Abstract
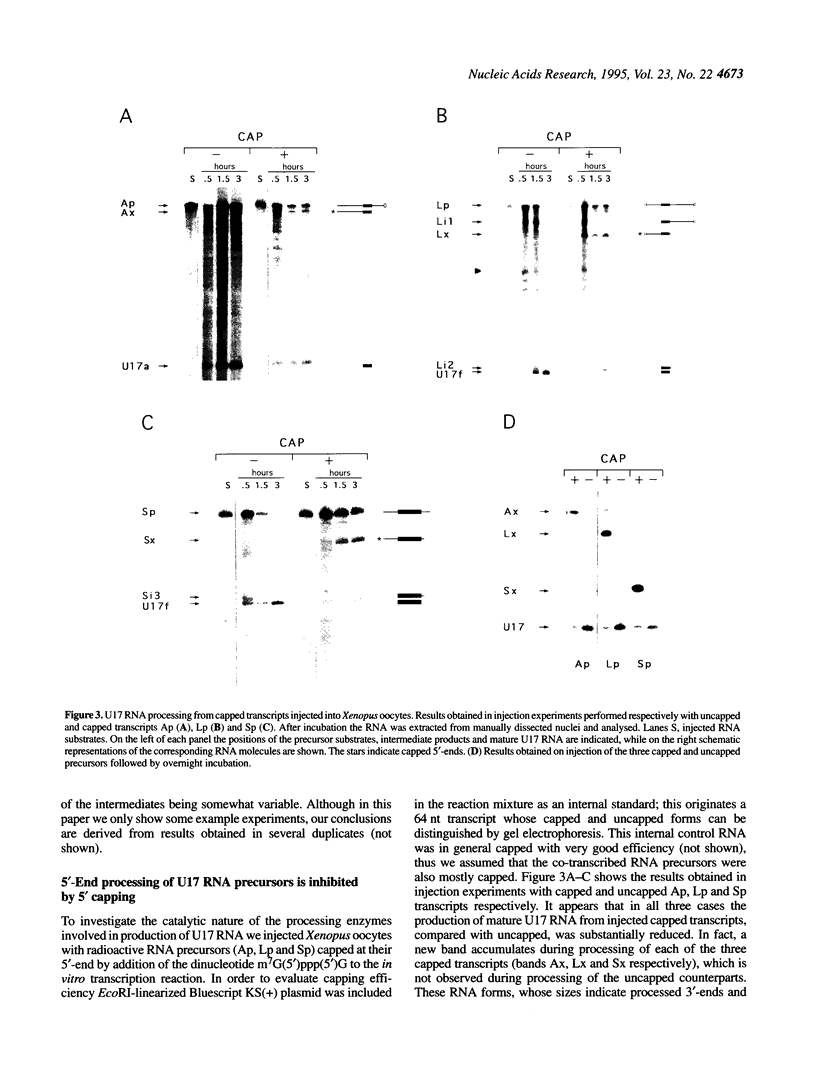
U17 is a small nucleolar RNA encoded in the introns of the Xenopus laevis gene for ribosomal protein S7 (formerly S8, see Note). To study the mechanisms involved in its in vivo processing from S7 transcripts, various in vitro synthesized RNAs embedding a U17 sequence have been microinjected into the germinal vesicle of Xenopus oocytes and their processing analysed. In particular, the Xenopus U17 gene copies a and f and a U17 gene copy from the pufferfish Fugu rubripes have been used. Information about the nature of the processing activities involved in U17 RNA maturation have been sought by injecting transcripts protected from exonucleolytic attack at their 5'-end by capping and/or lengthened at their 3'-end by polyadenylation. The results obtained indicate that U17 RNA processing is a splicing-independent event and that it is mostly or entirely due to exonucleolytic degradation at both the 5'- and 3'-ends of the precursor molecules. Moreover, it is concluded that the enzymes involved are of the processive type. It is suggested that the apparatus for U17 RNA processing is that responsible for the degradation of all excised and debranched introns. Protection from exonucleolytic attack, due to the tight structure and/or to the binding of specific proteins, would be the mechanism by which U17 RNA is produced.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein P., Ross J. Poly(A), poly(A) binding protein and the regulation of mRNA stability. Trends Biochem Sci. 1989 Sep;14(9):373–377. doi: 10.1016/0968-0004(89)90011-x. [DOI] [PubMed] [Google Scholar]
- Bozzoni I., Fragapane P., Annesi F., Pierandrei-Amaldi P., Amaldi F., Beccari E. Expression of two Xenopus laevis ribosomal protein genes in injected frog oocytes. A specific splicing block interferes with the L1 RNA maturation. J Mol Biol. 1984 Dec 25;180(4):987–1005. doi: 10.1016/0022-2836(84)90267-5. [DOI] [PubMed] [Google Scholar]
- Brenner S., Elgar G., Sandford R., Macrae A., Venkatesh B., Aparicio S. Characterization of the pufferfish (Fugu) genome as a compact model vertebrate genome. Nature. 1993 Nov 18;366(6452):265–268. doi: 10.1038/366265a0. [DOI] [PubMed] [Google Scholar]
- Caffarelli E., Arese M., Santoro B., Fragapane P., Bozzoni I. In vitro study of processing of the intron-encoded U16 small nucleolar RNA in Xenopus laevis. Mol Cell Biol. 1994 May;14(5):2966–2974. doi: 10.1128/mcb.14.5.2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecconi F., Mariottini P., Loreni F., Pierandrei-Amaldi P., Campioni N., Amaldi F. U17XS8, a small nucleolar RNA with a 12 nt complementarity to 18S rRNA and coded by a sequence repeated in the six introns of Xenopus laevis ribosomal protein S8 gene. Nucleic Acids Res. 1994 Mar 11;22(5):732–741. doi: 10.1093/nar/22.5.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasso M., Seki T., Azuma Y., Ohba T., Nishimoto T. A mutant form of the Ran/TC4 protein disrupts nuclear function in Xenopus laevis egg extracts by inhibiting the RCC1 protein, a regulator of chromosome condensation. EMBO J. 1994 Dec 1;13(23):5732–5744. doi: 10.1002/j.1460-2075.1994.tb06911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipowicz W., Kiss T. Structure and function of nucleolar snRNPs. Mol Biol Rep. 1993 Aug;18(2):149–156. doi: 10.1007/BF00986770. [DOI] [PubMed] [Google Scholar]
- Fournier M. J., Maxwell E. S. The nucleolar snRNAs: catching up with the spliceosomal snRNAs. Trends Biochem Sci. 1993 Apr;18(4):131–135. doi: 10.1016/0968-0004(93)90020-n. [DOI] [PubMed] [Google Scholar]
- Fragapane P., Prislei S., Michienzi A., Caffarelli E., Bozzoni I. A novel small nucleolar RNA (U16) is encoded inside a ribosomal protein intron and originates by processing of the pre-mRNA. EMBO J. 1993 Jul;12(7):2921–2928. doi: 10.1002/j.1460-2075.1993.tb05954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry Y., Wood H., Morrissey J. P., Petfalski E., Kearsey S., Tollervey D. The 5' end of yeast 5.8S rRNA is generated by exonucleases from an upstream cleavage site. EMBO J. 1994 May 15;13(10):2452–2463. doi: 10.1002/j.1460-2075.1994.tb06530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T., Filipowicz W. Exonucleolytic processing of small nucleolar RNAs from pre-mRNA introns. Genes Dev. 1995 Jun 1;9(11):1411–1424. doi: 10.1101/gad.9.11.1411. [DOI] [PubMed] [Google Scholar]
- Kiss T., Filipowicz W. Small nucleolar RNAs encoded by introns of the human cell cycle regulatory gene RCC1. EMBO J. 1993 Jul;12(7):2913–2920. doi: 10.1002/j.1460-2075.1993.tb05953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverette R. D., Andrews M. T., Maxwell E. S. Mouse U14 snRNA is a processed intron of the cognate hsc70 heat shock pre-messenger RNA. Cell. 1992 Dec 24;71(7):1215–1221. doi: 10.1016/s0092-8674(05)80069-8. [DOI] [PubMed] [Google Scholar]
- Mariottini P., Bagni C., Francesconi A., Cecconi F., Serra M. J., Chen Q. M., Loreni F., Annesi F., Amaldi F. Sequence of the gene coding for ribosomal protein S8 of Xenopus laevis. Gene. 1993 Oct 15;132(2):255–260. doi: 10.1016/0378-1119(93)90204-g. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlrad D., Parker R. Premature translational termination triggers mRNA decapping. Nature. 1994 Aug 18;370(6490):578–581. doi: 10.1038/370578a0. [DOI] [PubMed] [Google Scholar]
- Nicoloso M., Caizergues-Ferrer M., Michot B., Azum M. C., Bachellerie J. P. U20, a novel small nucleolar RNA, is encoded in an intron of the nucleolin gene in mammals. Mol Cell Biol. 1994 Sep;14(9):5766–5776. doi: 10.1128/mcb.14.9.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellizzoni L., Crosio C., Campioni N., Loreni F., Pierandrei-Amaldi P. Different forms of U15 snoRNA are encoded in the introns of the ribosomal protein S1 gene of Xenopus laevis. Nucleic Acids Res. 1994 Nov 11;22(22):4607–4613. doi: 10.1093/nar/22.22.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierandrei-Amaldi P., Beccari E., Bozzoni I., Amaldi F. Ribosomal protein production in normal and anucleolate Xenopus embryos: regulation at the posttranscriptional and translational levels. Cell. 1985 Aug;42(1):317–323. doi: 10.1016/s0092-8674(85)80127-6. [DOI] [PubMed] [Google Scholar]
- Pierandrei-Amaldi P., Beccari E. Messenger RNA for ribosomal proteins in Xenopus laevis oocytes. Eur J Biochem. 1980 May;106(2):603–611. doi: 10.1111/j.1432-1033.1980.tb04608.x. [DOI] [PubMed] [Google Scholar]
- Prislei S., Michienzi A., Presutti C., Fragapane P., Bozzoni I. Two different snoRNAs are encoded in introns of amphibian and human L1 ribosomal protein genes. Nucleic Acids Res. 1993 Dec 25;21(25):5824–5830. doi: 10.1093/nar/21.25.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu L. H., Nicoloso M., Michot B., Azum M. C., Caizergues-Ferrer M., Renalier M. H., Bachellerie J. P. U21, a novel small nucleolar RNA with a 13 nt. complementarity to 28S rRNA, is encoded in an intron of ribosomal protein L5 gene in chicken and mammals. Nucleic Acids Res. 1994 Oct 11;22(20):4073–4081. doi: 10.1093/nar/22.20.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff E. A., Rimoldi O. J., Raghu B., Eliceiri G. L. Three small nucleolar RNAs of unique nucleotide sequences. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):635–638. doi: 10.1073/pnas.90.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollner-Webb B. Novel intron-encoded small nucleolar RNAs. Cell. 1993 Nov 5;75(3):403–405. doi: 10.1016/0092-8674(93)90374-y. [DOI] [PubMed] [Google Scholar]
- Tycowski K. T., Shu M. D., Steitz J. A. A small nucleolar RNA is processed from an intron of the human gene encoding ribosomal protein S3. Genes Dev. 1993 Jul;7(7A):1176–1190. doi: 10.1101/gad.7.7a.1176. [DOI] [PubMed] [Google Scholar]
- Tycowski K. T., Shu M. D., Steitz J. A. Requirement for intron-encoded U22 small nucleolar RNA in 18S ribosomal RNA maturation. Science. 1994 Dec 2;266(5190):1558–1561. doi: 10.1126/science.7985025. [DOI] [PubMed] [Google Scholar]
- Wormington M. Preparation of synthetic mRNAs and analyses of translational efficiency in microinjected Xenopus oocytes. Methods Cell Biol. 1991;36:167–183. doi: 10.1016/s0091-679x(08)60277-0. [DOI] [PubMed] [Google Scholar]
- Yisraeli J. K., Melton D. A. Synthesis of long, capped transcripts in vitro by SP6 and T7 RNA polymerases. Methods Enzymol. 1989;180:42–50. doi: 10.1016/0076-6879(89)80090-4. [DOI] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]