Abstract
Neural systems that control movement maintain accuracy by adaptively altering motor commands in response to errors. It is often assumed that the error signal that drives adaptation is equivalent to the sensory error observed at the conclusion of a movement; for saccades, this is typically the visual (retinal) error. However, we instead propose that the adaptation error signal is derived as the difference between the observed visual error and a realistic prediction of movement outcome. Using a modified saccade-adaptation task in human subjects, we precisely controlled the amount of error experienced at the conclusion of a movement by back-stepping the target so that the saccade is hypometric (positive retinal error), but less hypometric than if the target had not moved (smaller retinal error than expected). This separates prediction error from both visual errors and motor corrections. Despite positive visual errors and forward-directed motor corrections, we found an adaptive decrease in saccade amplitudes, a finding that is well-explained by the employment of a prediction-based error signal. Furthermore, adaptive changes in movement size were linearly correlated to the disparity between the predicted and observed movement outcomes, in agreement with the forward-model hypothesis of motor learning, which states that adaptation error signals incorporate predictions of motor outcomes computed using a copy of the motor command (efference copy).
Keywords: saccade adaptation, forward model, motor learning
each day we make thousands of targeted movements: for example, several times a day we may press a button, look from a traffic light to oncoming cars, and step down from a curb before walking across the street. As we repeat these actions, the body's several motor systems constantly adjust motor commands to keep movement errors within an acceptable range. Motor systems adapt in response to long-term changes such as muscle atrophy or short-term changes such as fatigue to reduce movement errors. Such continuous adaptation implies that movements should always be nearly on target.
For most movements, adaptation does keep movement errors small; however, a notable exception involves saccadic eye movements. Saccades are rapid eye movements used to foveate objects of interest. Such actions are critical for acquiring detailed visual information about the environment before planning additional movements. Saccadic eye movements must be preprogrammed prior to their onset. Since saccades are so rapid (on the order of 70 ms), vision and proprioception are not available to modify saccadic movements midflight; it takes about the same amount of time to make a saccade as it does for retinal or proprioceptive information to reach cortex. Sensory feedback is only available at the conclusion of a saccade; this delayed feedback provides an observation of movement outcome that can be used for learning.
Despite their important function and the readily available presence of a well-defined visual error signal, saccades (at least in the laboratory) consistently fall short of the visual target (hypometria). It is surprising that the motor system does not adapt to completely minimize the postsaccadic retinal error of the primary saccade, since saccades are crucial for acquiring detailed information about specific portions of the visual scene. Rather than making accurate saccades, however, the motor system instead relies on corrective saccades to bring objects of interest onto the fovea (Bonnetblanc and Baraduc 2007). A typical saccadic gaze shift is composed of two parts: a large primary saccade that brings the eye about 90% of the distance to the target, followed by a small corrective movement (Becker 1989; Deubel et al. 1986; Irving et al. 2006). These corrective movements are not necessary for saccade adaptation to occur; it has previously been demonstrated that simply observing visual errors is enough to cause saccadic adaptation (Noto and Robinson 2001; Wallman and Fuchs 1998). Nevertheless, saccades remain hypometric, and the motor system may purposely maintain this situation. Henson (1978) demonstrated, using a device that tended to remove subjects' hypometria, that subjects decreased their saccade gain to restore their hypometria. In light of this observation, and noting that saccades are unlikely to be less optimally controlled than other goal-directed movements, this hypometria may be interpreted as intentional.
Several explanations have been proposed as to why primary saccades fall short and are generally related to the observation that saccade end points are variable. One possibility involves the fact that vision is suppressed during a saccade and that smaller saccades take less time to complete. Given that any single saccade is likely to be inaccurate due to variability, deliberately undershooting and then making a small forward correction minimizes the amount of time the eyes are in motion (relative to overshooting, which involves a saccade of longer duration). This maximizes the amount of time that stable vision is available to the brain (Harris 1995). Alternatively, purposely falling short prevents the eyes from overshooting the target, which has the advantage of keeping the target in the same visual hemifield and reducing the amount of cortical processing required (Robinson 1973). In agreement with the latter hypothesis, it has been demonstrated that corrective saccades made in the direction opposite that of the primary saccade tend to have longer latencies, suggestive of a heavier cortical load (Cohen and Ross 1978). On the contrary, it has been proposed that hypometria is simply the result of gain-decrease adaptation being more robust than gain-increase adaptation such that errors arising from a random distribution of saccade end-point scatter will tend to produce adaptation in favor of a decrease in gain (Robinson et al. 2003). However, this seems unlikely since making every saccade land exactly on target tends to produce a gain change (typically a gain decrease) even though retinal errors are eliminated (Henson 1978; Havermann and Lappe 2010). Thus, although the exact reason for saccade hypometria remains unknown, it is clear that the motor system intentionally produces saccades that fall short of the visual target.
Aside from this deliberate hypometria, previous work has suggested that adaptation does not simply work to reduce the postsaccadic retinal error between the target and fovea. Corrective saccades are suppressed when saccades land within the boundary of large target objects, suggesting that there may be only a poorly defined retinal error to drive a motor correction in this situation; however, adaptation may still be induced by stepping these large targets intrasaccadically (Bahcall and Kowler 2000). On the other hand, if the target is blanked during the saccade such that no postsaccadic visual error signal is available, primary saccades increase to become less hypometric, perhaps as a result of not being able to rely on a corrective saccade to ensure movement accuracy (Bonnetblanc and Baraduc 2007; but see Havermann and Lappe 2010 where the opposite tendency was found, perhaps due to fatigue). Furthermore, when subjects are asked to make saccades only partway to a target, saccade gain may be adapted by stepping the target as usual, even though the goal is no longer to reach the target (and reduce retinal error; Bahcall and Kowler 2000). During the no-step condition in their study, saccades did not adapt to become more accurate when subjects purposely made saccades that fell short of the visual target. Thus the saccade target does not have to be a visual one but can even be a goal to maintain some retinal error between the saccade end point and the target. In short, the evidence collectively suggests that the error signal that drives adaptation is not equivalent to postsaccadic retinal error.
We instead hypothesize that the adaptation error signal is derived from a difference between the observed outcome at the conclusion of the movement and a prediction of that outcome. The motor system has available to it not only the retinal error but also a fairly accurate copy of the saccade command in the form of an efference copy. This may be used to generate an estimate of the intended movement outcome (Collins et al. 2009; Mehta and Schaal 2002; Miall and Wolpert 1996; Tseng et al. 2007). Deliberate saccade hypometria suggests that the predicted end point may not be equivalent to the target position, but instead may be some location that lies closer to the actual saccade end point than to the visual target (see also Henson 1978). The difference between this predicted outcome and the observed outcome may then yield the adaptation error signal. Since it is possible that the predicted outcome may also become modified during adaptation, it is only appropriate to take saccade hypometria as an estimate of the expected movement outcome prior to adaptation. However, assuming that any potential changes to this predicted outcome are small, preadaptation saccade hypometria may be used as a surrogate measure of the predicted outcome across the session. In this study, we have taken advantage of this inherent hypometria to dissociate visual (retinal) error (the retinal distance from the eye to the target) from prediction error (the difference between the visual error and the expected hypometria). Such an approach allows us to examine the nature of the error signal that drives adaptation.
We have accomplished this using a modified version of the double-step adaptation paradigm to alter the amplitudes of primary saccades. In the typical saccade adaptation paradigm (McLaughlin 1967), a primary target is presented some distance from the eye, evoking a saccade to foveate the target. While the eye is moving, the target is shifted to a secondary position such that when the eye stops moving, the observed error is unexpected. The target shift leads to an adaptation of saccade gain such that after many repeated trials, the primary target evokes a saccade directed toward the secondary target. Typically, the size of the experimenter-induced target steps (that is, the locations of the primary and secondary saccade targets) are fixed so that over time, the magnitude of the visual error decreases as the primary-saccade amplitude changes. Eventually, learning reaches an asymptote and the gain stops changing, typically at a value that is less than that of the requested gain change. Even if the double-step size is not consistent on every trial, the saccade gain will still adapt in response to the most recently observed error (Srimal et al. 2008). Asymptotic learning can also take place if the secondary saccade target is placed relative to the eye at the end of each saccade such that the size of the horizontal postsaccadic error remains fixed: a constant-error trial (Robinson et al. 2003; Havermann and Lappe 2010). In other words, regardless of the size of the saccade generated in response to the primary target, the postsaccadic visual error is forced to a value that is selected prior to the experiment.
Assuming subjects are 10% hypometric, the size of this fixed postsaccadic error is chosen such that the secondary target appears closer to the eye than expected while keeping the eye hypometric. This does not mean the target always steps backward after every saccade; if a saccade happens to be very accurate or even hypermetric (land beyond the primary target), the target would step forward on that trial to make it appear that the saccade still fell short of the target by the preselected amount. Note, however, that the prediction error is not necessarily equivalent to the target movement. In making no assumptions about the source of variability (that is, whether or not the predicted movement outcome incorporates an estimate of movement noise), we treat the target behavior and the prediction error as independent parameters for analysis.
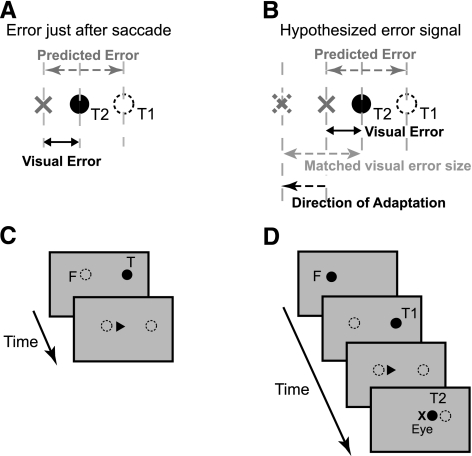
Using this technique of controlling the postsaccadic retinal error, we can make visual and prediction errors lie in opposite directions (Fig. 1) and provide conflicting error information for adaptation. When the motor system encounters a constant-error trial, it experiences an observed visual error (retinal distance from the eye to the target) that is much smaller than the predicted error the motor system would expect to see as a result of being hypometric (Fig. 1A). According to the prediction-based error-signal hypothesis, the motor system will determine that it is producing motor commands that cause saccades to be larger than expected. If the motor system adapts in the direction that moves the size of the postsaccadic visual error toward that of the predicted error, the resulting change in motor commands will cause saccades to become smaller (Fig. 1B). This means that subjects will experience a decrease in saccade gain as a result of a prediction error signal. Alternatively, if visual error drives adaptation, one would expect an increase in saccade gain, since there is still a forward-directed postsaccadic visual error (the eyes remain hypometric to the target). Thus we can achieve a dissociation between prediction error and visual error by examining the direction of adaptation in this paradigm relative to the preadaptation primary saccade hypometria. This enables us to discern which error constitutes the driving signal for adaptation.
Fig. 1.
Experiment design. A: hypothetical visual errors and predicted (expected) errors during constant-error adaptation trials as experienced by the motor system at the conclusion of the primary saccade, assuming that subjects make hypometric saccades. The predicted error is the retinal distance between the saccade end point and the target that the motor system expects to observe at the end of the movement, whereas the visual error is the actual retinal error observed as a result of displacing the target during the trial. T1, primary target; T2, secondary target; X, saccade end point. B: hypothesis for computing the adaptation error signal for a single trial based on the predicted saccade end point. For the visual error to match the size of the predicted error from A (that is, the difference between the observed error and predicted error is zero), the saccade should have been smaller (dashed X); thus, on the next trial saccade gain should become smaller (gain-decrease adaptation) even though both the visual error and the motor correction are directed in the positive (forward) direction. Note that the predicted error is not necessarily the same on every trial. C: target sequence for open-loop trials. Each trial began with a fixation point (F) at the current eye position. After a variable delay (1–2 s), a target (T) appeared 20° away and was extinguished at the onset of the saccade. D: target sequence for constant-error adaptation trials. A fixation point (F) appeared at the current eye position. After a variable delay (1–2 s), the target jumped 20° away (T1) but was extinguished upon saccade onset. When the primary saccade concluded, the jumped target (T2) was placed relative to the saccade end point (X, eye) such that the saccade remained hypometric but the visual error was modified.
MATERIALS AND METHODS
Twelve subjects participated in this study. Informed consent, according to the local institutional review board, was obtained from each participant. Of the 12 subjects, 11 underwent 1 experiment session (subjects A–K), whereas subject L participated in an initial pilot study involving three different sessions, spaced at least a week apart. Subject A was not naive to the purposes of this experiment and the specifics of the target manipulations; subject L was aware of the general goals of the study but was not informed of the specific target manipulations experienced in each session. Subjects A and H also participated in a control experiment to assess the effects of fatigue.
Eye movements were recorded using a scleral search coil in a magnetic field (Skalar Medical, Delft, The Netherlands; Chronos Vision, Berlin, Germany) to record horizontal and vertical eye movements from either the right or left eye (Robinson 1963). Data were acquired at 1,000 Hz on a personal computer running real-time experiment control software developed in-house. Subjects sat in a dark room in a stationary chair, and a bite bar was used to minimize head movements. Targets were presented using a mirror-controlled laser dot 2 mm in diameter that was rear-projected onto a screen 1 m in front of the subject.
Experimental Paradigm
As illustrated in Fig. 1, saccade adaptation was elicited using a modification of the conventional double-step adaptation paradigm in which the onset of a saccade causes the primary target (T1) to jump to a new location (T2). As a result of this displacement, an unexpected error is observed at the end of the primary saccade. Repetition of this process causes a change in saccade gain (Deubel et al. 1986; McLaughlin 1967).
Saccade gain before and after adaptation was assessed with a block of open-loop trials (Fig. 1C). At the start of each open-loop trial, the target jumped 20° away from the current point of fixation, alternating rightward and leftward target jumps on each trial. The onset of the subsequent saccade triggered the disappearance of the target so that the eye landed in darkness; 500 ms later, the next fixation target appeared at the current eye position to start the next trial. The delay of 500 ms was chosen because it provides enough separation between trials to minimize additional changes in saccade gain (Fujita et al. 2002). Open-loop trials provide an estimate of the initial motor command for the primary saccade; saccades are generally not modifiable midflight, nor is there a visual target present during or after the saccade to do so. Furthermore, subjects are not likely to adapt between subsequent open-loop trials because there is no postsaccadic error signal available to drive learning. Thus these trials estimate the saccade gain state while minimizing the decay of adaptation (Ethier et al. 2008b). Open-loop trials can be used to assess each subject's inherent hypometria. By using data from the preadaptation block before any changes of saccade gain have occurred, inherent hypometria is computed as the difference between the requested saccade size (20°) and the measured mean saccade amplitude.
A feature of these open-loop trials is that new fixation targets are placed relative to the position of the eye following a delay after the previous trial such that no saccade is necessary to begin the next trial. This means that fixation targets can appear anywhere along the horizontal axis; over time, a drift from center could develop. For example, amplitude asymmetries between rightward and leftward saccades could cause such a drift. To prevent saccades from drifting too far in either direction, a “reset” trial was presented to bring the eye back toward center if saccades went beyond ±20° in the horizontal direction. The vertical target location was fixed at 0° on all trials.
Adaptation proceeded in 6 blocks of 50 constant-error trials. Blocks were separated by a rest period of ∼30 s in which subjects remained in the dark with their eyes closed. Trials began when the primary saccade target, T1, moved 20° away from the current fixation target after some random fixation time (mean: 1,500 ms), alternating between rightward and leftward target jumps on each trial. This random fixation time was designed to reduce the tendency for subjects to make anticipatory saccades. The onset of the subsequent saccade, detected as the time when the eye exited a 3° window centered on the fixation target, triggered the T1 target to disappear. The target reappeared only after the conclusion of the saccade in a secondary location, T2. These constant-error trials, involving very small postsaccadic visual errors (<10% of the requested primary saccade size), are applied to induce adaptation (Fig. 1D). Both rightward and leftward saccades were adapted; data from saccades made in both directions were combined for analysis.
As with open-loop trials, slow drifts away from the center were possible due to directional asymmetries; thus reset trials were also employed during adaptation blocks as needed. However, reset trials that occurred during adaptation blocks included a target double-step such that the postsaccadic visual error remained consistent with all other constant-error adaptation trials; this prevented deadaptation on reset trials. There were typically no more than two reset trials per block. Adaptation targets could appear anywhere within a constrained horizontal range; however, since targets in open-loop trials could also fall anywhere in this same region of visual space, it was unlikely that open-loop gain measurements were conducted in a different region of space than the one in which adaptation occurred.
For the 11 subjects in the main experiment, the secondary target, T2, was placed 0.7° farther eccentric than the end point of the primary saccade. The initial pilot study performed with subject L consisted of three separate adaptation sessions where the target was placed for the entire session at a distance of 0.5°, 1.0°, or 2.0° from the end point of the primary saccade. Since subjects tended to be hypometric (see results; average inherent hypometria was 1.63°), this target placement resulted on majority of trials with a backward target jump toward the fovea but a forward corrective saccade (Table 1). Subjects were given no explicit instructions describing the nature of the task.
Table 1.
Size and frequency of corrective saccades made for each subject in comparison to the size of the presented visual error
| Corrective Saccades |
||||||
|---|---|---|---|---|---|---|
| Subject | Clamp Size, Degrees | Inherent Hypometria, Degrees | Gain Change, % | Amplitude | Frequency, % | Latency, ms |
| A | 0.7 | 0.80 | −4.13 | 0.49 ± 0.19 | 28 | 349.65 ± 64.65 |
| B | 0.7 | 0.66 | 6.51 | 0.38 ± 0.13 | 72 | 260.29 ± 57.11 |
| C | 0.7 | 1.84 | −4.90 | 0.48 ± 0.21 | 67 | 288.90 ± 95.38 |
| D | 0.7 | 2.66 | −11.90 | 0.39 ± 1.34 | 60 | 249.41 ± 63.25 |
| E | 0.7 | 1.02 | −6.25 | 0.59 ± 0.73 | 54 | 279.96 ± 99.64 |
| F | 0.7 | 4.83 | −12.55 | 0.41 ± 0.14 | 65 | 366.23 ± 72.55 |
| G | 0.7 | 1.23 | −7.53 | 0.59 ± 0.16 | 64 | 328.37 ± 82.34 |
| H | 0.7 | 0.71 | −0.27 | 0.42 ± 0.14 | 67 | 273.38 ± 72.77 |
| I | 0.7 | 0.93 | 0.71 | 0.56 ± 0.18 | 55 | 340.01 ± 71.68 |
| J | 0.7 | 2.98 | −11.68 | 0.57 ± 0.17 | 53 | 288.65 ± 79.78 |
| K | 0.7 | −0.53 | −10.07. | 0.38 ± 0.45 | 53 | 343.75 ± 80.79 |
| L | 0.5 | 1.86 | −6.14 | 0.31 ± 0.45 | 77 | 252.61 ± 57.36 |
| 1.0 | 2.75 | −0.50 | 0.42 ± 0.13 | 75 | 259.01 ± 56.04 | |
| 2.0 | 1.04 | 8.20 | 1.43 ± 0.21 | 99 | 191.33 ± 28.61 | |
Corrective saccade amplitude values are medians ± semi-interquartile deviations (SID) across the 6 adaptation blocks; latency values are means ± SD. Measured latencies are reasonable given the corresponding corrective saccade amplitudes (Becker 1989). As discussed in text, subject K had both hypometric and hypermetric saccades. When all hypermetric saccades were removed, the inherent hypometria was calculated to be 1.06° and the gain change to be −5.72%.
During the control task, subjects were presented with targets that behaved exactly as they did in the adaptation session except that the T2 target appeared at the conclusion of the saccade in the same location as the T1 target (that is, the target was not shifted). This task was designed to examine whether subjects experienced changes in saccade gain that could be attributed to fatigue as a result of performing a large number of saccades that ended in darkness before the stimulus reappeared.
We calculated the position of the eye precisely at all times using a piecewise linear calibration tailored to each subject, based on eye position while fixating 25 points over a range of ±20° horizontally and ±15° vertically. This, along with the spatially precise eye-position measurement acquired with a scleral coil, enabled us to place targets accurately with respect to the eye in real time (accuracy determined offline by using recomputed target positions to measure the ability to place targets 0.7° away from the postsaccadic eye position: median error of 0.02° with quartiles of −0.02° and 0.11°). The final eye position was calculated as the point after the eye remained within a 0.4° window for 45 ms at the conclusion of the primary saccade, approximately equivalent to a velocity threshold of 9°/s. The small 45-ms delay necessary to assess the final eye position and place the target relative to the eye is unlikely to affect the extent of adaptation, producing results comparable with traditional double-step adaptation paradigms in which T2 is present before the eye stops moving (Fujita et al. 2002; Shafer et al. 2000).
Data Analysis
Eye-tracking data were analyzed offline with an interactive computer program written in MATLAB (The MathWorks, Natick, MA) that selected primary-saccade and corrective-saccade start and end points using a velocity threshold (>30°/s). Saccade metrics including amplitude and latency were measured. When subjects blinked during a saccade, the resulting saccade was discarded from further analysis. Corrective saccades that were executed before or immediately after (within 80 ms) the presentation of T2 were excluded from analysis, because they reflect corrective saccades programmed before error information at the conclusion of the primary saccade became available. For the presented error sizes, the corrective saccade latency is typically ∼250 ms (Becker 1989). Thus corrective saccades made much earlier than this are likely to be saccades that were planned in parallel with the primary saccade and do not represent a correction to the jumped target (T2). Instead, these preplanned corrective movements are directed toward the original target (T1), often resulting in an additional corrective saccade to bring the eye back to T2. Since this only occurred on a few trials, and the net effect of these two corrective saccades is unclear, these pairs of corrective saccades were excluded from the set of corrective saccades whose metrics were analyzed. Offline, target positions were recomputed using the recorded output of the commands sent to the mirror-galvanometers responsible for adjusting the position of the laser dot on the screen.
Inherent hypometria for each subject was measured as the average visual error observed at the conclusion of the primary saccade across the pre-adaptation block of open-loop trials. Error was calculated as primary target position minus saccade end point; thus hypometric errors are positive. Gain for each trial was measured as the size of the primary saccade divided by the size of the initial target step. The change in saccade gain due to adaptation was calculated as
where the gains were computed using saccades made during the preadaptation and postadaptation blocks of open-loop trials.
RESULTS
Constant-Error Adaptation: Main Findings
Subjects made reactive saccades (latency: 247.0 ± 71.0 ms) throughout the constant-error adaptation paradigm. Before adaptation, subjects had an average inherent hypometria of 1.63 ± 1.33° for a 20° saccade. This means subjects were on average hypometric by about 8.2%, very close to the expected 10%. This average, however, is skewed by subject K, whose open-loop saccades tended to be hypermetric (postsaccadic error: −0.53°); all other subjects made saccades that consistently fell short of the requested 20° target step with an average inherent hypometria ranging from 0.66° to 4.82°.
Subjects were repeatedly presented with positive visual errors at the conclusion of their saccades. This was verified in an offline analysis by measuring the postsaccadic errors presented to subjects during adaptation trials. For the 11 subjects in the main experiment, the target was placed a median distance of 0.72° (quartiles of 0.68° and 0.81°) away from the eye position at the conclusion of the primary saccade.
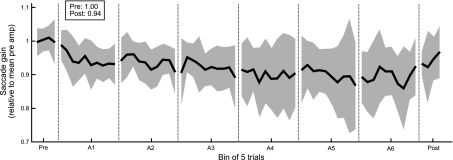
During constant-error adaptation, subjects tended to decrease their saccade gain despite positive visual errors at the conclusion of their primary saccades. Figure 2 shows the mean time course of adaptation across the 11 subjects in the main experiment. For each subject, saccade amplitudes were normalized by the mean saccade amplitude in the preadaptation block of open-loop trials such that all subjects had a normalized preadaptation saccade gain of 1.00. Although no subject actually displayed a saccade gain of 1.00 before adaptation, normalization enabled us to compare changes in gain across subjects. On average, subjects who experienced a 0.7° visual error exhibited a 6% decrease in saccade gain. This learning appears asymptotic, since saccade gains tended to decrease the most in the first block and largely stopped changing by the last adaptation block. The apparent increase in variability across the paradigm (Fig. 2) is caused by the fact that not all subjects adapted to the same extent or at the same rate; thus, as adaptation proceeds, across-subject averages become more variable. Furthermore, fatigue may produce some increased variability in the later portion of the paradigm. There also appears to be a drift in the postadaptation block back toward the baseline saccade gain; this may result from some deadaptation taking place during open-loop trials. It may alternatively represent the tendency for saccade amplitudes to increase when the saccade target is removed midflight and corrective saccades cannot be appropriately planned (Bonnetblanc and Baraduc 2007).
Fig. 2.
Saccade gain changes in response to small target perturbations that place predicted and visual errors in opposition. The target is stepped relative to the saccade end point, maintaining the directional hypometria of the primary saccade but reducing its magnitude from what would be expected given the size of the primary saccade. For a typical hypometric saccade, this means that the target will step backward toward the saccade end point by a small amount. Shown is the group average of the time course of adaptation for subjects presented with a 0.7° constant error. To assess the average gain change across all subjects, we normalized saccade amplitudes for each subject to their mean preadaptation saccade amplitude (mean pre amp) such that every subject had an average preadaptation saccade gain of 1.0; trials were then grouped in bins of five. Each bin was then averaged across subjects (n = 11) to obtain the mean saccade gain across subjects (solid line; shaded region denotes SD). Subjects experienced a mean 6% gain decrease as a result of the small, positive error presented.
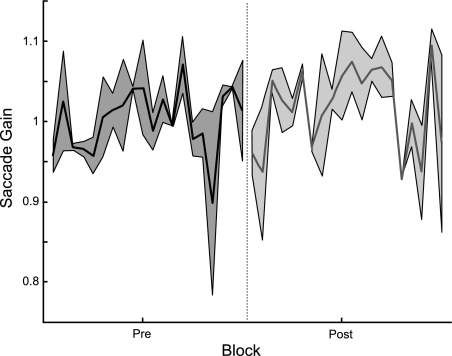
To verify that the observed changes in saccade gain were not due entirely to fatigue, we conducted a control experiment. During this experiment, the target reappeared in the same location as before the saccade; the postsaccadic retinal error was not manipulated by the experimenter. In this condition, subjects did not experience any significant change in saccade gain (Fig. 3; t-test, P = 0.20).
Fig. 3.
Control experiment to examine the effects of fatigue. Two subjects repeated the constant-error adaptation experiment, except the target did not shift its location after the primary saccade. The average time course of saccade gain during the preadaptation and postadaptation blocks from these subjects is plotted (shaded region denotes SD). Saccade gain did not change across the session; there was a small but not significant gain increase of 1.92% (P = 0.20).
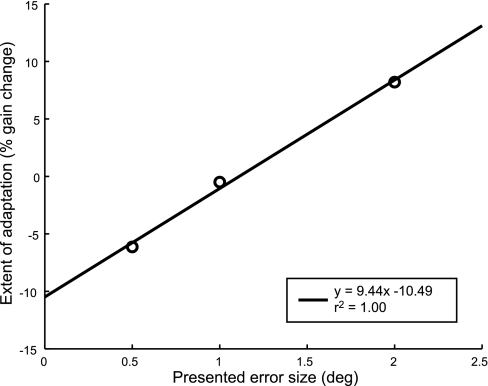
In the pilot study, subject L underwent three adaptation sessions, with a different visual error size presented in each session. This allowed an examination of the effect of positive visual error size on adaptation within the same person, to verify that both gain-increase and gain-decrease adaptation could be induced with the same paradigm depending on the magnitude of the visual error. Figure 4 shows the extent of adaptation (change in gain) achieved for each of the different error sizes. Subject L's data demonstrate that an error of fixed size following a very brief (45 ms) delay causes saccade gain to change in relation to the size of the error. Importantly, in all three sessions, the visual error was in the forward direction (the eye remained hypometric), but the saccade gain decreased in response to small errors and increased in response to large errors. There appears to be a null point in which errors of some small, positive size may actually cause no change in saccade gain. These pilot data show that the direction of adaptation is not affected by either the brief time after the eye lands when there is no target present or the fact that the error size remains constant relative to the saccade end point throughout the course of the adaptation session.
Fig. 4.
Effect of varying error size on the extent of adaptation achieved. Subject L received 3 different error sizes (0.5°, 1.0°, 2.0°) in 3 separate sessions. Plotted is the gain change that was induced in each session against the error size experienced. There is a clear linear relationship, showing that adaptation may proceed in a gain-up or gain-down manner depending on the size of the forward-directed error presented.
Exploration of the Potential Sources Driving Adaptation
There are several potential explanations why saccade gain tends to decrease in response to a fixed, positively directed visual error. In this study, we explored these possibilities in an attempt to understand the nature of the error signal that drives adaptation. We began with an examination of individual subject data according to the hypothesis that the error signal is composed of the difference between the observed and predicted outcomes of the movement. In other words, the adaptation error signal can be approximated as a difference between the postsaccadic error presented to the subject, in this case a 0.7° retinal error, and the subject's inherent hypometria, which is the expected movement outcome if the target had not been displaced during the saccade. Since the measured hypometria for each subject varied, we expected individual subjects to adapt their saccade gain to different extents in response to a constant error. For example, if a subject made very accurate saccades, a constant-error size of 0.7° for a 20° saccade might not produce a visual error size that is smaller than expected, which would affect the size and direction of the prediction error experienced on each trial (Fig. 1B).
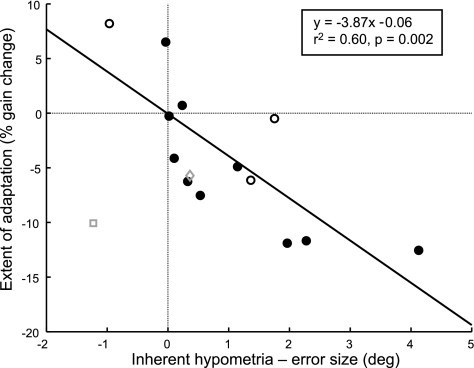
We examined this relationship by plotting, for all subjects, the extent of adaptation that occurred versus the difference between inherent hypometria and the presented error size. Figure 5 shows the three sessions from the pilot experiment (open circles) as well as the 11 subjects from the main experiment. For eight subjects who showed a decrease in saccade gain, the difference of their inherent hypometria to the presented error size is greater than zero. This means that the target appeared closer to the saccade end point than expected, which leads to a decrease in saccade gain. For two subjects (B and I), saccade gain increased at the conclusion of the constant-error adaptation task. These subjects exhibited a difference between inherent hypometria and the presented error that is close to or less than zero, meaning the target may have appeared farther from the saccade end point than expected; saccade gain in this case increases.
Fig. 5.
Relationship between the extent of adaptation and the difference between inherent hypometria and presented error size. Hypometria was measured as the difference between the requested saccade size (20°) and the average preadaptation primary saccade amplitude. The difference between hypometria and error size is related to adaptation: when hypometria exceeds the presented error, saccade gain decreases, and vice versa as expected (n = 12; 14 sessions). Data were included from subjects who experienced the 0.7° constant error (●) as well as the 3 sessions in the pilot study (○). One subject (□) made hypermetric and hypometric saccades in an inconsistent fashion; when the average of only the hypometric saccades was measured (◊), his data agreed with that of the other 11 subjects (r2 = 0.57, P = 0.002). Nevertheless, this subject was excluded from the linear regression (solid line; r2 = 0.60, P = 0.002) because the nature of his expectation of inherent hypometria was unclear.
Although the observed data are statistically correlated (r2 = 0.33, P = 0.03), one subject's data appear to be outliers (subject K; Fig. 5, open square). Subject K made very inconsistent saccades, some hypermetric and some hypometric. In fact, this subject was the only one to make hypermetric saccades, which occurred during the preadaptation block as well as during the calibration when targets were continuously illuminated and no intrasaccadic movement or target blanking occurred. When all hypermetric saccades in the preadaptation block were excluded and the data for this subject were reanalyzed, the relationship between the extent of adaptation and the difference between the inherent hypometria and presented error agreed with that of the other subjects (Fig. 5, open diamond; regression fit with this modified data point: r2 = 0.57, P = 0.002). Because of the inconsistency in saccade end points, it is unclear whether subject K expected to be hypermetric or hypometric, or even whether these predictions were consistent from trial to trial. Therefore, subject K's data were excluded from regression fits but were retained for further analysis.
Fitting a regression line to the data from the remaining 11 subjects (including the data from the pilot study), we found the adaptive change in saccade gain correlates well with the difference between subjects' inherent hypometria and the presented error size (r2 = 0.60, P = 0.002; regression fit without pilot data: r2 = 0.60, P = 0.008). This regression analysis informs us that when the inherent-hypometria to error-size difference (abscissa value) is zero, the expected extent of adaptation is −0.06%, which is not significantly different from zero (P = 0.97; if the pilot data is excluded, the intercept remains not statistically different from zero, P = 0.44). Such a finding suggests that if we could present a target error at exactly the size that subjects expect to fall short, there would be no change in saccade gain.
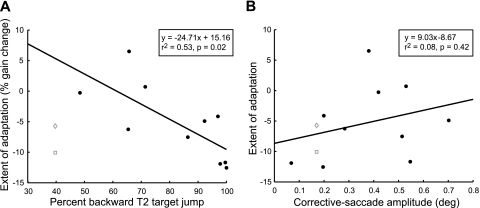
Although these data agree with our hypothesis, it is possible that subjects may have simply adapted in response to the direction of the target step. This is not equivalent to our hypothesis, because we do not assume that the predicted movement outcome necessarily includes an estimate of movement variability. Consider, for example, the case where on a given trial a subject expects to be hypometric to T1 when, in fact, variability results in hypermetria; in this situation, the target motion will be the sum of the overshoot plus the presented error, but the predicted error will be the difference between the expected hypometria and the presented error. Such situations suggest that we should consider the target behavior to be an independent parameter that may not necessarily be equivalent to an error signal derived from the difference between observed and predicted movement outcomes. Thus we show in Fig. 6A the extent of adaptation against the percentage of trials in which the target double step is directed in the backward direction (toward the previous fixation point). On average, for subjects who experienced a 0.7° error, the target stepped backward about 82% of the time. As before, when subject K is excluded from this regression analysis, a significant correlation (r2 = 0.53, P = 0.02) is observed. However, unlike with the prediction-error hypothesis, neither including subject K's data (r2 = 0.18, P = 0.19) nor using the analysis above where all hypermetric saccades are excluded (r2 = 0.32, P = 0.07) provides a significant correlation that can account for this outlier. Thus, although the prediction-error hypothesis can explain the apparent outlier in the data (subject K), the proposal that adaptation occurs in response to the target behavior is unable to do so.
Fig. 6.
Correlation of various parameters to the measured extent of adaptation for all subjects in the main experiment (0.7° constant error). A: correlation of achieved adaptation to the percentage of times T2 jumped in the backward direction. Correlation is significant (r2 = 0.53, P = 0.02), but a stepwise linear correlation excludes this regression in favor of the regression presented in Fig. 5. B: no correlation to the average corrective saccade amplitude (r2 = 0.08, P = 0.42), which agrees with previous studies suggesting that the corrective saccade is not necessary for adaptation. For both plots, data from subject K are displayed with all preadaptation saccades included (□) and with only hypometric preadaptation saccades analyzed (◊). As before, these data have been excluded from the correlation analyses; including either possible data point decreases the measured correlations to nonsignificant levels.
If we examine individual subjects, we see some important exceptions to the alternative hypothesis that the direction of the target double step determines the extent of adaptation. Subject H, who experienced forward target jumps on >50% of adaptation trials, exhibited a small decrease in saccade gain. Furthermore, the two subjects who showed increases in saccade gain both experienced forward-directed target steps on <50% of all trials (average: 32%). Since gain-increase adaptation is slower than gain-decrease adaptation, it is unlikely that these data could be explained if target motion was driving adaptation; according to the regression in Fig. 6A, a majority (61%) of backward target steps must be observed before gain decreases.
A stepwise linear regression (excluding subject K) was conducted to determine whether the extent of adaptation achieved in the main experiment can best be explained by either the difference between inherent hypometria and presented error size or by the percentage of backward-jumping T2 target steps. Only the difference between inherent hypometria and the presented error size was necessary to explain the adaptation achieved (regression coefficient = −3.55, P = 0.008), and no additional information was captured by including the target behavior (regression coefficient = −12.98, P = 0.22). This is reasonable, since the amount of hypometria a subject exhibits will dictate target behavior.
A second alternative explanation for these data is that adaptation is a result of the motor correction made in response to observed errors. We explored this possibility by examining the corresponding corrective saccades made during adaptation trials. On average, subjects made forward-directed corrective saccades toward the shifted target (median corrective-saccade amplitude: 0.46°, quartiles of 0.26° and 0.67°; Table 1). Although the median corrective saccade size is <0.7°, we showed above that the placement of T2 relative to the eye was reasonably accurate and consistently resulted in a forward-directed postsaccadic error. The fovea has been estimated to be about 0.5° in diameter, and the goal of a corrective saccade is simply to bring the target onto any part of the fovea. Thus a corrective saccade that is 0.46° will clearly bring the secondary target, which has a diameter of ∼0.2° and is placed 0.7° away from the center of the fovea, well onto the fovea. Corrective saccades were made on ∼60% of all adaptation trials; on the remaining 40% of trials, the target may have appeared close enough to the fovea that it did not trigger a motor correction. Since the laser dot was not a discrimination target, subjects may not have felt compelled to completely foveate the target on all trials. Nevertheless, the size of the average corrective saccade during adaptation for each subject is not significantly correlated with the extent of adaptation attained (r2 = 0.08, P = 0.42; Fig. 6B).
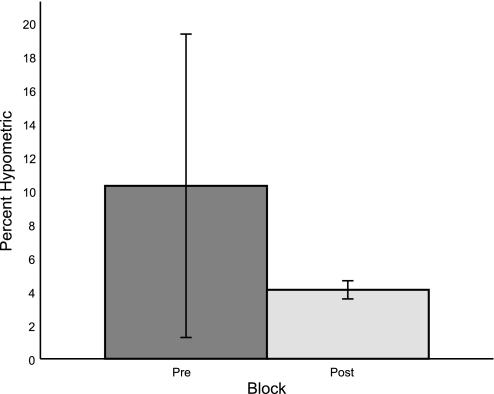
Finally, we investigated the possibility that the motor system seeks to maintain a constant proportion of hypometria to primary saccades, for example, to keep saccade end points 10% hypometric of the target (Becker 1989). For each subject, we computed the percentage of the primary saccade that was represented by the inherent hypometria (that is, inherent hypometria divided by mean primary saccade size in the preadaptation block). Assuming that saccade gains had stopped changing by the conclusion of the experiment, we then computed the percentage of the adapted saccade amplitude that was represented by the 0.7° visual error we presented. It is reasonable to assume that adaptation had stopped, since the data appear to have reached asymptotes by the end of the last block (Fig. 2; see also Robinson et al. 2003). Subject K was again excluded from this analysis. If the goal of adaptation is to maintain a constant-proportion bias, pre- and postadaptation hypometria percentages should be equal. Instead, subjects were found to be significantly more hypometric before adaptation than after adaptation (Fig. 7; rank sum test, P = 0.03). It is possible, however, that even though adaptation has stopped, it may not be complete; that is, some other mechanism may stop adaptation early before the error signal goes to zero. If adaptation is incomplete, the means of the pre- and postadaptation hypometria distributions may be different. Nevertheless, if adaptation is an effort to restore a constant-proportion bias, the driving signal for each subject is different, so subjects should adapt to different extents before the adaptation process is cut off; then, the variance of these two distributions (pre- and postadaptation) should be similar. We found this also not to be the case: the variance of the ratio of hypometria to primary saccade amplitude across subjects is significantly different before and after adaptation (F-test, P = 0.000). In contrast, the variance of the pre- and postadaptation primary saccade amplitudes are similar (F-test, P = 0.19), despite subjects exhibiting different changes in saccade gain. This suggests that the constant-proportion bias hypothesis is not likely and that adaptation is instead driven by an alternative error signal.
Fig. 7.
Comparison of the size of hypometria relative to that of the primary saccade before (Pre) and after (Post) adaptation. Values are means ± SD. Preadaptation, each subject's inherent hypometria was divided by their average primary saccade size prior to adaptation. Postadaptation, the presented visual error of 0.7° was divided by the average adapted saccade amplitude. The mean percentage of hypometria is significantly different before and after adaptation (rank sum test, P = 0.03), as is the variance pre- compared with postadaptation (F-test, P = 0.000).
DISCUSSION
Our results demonstrate that forward-directed visual errors that keep saccades hypometric can nonetheless induce a decrease in saccade gain. We propose that our paradigm achieves a separation of prediction errors from visual errors by giving subjects very small, constant postsaccadic errors; in this manner, we have shown that neither the visual error nor the motor correction is the source of the error signal for adaptation. Instead, this error signal may be the difference between the predicted (expected) and observed outcomes of a movement. Supporting this hypothesis, we found a good correlation between the extent of adaptation and the amount of error one expects (that is, the difference between the inherent hypometria and the presented error size). Thus, if a subject who is typically hypometric makes saccades that land exactly on target, one would anticipate his or her saccade gain to decrease.
Henson (1978) found evidence in favor of this by having subjects wear a device that tended to eliminate saccade hypometria; over time, subjects decreased their gain in response to this perturbation. Previously, it has been suggested that hypometria results from an imbalance between gain-increase and gain-decrease adaptation (Robinson et al. 2003), rather than from some intent to remain hypometric. However, this would imply that if a subject experienced an equal number of backward and forward target jumps, gain would on average tend to decrease. Instead, we observed that it requires a majority of target back steps (61%) before subjects adapt in a gain-decrease fashion (Fig. 6A). In addition, it has been demonstrated that the magnitude of adaptation that occurs on a trial-by-trial basis for forward or backward target jumps is the same (Srimal 2008). Thus it is unlikely that gain-decrease adaptation has such an advantage over gain-increase adaptation that it would cause saccades to be consistently hypometric.
Our findings also agree with results observed when a constant-error paradigm was applied in monkeys (Robinson et al. 2003). In contrast to our hypothesis, however, Robinson et al. suggested that the observed gain decreases they observed in response to small, constant errors may have simply been caused by fatigue. However, it has been shown that humans efficiently compensate for fatigue over several hundred saccades (Golla et al. 2008; Xu-Wilson et al. 2009). Furthermore, our control data suggest that the gain changes we observed were not simply due to fatigue. Thus the observed gain decreases of Robinson et al. (2003), as well as those observed in this study in response to small forward-directed errors, indicate that visual error is not likely to be the driving signal for adaptation. The findings of Havermann and Lappe (2010), who performed a foveal target step with zero retinal error, are similar to our data in finding a correlation of the extent of adaptation achieved with the difference between observed errors (zero retinal error) and predicted errors (initial hypometria). In our study, however, we presented a positive retinal error to our subjects, which enabled us to avoid any potential confounds that would result from having the eye land hypermetric to the target. In addition, any errors in target placement tend to make saccades appear more hypometric than the intended 0.7° (median error of 0.02° with quartiles of −0.02° and 0.11°). Nonetheless, even with this certainty that the eye is consistently hypometric to the target, we were still able to observe a surprising gain-decrease adaptation across subjects.
Bahcall and Kowler (2000) demonstrated that if subjects are instructed to make a saccade only partway to a target, their saccade gain may be adapted by stepping that target forward or backward even though the retinal error remains large. They suggest that adaptation occurs because subjects depend on the intended saccade end point rather than the actual target itself; this intention is akin to the predicted motor outcome we posit in the present study. Although the intention to aim partway to the target is a conscious objective, whereas subjects are not aware of their normal hypometria, such movement goals must nonetheless be incorporated into the adaptation error signal; otherwise, subjects would adapt even if they intended to land short of the target (Bahcall and Kowler 2000). Our results suggest that such expectations directly affect the learning signal for adaptation.
If expectation plays such a large role in adaptation, this suggests that presenting subjects with a target error exactly equal to their prediction for a given trial should result in no change of the saccade gain. This situation is well approximated when subjects make saccades to fixed targets with no intrasaccadic target jump, a situation that causes no change in saccade gain. In this case, visual errors agree with prediction errors; thus there is no need to adapt the saccade gain. As a result, saccades remain hypometric rather than adapting to bring future saccade end points exactly on target. In addition, as demonstrated by the control task, if the target reappears in the same location as before the saccade, there is no significant change in the saccade gain (see also Bonnetblanc and Baraduc 2007). Therefore, if the intention to remain hypometric (the reason for which is still unknown) is incorporated into the adaptation error signal via a calculated prediction error, this could explain why saccades do not adapt completely to always bring the target directly onto the fovea.
These findings are consistent with the forward-model hypothesis, a commonly accepted proposal regarding the way that motor learning may occur. The hypothesis posits that the brain uses a copy of the motor command (efference copy) to model the expected outcome of a movement; this prediction is compared with the observed movement outcome at the conclusion of the saccade, and the error between predicted and observed outcomes drives adaptation of movement gain (Mehta and Schaal 2002; Miall and Wolpert 1996; Tseng et al. 2007). This error signal for adaptation is exactly the one we presently propose, suggesting that adaptation may indeed be driven by a forward model.
Although the forward-model hypothesis explains our findings, a simpler explanation is that subjects adapt in response to the direction of the target jump, regardless of postsaccadic error. This could explain not only our data but also those of Bahcall and Kowler (2000). However, although the correlation between the percentage of forward target steps and the extent of adaptation is significant, we found that data from individual subjects seem to disagree with this hypothesis. Furthermore, a stepwise regression involving the group data suggests that target behavior provides no additional information beyond that provided by prediction errors (the difference between observed and expected movement outcomes) to explain the extent of adaptation achieved. Together, this suggests it is unlikely that subjects are simply adapting in response to the behavior of the stimulus.
Other potential explanations for our findings have also been considered. Our results are not consistent with the hypothesis that the motor system seeks to maintain a constant-proportion movement bias. This hypothesis posits that hypometria is a fixed movement offset proportional to the primary saccade, which the motor system seeks to maintain during adaptation. However, subjects do not appear to adapt to maintain such a bias. The data are also inconsistent with the notion that subjects adapt in response to the motor correction; we have shown that saccade gain decreases despite making appropriate, forward-directed corrective saccades. This agrees well with previous studies that suggest the motor correction does not drive adaptation (Noto and Robinson 2001; Tseng et al. 2007; Wallman and Fuchs 1998). Still, it is surprising that motor corrections can be made in the opposite direction from that of gain changes during adaptation in light of studies suggesting that the superior colliculus may be involved in driving adaptation (Kaku et al. 2009; Soetedjo et al. 2009), since the superior colliculus issues saccade commands to the oculomotor plant. Nevertheless, our data clearly indicate that the motor correction does not influence adaptation.
Finally, the gain changes we observed may be the result of fatigue, particularly because the magnitude of gain decreases tend to be small. However, this is unlikely since saccades are reasonably resistant to fatigue in humans (Golla et al. 2008; Xu-Wilson et al. 2009). In addition, saccade amplitudes made during the control experiment did not show a tendency to decrease, which suggests that having a large number of saccades that ended in darkness does not cause an unusual amount of fatigue (but see Havermann and Lappe 2010). If anything, we saw the hint of an increase in saccade gain, although this finding is not significant. This agrees with the results of Bonnetblanc and Baraduc (2007), who observed both that saccades that conclude with no postsaccadic visual target actually tend to produce a gain increase and that saccades to targets that are blanked at saccade onset and reilluminated at the end of the movement are just as accurate as saccades made to continuously illuminated targets. Although their finding was only significant for very large saccades, the tendency to see a gain increase was hinted at for saccades of smaller magnitude as well (see their Figs. 1 and 2). Thus it seems unlikely that our findings can be explained simply as a result of fatigue.
In our paradigm we present a constant visual error, but, surprisingly, saccades eventually stop adapting (see also Kaku et al. 2009; Robinson et al. 2003). That is, although subject performance does not improve (retinal error remains fixed), adaptation reaches an asymptote after some time. One possibility is that the motor system has a limit on the amount of adaptation that may occur, and once this limit is reached, saccade amplitudes cannot be further modified. Alternatively, we propose the reason for this incomplete adaptation may be that motor learning not only modifies the system that is issuing motor commands (the motor controller) but also changes the predictor that is estimating movement outcomes (the forward model). In other words, the brain decides whether credit for the error lies in the motor system issuing a poor motor command or in a change in the external environment that results in an unexpected perturbation (Kording et al. 2007; Kluzik et al. 2008). If the error is caused by the motor system, future motor commands should be modified to account for changes in effector dynamics, muscle strength, or neural dysfunction; if the error is caused by the environment, future predictions should be modified to account for external perturbations. Depending on the reliability of the error and the uncertainties associated with both the issued motor command (movement variability) and the changing external environment, the adaptive system credits more of the error to one cause or the other.
However, the motor system rarely assigns all credit to only one of the two sources. Thus not only does the motor system issue a different command to achieve the desired goal, but it also should modify the forward model to predict a different observed outcome. In the case of constant-error adaptation, for example, the forward model would learn to expect a fixed postsaccadic retinal error, regardless of the primary saccade amplitude. Once this is learned, the difference between the observed retinal error and the motor prediction goes to zero (presented errors are fully anticipated), and adaptation stops. In standard double-step adaptation in which the target step is fixed, on the other hand, the forward model may learn to expect a target double-step, or at least a larger postsaccadic error than was previously typical. As this learning proceeds in tandem with changes in saccade gain, the error signal is driven to zero faster than if only visual errors alone were driving learning. This could explain why adaptation reaches an asymptote that is typically less than the requested gain change and is not equal to a fixed proportion of hypometria with respect to the primary saccade.
Such learning by the forward model is analogous to changing the system parameters of a state-space model or an optimal control model of the form typically used to model adaptation (Ethier et al. 2008a; Smith et al. 2006) as learning proceeds. Nonfixed parameters may be necessary to account for phenomena such as savings after a long washout period (Zarahn et al. 2008). These modifications to the forward model affect adaptation only via changes to the predicted movement outcome, which contributes one term to the derived adaptation error signal. Changes to the actual issued movement are affected by adaptation of the motor controller, which may learn by using a completely different mechanism. Previous studies have suggested that the motor controller modifies saccade gain by either target remapping for gain-increase adaptation or by changing saccade trajectories for gain-decrease adaptation (Ethier et al. 2008a, Semmlow et al. 1989). Thus, although speculative, the forward-model hypothesis may explain why adaptation is incomplete.
What is evident is that saccade adaptation does not occur in response to either the motor correction (Noto and Robinson 2001; Wallman and Fuchs 1998) or the pure visual error signal (Bahcall and Kowler 2000, Bonnetblanc and Baraduc 2007, Henson 1978). Our results suggest that the adaptation error signal is instead derived from a difference between the observed and expected movement outcomes, which can explain the finding of gain-decrease adaptation despite observing forward-directed postsaccadic visual errors. Such findings are consistent with the popular forward-model hypothesis, which utilizes efference copy to generate a prediction of motor output that can be used to drive adaptation.
GRANTS
This work was supported by National Science Foundation Grant BCS-0615106 and National Institutes of Health Grants T32-DC-000023 and R21-EY-019713.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We gratefully acknowledge D. Roberts for technical assistance and D. Zee for a critical reading of the manuscript.
REFERENCES
- Bahcall DO, Kowler E. The control of saccadic adaptation: implications for the scanning of natural visual scenes. Vision Res 40: 2779–2796, 2000 [DOI] [PubMed] [Google Scholar]
- Becker W. Metrics. In: The Neurobiology of Saccadic Eye Movements, edited by Wurtz RH, Goldberg M. New York: Elsevier Science, 1989 [Google Scholar]
- Bonnetblanc F, Baraduc P. Saccadic adaptation without retinal postsaccadic error. Neuroreport 18: 1399–1402, 2007 [DOI] [PubMed] [Google Scholar]
- Cohen ME, Ross LE. Latency and accuracy characteristics of saccades and corrective saccades in children and adults. J Exp Child Psychol 26: 517–527, 1978 [DOI] [PubMed] [Google Scholar]
- Collins T, Rolfs M, Deubel H, Cavanagh P. Post-saccadic location judgments reveal remapping of saccade targets to non-foveal locations. J Vis 9: 29.1.9517–29, 2009 [DOI] [PubMed] [Google Scholar]
- Deubel H, Wolf W, Hauske G. Adaptive gain control of saccadic eye movements. Hum Neurobiol 5: 245–253, 1986 [PubMed] [Google Scholar]
- Ethier V, Zee DS, Shadmehr R. Changes in control of saccades during gain adaptation. J Neurosci 28: 13929–13937, 2008a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethier V, Zee DS, Shadmehr R. Spontaneous recovery of motor memory during saccade adaptation. J Neurophysiol 99: 2577–2583, 2008b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Amagai A, Minakawa F, Aoki M. Selective and delay adaptation of human saccades. Brain Res Cogn Brain Res 13: 41–52, 2002 [DOI] [PubMed] [Google Scholar]
- Golla H, Tziridis K, Haarmeier T, Catz N, Barash S, Thier P. Reduced saccadic resilience and impaired saccadic adaptation due to cerebellar disease. Eur J Neurosi 27: 132–144, 2008 [DOI] [PubMed] [Google Scholar]
- Harris CM. Does saccadic undershoot minimize saccadic flight time? A Monte-Carlo study. Vision Res 35: 691–701, 1995 [DOI] [PubMed] [Google Scholar]
- Havermann K, Lappe M. The influence of the consistency of postsaccadic visual errors on saccadic adaptation. J Neurophysiol 103: 3302–3310, 2010 [DOI] [PubMed] [Google Scholar]
- Henson DB. Corrective saccades: effects of altering visual feedback. Vision Res 18: 63–67, 1978 [DOI] [PubMed] [Google Scholar]
- Irving EL, Steinbach MJ, Lillakas L, Babu RJ, Hutchings N. Horizontal saccade dynamics across the human life span. Invest Ophthalmol Vis Sci 47: 2478–2484, 2006 [DOI] [PubMed] [Google Scholar]
- Kaku Y, Yoshida K, Iwamoto Y. Learning signals from the superior colliculus for adaptation of saccadic eye movements in the monkey. J Neurosci 29: 5266–5275, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluzik J, Diedrichsen J, Shadmehr R, Bastian AJ. Reach adaptation: what determines whether we learn an internal model of the tool or adapt the model of our arm? J Neurophysiol 100: 1455–1464, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kording KP, Tenenbaum JB, Shadmehr R. The dynamics of memory as a consequence of optimal adaptation to a changing body. Nat Neurosci 10: 779–786, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin SC. Parametric adjustment in saccadic eye movements. Percept Psychophys 2: 359–362, 1967 [Google Scholar]
- Mehta B, Schaal SJ. Forward models in visuomotor control. J Neurophysiol 88: 942–953, 2002 [DOI] [PubMed] [Google Scholar]
- Miall RC, Wolpert DM. Forward models for physiological motor control. Neural Netw 9: 1265–1279, 1996 [DOI] [PubMed] [Google Scholar]
- Noto CT, Robinson FR. Visual error is the stimulus for saccade gain adaptation. Brain Res Cogn Brain Res 12: 301–305, 2001 [DOI] [PubMed] [Google Scholar]
- Robinson DA. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng 20: 137–145, 1963 [DOI] [PubMed] [Google Scholar]
- Robinson DA. Models of the saccadic eye movement control system. Biol Cybern 14: 71–83, 1973 [DOI] [PubMed] [Google Scholar]
- Robinson FR, Noto CT, Bevans SE. Effect of visual error size on saccade adaptation in monkey. J Neurophysiol 90: 1235–1244, 2003 [DOI] [PubMed] [Google Scholar]
- Semmlow JL, Gauthier GM, Vercher JL. Mechanisms of short-term saccadic adaptation. J Exp Psychol Hum Percept Perform 15: 249–258, 1989 [DOI] [PubMed] [Google Scholar]
- Shafer JL, Noto CT, Fuchs AF. Temporal characteristics of error signals driving saccadic gain adaptation in the macaque monkey. J Neurophysiol 84: 88–95, 2000 [DOI] [PubMed] [Google Scholar]
- Smith MA, Ghazizadeh A, Shadmehr R. Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biol 4: 1035–1043, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soetedjo R, Fuchs A, Kojima Y. Subthreshold activation of the superior colliculus drives saccade motor learning. J Neurosci 29: 15213–15222, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srimal R, Diedrichsen J, Ryklin EB, Curtis CE. Obligatory adaptation of saccade gains. J Neurophysiol 99: 1554–1558, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng Y, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol 98: 54–62, 2007 [DOI] [PubMed] [Google Scholar]
- Wallman J, Fuchs AF. Saccadic gain modification: visual error drives motor adaptation. J Neurophysiol 80: 2405–2416, 1998 [DOI] [PubMed] [Google Scholar]
- Xu-Wilson M, Chen-Harris H, Zee DS, Shadmehr R. Cerebellar contributions to adaptive control of saccades in humans. J Neurosci 29: 12930–12939, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarahn E, Weston GD, Liang J, Mazzoni P, Krakauer JW. Explaining savings for visuomotor adaptation: linear time-invariant state-space models are not sufficient. J Neurophysiol 100: 2537–2548, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]