C and D). The labeled axons shown in Fig. 10 have much larger diameters (1.3 ± 0.5 μm; means ± SD), are smooth with little or no synaptic varicosities, and travel in the PCL. In rostral sections that included the attached Cb peduncle, labeled axons were observed bending ventrally towards the brainstem and entering the peduncle (Fig. 10F). In more caudal sections without peduncles, these labeled axons approached the lateral edges but would often leave the plane of section. A few axons were observed forming small branches with terminal boutons near the Purkinje cell somata (Fig. 10E, red asterisks).
In a separate study, immediately after removal from the cranium, the intact Cb/brainstem was immersed in fixative for histological processing for myelin (see methods). Sixteen sagittal sections were studied, from the midline (section A) to lateral edge at the widest point (section P). Many myelin profiles were observed in the midsagittal section immediately around the Purkinje cells, forming a band approximately twice as wide as the PCL itself (Fig. 11, see magnified insets of 3 of the 13 regions studied). The mean axon diameters (1.4 ± 0.5 μm, range 0.8–2.8 μm) were similar to the diameters of the HRP-labeled axons and much thicker than that of parallel fibers (0.6 μm; Tolbert et al. 2004). Myelination and large diameter are consistent with the optical recordings that show fast conduction observed between each vCb. The myelinated profiles surround large, elliptical cell bodies (mean diameter: 13.7 ± 2.4 by 29.1 ± 4.9 μm) that appear to be Purkinje cells in contrast to much smaller granule cells (diameter: ∼5 μm).
Fig. 11.
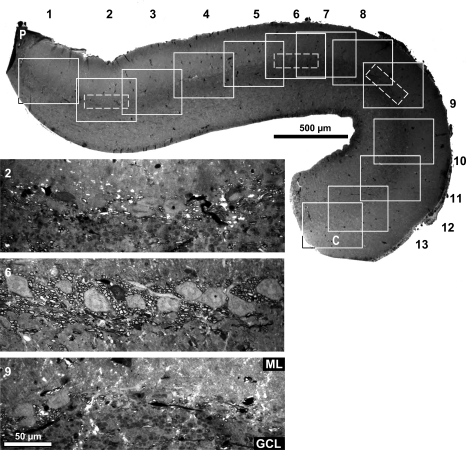
Low-power photograph of a 1-μm cerebellar section cut at its midsagittal plane (section A). Cerebellum was epoxy embedded and sections were stained with toluidine blue. Regions were numbered 1 through 13 (rostral to caudal, solid boxes). Within 3 regions (2, 6, and 9), dashed boxes were oriented parallel to the PC layer to indicate the location of the three magnified photographs below. Counts of myelinated axons in those 3 regions are graphed in Fig. 13A (section A). ML is up; rostral is left; P is peduncle; and C is caudal end of section.
The myelinated axons were not uniformly distributed along the rostrocaudal extent of the PCL but rather were more concentrated in the middle of the Cb. We divided the Cb into 13 regions from rostral (1) to caudal (13) and found the densest myelination in regions 5–7 (Fig. 11). These fibers extended as far caudally as region 9 in the midsagittal plane but were not apparent in regions 10–13. The increased density of myelinated fibers in the middle of the rostrocaudal extent of the Cb was also observed in the more lateral sagittal sections (Fig. 12). High density of myelinated fibers were even observed immediately around the Purkinje cells in very lateral sections where the caudal end of the Cb has curved, resulting in a shorter section (Fig. 12, section M, right column of the lowest row).
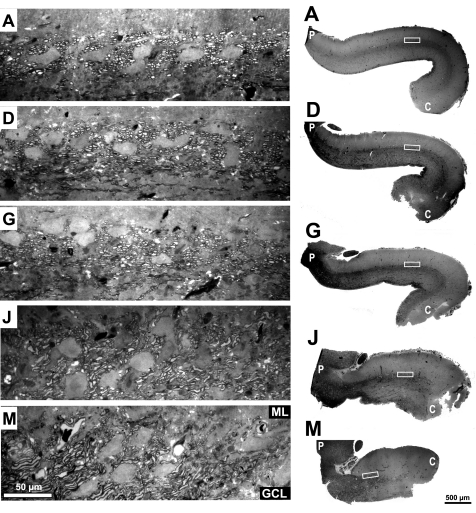
Fig. 12.
Photographs of 5 sagittal 1 μm sections (A, D, G, J, and M; representing 0, 20, 40, 60, and 80% of the distance from the midline to the lateral edge of the cerebellum, respectively). Right: low-power photographs with boxes indicating the location of the high-power photographs at left. High-power photographs have the same magnification as those in Fig. 11. Epoxy-embedded sections stained with toluidine blue.
To quantify these regional differences, myelin-profiles were counted at three rostrocaudal positions of the midsagittal Cb [Fig. 13A, regions 2 (rostral), 6 (middle), and 9 (caudal)] and four mediolateral levels (Fig. 13A, sections A, D, G, and J). Consistent with the localization of optical responses to nVIII stimulation, these fibers were not uniformly distributed rostrocaudally. Along 100 μm of Cb, the density of myelinated fibers was measured (rostral: 69 ± 20.4; middle: 139 ± 13.6; caudal: 67 ± 22.4; n = 4). We found that the density of myelin profiles in middle Cb was statistically higher than that found in both the rostral Cb (P < 0.01) and the caudal Cb (P < 0.01) by approximately twofold. The difference in the densities of myelinated fibers between the rostral Cb and the caudal Cb were not statistically different (two-tailed t-test). Relative to the density of Purkinje cells, the myelinated fibers were also not evenly distributed. The number of fibers per Purkinje cell averaged 21 rostrally, 37.3 in the middle, and 21.5 caudally. Thus the myelinated fibers in the middle region had double the density of either rostrocaudal extreme.
Fig. 13.
A: bar graph shows the density of myelinated fibers around PCs at the same 3 regions (2, 6, and 9) as in Fig. 11. Counts were made for the midsagittal section (A) and 3 sections more lateral (D, G, and J) as shown in Fig. 12. At each lateral position, myelinated fiber density is highest in the region 6. B: low and high magnification electron micrographs show axons in the midsagittal section A. Cell body at the top right is part of a PC. High-magnification image shows a detail of the myelin sheath of a ∼1 μm axon ensheathed by ∼16 layers of myelin.
Because these specimens were not first stretched to be pinned to a recording chamber or otherwise flattened, it was difficult to quantify very lateral sagittal sections (sections N through P). The lateral rim of the turtle Cb naturally curves ventrally and its cortex becomes thinner. In addition, the distance between the lateral edges gradually narrows towards the caudal end of the turtle Cb, so that middle and caudal Cb were no longer present in more lateral sagittal sections. In the context of these gross anatomical features, several interesting observations were made on myelinated fibers in the PCL of the lateral Cb. First, relative to the midline section (section A), the density of myelinated fibers in the PCL (Fig. 13A, middle bar) did not decrease much for more lateral sections (sections D, G, and J). The small decrease might be attributed to the loss of some axons that turn rostrally towards the peduncle (Fig. 10F). In contrast, fiber density in the rostral region, and to some extent in the caudal region as well, appears to increase somewhat with more lateral points.
Both the smooth axons labeled by HRP (Fig. 10) and these myelinated fibers are plentiful in the middle Cb between the two vCb regions. HRP-labeled fibers were seen within the PCL, first coursing towards the lateral edge, but then turning rostrally and ventrally near the lateral edge to connect with the peduncle. Here too, the myelinated fibers in the PCL are cut transversely in the most medial sections (Fig. 12, A, D, and G) yet are more often cut obliquely in the more lateral sections (Fig. 12, J and M). The presence of these myelinated fibers in lateral sections does not simply reflect Cb afferents and efferents connected to the nearby peduncle because those other fibers primarily connect through the white matter layer on the ventricular surface.
This analysis of myelinated axons was also extended to the electron microscopic level (Fig. 13B). Heavy myelin was observed at high magnification from typical myelinated axons in the midsagittal section. This amount of myelin surrounding these axons should ensure that they conduct action potentials very rapidly to connect the two vCbs without much delay, as was observed by optical recordings. Rapid conduction between the lateral edges of the Cb would help synchronize the neural activity of the cerebellar regions that are devoted to vestibular processing.
DISCUSSION
With the use of in vitro optical recordings of voltage-sensitive activity of the entire turtle Cb, a fast pathway between each hemisphere's vCb has been revealed for the first time. Robust responses to nVIII and cerebellar stimulation indicate that the region along each lateral edge of the Cb is activated by nVIII stimulation. That vestibulocerebellar region is distinct from Cb regions activated by current pulses to the ML or the IO in the brainstem. The same vestibulocerebellar region has been identified anatomically as receiving terminal arbors from vestibular afferents labeled by extracellular HRP application (E. H. Peterson, personal communication). Other species also show a direct connection from the vestibular periphery to the Cb (Kevetter et al. 2004; Korte and Mugnaini 1979; Precht and Llinas 1969a,b; Schwarz and Schwarz 1983).
A temporal analysis of the risetime of responses in each vCb indicated that a rapidly conducting excitatory path exists for communication between the two vCb within a few milliseconds. This commissure, which has never been described previously, travels roughly within the transverse plane yet does not activate the intervening Cb. Unlike parallel fibers that are thin unmyelinated axons, this path appears to be mediated by larger, unbranched axons that lack varicosities but are myelinated. The rapid connection between both vestibular components of the Cb may be necessary for the control and modification of rapid coordinated behaviors involving both sides of the body (Yamamoto et al. 2001). Because the white matter layer of the turtle Cb is very limited and interrupted by the midline raphe, the turtle's cerebellar commissure may run through the PCL. To our knowledge, such a pathway has not been described within the mammalian Cb (Shinoda and Yoshida 1975, Wu et al. 1999).
Two distinct functional topographies.
The standard Cb circuit of mammals involves inputs from climbing fibers and slow transverse communication via parallel fibers. The data presented here suggest that the vCb may differ significantly from the medial Cb, even though the turtle Cb has an apparently uniform Cb based on its cytoarchitectural characteristics. It lacks structurally distinct components like the corpus cerebelli and auricles found in fish and amphibians. Devor (2000) compared the standard mammalian Cb circuit with that of several “cerebellar-like” structures: the electroreceptive lateral-line lobe of fish, the octavolateral nuclei of amphibians, and the dorsal cochlear nucleus of mammals. She noted that the corpus cerebelli receives climbing fiber input from the IO, while Cb-like structures get primary afferent input from sensory ganglia. Devor suggested that climbing fibers also provide sensory signals to the Cb, yet those signals have been filtered within the IO by other excitatory or inhibitory nonsensory IO inputs. The presence of an additional level of sensory modification relayed to the Cb may allow for a more sophisticated learning process in the mammalian Cb (Devor 2000).
It has long been known that the vestibular system played an important role in the evolution of the Cb in early vertebrates (Hillman 1969; Larsell 1967; Llinas et al. 1971; Llinas et al. 1967a; Precht and Llinas 1969b). Early vertebrates exhibit two cerebellar regions, the medial corpus and the lateral auricles (Butler and Hodos 2005). To study cerebellar evolution, frogs were chosen among the early vertebrates that have both the corpus cerebelli and auricles yet lack lateral line sensation and its associated Cb-like structures (Precht and Llinas 1969a). Direct vestibular afferent input to the auricles was found to have many similarities to that of climbing fibers, and electrical stimulation evoked both ipsilateral and contralateral auricle responses (Llinas et al. 1967b).
Vestibular processing has evolved in vertebrates from being within a specialized brainstem structure to being just one region of a uniform multifunctional cortex. In the turtle Cb, apart from sagittal groves along its ventricular surface, there is no gross regional distinction characteristic of auricular structures and no regional zebrin bands (Hawkes, unpublished observation). Its cortex also has relatively uniform cellular distribution (Ariel et al. 2009) with its three-layered structure and neuronal morphologies that are typical of those found in mammalian cerebellum (Tolbert et al. 2004). On the other hand, the rim of the turtle cerebellum has two distinct features. First, the cortical thickness tapers within 0.5 mm of the edge. Second, its very edge is in contact with the brainstem and vasculature. Along that Cb rim, there is evidence of a proliferating zone of small cells that may display unique morphologies as they migrate (Ariel et al. 2009; Martin et al. 2008). It is not known whether these features of neurogenesis have any relationship to the lateral localization of the optical responses to nVIII stimulation or to the cerebellar processing that occurs in vCb.
The optical recordings of the turtle vCb shown here suggest some similarity to responses found in the frog auricles. The response to nVIII stimulation in the turtle vCb is fairly synchronized and often oriented sagittally, like that of IO stimulation in the medial Cb (Brown and Ariel 2009). Activating climbing fibers by IO stimulation evoked complex spikes in Purkinje cells, much like the complex spikes evoked by nVIII stimulation in the frog auricle, as reported by Llinas et al. (1967b). We predict that Purkinje cells in turtle vCb exhibit complex spikes to nVIII stimuli and suggest that the simple three-layered turtle vCb may correspond to an organization that bridged the evolutionary gap between the amphibian auricle and the mammalian vCb.
The results presented here indicate that, in spite of the uniformity of turtle Cb and its three-layered structure, the vCb may have a mode of processing that is distinct from the medial Cb. Evidence for different vCb processing comes from two features of the standard Cb topography, activity from transverse parallel fiber beams and sagittal olivocerebellar bands, are weak or absent in the vCb. The vCbs are also distinct in that a rapid commissure exists between the two vCbs. That commissure does not influence the intervening cortex in that there are no optical signals generated in the medial cortex, nor do well-myelinated axons that run adjacent to Purkinje cell bodies have boutons to make synaptic contact. One might conclude that the Cb of the turtle represents an evolutionary stage when any gross distinctions between auricles and corpus have disappeared, yet two distinct modes of processing still exist. Both modes result from their separate afferent and efferent connections but both utilize the same standard Cb module for cortical processing.
The existence of two distinct modes of processing is consistent with the strong lateral response to nVIII stimuli and our previous report (Brown and Ariel 2009) that noted a lack of a strong response in the lateral Cb to moderate levels of IO microstimulation (see Fig. 7). Although responses to IO and nVIII stimuli are spatially distinct, overlap can occur with very high stimulus currents. It is not known if the origin of climbing fibers in the vCb differs from that of the olivocerebellar path that primarily activates the medial Cb. It is also interesting that slowly conducting transverse activation (the “parallel fiber beam”) did not emanate from vCb stimulation. One can hypothesize that the parallel fibers found in the vCb are shorter and only synapse in or near the vCb, while parallel fibers from the medial Cb travel long distances only to end abruptly as they near the vCb on either side. Kunzle (1987) reported that parallel fibers projecting across the Cb midline towards the contralateral edge are mainly in the basal half of the ML, while lateral projections are more superficial. This too is consistent with our suggestion that the commissure between the vCbs run transversely near the Purkinje cells.
Comparing response timing to nVIII and lateral edge microstimulation.
These experiments have shown similarities between the responses evoked by current through a suction pipette on a cranial nerve and a bipolar stimulating electrode within the Cb. Because neither stimulus is natural, care should be taken when interpreting their ability to activate a functional pathway selectively. nVIII stimulation could affect a direct vestibular afferent pathway to the cerebellum or an indirect pathway via the vestibular nuclei. Either way, the optical localization of the vestibular cerebellum to the lateral edge of the middle cerebellum shown here is supported by spike responses of cerebellar neurons to head rotation (M. Ariel, unpublished results using the in vitro turtle brainstem preparation with the temporal bones attached; see Fan et al. 1997).
In contrast to Cb responses via cranial nerves, stimulation from an intracerebellar electrode cannot be as selective as a stimulus to the vestibular pathway. Optical responses after lateral edge microstimulation may evoke other circuits beyond those that connect the two vCb. For example, larger regions of Cb were activated as this bipolar electrode was moved more rostrally, presumably due to these fibers of passage. The proximity of the vCb to the Cb peduncle may allow current spread to the mossy and climbing fibers that enter the Cb along a sagittal trajectory. Current spread might account for the ipsilateral vCb's larger size and stronger response amplitudes, compared with the contralateral vCb. However, activation of the ipsilateral vCb is also larger and stronger than the contralateral vCb during nVIII stimulation, even though the peduncular fibers of passage are not affected.
Although the responses to nVIII and lateral edge microstimulation exhibited topographical similarities (Fig. 4), the measurements of response timing for the same data set are perplexing. Based on the difference in risetimes of diode responses in the two vCbs and the distance between those diodes for each measurement, the velocity between the vCbs evoked by nVIII stimulation was faster (7.65 m/s) than that evoked by vCb stimulation (1.86 m/s). One explanation is that the conduction path length and velocity are not the same. In a seemingly unrelated observation, it is also perplexing that the optical recording experiments involving transverse Cb slices often failed to evoke responses on the contralateral side of the slice. Only with thicker slices were both vCb activated. A third relevant observation is that the labeled axons that presumably mediate the rapid conduction across the Cb were seen turning towards the peduncle as they approached the lateral Cb and no cell bodies were backfilled in the processed Cb sections.
Based on these observations, we suggest that the rapid communication between each vCb is mediated by axons, the neurons of which are outside the Cb. From the timing of the responses, we hypothesize that, as those axons enter the Cb, they bifurcate forming two collaterals. One collateral, traveling within the PCL, is thick and myelinated so that it can rapidly conduct its spikes to the contralateral vCb. The shorter collateral conducts to the ipsilateral side very slowly to allow both responses to arrive nearly synchronously. By directly stimulating that shorter axon collateral, the timing between the two vCbs is a combination of the slow antidromic conduction until the ipsilateral branch point and the rapid orthodromic conduction to the contralateral vCb. This is one possible mechanism to explain why the mean risetime between the vCbs is 0.44 ms for nVIII stimulation while the mean risetime from the ipsilateral vCb to the contralateral vCb is 3.3 ms.
The aforementioned mechanism to control response timing between the vCb is consistent with other Cb analyses that show that the timing of afferent information in the Cb is synchronized at many stages within the cerebellum itself (Ariel 2005; Brown and Ariel 2009; Lang and Rosenbluth 2003; Shin et al. 2006). Response timing within the Cb may therefore be necessary to control complex motor behaviors (Llinas 2009; Yarom and Cohen 2002).
Anatomical findings.
In this study, we suggest that transversely oriented myelinated axons in the turtle PCL course between the two Cb regions that respond to nVIII stimulation. These axons travel along the same path as the axons labeled by HRP injections into the lateral Cb. The origin of these fast conducting transverse axons has not yet been determined, but one suggestion is that these axons are collaterals from brainstem neurons, perhaps even vestibular ganglion cells.
The role of myelination in synchronizing bilateral vestibular responses may be similar to its active role in synchronizing mammalian Cb responses to IO spike activity (Lang and Rosenbluth 2003). Complex spike synchronization differed when comparing Cb from normal rats and myelin-deficient rats that lacked virtually all CNS myelin. In addition, in those myelin-deficient rats, olivocerebellar fibers directed to different regions of the Cb had markedly different overall lengths and conduction times, as would be expected for asynchronous activation of Purkinje cells. In the normal rat, however, the olivocerebellar tract is myelinated, and synchronization is clearly greater in the normal compared with the myelin-deficient Cb (Lang and Rosenbluth 2003). Evidently, the longer fibers have thicker myelin and/or longer internodes with fewer nodes per unit length, both of which would serve to speed their spike conduction. Thus myelination may compensate for discrepancies in path length, resulting in more synchronous responses.
The myelinated axons quantified in this study run among the turtle Purkinje cell bodies and were transversely oriented (as briefly described by Mugnaini et al. 1974). In the cerebellar cortices of other species, myelin profiles are also found in infragranular plexus and supragranular plexus (Palay and Chan-Palay 1974). The transverse orientation of myelinated axons in the PCL make it unlikely that they represent horizontal, Lugaro, or basket cells, which ramify sagittally in those plexuses. The myelin profiles are also not similar to the recurrent axon collaterals of mammalian Purkinje cells, as described by Cajal (as referenced by Palay and Chan-Palay 1974). Recurrent collaterals are described as a thinly myelinated, tenuous beaded threads (diameter <1 μm) that repeatedly bifurcated before terminating in a burst of terminal endings, mainly in the parasagittal plane. The commissure that we describe comprise of myelinated axons (diameter 1.4 ± 0.5 μm), without varicosities or branches, that travel in the transverse plane above and below the Purkinje cell somata.
Not all the myelinated axons observed in the PCL may convey rapid vestibular signals. A portion of the myelinated profiles may represent parallel fibers because parallel fibers that are deeper in the ML are generally thicker than superficial parallel fibers (Palay and Chan-Palay 1974). They are sheathed with myelin 3–4 lamellae thick, which is much thinner than we observed adjacent to the Purkinje cells. Alternatively, some myelinated axons could be climbing fibers that occasionally reach the PCL with their thick myelin. In addition, glomerular collaterals of climbing fibers have been reported to course transversely before their myelin sheaths ends (Cajal 1889a, referenced by Palay and Chan-Palay 1974).
The role of myelin in the synchronization of cerebellar pathways and the usefulness of that synchronization in vestibular processing need further study. It is clear though that the vestibular pathways within the cerebellum remain topographically distinct from parallel fiber paths and climbing fiber inputs in the turtle even though the same basic cortical structure and cellular morphology exist across the Cb. Due to its position in evolutionary tree, the turtle Cb may provide further insights into the common functions of Cb that receive different sensory afferents.
GRANTS
This study was supported by National Institute of Neurological Disorders and Stroke Grants NS-46499 (to M. Ariel) and NS-037475 (to J. Rosenbluth) and National Multiple Sclerosis Society Grant RG3618 (to J. Rosenbluth).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Amanda J. Allen for help with HRP staining, Dr. Rolf Schiff and Chris Petzold for help with sectioning and myelinated fiber counts, Dr. Jason Organ for statistical help, and Dr. Eric Lang for helpful discussions and comments on the manuscript.
REFERENCES
- Ariel M. Latencies of climbing fiber inputs to turtle cerebellar cortex. J Neurophysiol 93: 1042–1054, 2005 [DOI] [PubMed] [Google Scholar]
- Ariel M, Brown ME. Origin and timing of voltage-sensitive dye signals within layers of the turtle cerebellar cortex. Brain Res 1357: 26–40, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariel M, Fan TX. Electrophysiological evidence for a bisynaptic retinocerebellar pathway. J Neurophysiol 69: 1323–1330, 1993 [DOI] [PubMed] [Google Scholar]
- Ariel M, Ward KC, Tolbert DL. Topography of Purkinje cells and other calbindin-immunoreactive cells within adult and hatchling turtle cerebellum. Cerebellum 8: 463–476, 2009 [DOI] [PubMed] [Google Scholar]
- Baker BJ, Kosmidis EK, Vucinic D, Falk CX, Cohen LB, Djurisic M, Zecevic D. Imaging brain activity with voltage- and calcium-sensitive dyes. Cell Mol Neurobiol 25: 245–282, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangma GC, ten Donkelaar H. Afferent connections of the cerebellum in various types of reptiles. J Comp Neurol 207: 255–273, 1982 [DOI] [PubMed] [Google Scholar]
- Brown ME, Ariel M. Topography and response timing of intact cerebellum stained with absorbance voltage-sensitive dye. J Neurophysiol 101: 474–490, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AB, Hodos W. Comparative Vertebrate Neuroanatomy: Evolution and Adaptation. Hoboken, NJ: Wiley-Interscience, 2005 [Google Scholar]
- Devor A. Is the cerebellum like cerebellar-like structures? Brain Res Brain Res Rev 34: 149–156, 2000 [DOI] [PubMed] [Google Scholar]
- Ebner TJ, Chen G. Use of voltage-sensitive dyes and optical recordings in the central nervous system. Prog Neurobiol 46: 463–506, 1995 [DOI] [PubMed] [Google Scholar]
- Eccles JC, Llinas R, Sasaki K. Parallel fibre stimulation and the responses induced thereby in the Purkinje cells of the cerebellum. Exp Brain Res 1: 17–39, 1966 [DOI] [PubMed] [Google Scholar]
- Fan TX, Rosenberg AF, Ariel M. Visual-response properties of units in the turtle cerebellar granular layer in vitro. J Neurophysiol 69: 1314–1322, 1993 [DOI] [PubMed] [Google Scholar]
- Fan TX, Scudder C, Ariel M. Neuronal responses to turtle head rotation in vitro. J Neurobiol 33: 99–117, 1997 [PubMed] [Google Scholar]
- Hillman DE. Light and electron microscopical study of the relationships between the cerebellum and the vestibular organ of the frog. Exp Brain Res 9: 1–15, 1969 [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Leonard RB, Newlands SD, Perachio AA, Kevetter GA, Leonard RB, Newlands SD, Perachio AA. Central distribution of vestibular afferents that innervate the anterior or lateral semicircular canal in the mongolian gerbil. J Vestib Res 14: 1–15, 2004 [PubMed] [Google Scholar]
- Kistler WM, van Hemmen JL, De Zeeuw CI. Time window control: a model for cerebellar function based on synchronization, reverberation, and time slicing. Prog Brain Res 124: 275–297, 2000 [DOI] [PubMed] [Google Scholar]
- Kitazawa S, Wolpert DM. Rhythmicity, randomness and synchrony in climbing fiber signals. Trends Neurosci 28: 611–619, 2005 [DOI] [PubMed] [Google Scholar]
- Konnerth A, Obaid AL, Salzberg BM. Optical recording of electrical activity from parallel fibres and other cell types in skate cerebellar slices in vitro. J Physiol 393: 681–702, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte GE, Mugnaini E. The cerebellar projection of the vestibular nerve in the cat. J Comp Neurol 184: 265–277, 1979 [DOI] [PubMed] [Google Scholar]
- Kunzle H. Non-uniform projections of granule cells to the cerebellar molecular layer. An autoradiographic tracing study in a turtle. Anat Embryol (Berl) 175: 537–544, 1987 [DOI] [PubMed] [Google Scholar]
- Lang EJ, Rosenbluth J. Role of myelination in the development of a uniform olivocerebellar conduction time. J Neurophysiol 89: 2259–2270, 2003 [DOI] [PubMed] [Google Scholar]
- Larsell O. The Comparative Anatomy and Histology of the Cerebellum from Myxinoids Through Birds. Minneapolis, MN: University of Minnesota Press, 1967, p. 175–195 [Google Scholar]
- Llinas R. Neurobiology of Cerebellar Evolution and Development. Chicago, IL: American Medical Association, 1969 [Google Scholar]
- Llinas R, Precht W, Clarke M. Cerebellar Purkinje cell responses to physiological stimulation of the vestibular system in the frog. Exp Brain Res 13: 408–431, 1971 [DOI] [PubMed] [Google Scholar]
- Llinas R, Precht W, Kitai ST. Cerebellar purkinje cell projection to the peripheral vestibular organ in the frog. Science 158: 1328–1330, 1967a [DOI] [PubMed] [Google Scholar]
- Llinas R, Precht W, Kitai ST. Climbing fibre activation of Purkinje cell following primary vestibular afferent stimulation in the frog. Brain Res 6: 371–375, 1967b [DOI] [PubMed] [Google Scholar]
- Llinas RR. Inferior olive oscillation as the temporal basis for motricity and oscillatory reset as the basis for motor error correction. Neuroscience 162: 797–804 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Chanasue L, Ariel M. Neurogenesis in the turtle cerebellum. Soc Neurosci Abstr 32: 2008 [Google Scholar]
- Mugnaini E, Atluri RL, Houk JC. Fine structure of granular layer in turtle cerebellum with emphasis on large glomeruli. J Neurophysiol 37: 1–29, 1974 [DOI] [PubMed] [Google Scholar]
- Palay SL, Chan-Palay V. Cerebellar Cortex. New York: Springer, 1974 [Google Scholar]
- Precht W, Llinas R. Comparative aspects of the vestibular input to the cerebellum. In: Neurobiology of Cerebellar Evolution and Development: Proceedings of the First International Symposium of the Institute for Biomedical Research, edited by Llinas R. Chicago, IL: American Medical Association, 1969, p. 677–702 [Google Scholar]
- Precht W, Llinas R. Functional organization of the vestibular afferents to the cerebellar cortex of frog and cat. Exp Brain Res 9: 30–52, 1969b [DOI] [PubMed] [Google Scholar]
- Rosenberg AF, Ariel M. Visual-response properties of neurons in turtle basal optic nucleus in vitro. J Neurophysiol 63: 1033–1045, 1990 [DOI] [PubMed] [Google Scholar]
- Saltzberg BM, Grinvald A, Cohen LB, Davila HV, Ross WN. Optical recording of neuronal activity in an invertebrate central nervous system: Simultaneous monitoring of several neurons. J Neurophysiol 40: 1977 [DOI] [PubMed] [Google Scholar]
- Schwarz IE, Schwarz DW. The primary vestibular projection to the cerebellar cortex in the pigeon (Columba livia). J Comp Neurol 216: 438–444, 1983 [DOI] [PubMed] [Google Scholar]
- Shin SL, De Schutter E, Shin SL, De Schutter E. Dynamic synchronization of Purkinje cell simple spikes. J Neurophysiol 96: 3485–3491, 2006 [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Yoshida K. Neural pathways from the vestibular labyrinths to the flocculus in the cat. Exp Brain Res 22: 97–111, 1975 [DOI] [PubMed] [Google Scholar]
- Tolbert DL, Conoyer B, Ariel M. Quantitative analysis of granule cell axons and climbing fiber afferents in the turtle cerebellar cortex. Anat Embryol 209: 49–58, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voogd J, Glickstein M. The anatomy of the cerebellum. Trends Neurosci 21: 370–375, 1998 [DOI] [PubMed] [Google Scholar]
- Voogd J, Wylie DR. Functional and anatomical organization of floccular zones: a preserved feature in vertebrates. J Comp Neurol 470: 107–112, 2004 [DOI] [PubMed] [Google Scholar]
- Wu HS, Sugihara I, Shinoda Y. Projection patterns of single mossy fibers originating from the lateral reticular nucleus in the rat cerebellar cortex and nuclei. J Comp Neurol 411: 97–118, 1999 [DOI] [PubMed] [Google Scholar]
- Wu JY, Lam YW, Falk CX, Cohen LB, Fang J, Loew L, Prechtl JC, Kleinfeld D, Tsau Y. Voltage-sensitive dyes for monitoring multineuronal activity in the intact central nervous system. Histochem J 30: 169–187, 1998 [DOI] [PubMed] [Google Scholar]
- Wyatt KD, Tanapat P, Wang SSH. Speed limits in the cerebellum: constraints from myelinated and unmyelinated parallel fibers. Eur J Neurosci 21: 2285–2290, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Liu T, Ashe J, Bushara KO. Role of the olivo-crebellar sstem in timing. J Neurosci 26: 5990–5995, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Fukuda M, Llinas R. Bilaterally synchronous complex spike Purkinje cell activity in the mammalian cerebellum. Eur J Neurosci 13: 327–339, 2001 [DOI] [PubMed] [Google Scholar]
- Yarom Y, Cohen D. The olivocerebellar system as a generator of temporal patterns. Ann NY Acad Sci 978: 122–134, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.