Abstract
One of the critical factors in determining network behavior of neurons is the influence of local circuit connections among interneurons. The short-term synaptic plasticity and the subtype of presynaptic calcium channels used at local circuit connections of inhibitory interneurons in CA1 were investigated using dual whole-cell recordings combined with biocytin and double immunofluorescence labeling in acute slices of P18- to 21-day-old rat stratum radiatum (SR) and stratum lacunosum moleculare (SLM). Two forms of temporally distinct synaptic facilitation were observed among interneuron connections involving presynaptic cholecystokinin (CCK)-positive cells in SR, frequency-dependent facilitation, and a delayed onset of release (45–80 ms) with subsequent facilitation (DORF). Inhibition at both these synapses was under tonic cannabinoid-type 1 (CB1) receptor activity. DORF synapses did not display conventional release-dependent properties; however, blocking CB1 receptors with antagonist AM-251 (10 μM) altered the synaptic transmission to frequency-dependent depression with a fast onset of release (2–4 ms). Presynaptic CCK-negative interneurons in SLM elicited inhibitory postsynaptic potentials (IPSPs) insensitive to CB1 receptor pharmacology displayed frequency-dependent depression. Release of GABA at facilitating synapses was solely mediated via N-type presynaptic calcium channels, whereas depressing synapses utilized P/Q-type channels. These data reveal two distinct models of neurotransmitter release patterns among interneuron circuits that correlate with the subtype of presynaptic calcium channel. These data suggest that endocannabinoids act via CB1 receptors to selectively modulate N-type calcium channels to alter signal transmission.
Keywords: cannabinoid type 1 receptors, cholecystokinin, short-term plasticity
gabaergic interneurons are important in generating rhythmic activity by creating regular and temporally coordinated spike timing and synaptic integration in populations of neurons (Mann et al. 2005; Miles et al. 1996). The diverse subclasses of interneurons are characterized by criteria such as neurochemical marker expression, dendritic and axonal morphology, and electrophysiological properties (Somogyi and Klausberger 2005; Freund and Buzsaki 1996). Anatomical studies reveal the existence of various subclasses of interneurons in stratum radiatum (SR) and stratum lacunosum moleculare (SLM) of CA1 (Somogyi and Klausberger 2005; Freund and Buzsaki 1996). Although these two subregions are rich in different subclasses of interneurons, the physiology and pharmacological properties of the connections among these various subclasses of interneurons need further investigation.
Physiologically, it has been shown that subclasses of interneurons in stratum pyramidale (SP) and stratum oriens contribute differentially to network oscillations at distinct frequencies in vivo (Tukker et al. 2009; Klausberger et al. 2003) and interestingly the same class of interneurons behave differently during different network oscillations (Klausberger et al. 2005). This suggests that there are important factors that control the timing of interneuron firing, and the existing knowledge of the patterns of transmission across excitatory synapses onto interneurons and the pattern of presynaptic activity is not enough to explain this behavior of subclasses of interneurons during specific network oscillations. The influence of local circuit connections among interneurons may be a critical factor in determining network behavior due to the diversity of the neuromodulators subclasses of interneurons contain. For example, cannabinoid type-1 (CB1) receptors are powerful modulators, often found presynaptically on presynaptic cholecystokinin (CCK) interneurons (Hajos et al. 2000; Katona et al. 1999). Recently it has been reported that communication among subclasses of CCK interneurons in SR and SLM of CA1 is selectively modulated via CB1 receptors, which disrupt the synchronicity of synaptic release at these connections (Ali and Todorova 2010). These CB1 receptor-sensitive synapses also showed synaptic facilitation, which was in contrast with synaptic depression typically observed at hippocampal interneuron to pyramidal cells and other SP interneurons reported previously (Ali et al. 1998; Ali et al. 1999). Whether synaptic facilitation is a property of synapses between CCK, CB1 receptor sensitive interneurons only needs further investigation.
There are a variety of processes that may lead to a decrease in synaptic strength resulting in depression or an enhancement of synaptic strength leading to facilitatory processes. These processes are thought to involve both pre- and postsynaptic properties (Heine et al. 2008; Thomson and Bannister 1999; Dobrunz and Stevens 1997), including several types of presynaptic calcium channels and G protein modulation of calcium channels. Calcium channels mediating release of neurotransmitter at central synapses includes N-, P/Q-, and R-types high voltage-activated channels. Evidence suggests that specific calcium channels seem to be selectively associated with specific types of synaptic connections; excitatory facilitating synapses predominantly utilize N-type, whereas depressing synapses utilize P/Q-type channels (Ali and Nelson 2006). Others have shown that the release of GABA involve N-type calcium channels at one synapse and P/Q-type mediating release at others (Zaitsev et al. 2007; Hefft and Jonas 2005; Wilson et al. 2001; Poncer et al. 1997).
The aim of this study was to investigate the short-term plasticity and the relationship between subtypes of presynaptic calcium channel with specific release patterns of identified CCK-positive and CCK-negative interneurons that target other interneurons in SR and SLM of CA1 using paired whole-cell recordings combined with biocytin and double immunofluorescence labeling.
METHODS
Slice preparation.
Male Wistar rats (postnatal day, P18–21) were anesthetized by an intraperitoneal injection of pentobarbitone sodium (60 mg kg−1; Euthatal, Merial, UK) and perfused transcardially with 50–100 ml ice-cold modified artificial cerebrospinal fluid (ACSF). This modified ACSF contained (in mM): 248 sucrose, 25.5 NaHCO3, 3.3 KCl, 1.2 KH2PO4, 1.0 MgSO4, 2.5 CaCl2, and 15 D-glucose, equilibrated with 95% O2-5% CO2. The animals were then decapitated, and the brain was removed. These procedures were approved by local ethical committee and the British Home Office.
Coronal sections of hippocampus, 300–330 μm thick, were cut using a vibratome (Leica, Germany). The slices were incubated for 1 h in standard ACSF containing (in mM): 121 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2, 26 NaHCO3, and 20 glucose and equilibrated with 95% O2-5% CO2. For recordings, slices were transferred to a submerged-style chamber and perfused at 1 to 2 ml/min with the standard aCSF.
Paired recordings.
Simultaneous dual whole-cell somatic recordings were made in current clamp between electrophysiologically identified CA1 SR and SLM interneurons. Cells of each recorded pair were visually selected using video microscopy under near-infrared differential interference contrast illumination. Interneurons were selected with round or oval somata and further characterized from their firing properties. Experiments were conducted at room temperature (20–22°C) with patch pipettes (resistance, 8–10 MΩ) pulled from borosilicate glass tubing and filled with an internal solution containing (in mM): 144 K-gluconate, 0.2 EGTA, 10 HEPES, 2 Na2-ATP, 0.2 Na2-GTP, and 0.02% wt/vol of biocytin (pH 7.2–7.4, 300 mOsm).
Singles and/or pairs (with an interspike interval of 50 ms) of presynaptic action potentials were elicited by injecting short (5–10 ms) pulses of depolarizing current into the presynaptic interneuron, repeated at 0.33 Hz (SEC 05L/H; npi electronics). Trains of presynaptic action potentials were elicited by injecting longer depolarizing pulses of 200 ms in presynaptic cells. Postsynaptic responses recorded in interneurons were amplified, low-pass filtered at 2 kHz, digitized at 5 KHz using a CED 1401 interface, and recorded on disc for offline analysis.
Drugs
Pharmacology was performed on IPSPs elicited by single presynaptic action potentials that were above 0.3 mV in average peak amplitude in order for the effect of the drug to be seen easily.
Data were collected in the presence of bath applied, CNQX and D-AP5 (50 μM), MCPG (1 mM), CPPG (100 μM) and CPG55845 (100 μM), naloxone (100 μM) to block AMPA/kainate, NMDA, mGluR, GABAB, and opioid receptors (all from Tocris, Bioscience).
The endogenous CB receptor agonist anandamide (in water soluble emulsion) (14 μM) and CB1 receptor inverse agonist/antagonists AM-251 (5 μM) (Tocris) were used to study the CB1 receptor pharmacology of IPSPs. Calcium channel blocker, ω-conotoxin GVIa (1 μM), and ω-agatoxin IVa (0.5 μM) were from Sigma.
Data analysis.
Data were acquired and analyzed using Signal software (Cambridge Electronic Design, Cambridge, UK) and in-house software. Individual sweeps were observed and either accepted, edited, or rejected according to the trigger points that would trigger measurements and averaging of the IPSPs during subsequent data analysis. IPSPs were selected within a narrow window of membrane potential of ±0.5 mV, and averaging was triggered from the rising phase of the presynaptic spike. The IPSP 10%-90% rise times and width at half amplitude were measured from averaged IPSPs. Single sweep IPSP amplitudes were measured from the baseline to the peak of the IPSP. Average IPSP amplitudes are given as means ± SD of measurements of 50–250 single sweeps. The paired-pulse ratio (PPR) was measured as the amplitude of the second response to that of the first (fixed time interval of 50 ms).
Apparent failures of synaptic transmission were identified by eye and confirmed when averaging of these apparent failures resulted in no measurable postsynaptic response. IPSP latencies were manually measured as the time delay between presynaptic action potential peaks to the onset of the detectable IPSPs. The fluctuations in the IPSP latencies were quantified in nonoverlapping time interval sets of 5 ms after each presynaptic action potential. The onset of IPSP from the first action potential is the time taken for the first IPSP to appear in a train of action potentials from the first action potential elicited.
The electrophysiological characteristics of the recorded cells were measured from their voltage responses to 500-ms current pulses between −0.2 and +0.1 nA in amplitude.
To determine whether the electrophysiological characteristics of the three groups of presynaptic interneurons studied differed, measurements of action potential (AP) characteristics, input resistance, and membrane time constants were compared (1-way ANOVA followed by pair-wise Student's unpaired t-test to determine which class differed). To determine whether a drug had a significant effect on a given population of IPSPs, the mean IPSP amplitudes obtained under control and under drug conditions were compared (Student's paired t-test).
Morphology.
Slices containing biocytin-filled cells were fixed overnight in 4% paraformaldehyde plus 0.2% saturated picric acid solution in 0.1 M phosphate buffer (PB), pH 7.2, at 4°C for immunofluorescence or in 1.25% glutaraldehyde and 2.5% paraformaldehyde in 0.1 M PB for standard Avidin-HRP-DAB processing (see below). Extensive rinses were carried out between each step using PBS (0.1 M). The sections were freeze-thawed over liquid nitrogen after cryoprotecting for 2 × 10 min in 10% sucrose, 2 × 20 min in 20% sucrose with 6% glycerol, and 2 × 30 min in 30% sucrose and 12% glycerol. The sections then followed either a double immunofluorescence (shorter recordings of 10–40 min) or a standard biocytin labeling protocol for longer recording (40–90 min, for a detailed protocols see Hughes et al. 2000).
Double immunofluorescence.
After rapid freeze-thawing, the sections were washed in PB and then incubated in 1% sodium borohydride (NaBH4) solution in 0.1 PB for 30 min. After further washes in PB to remove NaBH4, slices were incubated in normal blocking serum diluted in 0.1 M PBS for 30 min. Both fluorescent-tagged secondary antibodies used were raised in goat, 10% normal goat serum (Sigma). The cells recorded in this study were incubated in antibodies to vasoactive intestinal peptide (VIP) and CCK. These antibodies were raised against different antigenic targets, e.g., rabbit anti-VIP (B 34-1, source, Euro-Diagnostica B V, NETH, 1:1,000 dilutions) and mouse anti-gastrin/CCK (gift, source, Cure antibody laboratory, UCLA, 1:2,000 dilution). Primary antibody mixtures were made up with 3 mg BSA (Sigma) per milliliters of diluents containing peroxidase-labeled ABC Elite (ABC-peroxidase; Vector Laboratories) made in PBS and incubated overnight. The sections were washed in PBS before incubating in secondary antibodies and avidin macromolecules tagged with different fluorescent markers. This cocktail contained goat anti-rabbit IgG (diluted to 1:600; Molecular Probes) conjugated to Texas red, goat anti-mouse IgG (diluted 1:160; Sigma) conjugated to FITC and avidin conjugated to AMCA (1:250, Vector Laboratories). After 3 h of incubation at room temperature, the sections were washed in PBS and mounted onto glass slides in 50% glycerol in PBS and cover slipped. The sections were then examined for florescent labeling using a Leica DMR microscope with appropriate filter blocks to visualize FITC, Texas red, and AMCA, respectively, at ×40 magnification (see Hughes et al. 2000, for further details). After immunohistochemical detections the sections were processed to reveal the biocytin-labeled cells as described below.
Biocytin labeling.
The sections were incubated overnight in Vector ABC-peroxidase (1:200) at 4°C. The peroxidase group was revealed using 3′3-DAB as the chromogen (Vector DAB kit). The visualized cells were intensified with 0.1% osmium tetroxide, and the sections were cleared by dehydration in an ascending series of alcohols to 100% and then embedded in Durcupan resin (Agar Scientific). The cells were reconstructed using a Zeiss Axioskop with attached camera lucida. Morphometric analysis was performed manually from two-dimensional reconstructions of neurons.
The classification of interneurons was based on electrophysiology, neurochemistry, and on gross morphology (Freund and Buzsaki 1996; Somogyi and Klausberger 2005).
RESULTS
Paired whole-cell recordings were performed between two interneurons in SR and between interneurons in SR and SLM. The postsynaptic membrane potentials were in the range of −53 and −55 mV, and all recordings were somatic.
Anatomical and electrophysiology properties of interneurons in SR and SLM.
Interneurons that were CCK positive and VIP negative revealed by double immunofluorescence include Shaffer collateral associated (SCA) and lacunosum-moleculare-Schaffer collateral associated (LM-SCA) interneurons. Presynaptic interneurons, which were CCK- and VIP negative, resembled quadrilaminar cells. Table 1 shows morphological and electrophysiological properties of the three subtypes of interneurons studied. All three subtypes of interneurons studied displayed trains of action potentials with frequency adaptation and accommodation. Action potential widths were similar; however, time constants and input resistance increased with increasing length of basal dendrites from soma to termination.
Table 1.
Electrophysiological and morphological features of presynaptic interneurons in CA1
| Action Potential Properties |
Input Resistance, MΩ |
|||||||
|---|---|---|---|---|---|---|---|---|
| Presynaptic Cell | n | Distance of Basal Dendrites From Soma at Termination, μm | Location of Somata and Axonal Center | Spike HW, ms | AHP amplitude, mV | AHP HW, ms | Time Constant, ms | |
| LM-SCA | 9 | 100 ± 34* | Soma-SR/SLM border | 1.7 ± 0.38† | −4.5 ± 2.0* | 6.5 ± 2.0† | (Ih) | 18 ± 4.3* |
| Axon-SR | Start(Sag): 258 ± 109* | |||||||
| End: 139 ± 99* | ||||||||
| SCA | 12 | 308 ± 74 | Soma- SR | 1.6 ± 0.80 | −9.5 ± 2.60 | 6.5 ± 1.5 | Linear I/V | 30 ± 4.5 |
| Axon-SR | 352 ± 56 | |||||||
| Quadrilaminar | 6 | 510 ± 60* | Soma-SLM | 1.7 ± 0.40† | −9.3 ± 1.5† | 3.5 ± 1.5* | Linear I/V | 38 ± 2.8 |
| Axon-all layers and back-projecting to DG | 423 ± 87* | |||||||
Values are means ± SD. AHP, after hyperpolarization; HW, half width; LM-SCA, lacunosum-moleculare-Schaffer collateral associated; SR, stratum radiatum; SLM, stratum lacunosum moleculare; DG, dentate gyrus; I/V, current-voltage relation.
Significantly different from the other 2 subclasses of interneurons, 1-way ANOVA, P = <0.05;
not significantly different from SCA interneurons, 1-way ANOVA, P > 0.5.
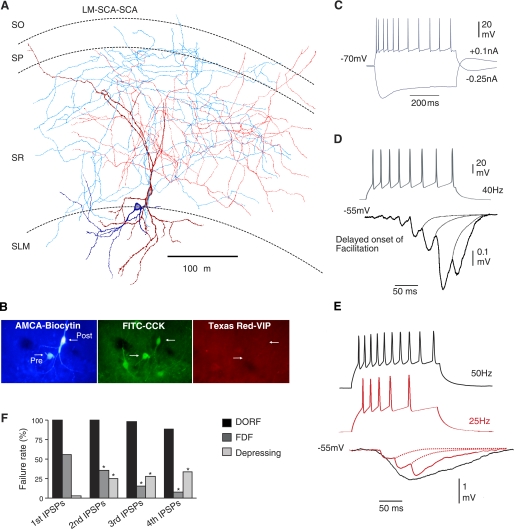
LM-SCA cells were presynaptic to other CCK-positive and VIP-negative interneurons (n = 9). These interneurons have not been described previously, and the name was given here because these interneurons had multipolar oval/round somata located in the border of radiatum and lacunosum moleculare with basal dendrites in conjunction with Schaffer collaterals. The smooth dendrites did not have a wide span and were often horizontal in orientation. The axonal arbor projected from basal dendrites and was centered in radiatum forming a dense plexus in stratum radiatum, with a few branches projecting to pyramidale and oriens. Figures 1, A and B, and 2A illustrate LM-SCA interneurons, which were connected to postsynaptic CCK-positive cells. These presynaptic interneurons often targeted SCA interneuron (Fig. 1A, n = 7) and postsynaptic basket cells (Fig. 2A, n = 2).
Fig. 1.
Temporally distinct synaptic facilitation that is delayed on the onset of release. A: reconstruction of a synaptically connected stratum lacunosum moleculare (SLM) interneuron, lacunosum moleculare-Shaffer collateral associated cell (LM-SCA; blue-soma, light blue-axon), and a stratum radiatum (SR), Shaffer collateral associated cell (SCA; deep red- dendrites, red-axon). B: double immunoflorescence labeling revealed the two recorded cells filled with biocytin (AMCA) immunopositive for CCK (FITC) and immunonegative for VIP (Texas red). C: intrinsic membrane properties of the presynaptic LM-SCA cell. In response to hyperpolarizing current injection this interneuron displayed pronounced sag, indicative of Ih current. In response to depolarizing current fast but adapting firing patterns were displayed. D: IPSPs elicited by LM-SCA cells displayed a delayed onset of synaptic facilitation (DORF). IPSPs were usually observed after third to fifth presynaptic spike in a train. E: another synaptic connection between a LM-SCA cell and SCA cell; the onset of first IPSP did not appear earlier in the train when increasing the firing frequency of the presynaptic interneuron from 25 Hz to 50 Hz. At higher presynaptic firing frequencies the IPSPs summated more readily, shown by the black trace. F: comparison of average failure rates of 1st, 2nd, 3rd and 4th IPSPs in control at DORF, frequency dependent facilitating (FDF), and depressing synapses studied. SP, stratum pyramidale, SO, stratum oriens.
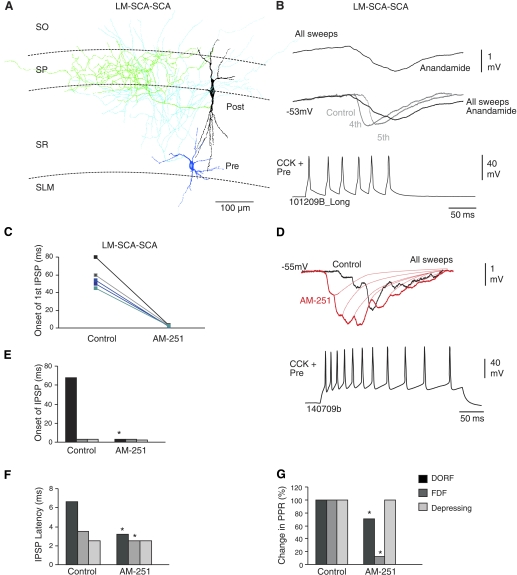
Fig. 2.
Temporary release-independence via CB1 receptors at synapses displaying DORF. A: reconstruction of a LM-SCA and a basket cell (black soma, green axon). B: bath application of 14 μM anandamide (CB receptor agonist) enhanced the facilitation. C: the change in the onset of release of GABA in control and in AM-251; data for individual pairs are shown (n = 5). D: the delayed release and suppression of inhibition was blocked by 10 μM CB1 receptor antagonist, AM-251, changing the synaptic efficacy and the onset of the IPSPs, which appeared earlier during the train of action potentials elicited. E and F: bar graphs comparing the onset of first IPSPs during a train of presynaptic action potentials and IPSP latency at DORF, FDF, and depressing synapse in control and after bath application of AM-251. Onset of the first IPSP seem to be delayed at connections between LM-SCA and SCA interneurons. G: comparison of change in paired-pulse ratios (PPRs) in control and AM-251. *Significantly different than control first IPSPs.
Electrophysiologically, these interneurons displayed a pronounced sag in response to hyperpolarizing current injection, indicative of Ih current (Fig. 1C). This sag is indicated by the higher input resistance at the start of the current pulse injected (see Table 1). This current was previously identified as containing a hyperpolarization-activated cyclic nucleotide-sensitive channel, a member of the voltage-gated potassium channel family permeable to both sodium and potassium ions (Lüthi and McCormick 1998). The Ih current was activated at hyperpolarizing currents; this is an inward current shifting the membrane potential to more positive values and also resulting in rebound depolarization (see also Lüthi and McCormick 1998; Ingram and Williams 1996). The input resistance measured at the peak of the voltage response to a −0.1 nA, hyperpolarizing current pulse delivered from a membrane potential of −70 mV was 258 ± 109 MΩ, and at the end of the 500 ms current pulse the input resistance was 139 ± 99 MΩ. The time constant for these cells was faster than the other three subtypes of interneurons studied (18 ± 4.3 ms; see Table 1).
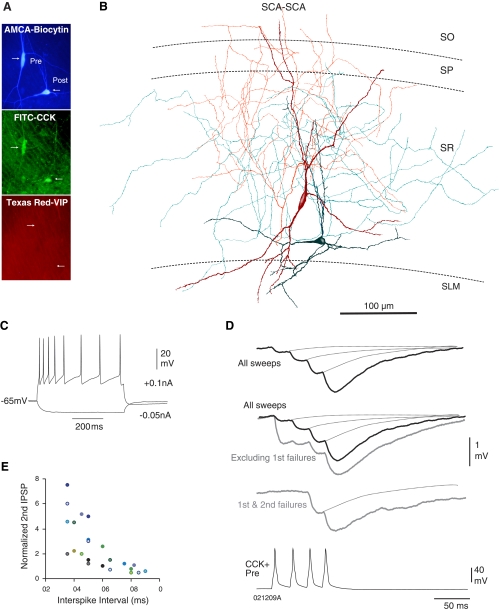
SCA interneurons (described previously; see Somogyi and Klausberger 2005) frequently made connections with each other and were postsynaptic to LM-SCA cells. The SCA interneurons in this study had irregular multipolar somata located in stratum radiatum. Their dendrites projected radially in all directions with main branches projecting to the oriens. The axons of SCA interneurons originated from the somata or a main dendritic branch close to the soma and ramified predominantly in SR with a few branches entering SP and oriens (n = 36). Figure 3, A and B, illustrates two SCA interneurons, which were synaptically connected, and an example of their intrinsic membrane properties is shown in Fig. 3C.
Fig. 3.
Synaptic facilitation between 2 CCK-positive, SCA interneurons is release dependent. A: immunofluorescence labeling showing both recorded cells as CCK positive and VIP negative. B: reconstruction of the biocytin-labeled cells shown in A, which displayed synaptic facilitation shown in D. C: firing properties of the presynaptic SCA interneuron (shown in red below) recorded in response to −0.05 and 0.1 nA current injection. SCA cells typically displayed adapting properties (see Table 1). D: averaged responses to trains of presynaptic action potentials (black traces include all sweeps in average; gray traces include specific subsets of data). These connections typically displayed synaptic facilitation, which was greater after excluding first and second IPSP failures or only first IPSP failures. Excluding first IPSP failures resulted in larger first and smaller second IPSPs (gray traces). E: graph to illustrate the recovery of second IPSP from synaptic facilitation at longer interspike intervals (each color represents an individual pair of connection, n = 12).
CCK-negative, quadrilaminar cells in SLM.
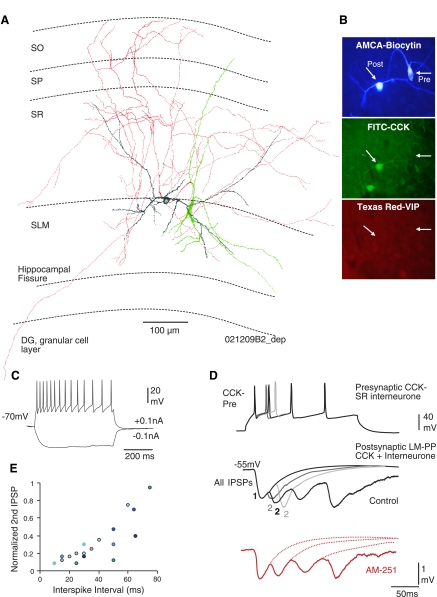
The name was given here because the axons of these cells crossed subregions of the hippocampus. Quadrilaminar cells located in SLM often targeted other CCK-positive interneurons in the SR/SLM border. These cells were CCK negative, and their multipolar, fusiform somata were located in SLM with the widest dendritic expansion (see Table 1). The dendritic fields expanded to all layers in CA1, sometimes terminating in the alveus. The axons originated from proximal dendrites or somata and innervated SR and SLM that extended to the dentate gyrus, a few axonal branches projected into SLM toward the CA3 subregion, passing the hippocampul fissures and innervating dentate gyrus granular cells (n = 6). Figure 4, A and B, illustrates an example of the presynaptic quadrilaminar cells studied. Electrophysiologically, these cells had the highest input resistance (510 ± 60 MΩ) and slowest time constant (38 ± 2.8 ms) compared with the other three subtypes of interneurons. Figure 4C illustrates an example of the intrinsic membrane properties of a quadrilaminar cell in SLM.
Fig. 4.
CCK-negative, long-range quadrilaminar cells display synaptic depression. A: reconstruction of a synaptically connected CCK-negative quadrilaminar cell (green soma, red axon) to a LM-SCA cell (gray). B: double immunoflorescence labeling reveals the 2 recorded cells filled with biocytin (AMCA) immunonegative for VIP (Texas red) and only the postsynaptic cell was immunopositive for CCK (FITC). C: intrinsic membrane properties of the presynaptic back projecting cell shown in A and B. D: this synapse displayed release of neurotransmitter at short latencies of 2.5 ms with precision. IPSPs elicited by a train of action potentials displayed synaptic depression. The gray traces are superimposed averages of second IPSPs at different spike intervals; the shorter the spike interval, the stronger the depression (illustrated by smaller IPSPs). These IPSPs were insensitive to AM-251 (red trace). E: graph to illustrate the recovery from synaptic depression at longer inter spike intervals (each color represents an individual pair, n = 8).
Short-term plasticity among interneuron connections.
Synaptic connections were subdivided according to the pattern of neurotransmitter release in control conditions; short-term synaptic plasticity observed was correlated with the presynaptic interneuron subtypes. From 32 synaptically connected pairs, three distinct short-term plasticity were observed: a delayed onset of release with subsequent facilitation (n = 9), paired pulse and brief train facilitation (n = 12), and paired pulse and brief train depression (n = 11). These synaptic release patterns were presynaptic interneuron dependent, and Table 2 summarizes the characteristics of these IPSPs.
Table 2.
Properties of IPSPs and short-term plasticity among interneuron-interneuron synapses in CA1
| First IPSP properties |
AM-251 | Calcium Channel Type |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Morphology of Synapse | Probability of Connection | Short-term Plasticity | n | Onset of IPSP from first AP, ms | Latency, ms | 1 s IPSP Amp., mV | 10–90% RT, mS | HW, ms | PPR, ms | Peak Amp., % | PPR change | First IPSP peak Apm. in toxin, % of control |
| CCK+ LM-SCA to SCA or BC | 1:5 | Delayed onset of facilitation | 9 | 68.6 ± 20† (cf. *3.3 ± 2 ms in AM-251) | 6.6 ± 3.3† | 0.13 ± 0.2†, on third AP | 7.4 ± 2.4† | 38 ± 2.7† | 2.3 ± 0.8† | 450 ± 120*†↑ | 0.7 ± 0.5*†↑ | ω-Conotoxin N-type 2 ± 0.2 |
| CCK+ SCA to SCA cell | 1:2 | Frequency-dependent facilitation | 12 | 3.2 ± 0.4 | 3.5 ± 1.7 | 0.6 ± 0.3 | 7.5 ± 1.9 | 40 ± 5.6 | 1.6 ± 1.1 | 200 ± 80*↑ | 1.4 ± 0.5↓ | ω-Conotoxin N-type 1 ± 0.4 |
| CCK- quadri- laminar to LM-SCA or LM-SCA cell | 1:4 | Frequency-dependent depression | 11 | 2.7 ± 0.3 | 2.5 ± 0.5 | 0.92 ± 0.5 | 5.6 ± 1.6 | 24 ± 5.5 | 0.48 ± 0.1 | No change | No change | ω-Agatoxin P/Q-type 2 ± 0.3 |
Values are means ± SD. For calcium channel type, n = 3; in AM-251 peak amplitude (Amp.), n = 5 for cholecystokinin (CCK) + LM-SCA to SCA or basket cell (BC) and CCK + SCA to SCA cell and n = 4 for CCK- quadri- laminar to LM-SCA or LM-SCA cell. RT, rise time; PPR, paired pulse ratio (2nd IPSP Amp./1st IPSP Amp.).
Significantly different from control, unpaired t-test, P = <0.05;
significantly different from frequency depressing synapses, paired t-test, P = <0.05.
Temporally distinct synaptic facilitation: delayed onset of facilitation.
The probability of finding a connection with a presynaptic LM-SCA cell was 1:5 pairs of interneurons tested. Synaptic connections involving presynaptic LM-SCA interneurons with characteristic Ih currents elicited IPSPs onto SCA cells (n = 7) and SP basket cells (n = 2) with a different form of facilitation that was delayed (DORF) on the onset of release of neurotransmitter GABA.
IPSPs elicited by trains of presynaptic action potentials resulting in enhanced inhibition were defined as facilitation. The average traces include all sweeps to include synaptic failures and only where there was no detectable first spike response in that average was taken as a failure. Single or double presynaptic action potentials could not elicit IPSPs. Trains of actions potentials at firing frequencies in the range of 25–50 Hz repeated at 0.33 Hz elicited a response resulting in facilitating IPSPs (Fig. 1, D and E; see Table 2). Even following total apparent failures of transmission after average first, second, third, or sometimes fourth IPSPs, the first small event was delayed in the range of 45–80 ms (Fig. 1F and Fig. 2, C and E). This delayed release observed in some postsynaptic interneurons was similar to CA3 mossy-fiber associated interneuron to pyramidal cells, which were activated at 50 Hz (Losonczy et al. 2004). The synapses studied here did not require as high a frequency to be activated. In contrast with Losonczy et al. 2004, DORF synapses reported here displayed very little frequency-dependency; increasing presynaptic firing frequency from 25 Hz to 50 Hz did not alter the appearance of the first IPSP in the postsynaptic interneurons. Figure 1E illustrates that IPSPs were not elicited by earlier action potentials in the train during higher firing frequency of the presynaptic LM-SCA cells.
The second IPSPs in the train were on average larger than first IPSPs by 150 ± 20% (n = 9) at all spike intervals, and third IPSPs in the train were larger than the second IPSPs by 200 ± 23% (n = 9).
These IPSPs were not sensitive to somatic voltage changes, suggesting that the inputs may be a distance away from the soma. On average 2–5 close apposition of presynaptic boutons on postsynaptic dendrites were observed at the light microscope level. In alignment with this observation IPSP rise times and widths at half amplitudes were slower than IPSPs, which showed synaptic depression (see Table 2).
Interestingly (and in contrast with the synapses studied by Losonczy et al. 2004), these synapses showed a significant amount of jitter in their latencies, which were on average longer than the other two types of connections studied here (Fig. 2F). Asynchronous release of GABA at interneuron-interneuron connections in SR/SLM of CA1 was correlated with the tonic suppression of inhibition via CB1 receptors reported as previously (Ali and Todorova 2010).
CB1 receptors allow release-independence at DORF synapses.
To determine whether endocannabinoids played a role in causing the low probability of release and subsequent facilitation at DORF synapses, the CB receptor agonist anandamide (14 μM) and CB1 selective antagonist AM-251 (10 μM) were bath applied. At synapses displaying DORF, anandamide occluded release of neurotransmitter and enhanced the delay of facilitation (decrease of 5–15% of control IPSPs, n = 3) shown in Fig. 2B. Interestingly, at DORF synapses bath application of AM-251 increased IPSP amplitudes (increase of 100–500% of control IPSPs, n = 5); the appearance of the IPSPs shifted to the first action potentials elicited, which changed the onset of release of GABA resulting in an IPSP (Fig. 2, C–E). The onset of release changed to 3.3 ± 2 ms (from first action potential to onset of first IPSP), suggesting a dramatic change in synaptic efficacy to a high probability of release when blocking tonic CB1 receptors. In AM-251, the average second and third IPSP amplitudes were between 60% and 40% of the first IPSP amplitudes and the third IPSP amplitudes were between 15% and 35% of the second IPSP amplitudes (n = 5).
In AM-251 the IPSP latency and the PPR ratio decreased (due to a decreased failure rate of synaptic transmission) at DORF synapses in comparison with frequency dependent facilitation (FDF) synapses (see Table 2 and Fig. 2G).
Frequency dependent facilitation.
The probability of finding a connection between two CCK-positive SCA cells was 1:2 pairs of interneurons tested. SCA-SCA interneuron connections always displayed FDF; the second IPSP amplitude was inversely related to the first IPSP amplitude at short interspike intervals, and on average second IPSPs were 50–200% larger than first IPSPs (n = 12). At connections where paired pulse and brief train facilitation dominated, facilitation was greatest at short spike intervals of 10–30 ms. This facilitation gradually declined at longer spike intervals of 50–70 ms (Fig. 3, D and E). CB1 receptor pharmacology at SCA-SCA has been reported previously (Ali and Todorova 2010; Ali 2007); in brief at SCA-SCA connections anandamide reduced the amplitude of average IPSPs by ∼50 ± 5.6% of control and increased the number of apparent failures of transmission and bath application of AM-251 increased IPSP amplitudes, suggesting a tonic inhibition via CB1 receptors at these synapses.
Synaptic depression elicited by CCK-negative interneuron in SLM.
The probability of finding a connection with a CCK-negative quadrilaminar cell with another interneuron was 1:4 interneuron pairs tested. Quadrilaminar cells presynaptic to CCK-positive LM-SCA (n − 3) or SCA (n = 8) cells elicited short latency IPSPs with precision and displayed a high probability of release resulting in synaptic depression. These IPSPs had fast rise times and widths at half amplitude (see Table 2) and were sensitive to somatic voltage changes, suggesting proximal contacts of release sites. At the light microscope level, three to five close appositions of presynaptic axon on postsynaptic soma or proximal dendrites were seen. The average second IPSPs were between 10% and 23% of the first, and third IPSPs were between 12% and 25% of second IPSPs. Synaptic depression observed at these connections recovered following spikes elicited at longer intervals; the decay of depression was around 50–70 ms (Fig. 4E). Following larger first IPSPs, release-dependent depression was apparent shown in Fig. 4D. At connections among SP baskets cells, similar type of synaptic depression of IPSPs was reported (Ali et al. 1999). These IPSPs were insensitive to bath application of AM-251, consistent with the observation that these cells were CCK negative (Fig. 4D).
Presynaptic calcium channel subtype is correlated with short-term synaptic plasticity.
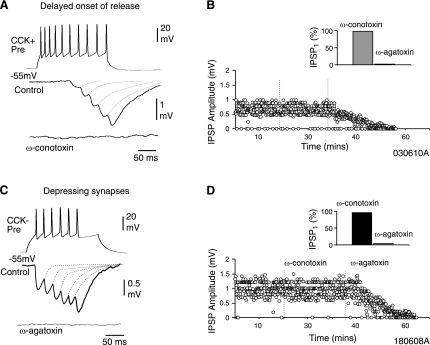
Evidence suggests that specific calcium channels are selectively associated with specific types of synaptic connections (Poncer et al. 1997; Ali and Nelson 2006). It is unknown whether the same type of presynaptic calcium channels type is associated with specific short-term dynamics at interneuron-interneuron connections. Furthermore, to investigate whether the activation of specific presynaptic calcium channels is involved at synapses associated with presynaptic CB1 receptors, the effects of ω-conotoxin GVIa (1 μM), a selective N-type channel blocker was studied. In CCK-positive interneuron-interneuron connections that displayed FDF or DORF, the N-type blocker completely abolished the IPSPs (paired t-test; P = <0.05, n = 6; Fig. 5, A and B). ω-Agatoxin IVa (0.5 μM), a selective P/Q type calcium channels blocker, had no effect at facilitating inhibitory synapses; however, at depressing synapses involving CCK-negative presynaptic interneurons ω-agatoxin IVa completly abolished the IPSPs (paired t-test; P = <0.05, n = 3; Fig. 5, C and D). This suggests that synapses that display synaptic facilitation and utilize presynaptic CB1 receptors involve N-type presynaptic calcium channels, whereas P/Q-type calcium are involved at depressing synapses that were insensitive to CB1 receptor pharmacology. Table 2 summarizes the change in IPSPs in bath application of the toxins for the different types of short-term plasticity studied.
Fig. 5.
Different calcium channels mediate synaptic transmission at depressing, CB1-insensitive, and facilitating, CB1-sensitive synapses. A: ω-conotoxin GVIa (1 μM) blocks transmission at CCK-positive presynaptic interneurons that demonstrate a delayed onset of release and synaptic facilitation, suggesting the involvement of N-type calcium channels. Facilitating synapses sensitive to CB1 receptor pharmacology were not sensitive to ω-agatoxin Iva. B: plot of peak amplitudes of second IPSPs during an experiment in control and in bath application of calcium channel blockers. B, Inset: summary of normalized unitary IPSP amplitudes in ω-agatoxin and ω-conotoxin (n = 4). C: presynaptic CCK-negative interneurons elicited depressing IPSPs and was insensitive to ω-conotoxin GVIa (1 μM), but transmission was blocked by ω-agatoxin IVa (0.5 μM), suggesting the involvement of P/Q-type calcium channels. D: plot of peak amplitudes of first IPSPs during an experiment in control and in bath application of calcium channel blockers. D, Inset: summary of normalized unitary IPSP amplitudes in ω-conotoxin and ω-agatoxin (n = 3).
DISCUSSION
The time-dependent release patterns of neurotransmitter reveal information about how synapses may be activated during specific brain states. A target-dependent synaptic plasticity is well documented at excitatory synapses (Ali et al. 1998; Markram et al. 1998; Reyes et al. 1998). Here I report the release patterns at inhibitory synapses involving interneurons only. The short-term synaptic plasticity observed (FDF, DORF, and synaptic depression) correlates with the class of presynaptic interneuron and the subtype of presynaptic calcium channel.
Variations of synaptic facilitation among local circuit interneurons were confined to SR, and interestingly, unlike previously well-documented forms of presynaptic facilitation, presynaptic cells with a prominent Ih current correlated with a delayed onset of facilitation, independent of the recent history of release, which was under the influence of CB1 receptors. DORF synapses also received strong tonic reduction in inhibition (although not completely silenced) and displayed asynchronous releases of GABA similar to inhibitory connections between lacunosum moleculare perforant path associated cells and SCA cells reported previously (Ali and Todorova 2010). The tonic reduction of release/inhibition at DORF synapses may be a result of two possibilities here; constitutive activity of CB1 receptors in the absence of agonists, as the inverse agonist/antagonist AM-251 reduced the constitutive activity and enhanced synaptic transmission (Guo and Ikeda 2004; Vásquez and Lewis 1999) or by enhanced background levels of endocannabinoids released by the postsynaptic cell (Neu et al. 2007).
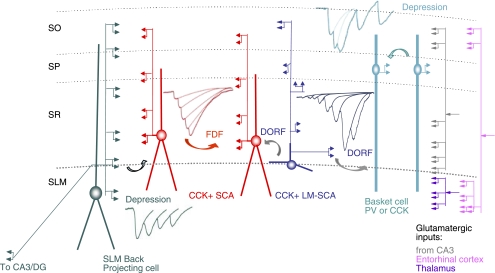
In contrast, local circuit connections among interneurons involving CCK negative, quadrilaminar presynaptic interneurons in SLM with axonal projections that expanded across subregions displayed strong depression. Figure 6 is a schematic of the local circuit interneuron connections discussed here and summarizes the presynaptic-dependent release patterns observed.
Fig. 6.
Three types of short-term synaptic plasticity at local circuit interneuron connections in CA1. CCK-positive interneurons in SR (where glutamatergic input from CA3 enters) receive facilitating IPSPs, which are temporally distinct (FDF and DORF), mediated by N-type calcium channels and under the modulation via CB1 receptors. Synaptic depression is observed in SP and SLM (mediated by P/Q-type calcium channel and insensitive to CB1 receptor pharmacology) where excitatory input from entorhinal cortex and the thalamus are received. This localized short-term plasticity among interneuron synapses probably plays important roles in synaptic integration while rapidly shunting some inhibitory signals; other signals are allowed a wider time window for synaptic integration. PV, parvalbumin.
Factors influencing short-term plasticity.
The differences in the synaptic dynamics could be due to several presynaptic factors including calcium availability and, therefore, the probability of release, the number of release sites, the type of presynaptic calcium channels, and G protein modulation of CB1 receptors; these concepts are well documented at excitatory synapses (Scott et al. 2008; Ali and Nelson 2006; Koester and Johnston 2005; Sun et al. 2005; see Pelkey and McBain 2008; Bischofberger et al. 2006, and Thomson 2003 for reviews). At low firing frequencies, synapses that strongly facilitate are thought to display low release probabilities and act as high-pass filters, but the calcium influx is thought to prime the release machinery and increase subsequent release probabilities (Katz and Miledi 1970). In contrast, depressing synapses with high release probabilities are thought to act as low-pass filters and at low frequency may enter a state of release site refractory (Betz 1970), resulting in depression after subsequent action potentials. Facilitation may not be absent at depressing synapses, but masked by depression.
At DORF synapses priming of release via preceding action potentials is neither a strong factor nor the recent history of release; therefore another mechanism is involved here.
The present results suggest that N-type calcium channels were dominant at facilitating synapses, which utilized presynaptic CB1 receptors, whereas P/Q-type calcium channels were restricted to the CB1 insensitive, depressing synapses among local interneuron circuits. This is consistent with previous studies at excitatory and inhibitory synapses (Ali and Nelson 2006; Poncer et al. 1997). The present study is also consistent with previous studies reporting P/Q-type, but not N-type, channels mediate GABA release from fast-spiking interneurons to pyramidal cells (Zaitsev et al. 2007; Hefft and Jonas 2005), since the CCK-negative interneurons here displayed fast IPSPs, insensitive to CB1 pharmacology.
N-type calcium channels are thought to be modulated by cannabinoid, muscarinic, and metabotropic glutamate receptor agonists (Qian and Saggau 1997; Vásquez and Lewis 1999). Only when CB1 receptors were blocked, strong synaptic facilitation that was delayed switched to high fidelity depression synapses, suggesting CB1 receptors modulate synaptic transmission probably via G protein modulation of N-type channels and causing a state of release-independence.
Both types of facilitating synapses (FDF and DORF) involved N-type calcium channels, which suggests that the differences in the time course of facilitation at these two synapses could be due to other factors such as the differential coupling strength between calcium source and sensor, the location of release sites (Farrant and Nusser 2005; Nusser et al. 1998), or different affinity for calcium sensor of exocytosis (Hui et al. 2005; Hefft and Jonas 2005).
Functional implications.
Previous studies have demonstrated that CCK-containing cells allow more temporal and spatial flexibility; CCK-positive cells have been shown to release GABA asynchronously to cause a long lasting inhibition in pyramidal cells (Ali and Todorova 2010; Karson et al. 2009; Hefft and Jonas 2005). CCK-positive cells are associated with presynaptic CB1 receptors to attenuate inhibition via retrograde signaling. This type of redistribution of synaptic efficacy may have a powerful effect in altering synaptic dynamics during specific frequencies and hence subtly altering the content of the signal transferred between neurons. The SR receives major excitatory inputs from CA3, whereas the SLM receives inputs from the thalamus and the entorhinal cortex (see Fig. 6); perhaps the localized short-term plasticity among interneurons plays important roles in differentially responding to these excitatory signals.
DORF synapses with a delay in the release of neurotransmitter and low release probability at low frequencies and periods of depression when CB1 receptors are switched off may act as a band-pass filter since they allow changes to be detected at frequencies within a certain range and attenuate frequencies outside that range.
In summary, this study demonstrates that presynaptic-dependent short-term synaptic plasticity give rise to inhibition that may differentially entrain the output of postsynaptic cells and is correlated with specific calcium channel subtype. CB1 receptors, which modulate synapses using N-type calcium channels, have the ability to delay synaptic transmission at CCK-positive synapses, allowing other synapses a greater time window for synaptic integration.
GRANTS
This work was supported by the Medical Research Council (UK) (New Investigators Award).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
I thank Marina Todorova and Jonathan Iball for technical assistance. I also thank Dr. Gordon Ohning at Cure antibodies (UCLA) for the kind gift of anti-CCK.
REFERENCES
- Ali AB. Presynaptic inhibition of GABAA receptor-mediated unitary IPSPs by cannabinoid receptors at synapses between CCK-positive interneurons in rat hippocampus. J Neurophysiol 98: 861–869, 2007 [DOI] [PubMed] [Google Scholar]
- Ali AB, Bannister AP, Thomson AM. IPSPs elicited in CA1 pyramidal cells by putative basket cells in slices of adult rat hippocampus. Eur J Neurosci 11: 1741–1753, 1999 [DOI] [PubMed] [Google Scholar]
- Ali AB, Deuchars J, Pawelzik H, Thomson AM. CA1 pyramid to basket and bistratified cells EPSPs: dual intracellular recordings in rat hippocampal slices. J Physiol 507.1: 201–217, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AB, Nelson C. Distinct Ca2+ channels mediate transmitter release at excitatory synapses displaying different dynamic properties in rat neocortex. Cereb Cortex 6: 386–393, 2006 [DOI] [PubMed] [Google Scholar]
- Ali AB, Todorova M. Asynchronous release of GABA via tonic cannabinoid receptor activation at identified interneuron synapses in rat CA1. Eur J Neurosci 31: 3–12, 2010 [DOI] [PubMed] [Google Scholar]
- Betz WJ. Depression of transmitter release at the neuromuscular junction of the frog. J Physiol 206: 620–644, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischofberger J, Engel D, Frotscher M, Jonas P. Timing and efficacy of transmitter release at mossy fiber synapses in the hippocampal network. Pflügers Arch 453: 361–372, 2006 [DOI] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron 18: 995–1008, 1997 [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci 6: 215–229, 2005 [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus 6: 347–470, 1996 [DOI] [PubMed] [Google Scholar]
- Guo J, Ikeda SR. Endocannabinoids modulate N-type calcium channels and G-protein-coupled inwardly rectifying potassium channels via CB1 cannabinoid receptors heterologously expressed in mammalian neurons. Mol Pharmacol 65: 665–674, 2004 [DOI] [PubMed] [Google Scholar]
- Hajos N, Katona I, Naiem SS, MacKie K, Ledent C, Mody I, Freund TF. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci 12: 3239–3249, 2000 [DOI] [PubMed] [Google Scholar]
- Hefft S, Jonas P. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat Neurosci 8: 1319–1328, 2005 [DOI] [PubMed] [Google Scholar]
- Heine M, Groc L, Frischknecht R, Béïque JC, Lounis B, Rumbaugh G, Huganir RL, Cognet L, Choquet D. Surface mobility of postsynaptic AMPARs tunes synaptic transmission. Science 320: 201–205, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DI, Bannister AP, Pawelzik H, Thomson AM. Double immunofluorescence, peroxidase labelling and ultrastructural analysis of interneuronss following prolonged electrophysiological recordings in vitro. J Neurosci Methods 101: 107–116, 2000 [DOI] [PubMed] [Google Scholar]
- Hui E, Bai J, Wang P, Sugimori M, Llinas RR, Chapman ER. Three distinct kinetic groupings of the synaptotagmin family: candidate sensors for rapid and delayed exocytosis. Proc Natl Acad Sci USA 102: 5210–5214, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram SL, Williams JT. Modulation of the hyperpolarisation-activated current (I h) by cyclic nucleotides in guinea-pig primary afferent neurons. J Physiol 492: 97–106, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karson MA, Tang AH, Milner TA, Alger BE. Synaptic cross talk between perisomatic-targeting interneuron classes expressing cholecystokinin and parvalbumin in hippocampus. J Neurosci 29: 4140–4154, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci 19: 4544–4558, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Miledi R. Further study of the role of calcium in synaptic transmission. J Physiol 207: 789–801, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Marton LF, O'Neill J, Huck JH, Dalezios Y, Fuentealba P, Suen WY, Papp E, Kaneko T, Watanabe M, Csicsvari J, Somogyi P. Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J Neurosci 19: 9782–9793, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Magill PJ, Marton LF, Roberts JD, Cobden PM, Buzsaki G, Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature 421: 844–848, 2003 [DOI] [PubMed] [Google Scholar]
- Koester HJ, Johnston D. Target cell-dependent normalization of transmitter release at neocortical synapses. Science 308: 863–866, 2005 [DOI] [PubMed] [Google Scholar]
- Losonczy A, Biró AA, Nusser Z. Persistently active cannabinoid receptors mute a subpopulation of hippocampal interneurons. Proc Nat Acad Sci USA 3: 1362–1367, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthi A, McCormick DA. H-current: properties of a neuronal and network pacemaker. Neuron 21: 9–12, 1998 [DOI] [PubMed] [Google Scholar]
- Mann EO, Suckling JM, Hajos N, Greenfield SA, Paulsen O. Perisomatic feedback inhibition underlies cholinergically induced fast network oscillations in the rat hippocampus in vitro. Neuron 45: 105–117, 2005 [DOI] [PubMed] [Google Scholar]
- Markram H, Gupta A, Uziel A, Wang Y, Tsodyks M. Information processing with frequency-dependent synaptic connections. Neurobiol Learn Mem 70: 101–112, 1998 [DOI] [PubMed] [Google Scholar]
- Miles R, Toth K, Gulyas AI, Hajos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron 16: 815–823, 1996 [DOI] [PubMed] [Google Scholar]
- Neu A, Földy C, Soltesz I. Synaptic origin of CB1-dependent tonic inhibition of GABA release at cholecystokinin-positive basket cell to pyramidal cell synapses in the CA1 region of the rat hippocampus. J Physiol 578: 233–247, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci 18: 1693–1703, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkey KA, McBain CJ. Target-cell-dependent plasticity within the mossy fibre-CA3 circuit reveals compartmentalized regulation of presynaptic function at divergent release sites. J Physiol 586: 1495–1502, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncer JC, McKinney RA, Gahwiler BH, Thompson SM. Either N- or P-type calcium channels mediate GABA release at distinct hippocampal inhibitory synapses. Neuron 18: 463–472, 1997 [DOI] [PubMed] [Google Scholar]
- Qian J, Saggau P. Presynaptic inhibition of synaptic transmission in the rat hippocampus by activation of muscarinic receptors: involvement of presynaptic calcium influx. Br J Pharmacol 122: 511–519, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nature Neurosci 1: 279–285, 1998 [DOI] [PubMed] [Google Scholar]
- Scott R, Lalic T, Kullmann DM, Capogna M, Rusakov DA. Target-cell specificity of kainate autoreceptor and Ca2+-store-dependent short-term plasticity at hippocampal mossy fiber synapses. J Neurosci 28: 13139–13149, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Klausberger T. Defined types of cortical interneuron structure space and spike timing in the hippocampus. J Physiol 562: 9–26, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HY, Lyons SA, Dobrunz LE. Mechanisms of target-cell specific short-term plasticity at Schaffer collateral synapses onto interneurons versus pyramidal cells in juvenile rats. J Physiol 568: 815–840, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM. Presynaptic frequency- and pattern-dependent filtering. J Comput Neurosci 15: 159–202, 2003 [DOI] [PubMed] [Google Scholar]
- Thomson AM, Bannister AP. Release-independent depression at pyramidal inputs onto specific cell targets: dual recordings in slices of rat cortex. J Physiol 519: 57–70, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukker JJ, Fuentealba P, Hartwich K, Somogyi P, Klausberger T. Cell type-specific tuning of hippocampal interneuron firing during gamma oscillations in vivo. J Neurosci 27: 8184–8189, 2007. [Erratum. J Neurosci 29: 3009, 2009.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron 31: 453–462, 2001 [DOI] [PubMed] [Google Scholar]
- Vásquez C, Lewis DL. The CB1 cannabinoid receptor can sequester G-proteins, making them unavailable to couple to other receptors. J Neurosci 19: 9271–9280, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitsev AV, Povysheva NV, Lewis DA, Krimer L. P/Q-type, but not N-type, calcium channels mediate GABA release from fast-spiking interneurons to pyramidal cells in rat prefrontal cortex. J Neurophysiol 97: 3567–3573, 2007 [DOI] [PubMed] [Google Scholar]