Abstract
Previously, we reported a pronounced reduction in transmission through surviving axons contralateral to chronic hemisection (HX) of adult rat spinal cord. To examine the cellular and molecular mechanisms responsible for this diminished transmission, we recorded intracellularly from lumbar lateral white matter axons in deeply anesthetized adult rats in vivo and measured the propagation of action potentials (APs) through rubrospinal/reticulospinal tract (RST/RtST) axons contralateral to chronic HX at T10. We found decreased excitability in these axons, manifested by an increased rheobase to trigger APs and longer latency for AP propagation passing the injury level, without significant differences in axonal resting membrane potential and input resistance. These electrophysiological changes were associated with altered spatial localization of Nav1.6 sodium channels along axons: a subset of axons contralateral to the injury exhibited a diffuse localization (>10 μm spread) of Nav1.6 channels, a pattern characteristic of demyelinated axons (Craner MJ, Newcombe J, Black JA, Hartle C, Cuzner ML, Waxman SG. Proc Natl Acad Sci USA 101: 8168–8173, 2004b). This result was substantiated by ultrastructural changes seen with electron microscopy, in which an increased number of large-caliber, demyelinated RST axons were found contralateral to the chronic HX. Therefore, an increased rheobase, pathological changes in the distribution of Nav1.6 sodium channels, and the demyelination of contralateral RST axons are likely responsible for their decreased conduction chronically after HX and thus may provide novel targets for strategies to improve function following incomplete spinal cord injury.
Keywords: in vivo intra-axonal recording, rheobase, demyelination
using in vivo intracellular recordings from motoneurons in adult rats, we recently recorded synaptic responses from motoneurons after unilateral hemisection (HX) of the spinal cord at T10, when one side of the cord is lesioned and the other half remains intact (Arvanian et al. 2009). We found that synaptic responses recorded from individual motoneurons in the L5 ventral horn and evoked by electrical stimulation of white matter axons at T7, contralateral to HX, began to decline 1 wk post-spinal cord injury (SCI). This diminished transmission plateaued at 2 wk post-HX and remained reduced for at least 14 wk postlesion. The decline of synaptic responses was associated with a decrease in the amplitude of the volley responses recorded extracellularly from the L5 ventral horn (Arvanian et al. 2009; Hunanyan et al. 2010), suggesting that the decay in synaptic transmission during the chronic stage of HX could be determined by conduction deficits in fibers contralateral to HX. Consistent with the decline of responses that we recorded from the intact side below chronic HX and evoked by electric stimulation above, Hubscher and Johnson (2002) reported that in cases of chronic (4 wk) lateral HX at T8, neuronal responses on both sides of the medullary reticular formation to pinching of the hind paw on the side opposite the lesion (intact side) were weak or completely absent, depending on the lesion extent. However, extracellular recording cannot distinguish whether conduction deficits are the result of changes in the axonal resting membrane potential, membrane resistance, or the excitability of individual axons. Thus the mechanisms that underlie conduction deficits at the level of the single axon after unilateral chronic HX were not examined.
Intra-axonal recordings, however, have been shown to be a more direct technique for the assessment of an individual axon's electrical properties (Baker et al. 1987; Blight and Someya 1985; David et al. 1995; Honmou et al. 1996; Kocsis and Waxman 1982; Kriz and Padjen 2003). Importantly, intra-axonal recording allows the measurement of rheobase current, which correlates directly with the excitability of the axon. In the present study, to characterize the electrical properties of individual axons contralateral to the HX and to measure the propagation of action potentials (APs) through axons passing across the injury level, we recorded in vivo intra-axonally from lumbar rubrospinal/reticulospinal tract (RST/RtST) axons at 2–6 wk after HX. The RST/RtST axons are known to be heavily myelinated and to be important for rodent locomotion (Ballermann and Fouad 2006; Brown 1974; Kanagal and Muir 2008; Waldron and Gwyn 1969; Webb and Muir 2003). We found increased rheobase (i.e., larger depolarization pulse through the intra-axonal recording electrode required to trigger APs), as well as pathological changes in propagation of APs through individual uncut axons across from chronic HX.
Our ultrastructural analysis of the RST axons across from HX revealed an increased number of demyelinated axons on the uninjured side of the spinal cord in chronic injured rats. Demyelination of axons has been attributed to the loss of axonal function and development of neurological deficits in SCI and other types of neurotrauma, including multiple sclerosis and stroke (Blight and Decrescito 1986; McDonald et al. 2000; Nashmi and Fehlings 2001). In the models of multiple sclerosis and contusion injury, demyelination of axons has been associated with diffuse distribution of Nav1.6 sodium channels along the axons (Craner et al. 2004b). In this study we have shown similar changes in the distribution pattern of Nav1.6 channels in lateral white matter axons, opposite the injury, after chronic HX. In the uninjured spinal cord, Nav1.6 channels were clustered along the axons, specifically mostly at the nodes of Ranvier (as identified by flanking paranodal staining with anti-Caspr antibody), thus supporting rapid propagation of AP. However, in chronic HX rats, there was diffuse expression of Nav1.6 along a subset of spared axons in the contralateral white matter. Together, our findings of increased rheobase, demyelination, and abnormal distribution of Nav1.6 sodium channels in RST axons likely underlie the decreased excitability of contralateral axons in chronically HX adult rats.
MATERIALS AND METHODS
These studies were performed in accordance with protocols approved by the Institutional Animal Care and Use Committees at the State University of New York at Stony Brook and the Veterans Affairs Medical Center.
Surgical procedure for the unilateral HX.
Experiments were carried out on adult (∼210 g) female Sprague-Dawley rats (n = 37 rats). Animals were deeply anesthetized in an induction chamber with 3% isoflurane in 100% O2. After deep anesthesia, the animals were transferred to a facemask, where they were kept during surgery in 1.5% isoflurane and 100% O2. Dorsal laminectomy was performed to expose spinal cord segments T9–T11, and HX was performed as previously described (Arvanian et al. 2009). Briefly, one tip of the iridectomy scissors was passed through the entire thickness of the T10 spinal cord dorsal to ventral at the midline, and the left dorsal and ventral columns were cut from lateral to the midline. The muscle and skin were closed in layers, and antibiotic (Baytril, 5 mg/kg, 0.1 ml), analgesic (buprenorphine, 0.5 mg/kg, 0.1 ml), and sterile saline (10 ml) were administered subcutaneously. Control animals received a similar laminectomy procedure but no lesion.
In vivo intra-axonal and extra-axonal recordings.
Noninjured rats or rats 2–6 wk postinjury were deeply anesthetized with a ketamine (80 mg/kg, 0.5 ml)-xylazine (10 mg/kg, 0.5 ml) mixture administered by intraperitoneal injection. Heart rate and expired CO2 were monitored continuously. Two dorsal laminectomies of the spinal cord were performed to expose T6–T8 (for placement of the stimulation electrode) and L1–L6 (for placement of the recording electrodes). L1–L6 ventral spinal segments were placed tightly between custom-made bars, and the dorsal surface of the cord was embedded in a 3-mm-thick agar layer to ensure lack of movement of the cord during recordings. An opening in the agar layer above the recording site was filled with mineral oil, and dura was carefully removed to access recording electrodes from the dorsal surface of the cord. This procedure ensured stable intra-axonal recordings from the L5 segment. Several attempts to achieve stable intra-axonal recordings from the thoracic level were not successful, because the agar layer alone was not sufficient to immobilize the cord, while tightening of thoracic spinal segments within the bars resulted in breathing problems, and stable recordings could not be made by using paralytics along with a respirator.
Intra-axonal and extra-axonal recordings were performed simultaneously from L5 lateral white matter contralateral to the side of the injury in response to electrical stimulation at T7 lateral white matter ipsilateral to recording. The electrical stimulation electrode was positioned between the dorsal root entry zone and lateral edge at T7 via a micromanipulator and lowered to the depth of 1.3 mm to recruit axons of RST/RtST. The insulation coat of the stimulating electrode was removed from the tip that was inserted into the cord (1.3 mm). Electrical stimulation had 50-μs duration and was delivered at 1 Hz.
For intra-axonal recordings (Axoprobe amplifier; Molecular Devices), axons were impaled with sharp glass microelectrodes with resistances of 25–50 MΩ, filled with 3 M K-acetate. A glass microelectrode was attached to a hydraulic microdrive (David Kopf Instruments), which precisely (with accuracy to 1 μm) measures the depth of the tip of the electrode. Intra-axonal recordings were performed starting from the dorsal column of the right lateral edge of L5 to a depth of ∼1.2 mm. Intra-axonal impalements were identified by an abrupt drop of membrane potential, no changes of AP amplitude when the stimulus intensity was changed above threshold to evoke a response (“all or none”), and no underlying synaptic potential (Kocsis and Waxman 1980). An active bridge circuit allowed simultaneous current injection and recording through the same electrode. Axons with membrane potentials more negative than −55 mV were used for analysis. Usually, we recorded from 6–10 axons in each rat. Intra-axonal recordings were performed in the following sequence: after impalement of the axons, the resting membrane potential was measured and the rheobase (the minimum current that induced APs 100% of the time) was determined by delivering depolarizing current of 10-ms duration and 0.1-nA increments through the recording electrode; hyperpolarizing current steps were then applied through the recording electrode for measurement of axon input resistance and to evoke APs at anode break. After concluding examination of the axon by applying currents through the recording electrode, we examined in the same axon properties of APs evoked by electrical stimulation of the lateral white matter at T7 ipsilateral to recording. We measured minimum stimulus intensity of current at T7 that was required to induce AP at L5. Latency, maximum rise and decay slopes, and, finally, peak amplitude of the AP were measured using stimulus of 1.5× minimum intensity.
The input resistance (Rin) of the axons was measured by plotting voltage responses vs. hyperpolarization current steps and fitting the resulting curve using linear regression (SigmaPlot 11.0). Peak amplitude of APs was averaged from 20–30 consecutive responses/axon. The latency was measured from the beginning of the stimulus artifact to the rising phase of response. Axons with the same resting membrane potentials were used to compare peak amplitudes, rheobase, rise/decay slopes, and duration (measured as width at half-height) (Jackson et al. 1991) of APs in individual axons in normal and chronically injured rats. Measurement of the maximal rise and decay slope of the AP (dV/dtmax) was obtained using Clampfit 10.2 software. Axons with matched AP amplitudes were used to compare the duration and the maximum rise and decay slopes of the AP.
For extra-axonal recordings, we used tungsten electrodes (FHC, resistance 300 KΩ). The recording tungsten electrode was lowered into the lumbar L5 lateral white matter spinal cord to a depth of 1 mm, corresponding to the location of the RST (Waldron and Gwyn 1969; Webb and Muir 2003). The electrode was positioned to enter the lateral white matter of the cord at an angle of 20–22° from vertical in the sagittal plane (tip directed rostrally). The same stimulation electrode in T7 was used to evoke the APs (intra-axonal) and the volley (extra-axonal) responses. Extra-axonal composed responses representing the volley of AP consisted of a biphasic component, and peak amplitude was measured between up-going and down-going peaks (see arrows in Fig. 5A). For extra-axonal evoked responses, the responses to 50 consecutive stimuli were averaged. All signals were digitized at 100 kHz, stored on a personal computer, and analyzed off-line using PClamp 10.
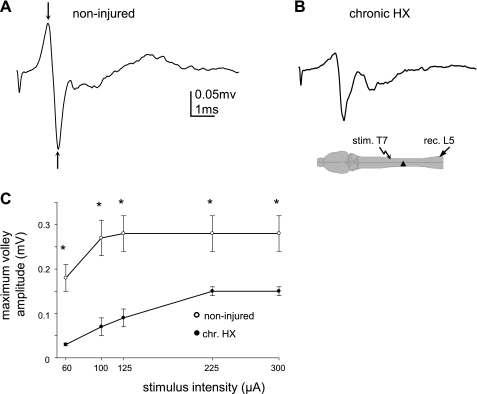
Fig. 5.
Diminished extracellular responses recorded from the L5 lateral white matter and evoked by electrical stimulation of the lateral white matter ipsilaterally at T7 in chronic HX SCI rats. A and B: representative traces of the volley of APs in noninjured (A) and chronic HX SCI rats (B). Diagram shows the configuration of the stimulation and recording electrodes. C: summary of results indicating the diminished maximum volley responses and the higher stimulus intensity required to induce these responses in chronic HX SCI rats. *P < 0.05.
Immunostaining of Caspr and Nav1.6 channels and quantitative analysis.
Spinal cords were removed, immersion fixed for 2 h in freshly prepared 4% paraformaldehyde in PBS, and then transferred into a PBS-sucrose solution (30%) for cyroprotection. The spinal cord segments containing the lesion sites (T10) were sectioned horizontally on a cryostat, and 20-μm-thick sections were collected serially onto substrated glass slides. Frozen sections were blocked for 2 h in 0.3% Triton (X-100) plus 6% normal goat serum in PBS and then incubated in monoclonal mouse anti-Caspr (1:1,000; NeuroMab) and polyclonal rabbit anti-Nav1.6 (1:300; gift from James Trimmer) overnight in the refrigerator. Sections were then washed with PBS three times for 10 min, followed by incubation in secondary AlexaFluor antibodies (Invitrogen), goat anti-rabbit IgG (AF 488) and goat anti-mouse (AF 594), for 45 min at room temperature and protected from light. After incubation in secondary antibodies, the sections were washed in PBS, rinsed in distilled H2O, and coverslipped with Fluoromount-G (Southern Biotech). After double staining for Nav1.6 and Caspr, confocal images were captured with a Zeiss LSM 510 microscope. Quantitative analysis of Nav1.6 staining was performed using ImageJ software (version 1.41) on sections from T10 right lateral white matter from normal and chronic HX rats.
Examination of tissue (sections taken at ∼1.2 mm depth dorsal to ventral) from control animals showed Nav1.6 staining confined to the nodes of Ranvier as identified by paranodal staining with anti-Caspr antibody, as expected. In contrast, in the tissue from HX cases, an obvious appearance of many profiles with diffuse, extranodal Nav1.6 staining along their lengths was noted. Those “wormlike” profiles, with areas of diffuse Nav1.6 staining extending more than 10 μm, were analyzed using ImageJ software for quantitative analysis. We measured the mean signal intensities of manually outlined profiles and compared them with background. A profile was considered immunopositive if the mean signal intensity was at least two times higher than background levels (Craner et al. 2004b). Many of these profiles contained examples of Nav1.6-positive staining in nodes identified by Caspr staining, identifying these wormlike profiles as axons.
Estimation of the number and percentage of myelinated axons.
Spinal cords from noninjured and chronic HX (2–6 wk post-HX) groups were removed after transcardial perfusion and prepared for morphological evaluation of the axons. Plastic embedment of a 2-mm-long piece of spinal cord containing the lesion was prepared as described previously (Pearse et al. 2005). With the use of Stereoinvestigator software (MicroBrightfield), the total number of myelinated axons in the area corresponding to the RST in the white matter across from the lesion was stereologically quantified (Schaal et al. 2007). Calculations were performed on three 1-μm plastic toluidine blue-stained semithin transverse sections taken from the injury epicenter within the tissue block. The mean data from these three sections were used for the final count. For myelinated-to-unmyelinated axon ratios in the RST opposite to the HX, thin sections from the tissue block were examined and photographed using a Philips CM10 electron microscope. Total fiber number was calculated by multiplying the myelinated axon count by the ratio of unmyelinated to myelinated axons and adding the resulting number to the myelinated axon count. The percentage of myelinated fibers was than calculated as a percentage of the total number of axons.
Statistical analysis.
For electrophysiological and immunostaining data analyses, a one-way ANOVA or one-way ANOVA on ranks (SigmaPlot 11.0) was employed. If significant differences were observed between groups, we used either a Student-Newman-Keuls test for pairwise multiple comparisons (normal distribution) or Dunn's method (nonparametric distribution). For estimation of the number and percentage of myelinated axons, we used a one-way ANOVA followed by Tukey's multiple comparisons posttest to compare groups. Data are means ± SE. A P < 0.05 was considered statistically significant.
RESULTS
Properties of single-axon APs recorded intra-axonally in L5 lateral white matter in response to stimulation of ipsilateral white matter at T7 in noninjured rats.
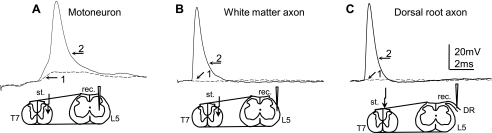
Since the region comprising white matter is very limited compared with gray matter within the lumbar spinal cord, it was important to confirm that we recorded from axons within the white matter axon and not from neurons in the gray matter during “blind” intracellular recording. Therefore, we carefully positioned recording electrodes by using a micromanipulator and compared the properties of APs recorded from 1) gray matter motoneurons (Fig. 1A; electrode positioned in the ventral horn and motoneurons identified by antidromic APs evoked by electrical stimulation of the corresponding ventral root; described in Arvanian et al. 2009), 2) lateral white matter axons (Fig. 1B; electrode positioned close to the lateral edge), and 3) dorsal root axons close to the dorsal root entry zone (Fig. 1C) that contains axons only and no neurons. Our results revealed that the best test to distinguish APs in motoneurons vs. APs in axons was to evoke APs in L5 by stimulation of the T7 lateral white matter and to examine dependence of these responses on stimuli of rising intensity. In the case of recording from a L5 ventral horn motoneuron in response to electrical stimulation at T7 lateral white matter ipsilateral to the recording electrode, the increasing stimulus intensity first evoked an excitatory postsynaptic potential (EPSP) (Fig. 1A, trace 1). A further increase of stimulus intensity induced an AP on top of some EPSP responses (Fig. 1A, trace 2). In contrast, in the case of intra-axonal recordings from the L5 lateral white matter, the EPSP phase was absent and increasing the intensity of stimulation at T7 evoked an AP in the L5 axons following the all-or-none rule (Fig. 1B). Note that in the case of stimulation of the lateral white matter and intracellular recording from L5 motoneurons, the EPSP phase of different amplitude was observed in all motoneurons. However, the AP could not be evoked on the top of EPSP in all motoneurons, although in the same motoneurons, AP could be triggered by applying a depolarization current through the recording electrode or by antidromic stimulation of the ventral root. As expected, APs recorded from the L5 dorsal root axons and evoked by electrical stimulation of fasciculus gracilis at T7 displayed a similar shape of AP and a similar dependency on stimulus intensity as the AP recorded from the L5 white matter axon from the same rat (Fig. 1C).
Fig. 1.
Comparison of the action potentials (APs) measured intracellularly from an L5 motoneuron (MN) and intra-axonally from a lateral white matter axon and a dorsal root (DR) axon in the spinal cord of the same adult rat. A: intracellular recording from the L5 MN in response to electrical stimulation of the lateral white matter ipsilaterally at T7 with rising stimulus intensity; the intensity of electrical stimulation was 30 μA [1, dotted trace; stimulus intensity was at maximum to evoke the maximum excitatory postsynaptic potential (EPSP) response] and 80 μA (2, solid trace; stimulus intensity was the minimum required to evoke an AP), respectively. B: intra-axonal recording from a L5 rubrospinal tract (RST) axon in response to electrical stimulation of the lateral white matter ipsilaterally at T7; the intensity of electrical stimulation was 80 μA (1) and 90 μA (2; minimum stimulus intensity required to trigger AP in this particular axon), respectively. C: intra-axonal recording from a L5 dorsal root axon in response to electrical stimulation of the fasciculus gracilis at T7; the intensity of electrical stimulation was 140 μA (1) and 150 μA (2; minimum stimulus intensity required to trigger AP), respectively. Diagrams below the traces show the positions of the stimulating (st.) and recording (rec.) electrodes. Note the EPSP phase is before the AP when recorded from MNs, but there is an absence of such an EPSP phase when the recording is made either from the lateral white matter or from DR axons.
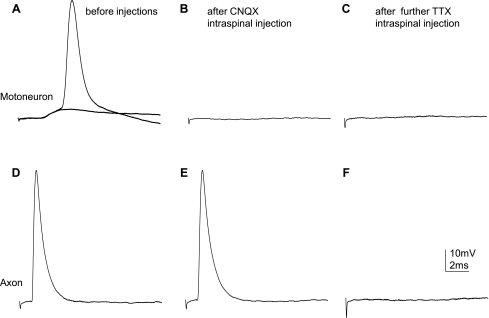
To find out more differences between motoneurons and axons in AP responses, we compared the pharmacological properties of these responses. It was shown previously that administration of 6-cyano-7-nitroquino-xaline-2,3-dione (CNQX; a dl-α-amino-3-hydroxy-5-methylisoxazole propionic acid/kainate receptor blocker that selectively blocks the initial monosynaptic response of motoneurons) blocked EPSP responses recorded intracellularly from ventral horn motoneurons in neonatal (Arvanian and Mendell 2001) and adult rats (Hunanyan et al. 2010). We compared effects of intraspinal injections of CNQX near the recording electrode with the responses recorded intracellularly from L5 motoneurons and white matter axons and evoked by stimulation of the T7 white matter in the same spinal cord. After injection of CNQX (30 μm/0.8 μl) near the recording electrode, the motoneuron EPSP response and AP on the top of this response were abolished (Fig. 2, A and B; details of intraspinal injection procedures are described in Hunanyan et al. 2010). Our attempts to record from other motoneurons in the vicinity of the CNQX injection site revealed a lack of EPSP responses in that region, although stimulation of the ventral root still triggered antidromic AP in these motoneurons. Further recordings from white matter axons in the same cord revealed that APs could be obtained from adjacent white matter after injection of CNQX (Fig. 2, D and E). After injection of tetrodotoxin (TTX; 20 μm/0.8 μl), an inhibitor of voltage-gated sodium channels, into the lateral white matter within the vicinity of the recording electrode, we could not find any AP responses (Fig. 2F).
Fig. 2.
Comparison of the pharmacological properties of EPSPs recorded from L5 MNs vs. APs recorded from L5 lateral white matter axons, both evoked by electrical stimulation of ipsilateral white matter at T7. A–C: EPSP responses from a MN before (A) and after 6-cyano-7-nitroquino-xaline-2,3-dione (CNQX) injections into the gray matter close to the recording electrode (B) and after tetrodotoxin (TTX) injections into the white matter rostral/ipsilateral to the recording electrode (C). D–F: axonal AP before (D) and after CNQX injections close and rostral to the recording electrode (E) and after TTX injections into the white matter rostral/ipsilateral to the recording electrode (F). Note that CNQX injections blocked EPSPs in the MN but did not affect the propagation of an axonal AP. Further TTX injection into the white matter blocked the axonal AP.
Thus APs recorded intracellularly from white matter axons and evoked by stimulation of the T7 white matter could be identified by the following: 1) the all-or-none rule, when recorded in L5 in response to increasing stimulation intensity when the stimulation electrode was positioned in T7 (Fig. 1; used routinely to identify axonal APs), and 2) resistance to CNQX intraspinal injections (Fig. 2).
Effect of chronic HX on APs recorded intra-axonally from L5 lateral white matter and triggered by depolarization current.
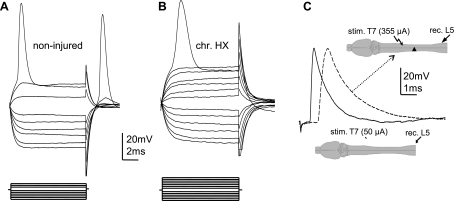
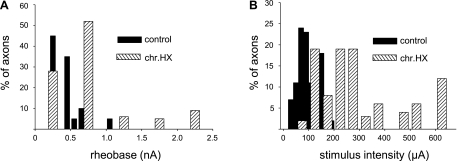
To examine the effect of chronic HX on the excitability of axons within the spared lateral white matter contralateral to the lesion, we compared properties of APs triggered by depolarization current steps in noninjured and chronically HX spinal cords. After penetration of the axon, we first examined the properties of APs as described in Fig. 1, to ensure that the recordings were intra-axonal. Next, to examine the axon's membrane properties in normal and chronic HX rats, we applied both hyperpolarizing and depolarizing current steps through the recording electrode (Fig. 3). Depolarizing steps were applied to determine rheobase, i.e., the minimum depolarization current required to trigger APs (Fig. 3, A and B). We found that in chronically injured animals, the mean rheobase increased significantly compared with that in noninjured animals (noninjured: 0.4 ± 0.04 nA, n = 9 rats/64 axons vs. injured: 0.8 ± 0.1 nA, n = 10 rats/55 axons, P < 0.01). In other words, a higher intensity current passing through the recording electrode was required to trigger APs in the axons of lesioned rats, indicating a decreased excitability of surviving axons in chronic HX rats. An overall comparison revealed more axons with a higher mean rheobase in chronic HX compared with noninjured rats (presented in histogram form in Fig. 4A).
Fig. 3.
Intra-axonal recording from the lateral white matter axons was done to demonstrate the decreased excitability (higher rheobase current), decreased conduction velocity (increased latency), and absence of changes in the resting membrane potential (RMP) in RST axons passing across from chronic hemisection (chr. HX). Changes in the properties of APs recorded intra-axonally from RST axons contralateral to the chronic HX spinal cord injury (SCI) are shown. A and B: representative traces show voltage membrane responses in single axons to current steps passed through the recording electrode in noninjured (A) and chronic HX SCI rats (B). Both axons had a RMP of −60 mV. Current steps (displayed below the voltage traces) of a 0.1-nA increment were applied through the recording electrode in both hyperpolarizing (to measure membrane resistance) and depolarizing directions (to trigger an AP). Note the higher rheobase but similar membrane resistance in the axon from chronic HX spinal cord. C: superimposed examples of APs in normal (solid trace) and chronic HX SCI rats (dashed trace) to demonstrate a longer latency of the AP recorded from L5 lateral white matter axons in response to electrical stimulation of T7 ipsilateral white matter. Both axons displayed a RMP of −63 mV. Diagram shows the positions of the recording (rec.) and stimulating (stim.) electrodes in normal (bottom) and chronic HX cord (arrow, top).
Fig. 4.
Histograms show the difference in the electrical properties of single axons recorded intra-axonally within the L5 lateral white matter in noninjured and chronic HX SCI rats. A: the rheobase current at the recording electrode required to trigger an AP. B: the minimum stimulus intensity at the T7 stimulating electrode required to evoke an AP in L5 lateral white matter axons. Note an increased percentage of axons that have a relatively high rheobase (A) and require a relatively high stimulus intensity to evoke an AP (B) in HX SCI rats. For comparison of the noninjured control and chronic HX SCI animals, we used axons that displayed a similar RMP.
Despite the changes in rheobase, the mean Rin of the axons, acquired through hyperpolarizing current pulses (Fig. 3, A and B), revealed no statistically significant difference between noninjured (81 ± 8.1 MΩ) and chronic HX rats (82 ± 7.3 MΩ, P > 0.05). Note that the termination of the hyperpolarizing current (anode break) triggered an AP with an increased amplitude in many axons when recorded in the noninjured spinal cord (in 47 of 64 axons recorded, Fig. 3A), which is consistent with properties of APs recorded in vitro from lumbosacral axons (Kocsis and Waxman 1982) and dorsal root axons (Novak et al. 2009) in rats. However, few axons contralateral to chronic HX (14 of 55 axons recorded) showed an anode break excitation after termination of the hyperpolarizing current. The mean resting membrane potential of the axons from both groups of animals was not significantly different (noninjured: −63 ± 2.1 mV vs. chronic HX: −60 ± 1.7 mV, P > 0.05). A similar percentage of axons (∼20%) was rejected because of depolarized resting potentials (greater than −55 mV) in the two groups (P > 0.05). The mean peak amplitude of the APs was averaged for all axons recorded in each group and was not significantly changed (noninjured: 57 ± 2.5 mV vs. chronic HX: 55 ± 2.2 mV, P > 0.05). Note that although many APs that we recorded did overshoot (∼60% of axons), the APs recorded from other axons did not overshoot, particularly when we recorded from axons with resting potential of −70 mV and more negative.
Other parameters that we measured were the maximum rise slope (dV/dt) and the decay slope of APs. Since rise slope is highly dependent on the peak amplitude of the AP (Renganathan et al. 2001), for comparison we used APs of the same peak amplitude at the same resting membrane potential in both noninjured and chronic HX rats. Although we observed decreased dV/dt of AP in some axons in chronic HX rats, the overall difference was not statistically significant (P > 0.05). However, when combined, the AP duration in chronic HX rats broadened compare with noninjured rats (noninjured: 0.97 ± 0.03 ms vs. chronic HX: 1.13 ± 0.04 ms, P < 0.05). A summary of these results is presented in Table 1.
Table 1.
Electrical properties of lateral white matter axons as determined by passing current through the recording electrode
| Resting Membrane Potential, mV | Input Resistance, MΩ | Rheobase, nA | AP Amplitude, mV | Rise Slope/Decay Slope, mV/ms | AP Duration at Half-Height, ms | |
|---|---|---|---|---|---|---|
| Control rats | −63 ± 2.1 | 81 ± 8.1 | 0.4 ± 0.04 | 57 ± 2.5 | 220 ± 13/123 ± 8 | 0.97 ± 0.03 |
| Chronic HX rats | −60 ± 1.7 | 82 ± 7.3 | 0.8 ± 0.1† | 55 ± 2.2 | 181 ± 19/118 ± 6 | 1.13 ± 0.04* |
Values are means ± SE for control (n = 9 rats/64 axons) and chronic hemisection (HX) rats (n = 10 rats/55 axons). Depolarization and hyperpolarization current steps were applied to trigger an action potential (AP) and to measure input resistance, respectively.
P < 0.05;
P < 0.01.
Propagation of APs through single axons in white matter contralateral to chronic HX (intra-axonal recording).
To examine AP propagation through white matter axons across from the HX (HX at T10), we recorded APs intra-axonally from white matter contralateral to the HX at L5 (caudal to HX) in response to stimulation of white matter at T7 (rostral to HX). Consistent with the measurements of peak amplitude of APs triggered by depolarization current steps (described above), the peak amplitude of the APs evoked by stimulation of T7 was not changed significantly from normal (normal: 55 ± 2.3 mV vs. chronic injured: 53 ± 1.5 mV, P > 0.05). However, the latency of the responses increased after chronic HX compared with noninjured spinal cord (noninjured: 1.6 ± 0.1 ms vs. chronic injured: 2.5 ± 0.1 ms, P < 0.01; Fig. 3C). Conversely, the mean conduction velocity, which was calculated as the ratio between the latency of the responses and the distance between the stimulating and recording electrodes, significantly decreased in chronic HX rats (noninjured: 31 ± 1.6 m/s vs. chronic injured: 21 ± 1.5 m/s, P < 0.01). Moreover, the minimum stimulus intensity required to induce an AP was markedly higher in chronic HX rats (control: 89 ± 12.8 μA vs. chronic HX: 288 ± 45 μA, P < 0.01). Overall, the comparison of the stimulus intensity required to evoke a response from T7 in L5 axons revealed that more axons required a higher stimulus intensity to induce an AP in chronic HX rats (Fig. 4B). A summary of these results is presented in Table 2.
Table 2.
Effect of chronic HX on propagation of APs along surviving fibers
| Intra-axonal Recording |
Extra-axonal Recording |
|||||
|---|---|---|---|---|---|---|
| Stimulus Intensity, μA | Latency, ms | Conduction Velocity, m/s | AP Amplitude, mV | Amplitude of Volley Response, mV | Stimulus Intensity, μA | |
| Control rats | 89 ± 12.8 | 1.6 ± 0.1 | 31 ± 1.6 | 55 ± 2.3 | 0.29 ± 0.04 | 100 ± 17 |
| Chronic HX rats | 288 ± 45† | 2.5 ± 0.1† | 21 ± 1.5† | 53 ± 1.5 | 0.15 ± 0.01* | 225 ± 16* |
Values are means ± SE for control (n = 9 rats/64 axons) and chronic HX rats (n = 10 rats/55 axons). AP and volley responses were recorded from L5 lateral white matter axons (right side) after electrical stimulation of the ipsilateral white matter at T7 in normal and chronic HX spinal cord injury rats (left-side HX at T10).
P < 0.05;
P < 0.01.
Composite volley response through lateral white matter axons contralateral to chronic HX (extra-axonal recordings).
To examine whether the changes in excitability of single axons as described above was associated with changes in composite volley responses within the white matter of the L5 region, we conducted extracellular recordings from the L5 axonal pool. Previously we have shown that extracellular responses recorded in noninjured rats from the L5 ventral horn in response to stimulation of the ventrolateral funiculus at T7 consist of an early biphasic component, representing the volley of APs (∼0.3 mV measured at first down-going peak; this component was not sensitive to the intraspinal injections of CNQX, but it was blocked after TTX injections) and a later component, representing the synaptic response (∼0.3 mV measured from baseline to second down-going peak; CNQX selectively blocked this component) (Arvanian et al. 2009; Hunanyan et al. 2010). Our present recordings of the extracellular responses recorded in noninjured rats from the L5 lateral white matter in response to the stimulation of the ipsilateral white matter at T7 showed mainly an early biphasic AP volley component (first down-going peak), whereas the later synaptic component (measured from baseline to second down-going peak; Fig. 5) was small and in many cases did not exceed the level of the background noise (later synaptic component was 0.04 ± 0.008 mV in noninjured vs. 0.02 ± 0.01 mV in chronic HX, P > 0.05). In noninjured rats, the average amplitude of the AP volley was 0.29 ± 0.04 mV (measured at arrows in Fig. 5A, n = 9 rats), whereas in the chronic HX rats, the amplitude volley response diminished significantly (0.15 ± 0.01 mV, n = 10, P < 0.05; Fig. 5B). In addition, the stimulus intensity required to induce maximum volley responses in chronic HX rats was significantly higher than in noninjured rats (chronic HX: 225 ± 16 μA vs. noninjured: 100 ± 17 μA, P < 0.05; Table 2). The relationship of the amplitudes of composite volley responses at L5 to electrical stimulation intensity at T7 in noninjured and chronic HX rats is presented in Fig. 5C. The diminished amplitude of the volley response and the higher stimulus intensity required for evoking this response both suggested that fewer numbers of spared fibers could be recruited for propagation of the composite response from T7 to L5 in chronic HX compared with normal rats. Together, the results of the intra-axonal and extra-axonal recordings indicate that there is a diminished excitability of the spared fibers across from the lesion in chronic HX rats.
Nav1.6 and Caspr expression patterns in normal and chronic HX spinal cord.
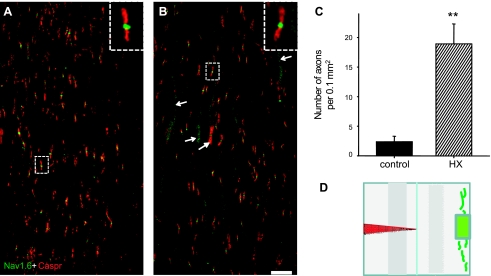
To determine whether the changes in axonal excitability revealed by our electrophysiological recordings were associated with changes in the distribution of voltage-gated sodium channels, we visualized the distribution of Nav1.6-type sodium channels in white matter axons of noninjured and chronic HX rats. In the normal rat central nervous system, Nav1.6 voltage-gated sodium channels are the major type of sodium channel that clusters at essentially all nodes of Ranvier, thus supporting rapid propagation of APs (Caldwell et al. 2000). To study the distribution of this type of voltage-gated sodium channel, sections from lateral white matter across from chronic HX and a corresponding area in control, uninjured tissues were immunostained with an anti-Nav1.6 antibody. For labeling of the paranodal regions, we used an antibody against Caspr. In noninjured spinal cords, double immunostaining with Nav1.6 and Caspr revealed that Nav1.6 was clustered at nodes of Ranvier as identified by paranodal staining with anti-Caspr antibody (Fig. 6A). This observation is consistent with previous reports in rodent and human spinal cord (Craner et al. 2004b; Sasaki et al. 2006). Consistent with noninjured rats, in chronic HX rats, the sodium channel immunostaining could be detected within most of the Caspr-stained nodes (see higher magnification insets in Fig. 6, A and B). However, in contrast with noninjured rats, in chronic HX rats Nav1.6 showed an extensive nonnodal (>10 μm) pattern of diffuse expression along numerous white matter axons (small arrows, Fig. 6B). In some cases after chronic HX, the diffuse Nav1.6 staining was bounded by Caspr (large arrow, Fig. 6B), which identified the wormlike profile as an axon. In chronic HX, the number of axons with dispersed Nav1.6 immunostaining increased significantly compared with noninjured controls (chronic injured: 19 ± 3.3/0.1 mm2 vs. control: 2.2 ± 0.9/0.1 mm2, P < 0.01; Fig. 6C). It is important to note that axons with diffuse Nav1.6 staining were localized within the area just across from chronic HX. From previous studies, a similar dispersed pattern of Nav1.6 channels localization was shown to occur along demyelinated axons (Black et al. 2002; Craner et al. 2004a,b), which may suggest that the diffuse staining contralateral to chronic HX represents demyelination.
Fig. 6.
Double immunostaining with Nav1.6 and Caspr to show their axonal localization at the T10 level on right lateral white matter of noninjured rats and left lateral white matter of chronic HX SCI rats. A: normal spinal cord. Note the expression of Nav1.6 was found mainly within the nodes of Ranvier. B: a diffuse localization pattern of Nav1.6 channels was observed after chronic HX SCI. Small arrows point to “wormlike” axonal profiles with diffuse (>10 μm) Nav1.6 sodium channel immunostaining. Large arrow shows the localization of Nav1.6 channels within the nodes of Ranvier on an axon also with diffuse Nav1.6 sodium channel expression. Insets in A and B are micrographs showing sodium channel immunostaining within the nodes at 10 times higher magnification. Scale bar, 20 μm. C: the number of axons with a diffuse localization of Nav1.6 in normal (n = 3) and chronic HX SCI rats (n = 3). Data are means ± SE. **P < 0.01. D: the highlighted area contralateral to the HX SCI on the diagram represents the region from which the images in A and B were taken.
Electron microscopic analysis of ultrastructural changes of axons across from HX.
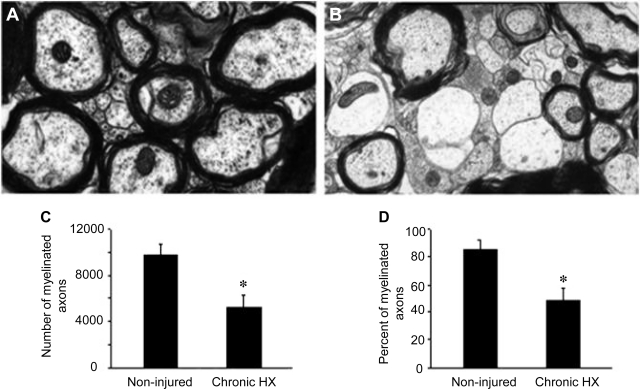
We examined ultrastructural changes of the axons on the uninjured side of the spinal cord within an area corresponding to the RST across from HX. In the chronic stage of injury, the appearance of demyelinated axons could be seen (Fig. 7B) compared with noninjured cord (Fig. 7A), and the number of myelinated axons was reduced significantly (9,808 ± 972, n = 3 noninjured vs. 5,215 ± 652, n = 6 at 6 wk post-HX, P < 0.01, Fig. 7C). The percentage of myelinated axons, calculated as a percentage of the total number of axons, at 6 wk postinjury also significantly decreased (84 ± 7% noninjured vs. 48 ± 9% 6 wk post-HX, P < 0.01; Fig. 7D).
Fig. 7.
Number and percentage of myelinated axons in an area corresponding to the RST within the contralateral white matter to the HX SCI shows that they are reduced in number during the chronic stage of injury. Compared with noninjured controls (A), there were significantly fewer myelinated axons at 6 wk postinjury (B). C and D: quantification of the numbers of myelinated axons (C) and the percentage of myelinated axons (D) within the region corresponding to the RST in noninjured controls (n = 3) vs. 6 wk post-HX SCI rats (n = 6). Data are means ± SE. *P < 0.05.
DISCUSSION
In this study we examined the possible mechanisms involved in the recently reported reduction of the synaptic responses of lumbar motoneurons through lateral white matter axons contralateral to chronic HX SCI in adult rat (Arvanian et al. 2009; Hunanyan et al. 2010). Our data show that uncut RST/RtST axons in the white matter, contralateral to HX, exhibit higher rheobase for intra-axonal current injection and thus reduced excitability, as well as higher required current intensity at an extracellular stimulation electrode placed at T7 for propagation of APs. The latency of these APs was longer, thus indicating a reduced conduction velocity in surviving axons. These results suggest a decreased excitability of a fraction of those axons contralateral to the HX and thus a lower number of axons that could be recruited for the propagation of APs after chronic HX. Such reduced excitability of individual axons would then account for the lower amplitude of the averaged volley responses as revealed by the extra-axonal recordings after chronic HX. These changes were not associated with changes in the axonal resting membrane potential, input resistance, or maximum peak amplitude of APs and suggested that there may be an altered functioning of the voltage-gated sodium channels along the axons surviving chronic HX. In support of our electrophysiological data, immunohistochemical results revealed a diffuse pattern of expression of voltage-gated Nav1.6 channels along numerous axons on the side opposite to the injury in chronic HX rats. Electron microscopy studies showed a significant reduction in the number and percentage of myelinated axons in the white matter across from chronic HX SCI.
Despite the reported ability of intrinsic spinal circuits to reorganize anatomically during the chronic stage of incomplete SCI (Courtine et al. 2008, 2009; Lane et al. 2009), many spinal tracts suffer delayed secondary injury after acute mechanical trauma. Functional loss within surviving axons is thought to be due to impaired axonal conduction, which in turn may be a result of distorted functional activity of ion channels involved in the propagation of AP along the axons (Blight 1983; Smith and Hall 2001). In support of this view, results of our in vivo recording from individual axons in injured spinal cord demonstrate the diminished excitability of the spared axons and pathological changes in AP propagation through these axons after chronic HX. Consistent with our in vivo intra-axonal recording results, several recent extracellular studies have shown a reduction of the compound AP amplitude and a diminished conduction velocity following chronic spinal cord contusion (Hains et al. 2004) or compression (Nashmi and Fehlings 2001), as well as HX injuries (Arvanian et al. 2009). Functional loss within surviving axons has been attributed to demyelination and inflammatory damage that occurs subsequent to mechanical trauma (Blight 1983; Blight and Young 1989; Guest et al. 2005; Park et al. 2004; Smith and Hall 2001; Totoiu and Keirstead 2005). Several mechanisms may be responsible for the reduction in the excitability of uncut axons, such as 1) a decreased membrane resistance, 2) depolarization of the resting membrane potential, which determines the number of active voltage-dependent sodium channels participating in the generation of an AP, and/or 3) a decrease of the number or property of the voltage-dependent sodium channels available to open during an AP.
To elucidate which of these mechanisms was responsible for the observed reduction in axon excitability, we performed long-lasting in vivo intra-axonal recordings in white matter contralateral to the chronic HX. Parameters of AP that were recorded from these axons in noninjured rats were similar to those described in one previous study (Kocsis and Waxman 1982). From our investigations, a statistically significant change in either the input membrane resistance or the resting membrane potential in axons contralateral to chronic HX was not found (see Table 1), thus suggesting that our finding of a pathological decrease in axonal excitability after chronic HX could be due to an altered property of voltage-gated sodium channels.
The results of our extracellular recordings of composite responses that we recorded from the axonal pool at L5 (caudal and contralateral to HX) and evoked by electric stimulation at T7 (rostral to HX) revealed dramatic deficits of conduction in surviving contralateral fibers across from the HX (Fig. 5). Our results of intra-axonal recordings revealed that the major electrophysiological changes induced by chronic HX were a higher rheobase and thus reduced excitability, a higher threshold, reduced propagation of APs along axons across from the HX, and decreased conduction velocity. These results are consistent with the results of our recent report (Arvanian et al. 2009), where we recorded synaptic responses from L5 motoneurons evoked by stimulation of either T7 or L1 and found that in chronic HX rats (HX at T10), the responses from T7 are markedly depressed, whereas the responses from L1 are maintained. Together, these results suggest that the major decline of conduction in surviving contralateral axons occurs at the level of HX. These results are consistent with the recent work of Siegenthaler et al. (2007), who demonstrated that after thoracic HX injury, the demyelination of axons is profound, but their work focused predominantly around the epicenter of the HX.
Voltage-gated sodium channels are known to cluster at the nodes of Ranvier in myelinated axons (Black et al. 2006; Caldwell et al. 2000). Myelin plays an important role in reducing an axon's membrane capacitance, preventing the leak current, increasing conduction velocity, and thus reducing the current for triggering an AP. In light of the original “submarine cable” assumption of Ranvier (Ranvier 1878), the myelin sheath represents a high-impedance shield around the internode that reduces the radial leakage of current. Consistent with this view, a low-resistance pathway through the myelin sheath in vertebrate peripheral axons (Barrett and Barrett 1982) and in axons of rat spinal cord has been reported (Blight and Someya 1985). From other reports, transplantation of glial cells into the dorsal columns of neonatal myelin-deficient rat spinal cords leads to myelination, and this was associated with a threefold increase in conduction velocity (Utzschneider et al. 1994). Demyelination of the axons related to many neurological deficits, including SCI, results in abnormal distribution of the nodal/paranodal ion channels, which in turn results in impaired propagation of APs (Boyle et al. 2001; Coetzee et al. 1996; Craner et al, 2004a, 2004b; Nashmi and Fehlings 2001; Poliak and Peles 2003). Several studies have shown that inhibition of voltage-gated sodium or potassium channels enhances axonal conduction, reduces axoplasmic pathology and damage to myelin, and improves motor recovery after different types of thoracic SCI in adult rat (Hains et al. 2004; Kobrine et al. 1984; Nashmi et al. 2000; Rosenberg et al. 1999; Schwartz and Fehlings 2001; Shi and Blight 1996; Teng and Wrathall 1997). Other studies have shown a dispersed pattern of expression of sodium Nav1.6 and Nav1.2 channels in demyelinated axons in the adult rat spinal cord in models of multiple sclerosis and after chronic compression injury (Black et al. 2002; Craner et al. 2004a,b; Nashmi et al. 2000). In the current study, the dispersed expression of Nav1.6 sodium channels outside of the nodes of Ranvier (Fig. 6) and the increased number of demyelinated fibers in the lateral white matter across from the chronic HX SCI (Fig. 7) are in a good agreement with a proposed distortion of the functional activity of these sodium channels along the axons and the observed reduction in the propagation of APs through this region (Figs. 3C and 5).
While analyzing the results of intra-axonal recordings from lateral white matter, one of our concerns was that the absolute mean for AP peak amplitude (55–57 mV) is similar to the mean of the resting membrane potential (−60 to −63 mV). This could be explained by our observation that not all APs overshoot, particularly when triggered from a resting membrane potential of −70 mV and more negative. This view is consistent with the reported results of in vivo intra-axonal recordings from the adult rat L5 dorsal root axons, which show that not all APs exceeded 0 mV (Novak et al. 2009). However, other in vivo studies performed in rat dorsal column axons from lumbosacral spinal cord reported that axonal APs overshoot (Kocsis and Waxman 1980, 1982). The disparities in degree of overshoot among these studies could reflect differences between axons of lateral and dorsal white matter, technical differences, or both. Regardless, the comparisons in the current study between HX and control axons were performed under the same recording conditions, and the changes we observed in electrophysiological parameters of axons contralateral to HX were also corroborated by anatomical observations.
In addition to the diminished propagation of APs through the intact side at the level of HX (T10), we also found that axons in the L5 region, i.e., caudal to the chronic HX, exhibit a broadened duration of AP and a higher rheobase. The current findings support our previous observation that although stimulation of lateral white matter at L1, caudal to HX, evoked synaptic responses in L5 motoneurons (which were not responding to stimulation from T7), these responses from L1 were significantly smaller compared with responses from L1 in noninjured rats (Arvanian et al. 2009). Examination of demyelination and Nav1.6 expression pattern below the level of injury needs to be conducted to understand how far below the injury level these axonal changes occur.
Together, our results suggest that the dispersed expression of Nav1.6 channels, a phenotype produced by partial demyelination of these axons, and the reduced number of myelinated axons in the region across from the HX could be important mechanisms involved in the reduced propagation of APs along these axons but cannot explain all the electrophysiological changes that we have observed after chronic HX SCI. Our results also suggest that changes in the electrical properties of spared axons across from chronic HX SCI could be multifactorial.
One possible alternative mechanism for the conduction block of spared fibers is an accumulation of chondroitin sulfate proteoglycans (CSPGs) within the glial scar region in and around the injury, which could be present during the chronic stage of SCI (Kwok et al. 2008; Lemons et al. 1999; Snow et al. 1990). Recently, we found that the enzymatic removal of CSPGs improves conduction after HX SCI, whereas intraspinal injection of one CSPG, NG2, into the lateral white matter inhibited conduction of noninjured axons (Hunanyan et al. 2010). Thus another possible mechanism involved in the conduction block of spared fibers after chronic unilateral HX injury could be a change in the properties of voltage-gated sodium channels through an interaction with CSPG, a mechanism that needs further examination.
In conclusion, this study is the first demonstration of the properties of APs of individual RST/RtST axons recorded intra-axonally after chronic HX spinal cord injury in adult rats. An increased rheobase, pathological changes in the propagation of APs and the distribution of Nav1.6 sodium channels, and the demyelination of contralateral RST axons are likely responsible for their decreased conduction chronically after HX and thus may provide novel targets for strategies to improve function following incomplete spinal cord injury.
GRANTS
This research was supported by Merit Review Funding from the Department of Veterans Affairs (to V. L. Arvanian), the New York State Spinal Cord Injury Research Board (to V. L. Arvanian), and National Institutes of Health Grants R01 EY003821 (to G. Matthews) and R01 NS056281 (to D. D. Pearse).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Lorne Mendell for valuable discussions and Margaret Bates of the Electronmicroscopy Core Facility (Miami) for help with the preparation of samples for electron microscopic analysis.
REFERENCES
- Arvanian VL, Mendell LM. Removal of NMDA receptor Mg2+ block extends the action of NT-3 on synaptic transmission in neonatal rat motoneurons. J Neurophysiol 86: 123–129, 2001 [DOI] [PubMed] [Google Scholar]
- Arvanian VL, Schnell L, Lou L, Golshani R, Hunanyan A, Ghosh A, Pearse DD, Robinson JK, Schwab ME, Fawcett JW, Mendell LM. Chronic spinal hemisection in rats induces a progressive decline in transmission in uninjured fibers to motoneurons. Exp Neurol 216: 471–480, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M, Bostock H, Grafe P, Martius P. Function and distribution of three types of rectifying channel in rat spinal root myelinated axons. J Physiol 383: 45–67, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballermann M, Fouad K. Spontaneous locomotor recovery in spinal cord injured rats is accompanied by anatomical plasticity of reticulospinal fibers. Eur J Neurosci 23: 1988–1996, 2006 [DOI] [PubMed] [Google Scholar]
- Barrett EF, Barrett JN. Intracellular recording from vertebrate myelinated axons: mechanism of the depolarizing afterpotential. J Physiol 323: 117–144, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JA, Renganathan M, Waxman SG. Sodium channel Na(v)1.6 is expressed along nonmyelinated axons and it contributes to conduction. Brain Res Mol Brain Res 105: 19–28, 2002 [DOI] [PubMed] [Google Scholar]
- Black JA, Waxman SG, Smith KJ. Remyelination of dorsal column axons by endogenous Schwann cells restores the normal pattern of Nav1.6 and Kv1.2 at nodes of Ranvier. Brain 129: 1319–1329, 2006 [DOI] [PubMed] [Google Scholar]
- Blight AR, Decrescito V. Morphometric analysis of experimental spinal cord injury in the cat: the relation of injury intensity to survival of myelinated axons. Neuroscience 19: 321–341, 1986 [DOI] [PubMed] [Google Scholar]
- Blight AR, Someya S. Depolarizing afterpotentials in myelinated axons of mammalian spinal cord. Neuroscience 15: 1–12, 1985 [DOI] [PubMed] [Google Scholar]
- Blight AR, Young W. Central axons in injured cat spinal cord recover electrophysiological function following remyelination by Schwann cells. J Neurol Sci 91: 15–34, 1989 [DOI] [PubMed] [Google Scholar]
- Blight AR. Axonal physiology of chronic spinal cord injury in the cat: intracellular recording in vitro. Neuroscience 10: 1471–1486, 1983 [DOI] [PubMed] [Google Scholar]
- Boyle ME, Berglund EO, Murai KK, Weber L, Peles E, Ranscht B. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron 30: 385–397, 2001 [DOI] [PubMed] [Google Scholar]
- Brown LT. Rubrospinal projections in the rat. J Comp Neurol 154: 169–187, 1974 [DOI] [PubMed] [Google Scholar]
- Caldwell JH, Schaller KL, Lasher RS, Peles E, Levinson SR. Sodium channel Na(v)1.6 is localized at nodes of ranvier, dendrites, and synapses. Proc Natl Acad Sci USA 97: 5616–5620, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee T, Fujita N, Dupree J, Shi R, Blight A, Suzuki K, Popko B. Myelination in the absence of galactocerebroside and sulfatide: normal structure with abnormal function and regional instability. Cell 86: 209–219, 1996 [DOI] [PubMed] [Google Scholar]
- Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, Roy RR, Sofroniew MV, Edgerton VR. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci 12: 1333–1342, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, Qi J, Edgerton VR, Sofroniew MV. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med 14: 69–74, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craner MJ, Hains BC, Lo AC, Black JA, Waxman SG. Co-localization of sodium channel Nav1.6 and the sodium-calcium exchanger at sites of axonal injury in the spinal cord in EAE. Brain 127: 294–303, 2004a [DOI] [PubMed] [Google Scholar]
- Craner MJ, Newcombe J, Black JA, Hartle C, Cuzner ML, Waxman SG. Molecular changes in neurons in multiple sclerosis: altered axonal expression of Nav1.2 and Nav1.6 sodium channels and Na+/Ca2+ exchanger. Proc Natl Acad Sci USA 101: 8168–8173, 2004b [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G, Modney B, Scappaticci KA, Barrett JN, Barrett EF. Electrical and morphological factors influencing the depolarizing after-potential in rat and lizard myelinated axons. J Physiol 489: 141–157, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest JD, Hiester ED, Bunge RP. Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp Neurol 192: 384–393, 2005 [DOI] [PubMed] [Google Scholar]
- Hains BC, Saab CY, Lo AC, Waxman SG. Sodium channel blockade with phenytoin protects spinal cord axons, enhances axonal conduction, and improves functional motor recovery after contusion SCI. Exp Neurol 188: 365–377, 2004 [DOI] [PubMed] [Google Scholar]
- Honmou O, Felts PA, Waxman SG, Kocsis JD. Restoration of normal conduction properties in demyelinated spinal cord axons in the adult rat by transplantation of exogenous Schwann cells. J Neurosci 16: 3199–3208, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubscher CH, Johnson RD. Differential effects of chronic spinal hemisection on somatic and visceral inputs to caudal brainstem. Brain Res 947: 234–242, 2002 [DOI] [PubMed] [Google Scholar]
- Hunanyan AS, Garcia-Alias G, Valentina Alessi Levine JM, Fawcett JW, Mendell LM, Arvanian VL. Role of chondroitin sulfate proteoglycans (CSPGs) in axonal conduction in mammalian spinal cord. J Neurosci 30: 7761–7769, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Konnerth A, Augustine GJ. Action potential broadening and frequency-dependent facilitation of calcium signals in pituitary nerve terminals. Proc Natl Acad Sci USA 88: 380–384, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagal SG, Muir GD. Effects of combined dorsolateral and dorsal funicular lesions on sensorimotor behaviour in rats. Exp Neurol 214: 229–239, 2008 [DOI] [PubMed] [Google Scholar]
- Kobrine AI, Evans DE, LeGrys DC, Yaffe LJ, Bradley ME. Effect of intravenous lidocaine on experimental spinal cord injury. J Neurosurg 60: 595–601, 1984 [DOI] [PubMed] [Google Scholar]
- Kocsis JD, Waxman SG. Absence of potassium conductance in central myelinated axons. Nature 287: 348–349, 1980 [DOI] [PubMed] [Google Scholar]
- Kocsis JD, Waxman SG. Intra-axonal recordings in rat dorsal column axons: membrane hyperpolarization and decreased excitability precede the primary afferent depolarization. Brain Res 238: 222- 227, 1982 [DOI] [PubMed] [Google Scholar]
- Kriz J, Padjen AL. Intra-axonal recording from large sensory myelinated axons: demonstration of impaired membrane conductances in early experimental diabetes. Diabetologia 46: 213–221, 2003 [DOI] [PubMed] [Google Scholar]
- Kwok JC, Afshari F, García-Alías G, Fawcett JW. Proteoglycans in the central nervous system: plasticity, regeneration and their stimulation with chondroitinase ABC. Restor Neurol Neurosci 26: 131–145, 2008 [PubMed] [Google Scholar]
- Lane MA, Lee KZ, Fuller DD, Reier PJ. Spinal circuitry and respiratory recovery following spinal cord injury. Respir Physiol Neurobiol 169: 123–132, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemons ML, Howland DR, Anderson DK. Chondroitin sulfate proteoglican immunoreactivity increases following spinal cord injury and transplantation. Exp Neurol 160: 51–65, 1999 [DOI] [PubMed] [Google Scholar]
- McDonald JW, Gottlieb DI, Choi DW. Reply to “What is a functional recovery after spinal cord injury?” Nat Med 6: 358, 2000 [DOI] [PubMed] [Google Scholar]
- Nashmi R, Fehlings MG. Changes in axonal physiology and morphology after chronic compressive injury of the rat thoracic spinal cord. Neuroscience 104: 235–251, 2001 [DOI] [PubMed] [Google Scholar]
- Nashmi R, Jones OT, Fehlings MG. Abnormal axonal physiology is associated with altered expression and distribution of Kv1.1 and Kv1.2 K+ channels after chronic spinal cord injury. Eur J Neurosci 12: 491–506, 2000 [DOI] [PubMed] [Google Scholar]
- Novak KR, Nardelli P, Cope TC, Filatov G, Glass JD, Khan J, Rich MM. Inactivation of sodium channels underlies reversible neuropathy during critical illness in rats. J Clin Invest 119: 1150–1158, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma 21: 754–774, 2004 [DOI] [PubMed] [Google Scholar]
- Pearse DD, Lo TP, Jr, Cho KS, Lynch MP, Garg MS, Marcillo AE, Sanchez AR, Cruz Y, Dietrich WD. Histopathological and behavioral characterization of a novel cervical spinal cord displacement contusion injury in the rat. J Neurotrauma 22: 680–702, 2005 [DOI] [PubMed] [Google Scholar]
- Poliak S, Peles E. The local differentiation of myelinated axons at nodes of Ranvier. Nat Rev Neurosci 4: 968–980, 2003 [DOI] [PubMed] [Google Scholar]
- Ranvier ML. Lecons sur I'Histoiogie du Systeme Nerveux. Paris: Libraire F. Savy, 1878 [Google Scholar]
- Renganathan M, Cummins TR, Waxman SG. Contribution of Na(v)1.8 sodium channels to action potential electrogenesis in DRG neurons. J Neurophysiol 86: 629–640, 2001 [DOI] [PubMed] [Google Scholar]
- Rosenberg LJ, Teng YD, Wrathall JR. Effects of the sodium channel blocker tetrodotoxin on acute white matter pathology after experimental contusive spinal cord injury. J Neurosci 19: 6122–6133, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Black JA, Lankford KL, Tokuno HA, Waxman SG, Kocsis JD. Molecular reconstruction of nodes of Ranvier after remyelination by transplanted olfactory ensheathing cells in the demyelinated spinal cord. J Neurosci 26: 1803–1812, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaal SM, Kitay BM, Cho KS, Lo TP, Jr, Barakat DJ, Marcillo AE, Sanchez AR, Andrade CM, Pearse DD. Schwann cell transplantation improves reticulospinal axon growth and forelimb strength after severe cervical spinal cord contusion. Cell Transplant 16: 207–228, 2007 [DOI] [PubMed] [Google Scholar]
- Schwartz G, Fehlings MG. Evaluation of the neuroprotective effects of sodium channel blockers after spinal cord injury: improved behavioral and neuroanatomical recovery with riluzole. J Neurosurg 94: 245–256, 2001 [DOI] [PubMed] [Google Scholar]
- Shi R, Blight AR. Compression injury of mammalian spinal cord in vitro and the dynamics of action potential conduction failure. J Neurophysiol 76: 1572–1580, 1996 [DOI] [PubMed] [Google Scholar]
- Siegenthaler MM, Tu MK, Keirstead HS. The extent of myelin pathology differs following contusion and transection spinal cord injury. J Neurotrauma 24: 1631–1646, 2007 [DOI] [PubMed] [Google Scholar]
- Smith KJ, Hall SM. Factors directly affecting impulse transmission in inflammatory demyelinating disease: recent advances in our understanding. Curr Opin Neurol 14: 289–298, 2001 [DOI] [PubMed] [Google Scholar]
- Snow DM, Lemmon V, Carrino DA, Caplan AI, Silver J. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp Neurol 109: 111–130, 1990 [DOI] [PubMed] [Google Scholar]
- Teng YD, Wrathall JR. Local blockade of sodium channels by tetrodotoxin ameliorates tissue loss and long-term functional deficits resulting from experimental spinal cord injury. J Neurosci 17: 4359–4366, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totoiu MO, Keirstead HS. Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol 486: 373–383, 2005 [DOI] [PubMed] [Google Scholar]
- Utzschneider DA, Archer DR, Kocsis JD, Waxman SG, Duncan ID. Transplantation of glial cells enhances action potential conduction of amyelinated spinal cord axons in the myelin-deficient rat. Proc Natl Acad Sci USA 91: 53–57, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron HA, Gwyn DG. Descending nerve tracts in the spinal cord of the rat. I. Fibers from the midbrain. J Comp Neurol 137: 143–153, 1969 [DOI] [PubMed] [Google Scholar]
- Webb AA, Muir GD. Unilateral dorsal column and rubrospinal tract injuries affect overground locomotion in the unrestrained rat. Eur J Neurosci 18: 412–422, 2003 [DOI] [PubMed] [Google Scholar]