Abstract
In the absence of brain input, spinal systems can adapt to new environmental relations. For example, spinally transected rats given a legshock each time the leg is extended exhibit a progressive increase in flexion duration that minimizes net shock exposure, a simple form of instrumental learning. This capacity for learning is modulated by prior stimulation; both variable shock and inflammation produce a lasting inhibition of learning. An extended exposure to fixed spaced shock has no adverse effect on learning and opposes the consequences of variable shock. The present studies expand on these findings and demonstrate that fixed stimulation ameliorates the impact of peripheral inflammation. Spinally transected rats were administered 900 fixed spaced legshocks prior to (Experiment 1) or 1800 legshocks following (Experiment 2) a subcutaneous hindpaw injection of capsaicin. Learning was assessed 24 hr later. Treatment with fixed shock attenuated the capsaicin-induced inhibition of learning. These findings suggest that fixed stimulation promotes adaptive plasticity and may foster recovery after injury.
Keywords: spinal cord injury, instrumental learning, capsaicin, central sensitization, recovery
Over the past few decades, research has shown that the spinal cord is not merely a passive conduit of information to and from the periphery. Rather, when isolated from descending brain systems, the spinal cord can support a dynamic range of adaptive behaviors. For example, the spinal cord can reacquire the ability to support rhythmical stepping behavior (Edgerton et al., 1997), even gaining the ability to reduce disruptions from external perturbing forces (Heng & de Leon, 2007; Hodgson, Roy, de Leon, Dobkin, & Edgerton, 1994). Spinal neurons are also capable of supporting other forms of adaptive behavioral modifications, including a variety of basic learning phenomena such as habituation, sensitization, classical conditioning, and instrumental learning (Beggs, Steinmetz, & Patterson, 1985; Durkovic, 1975; Grau, Barstow, & Joynes, 1998; Groves & Thompson, 1970; Joynes & Grau, 1996; Spencer, Thompson, & Neilson, 1966; Wolpaw, Lee, & Carp, 1991).
Our laboratory has focused on the capacity of the isolated spinal cord to demonstrate instrumental learning (Grau et al., 1998). Using a master-yoke preparation, one group of spinally transected rats (master) receives hindlimb shock whenever that limb is extended (controllable shock). As a result of this response-contingent shock treatment, rats learn to maintain their limbs in a flexed position that minimizes net shock exposure. A second group of rats (yoke) is experimentally coupled to rats in the controllable shock condition and receives stimulation at the same time and duration, but independent of leg position (uncontrollable shock). Yoked rats do not exhibit an increase in flexion duration during training and fail to learn when tested later with controllable shock, a learning deficit that is reminiscent of learned helplessness (Grau et al., 1998; Maier & Seligman, 1976; Overmier & Seligman, 1967). While uncontrollable stimulation disables the capacity to learn, controllable stimulation engages an opponent process that enables learning (Crown, Ferguson, Joynes, & Grau, 2002a) and can both prevent and reverse the learning deficit observed after uncontrollable shock (Crown & Grau, 2001).
Recent work has attempted to elucidate the mechanisms that underlie instrumental learning and the learning deficit. Evidence suggests that instrumental learning is reinforced by shock onset (Grau et al., 1998), involves a form of NMDA receptor (NMDAR) mediated plasticity (Joynes, Janjua, & Grau, 2004), and exerts a protective effect through the neurotrophin BDNF (Gómez -Pinilla et al., 2007). Paradoxically, the induction of the learning deficit is also NMDAR-dependent (Ferguson, Crown, & Grau, 2006), leading us to suggest that it may reflect a diffuse state of over-excitation akin to central sensitization (Baumbauer, Young, Hoy, & Joynes, 2007b; Baumbauer, Young, & Joynes, 2009; Ferguson et al., 2006; Grau et al., 2006; Hook, Huie, & Grau, 2008). Central sensitization is an NMDAR mediated increase in neural excitability within the spinal cord that is associated with an increase in pain-like behavior to mechanical stimulation (allodynia; Coderre & Melzack, 1992; Malan et al., 2000; Urban & Gebhart, 1999). This over-excitation could saturate plasticity and thereby inhibit the acquisition of selective response modifications (Baumbauer, Young et al., 2007b; Baumbauer, Young et al., 2009; Ferguson et al., 2006; Grau et al., 2006; Hook et al., 2008). Supporting this, we have shown that uncontrollable shock enhances mechanical reactivity (Ferguson et al., 2006) and that manipulations which induce central sensitization, such as peripheral inflammation (e.g. from capsaicin administration) or tissue damage, also inhibit instrumental learning (Hook et al., 2008; Young, Baumbauer, Hillyer, & Joynes, 2007; Young et al., 2008). Further, just as controllable stimulation can both prevent and reverse the learning deficit induced by uncontrollable shock, it has a protective/restorative effect on the inflammation-induced allodynia and learning impairment (Hook et al., 2008).
Additional studies have sought to detail the stimulus conditions that interfere with spinal learning. To accomplish this, we developed a computer program to emulate the distribution of shocks produced by master rats during acquisition (the first 5–10 min of instrumental training; Crown, Ferguson, Joynes, & Grau, 2002b). Analyses revealed that master rats typically received thirty 80-ms shocks per minute, yielding an average interstimulus interval (ISI) of 2 s. Because the duration of each response varied, yoked rats received shocks at varying intervals. Given these parameters, the program was designed to administer shock on a variable time (VT) schedule, with an average ISI of 2 s and a range of 0.2 s to 3.8 s (rectangular distribution). Using this program, we found that just 180 shocks delivered to the leg, tail, or sciatic nerve produced a learning deficit that persisted for up to 48 hr (Baumbauer et al., 2008; Baumbauer, Huie, Hughes, & Grau, 2009; Crown et al., 2002b; Ferguson et al., 2006). To derive the optimal frequency, we switched from variable stimulation to regular (fixed time [FT]) shock (Baumbauer et al., 2008). When subjects received just 180 shocks spaced 2 s apart, FT stimulation produced a learning deficit. However, when shock number was increased from 180 to 900 shocks, a surprising outcome was observed. Under these conditions, only VT stimulation produced a learning deficit, and this was true independent of how shock was applied (Baumbauer et al., 2008; Baumbauer et al., 2009). Moreover, an extended exposure to FT shock not only failed to yield a learning deficit, it induced a restorative effect similar to that produced by controllable stimulation, both preventing and reversing the learning deficit induced by uncontrollable VT shock (Baumbauer et al., 2009). We have now shown that the protective effect of FT shock depends on an NMDAR-mediated form of plasticity, the release of the BDNF, and de novo protein synthesis (Baumbauer et al., 2009).
Our results suggest that FT stimulation fosters adaptive plasticity, while VT stimulation has a disabling effect. Interestingly, FT and VT stimulation also have opposing effects on tactile reactivity. Whereas VT stimulation enhances tactile reactivity, exposure to FT stimulation causes a decrease in reactivity (Baumbauer et al., under review). Furthermore, like controllable shock, FT stimulation prevents and reverses the allodynia associated with hindpaw inflammation. Here, we expand on our previous work detailing the acute impact of FT stimulation and focus on its long-term effect. Specifically, we explore whether treatment with FT stimulation can preserve (Experiment 1) and restore (Experiment 2) the capacity to learn when performance was examined 24 hr following shock and capsaicin treatment. We show that FT, but not VT, stimulation protects spinal neurons against the deficit produced by capsaicin. Exposure to FT stimulation also reinstated learning, suggesting that FT stimulation engages mechanisms that mitigate the long-term impact of inflammatory stimulation.
General Method
Subjects
Subjects were male, Sprague-Dawley rats obtained from Harlan (Houston, TX). Rats were 70–90 days old and weighed 300–350g at the time of spinal cord transection. They were housed in pairs with free access to food and water, and were maintained on a 14–10 hr light-dark cycle. All experiments were carried out in accordance with NIH standards for the care and use of laboratory animals (NIH publications No. 80–23), and were approved by the University Laboratory Animal Care Committee at Texas A&M University. Every effort was made to minimize suffering and limit the number of animals used.
Spinalization Surgery
Prior to surgery, the fur over the thoracic portion of the vertebral column was shaved and disinfected with betadine solution (H-E-B, San Antonio, TX). Rats were anesthetized with isoflurane gas. The rat’s head was rendered immobile in a stereotaxic apparatus with a small (5 X 4 X 2.5 cm) gauze pillow under the subject’s chest. An anterior to posterior incision over the second thoracic vertebrae (T2) was made, the tissue just rostral to T2 was cleared using rongeurs, and the cord exposed and cauterized. The remaining gap in the cord was filled with Gelfoam (Pharmacia Corp., Kalamazoo, MI) and the wound was closed with Michel clips (Fisher Scientific, Waltham, MA). Following closure of the wound, the surface of each leg was shaved for electrode placement. Intraperitoneal injections (3 mL) of 0.9% saline solution were administered post-operatively to prevent dehydration. Following surgery, rats were placed in a temperature-controlled environment (25.5 °C) and monitored until awake. All rats were checked every six to eight hours during the 18–24 hr post-surgical period. During this time, hydration was maintained with supplemental injections of saline, and the rats’ bladders and colons were expressed as necessary.
Spinal transections were confirmed by inspecting the cord under a 10x dissection scope, and observing the behavior of the subjects after they recovered to ensure that they exhibited paralysis below the level of the forepaws and did not exhibit any supraspinally-mediated pain responses to legshock.
Stimulation Procedures
Shock was applied using intramuscular shock electrodes constructed from stainless steel wire (0.01 mm2 [36 AWG], magnet wire single beldsol) that was inserted into the tibialis anterior muscle. Shock was administered using a constant current AC shock generator (Model SG-903; BRS/LVE, Laurel MD), with the intensity set to a value that produced a 0.4 Newton (N flexion force (see Instrumental Testing). To determine how stimulation delivery affected subsequent behavior, subjects were administered 80 ms tailshocks on a variable (range = 0.2–3.8 s, mean ISI = 2 s) or fixed (2 s ISI) schedule. Prior work has shown that variable shock undermines learning (Baumbauer et al., 2008; Baumbauer et al., 2009; Baumbauer et al., 2007b; Crown et al., 2002b; Ferguson et al., 2008), while fixed shock does not (Baumbauer et al., 2008; Baumbauer et al., 2009).
Capsaicin Injections
1% Capsaicin (Sigma-Aldrich, St. Louis, MO) was dissolved in 50 μL of vehicle (Tween 20 [7%] and saline [93%]) and injected subcutaneously into the dorsal surface of the foot. Using the dorsal surface of the paw as the site of injection ensured that the resulting edema did not impact any of our assessment procedures (Hook et al., 2008).
Tactile reactivity
To determine whether our manipulations produced any changes in tactile reactivity, thresholds were assessed 24 hr following treatment (immediately prior to instrumental testing) using von Frey filaments (Stoelting, Wood Dale, IL). Sensitivity was determined by stimulating the mid-plantar surface of each hindpaw in an ascending order until a flexion response was elicited. Stimuli were presented twice to each paw in an ABBA counterbalanced fashion (A = left, B = right), with testing on the same leg separated by a 2 min interval. Filament thickness/force was related to behavior using the transformation provided by the manufacturer: Intensity = log10 (10,000 ·g). This transformation yields a scale that is approximately linear and amenable to parametric analyses. For purposes of analysis, data were converted to change from baseline scores using subjects’ prestimulation (Experiment 1) or preinjection (Experiment 2) response thresholds as baseline reactivity.
Apparatus
The apparatus used was similar to that described elsewhere (Grau et al., 1998). Briefly, during instrumental training, all subjects were loosely restrained in Plexiglas tubes, with their hindlimbs suspended above a saline solution contained in a rectangular plastic dish (11.5 cm [w] X 19 cm [l] X 5 cm [d]) positioned 7.5 cm below the restraining tube. Holes were drilled into the anterior portion of the tubes to allow for ventilation. Two slots were cut 4 cm apart and 1.5 cm from the posterior end of the tube to allow both hind legs to hang freely. To monitor leg position, a stainless steel rod (7 cm [l], 0.46 mm [w]) was attached to the pad of one foot (contact electrode) extending past the toes. The contact electrode was taped to the plantar surface of the rat’s foot (Orthaletic, 1.3 cm [width]; Johnson & Johnson, New Brunswick, NJ) with the end positioned directly in front of the plantar protuberance. Heat-shrink tubing electrically insulated the rod from the paw. A fine wire (0.01 mm2 [36 AWG], magnet wire single beldsol) was attached to the end of the rod at a point under the insulation. This wire extended from the rear of the foot and was connected to a digital input board that was monitored by a Macintosh G4 computer. To minimize lateral leg movements, a piece of porous tape (Orthaletic, 1.3 cm [width]) was wrapped around the leg above the tarsus and attached under the front panel of the restraining tube.
Procedure
Two electrodes were inserted into one hindleg. The first electrode was constructed of stainless steel wire (0.05 mm2 [30 AWG]) and was inserted through the skin over the tibia, 1.5 cm from the tarsus. The second was made of fine wire (0.01 mm2 [36 AWG], magnet wire single beldsol) and was inserted perpendicular to the leg, through the body of the tibialis anterior muscle, 1.7 cm above the first electrode. Legshock was applied by attaching one lead from a constant current AC shock generator (Model SG-903; BRS/LVE, Laurel MD) to the electrode inserted into the tibialis anterior muscle. The second lead was attached to the wire implanted in the skin over the tibia. Shock (60 Hz, AC) intensity was adjusted for each subject to a level that produced a 0.4 N flexion response. This value was determined prior to instrumental training by looping a monofilament plastic line (“6 lb.” test strength; Du Pont, Wilmington DE) around the rat’s ankle. The end of the line was attached to a strain gauge (Fort-1000; World Precision Instruments, New Haven, CT) fastened to a ringstand. The strain gauge output was fed through a calibrated multimeter that allowed for a conversion from voltage to force in N. To determine the necessary flexion force, a single 300 ms shock was applied to the leg and the shock intensity was adjusted in a manner that resulted in the prescribed flexion force. After flexion force was set, the monofilament line was removed from the rat’s paw and the saline solution was adjusted so that the contact electrode sat 4 mm beneath the surface of the salt solution. Once the animals were prepared, the 30 min instrumental testing session began. Whenever the subjects’ legs were in the down position, the end of the rod contacted the saline solution and completed an electrical circuit. When the circuit was closed, shock was delivered to the tibialis anterior muscle that elicited a flexion response. The flexion response broke the circuit and terminated the shock.
Behavioral Measures of Learning
Training and testing sessions were divided into 30, 1-min bins to examine learning across trials. Response number and response duration were collected by the computer during these sessions, and were separately averaged across each 1-min bin. Every time the contact electrode left the solution, the number of responses was increased by one. The computer also recorded the amount of time the electrode remained out of the solution. Response duration served as the primary measure of learning and was calculated for each 1-min bin using the equation: response duration = (time out of solution) |(response number + 1). Focusing on this measure allowed us to avoid a number of interpretative problems that have plagued other studies associated with using other measures such as response number (Buerger, Eisenstein, & Reep, 1981; Church, 1964; Grau et al., 1998).
Elsewhere, we have shown that treatments that disrupt learning (as indexed by a decrease in response duration) do not undermine subjects’ capacity to perform the target response. Indeed, subjects that fail to learn typically exhibit the highest rates of responding. A similar pattern was observed in the present study. For example, as reported elsewhere (Hook et al., 2008), peripheral treatment with capsaicin alone (without FT stimulation) impaired instrumental learning (as indexed by an increase in flexion duration) relative to the vehicle treated controls (Experiments 1 and 2). Rats that received capsaicin alone also exhibited the highest rate (mean ± SEM) of responding (vehicle = 37.95 ± 21.06; capsaicin treated = 180.99 ± 38.28). Even though capsaicin treated rats repeatedly experienced the response-outcome relation, it did not produce an increase in flexion duration.
Statistics
All behavioral measures were analyzed using mixed-design analysis of variance (ANOVA) or analysis of covariance (ANCOVA). Where appropriate, Tukey’s Honestly Significant Difference (HSD) was used to conduct post hoc analyses. Significant group differences are indicated in the figures with an ‘*’. In all cases, p < .05 was used to determine statistical significance.
Experiment 1
Previous work has shown that FT stimulation attenuates inflammation-induced allodynia when tactile thresholds are assessed shortly after drug treatment (Baumbauer et al., under review). The present experiment examined whether FT stimulation administered prior to hindpaw capsaicin treatment has a lasting protective effect on mechanical reactivity and whether FT stimulation preserved the ability to learn 24 hr after capsaicin treatment.
Method
Forty-eight subjects (n = 8 per condition) received 900 FT legshocks, 900 VT legshocks, or nothing, immediately prior to subcutaneous injection of 1% capsaicin or physiological saline (50 μL vol) into one hindpaw. Shock and capsaicin treatment always occurred on the same limb, and the limb treated was counterbalanced across subjects. Tactile reactivity was tested for 3 hr following capsaicin administration. These data have been published elsewhere (Baumbauer et al., under review), and showed that capsaicin increased tactile reactivity and that this effect was prevented by prior treatment with fixed stimulation. Twenty-four hr later, tactile reactivity was assessed (see below) and rats were prepared for instrumental testing. To reduce the impact of edema on electrode placement or performance, instrumental testing occurred on the limb contralateral to capsaicin/shock treatment.
Results
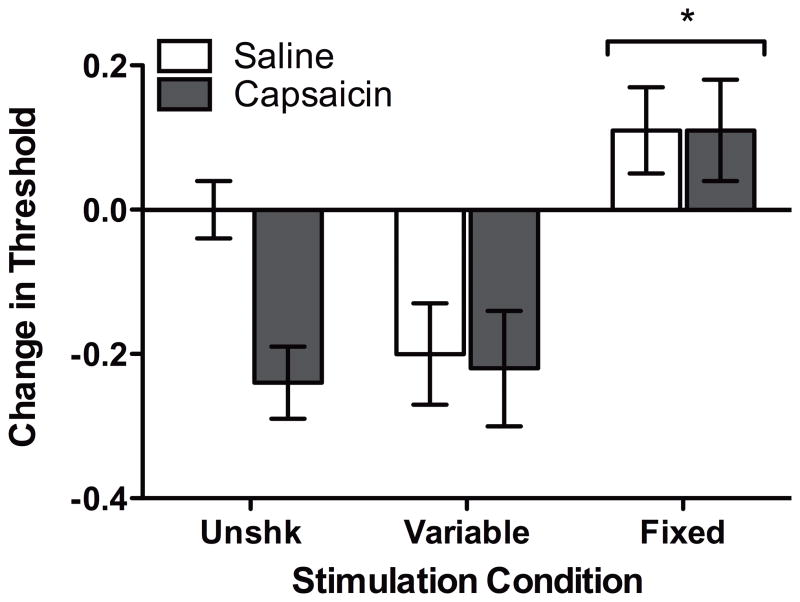
Twenty-four hr following shock treatment, rats were returned to the testing apparatus and response thresholds were assessed. Rats in the FT condition were less responsive to mechanical stimulation when compared to subjects in all other groups (Figure 1). Analysis failed to detect differences based on leg treated, F(1, 42) < 1.0, p > .05. Consequently, we collapsed our data across this variable for subsequent analysis. An ANOVA revealed a significant main effect of Shock condition, F(2, 42) = 12.20, p < .001. No other effects approached statistical significance, all Fs < 2.63, p > .05. Post hoc analysis confirmed that subjects in the FT condition exhibited greater response thresholds than subjects in the other shock conditions (p < .05).
Figure 1.
The effect of stimulation on tactile reactivity 24 hr following capsaicin treatment. Twenty-four hr following administration of 1% capsaicin or saline and fixed or variable shock treatment, rats received a final assessment of mechanical reactivity. Response values are depicted as changed from baseline scores. Asterisks indicate statistically significant differences (p < .05), and error bars depict ± SEM.
Immediately following assessment of tactile reactivity, rats were prepared for instrumental testing. To determine if our experimental procedures affected subjects’ capacity to perform the target flexion response, we analyzed the amount of stimulation required to produce a 0.4 N flexion force, as well as subjects’ initial flexion durations. Shock intensities (mean ± SEM) ranged from 0.47 ± 0.02 mA to 0.50 ± 0.02 mA, and initial flexion durations ranged from 0.13 ± 0.01 s to 0.17 ± 0.01 s. Individual ANOVAs performed on each measure failed to detect any significant differences in shock intensity, all Fs < 1.00, p > .05, but did reveal significant differences in initial response duration based on shock condition, F(2, 42) = 4.10, p < .05. Post hoc comparisons of group means demonstrated that rats in the FT shock condition had significantly longer initial flexion durations than rats in the VT shock condition. To evaluate whether this difference contributed to the results reported below, we analyzed the test data using an ANCOVA, entering initial response duration as a cofactor. Importantly, the ANCOVA revealed that initial response duration did not account for a significant proportion of the variance, F(1, 19) < 1.00, p < .05.
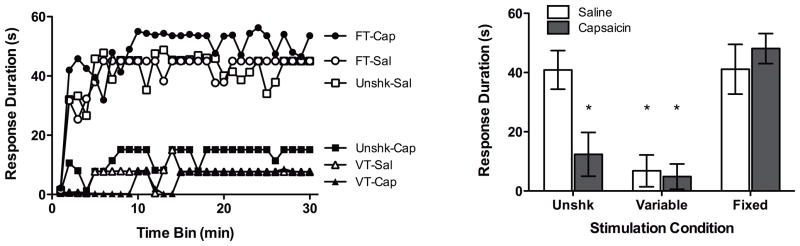
Subcutaneous injection of capsaicin undermined learning in unshocked rats (Figure 2). Rats administered VT shock exhibited a learning deficit at test, irrespective of drug treatment. Treatment with FT shock prior to capsaicin administration prevented the drug-induced learning deficit. An ANCOVA revealed significant main effects of Shock Condition and Trials, as well as significant Shock Condition X Trials and Shock Condition X Drug Condition interactions, all Fs > 1.60, p < .05. No other effects approached statistical significance, all Fs < 2.00, p > .05. Post hoc comparisons of group means demonstrated that unshocked subjects administered saline, as well as rats in the FT shock condition, maintained significantly longer response durations when compared to all other rats (p < .05).
Figure 2.
The effect of shock treatment on the capsaicin-induced learning deficit. Twenty-four hr following receiving capsaicin or saline and fixed or variable shock treatment, rats were tested for the capacity to demonstrate instrumental learning. The panel on the left depicts subjects’ response durations over time and the panel on the right depicts response duration as a mean value collapsed across time. Asterisks indicate statistically significant differences (p < .05), and error bars depict ± SEM.
Experiment 2
Experiment 1 showed that preexposure to FT stimulation has an effect similar to training with controllable shock, inducing a protective effect that blocks induction of the capsaicin-induced learning deficit. In addition to preventing deficit induction, controllable shock attenuates inflammation-induced allodynia and reinstates learning when administered following capsaicin (Hook et al., 2008). Elsewhere, we have shown that FT stimulation reverses capsaicin-induced allodynia (Baumbauer et al., under review), but this effect was not evident until the amount of stimulation was increased from 900 to 1800 FT shocks, suggesting that the consequences of FT stimulation grow with training (Baumbauer et al., 2008; Baumbauer et al., 2009). The current experiment explores whether extended exposure (1800 shock) to FT stimulation also reverses the capsaicin-induced learning deficit.
Method
Thirty-two rats (n = 8 per condition) received subcutaneous hindpaw injections of 1% capsaicin or physiological saline (50 μL vol). Tactile reactivity was monitored for the next 3 hr. Rats then received 0 or 1800 FT legshocks. Shock and capsaicin treatment always occurred on the same limb, and the limb treated was counterbalanced across subjects. A VT shock treated condition was not included because we have previously shown that uncontrollable VT shock has an adverse, rather than restorative, effect on subsequent learning (Baumbauer et al., 2008; Baumbauer et al., 2009; Crown & Grau, 2001). Day 1 tactile reactivity is reported elsewhere and showed that FT stimulation can attenuate allodynia after capsaicin treatment (Baumbauer et al., under review). Here, we focus on the results obtained 24 hrs later. Rats received an additional assessment of tactile reactivity and were prepared for instrumental testing. To reduce the impact of edema on electrode placement on performance, instrumental testing occurred on the contralateral limb to capsaicin/shock treatment.
Results
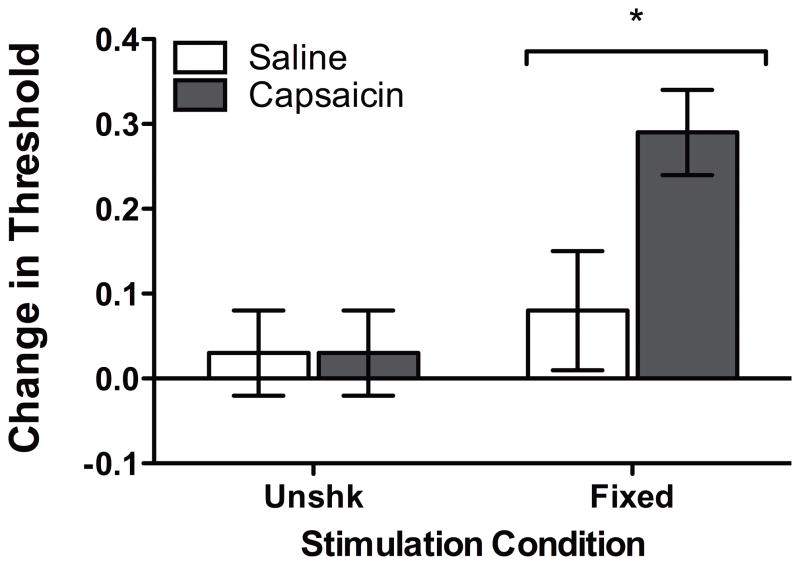
Rats administered FT stimulation exhibited significantly higher response thresholds than unshocked rats (Figure 3). An ANOVA performed on the overall data failed to detect any differences based on leg treated, F(1, 28) < 1.0, p > .05. Consequently, we collapsed the data across this variable. The subsequent ANOVA revealed a significant main effect of Shock Condition, F(1, 32) = 6.71, p < .05. No other statistical effects approached significance, all Fs < 3.10, p > .05. Post hoc analysis of group means confirmed that rats in the FT condition were less responsive relative to unshocked rats (p < .05).
Figure 3.
The effect of fixed legshock on tactile reactivity 24 hr following capsaicin administration. Twenty-four hr following capsaicin and shock treatment, rats received a final assessment of tactile reactivity. Values were converted to change from baseline scores. Asterisks indicate statistically significant differences (p < .05), and error bars depict ± SEM.
To determine if our experimental manipulations affected subjects’ ability to perform a flexion response, we analyzed the amount of stimulation required to produce a 0.4 N flexion force and subjects’ initial flexion durations. Shock intensities (± SEM) ranged from 0.51 ± 0.02 mA to 0.56 ± 0.01 mA, and initial response durations ranged from 0.13 ± 0.01 s to 0.17 ± 0.01 s. Individual ANOVAs revealed significant differences based on drug condition on both measures. Post hoc analysis of group means demonstrated that saline treated rats required significantly more stimulation to elicit a 0.4 N flexion force and maintained significantly longer initial flexion durations. Given these differences, subsequent analyses were performed using an ANCOVA, entering shock intensity and initial flexion duration as cofactors, to determine whether differences on either variable affected our experimental findings. Importantly, the ANCOVA revealed that neither covariate accounted for a significant proportion of the variance, both Fs < 1.0, p > .05.
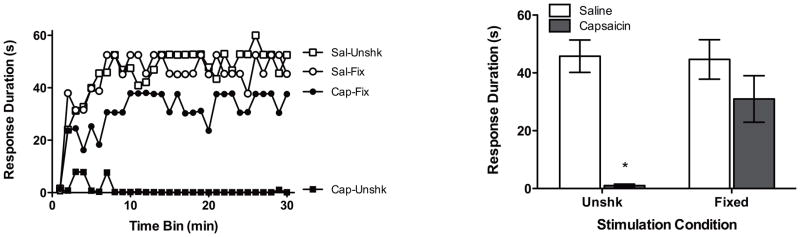
As observed in the previous experiment, unshocked rats given capsaicin alone exhibited a learning deficit (Figure 4). Interestingly, treatment with fixed stimulation after capsaicin restored learning. An ANCOVA revealed significant main effects of Shock Condition and Drug Condition, as well as significant Shock Condition X Drug Condition interactions, all Fs > 4.23, p < .05. The significant two-way interaction emerged because unshocked subjects administered capsaicin exhibited significantly shorter response durations than rats in all other conditions (p < .05).
Figure 4.
The effect of fixed legshock on the capsaicin-induced learning deficit. Twenty-four hr following capsaicin and shock treatment rats were assessed for instrumental learning. The panel on the left depicts subjects’ response durations over time, while the panel on the right depicts response durations as means collapsed across time. Asterisks indicate statistically significant differences (p < .05), and error bars depict ± SEM.
General Discussion
We have shown elsewhere that FT stimulation engages a process that can attenuate the acute allodynia induced by peripheral capsaicin treatment (Baumbauer et al, under review). FT stimulation was effective whether given before or after capsaicin treatment. However, to reverse the consequences of inflammation required additional training (1,800 rather than 900 FT shocks), presumably because inflammation (relative to uncontrollable shock) provides a more robust stimulus. The present study focused on a long-term consequence of capsaicin treatment, its impact on instrumental learning. As previously reported (Hook et al., 2008), we found that capsaicin-treated subjects exhibited impaired instrumental learning when tested on the contralateral leg 24 hrs after the inflammatory stimulus was applied. This observation is important because it suggests that inflammation has a residual long-term effect that impacts spinal plasticity and that the capacity for instrumental learning is a sensitive measure of this effect. Pretreatment with FT stimulation blocked the induction of the capsaicin-induced learning impairment (Experiment 1). Similarly, exposure to FT stimulation 3 hrs after capsaicin was applied reversed its long-term effect on learning (Experiment 2). Together, these results imply that FT stimulation counters both the induction and maintenance of the acute and long-term consequences of capsaicin treatment.
Our results provide further evidence that behavioral control and FT stimulation have parallel effects. Both do far more than prevent the induction of a learning impairment. Instead, they appear to engage a protective/restorative effect that counters the consequences of uncontrollable stimulation and peripheral inflammation (Baumbauer et al., under review; Hook et al., 2008). Further, both types of stimulation can enable learning when subjects are tested with a more difficult response criterion (Baumbauer, Young, Hoy, & Joynes, 2007a; Crown et al., 2002a; Gómez -Pinilla et al., 2007; Huie, Baumbauer, Hughes, & Grau, 2008) and, in both cases, these beneficial effects have been linked to the neurotrophin BDNF (Baumbauer et al., 2009; Gómez-Pinilla et al., 2007). Finally, both instrumental learning and FT stimulation depend on a form of NMDAR-mediated plasticity (Baumbauer et al., 2009; Joynes et al., 2004).
Given that FT stimulation and instrumental control have parallel effects, and depend on common neurochemical systems (NMDAR and BDNF), one might posit that they depend on common functional systems. Within the learning literature, learning about temporal regularity is sometimes characterized as a form of Pavlovian (temporal) conditioning (Pavlov, 1927), wherein the time between events functions as a kind of Pavlovian conditioned stimulus (CS) and shock onset operates as the unconditioned stimulus (US). Conceiving of the FT effect as a type of learning would help to explain why the emergence of the FT effect requires training (and that early in training VT and FT stimulation have similar effects). A learning-based account is also supported by the observations that the long-term consequences of FT training depend on NMDAR-mediated plasticity and protein synthesis (Baumbauer et al., 2009). Finally, if instituting a regular relation has a beneficial effect because it introduces a kind of Pavlovian cue (the time between shocks), then an explicit Pavlovian cue (a cutaneous stimulus) should also have a protective effect. Supporting this, we have recently obtained data have demonstrated that an external CS (200 ms legshock) that precedes the onset of tailshock prevents deficit induction.
If the FT effect is a kind of stimulus-stimulus (S-S; Pavlovian) learning, then the question of functional commonality with spinally-mediated instrumental (response-outcome [R-O]) learning can be rephrased. In particular, can spinal instrumental learning be treated as a type of Pavlovian conditioning? Decades ago, Konorski (1948) suggested that the acquisition of a similar motoric effect could be mediated by a form of S-S learning, wherein an afferent signal (indicating limb position) serves as a Pavlovian CS. Pairing this CS with shock onset (the US) could strengthen the flexion response (the conditioned response [CR]). Alternatively, one could argue that a cue related to the temporal dynamics of performing a motor response (R) provides a cue (a kind of hour glass) that could support a form of temporal conditioning. In either case, one could question whether the difference between instrumental and Pavlovian conditioning in this system has more to do with experimenter-defined variables than mechanistic differences within the organism (c.f. Timberlake, 1999; Timberlake & Lucas, 1989). Nonetheless, being aware that both instrumental control and the introduction of stimulus regularity can have beneficial effects may be important to the development of clinical therapies, because they provide alternative routes for engaging a beneficial effect. Yet, within this reduced preparation, both environmental contingencies may be mediated by common systems.
Independent of how we characterize the relation between FT stimulation and instrumental control, it is clear that spinal mechanisms can discriminate regular versus irregular stimuli and that these two forms of stimulation have divergent effects. At a physiological level, how could the system discriminate the stimuli? One possibility is that FT and VT stimulation engage distinct fiber pathways, providing a kind of “filter” that allows each to have a divergent effect (Baumbauer et al., 2009). Indirect support for this view comes from the recent observation that low frequency stimulation can induce either long-term potentiation (LTP) or long-term depression (LTD) in the spinal cord depending upon the spinal tract stimulated (Ikeda et al., 2006). However, from this perspective, it is not clear why FT and VT stimulation have similar effects after 36–180 shocks (inducing both an allodynia and a learning deficit). The fact they have common effects suggests that common mechanisms may be initially engaged and that the benefit of FT stimulation depends on a more central mechanism and extended training. Alternatively, as suggested above, introducing a FT relation may be important because it provides a new cue that accurately predicts the occurrence of shock onset. This cue could be provided by a physiological process that decays at a regular rate, yielding a physiological hourglass (Boulos & Terman, 1980). Alternatively, a sense of time could be gained through the entrainment of the central pattern generator (CPG) used to drive the rhythmical stepping pattern (Baumbauer et al., 2008; Baumbauer et al., 2009; Kiehn, 2006; McCrea & Rybak, 2008). Indirect evidence for this perspective comes from research demonstrating that the stimulus frequencies that generate a FT effect lie within the frequency range of stepping (de Leon, Hodgson, Roy, & Edgerton, 1994; Roy, Hutchinson, Pierotti, Hodgson, & Edgerton, 1991). Our data are in agreement with other studies suggesting that simple systems can exhibit timing-like behavior. Experiments using the isolated salamander retina have shown that delivering alternating patterns of light and dark stimulation can entrain the retina to respond in a predictable manner when a violation in the pattern of presentation occurs (Schwartz, Harris, Shrom, & Berry, 2007). Specifically, after the pattern has been entrained, omission of a stimulus leads to an increase in spike bursting that is precisely timed to when the stimulus should have occurred. Similarly, temporal regularity influences plasticity in the visual cortex (Perrett, Dudek, Eagleman, Montague, & Friedlander, 2001). Low frequency (1 Hz) stimulation administered in a regular (FT) manner leads to LTD while administering the same average frequency of stimulation in a variable (VT) manner leads to LTP. Taken together, these results suggest that the ability to engage in timing-like behavior may be a fundamental attribute of neural ensembles and may not be relegated to specific neuroanatomical regions.
Our data are also in agreement with studies examining the impact of uncontrollable stimulation in intact subjects. Uncontrollable stimulation is frequently used to model stress phenomena (Christianson, Thompson, Watkins, & Maier, 2009; Maier & Seligman, 1976; Overmier & Seligman, 1967, and work has shown that regular and variable stimulation have a divergent impact on behavior. For example, human participants will rate regularly presented aversive stimulation (shock administered every 2 s) as evoking less anxiety and having less negative valence and lower pain intensity, than unpredictable stimuli (variable shock administered, on average, every 2 s; range = 0.2 – 3.8 s; Carlsson et al., 2006). Research using animal models has shown similar effects, but only under extended stimulation conditions. In particular, rats exposed to one session of variable or fixed spaced stimulation exhibited decreased exploratory behavior, increased hot plate paw lick latencies, and increased inhibition of brain monoamine oxidase (MAO) relative to controls (Lemoine, Armando, Brun, Barontini, & Segura, 1994). However, when the rats were given 10 sessions of fixed or variable stimulation, subjects in the fixed spaced conditioned did not differ from untreated controls. Like the results reported here, these finding imply that fixed and variable stimulation can have divergent behavioral/physiological effects and that these differences may only emerge after extended training.
In the pain literature, researchers have generally used regular stimulation to study phenomena such as “wind-up” and “wind-down”. These phenomena are induced using regular (e.g., 1 Hz) electrical stimulation of an afferent nerve (e.g., the sciatic). After a relatively small number of stimuli (e.g., 10–16 pulses), spinal neurons exhibit an increase in excitability (wind-up) and tactile allodynia (Mendell, 1966; Woolf, 1996). If stimulation is continued, neuronal excitability is depressed (wind-down) and behavioral reactivity diminishes (Price et al., 2007). These results parallel the effects we observe after FT stimulation. What our findings suggest is that the transition from wind-up to wind-down may depend on the imposition of stimulus regularity, and that the emergence of wind-down could reflect a NMDAR/protein synthesis dependent process. Further, just as irregular and regular low frequency stimulation (900 pulses) within the visual cortex has divergent effects (inducing LTP versus LTD, respectively), continued irregular stimulation could induce central sensitization. Indeed, this could help address what is otherwise a puzzling observation. Because wind-up and central sensitization depend on some common neurochemical systems (e.g., NMDAR), and have a common behavioral effect (allodynia), researchers posited that the two phenomena are related (Baranauskas & Nistri, 1998; Urban, Thompson, & Dray, 1994; Woolf & Thompson, 1991). Yet, continued exposure to the stimulus train used to induce wind-up yields wind-down (Herrero, Laird, & Lopez-Garcia, 2000; Traub, 1997). Our results suggest that using VT stimulation (a pattern of stimulation that emulates the erratic firing of C fibers elicited by peripheral inflammation) will produce a central sensitization-like effect, with no evidence of wind-down.
Our results have important clinical implications, demonstrating that FT stimulation can not only reverse the allodynia associated with peripheral inflammation (Baumbauer et al., under review), but can also reinstate the ability to learn. FT stimulation may be a viable treatment alternative to surgical procedures or opiate-derivatives for the treatment of neuropathic pain. While FT and controllable stimulation both have a beneficial antiallodynic effect, FT stimulation may be more easily translated to clinical settings because it can be applied cutaneously without the need for intramuscular implantation of electrodes or the execution of a motor response. Moreover, given that FT stimulation enhances motor output (e.g. acquiring the prolonged foot flexion response; Huie et al., 2008), FT stimulation could also benefit the recovery of locomotor function following spinal injury.
Acknowledgments
We would like to thank Drs. Michelle Hook and Sandra Garraway, as well as Denise Puga, Kevin Hoy, Sarah Woller, and Kuan Lee for their helpful comments. The present work was supported by HD058412 and NS041548.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/BNE
References
- Baranauskas G, Nistri A. Sensitization of pain pathways in the spinal cord: cellular mechanisms. Progress in Neurobiology. 1998;54:349–365. doi: 10.1016/s0301-0082(97)00067-1. [DOI] [PubMed] [Google Scholar]
- Baumbauer KM, Hoy KC, Jr, Huie JR, Hughes AJ, Woller SA, Puga DA, Setlow B, Grau JW. Timing in the absence of supraspinal input I: variable, but not fixed, spaced stimulation of the sciatic nerve undermines spinally-mediated instrumental learning. Neuroscience. 2008;155:1030–1047. doi: 10.1016/j.neuroscience.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumbauer KM, Huie JR, Hughes AJ, Grau JW. Timing in the absence of supraspinal input II: regularly spaced stimulation induces a lasting alteration in spinal function that depends on the NMDA receptor, BDNF release, and protein synthesis. Journal of Neuroscience. 2009;29:14383–14393. doi: 10.1523/JNEUROSCI.3583-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumbauer KM, Puga DA, Lee KH, Woller SA, Hughes AJ, Grau JW. Timing in the absence of supraspinal input III: Introducing temporal regularity reduces stimulation-induced allodynia and counters the behavioral consequences of inflammation. Journal of Neuroscience (under review) [Google Scholar]
- Baumbauer KM, Young EE, Hoy KC, Jr, Joynes RL. Intrathecal administration of neurokinin 1 and neurokinin 2 receptor antagonists undermines the savings effect in spinal rats seen in an instrumental learning paradigm. Behavioral Neuroscience. 2007a;121:186–199. doi: 10.1037/0735-7044.121.1.186. [DOI] [PubMed] [Google Scholar]
- Baumbauer KM, Young EE, Hoy KC, Jr, Joynes RL. Neurokinin receptors modulate the impact of uncontrollable stimulation on adaptive spinal plasticity. Behavioral Neuroscience. 2007b;121:1082–1094. doi: 10.1037/0735-7044.121.5.1082. [DOI] [PubMed] [Google Scholar]
- Baumbauer KM, Young EE, Joynes RL. Pain and learning in a spinal system: contradictory outcomes from common origins. Brain Research Reviews. 2009;61:124–143. doi: 10.1016/j.brainresrev.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Beggs AL, Steinmetz JE, Patterson MM. Classical conditioning of a flexor nerve response in spinal cats: Effects of tibial nerve CS and a differential conditioning paradigm. Behavioral Neuroscience. 1985;99:496–508. doi: 10.1037//0735-7044.99.3.496. [DOI] [PubMed] [Google Scholar]
- Boulos Z, Terman M. Food availability and daily biological rhythms. Neuroscience and Biobehavioral Reviews. 1980;4:119–131. doi: 10.1016/0149-7634(80)90010-x. [DOI] [PubMed] [Google Scholar]
- Buerger AA, Eisenstein EM, Reep RL. The yoked control in instrumental avoidance conditioning: An empirical and methodological analysis. Physiological Psychology. 1981;9:351–353. [Google Scholar]
- Carlsson K, Andersson J, Petrovic P, Petersson KM, Olman A, Ingvar M. Predictability modulates the affective and sensory-discriminative neural processing of pain. Neuroimage. 2006;32:1804–1814. doi: 10.1016/j.neuroimage.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Christianson JP, Thompson BM, Watkins LR, Maier SF. Medial prefrontal cortical activation modulates the impact of controllable and uncontrollable stressor exposure on a social exploration test of anxiety in the rat. Stress. 2009;12:445–450. doi: 10.1080/10253890802510302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church RM. Systematic effect of random error in the yoked control design. Psychological Bulletin. 1964;62:122–131. doi: 10.1037/h0042733. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Melzack R. The contribution of excitatory amino acids to central sensitization and persistent nociception after formalin-induced tissue injury. Journal of Neuroscience. 1992;12:3665–3670. doi: 10.1523/JNEUROSCI.12-09-03665.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crown ED, Ferguson AR, Joynes RL, Grau JW. Instrumental learning within the spinal cord: II. Evidence for central meditation. Physiology & Behavior. 2002a;77:259–267. doi: 10.1016/s0031-9384(02)00859-4. [DOI] [PubMed] [Google Scholar]
- Crown ED, Ferguson AR, Joynes RL, Grau JW. Instrumental learning within the spinal cord: IV. Induction and retention of the behavioral deficit observed after noncontingent shock. Behavioral Neuroscience. 2002b;116:1032–1051. doi: 10.1037//0735-7044.116.6.1032. [DOI] [PubMed] [Google Scholar]
- Crown ED, Grau JW. Preserving and restoring behavioral potential within the spinal cord using an instrumental training paradigm. Journal of Neurophysiol. 2001;86:845–855. doi: 10.1152/jn.2001.86.2.845. [DOI] [PubMed] [Google Scholar]
- de Leon R, Hodgson JA, Roy RR, Edgerton VR. Extensor- and flexor-like modulation within motor pools of the rat hindlimb during treadmill locomotion and swimming. Brain Res. 1994;654:241–250. doi: 10.1016/0006-8993(94)90485-5. [DOI] [PubMed] [Google Scholar]
- Durkovic RG. Classical conditioning, sensitization and habituation in the spinal cat. Physiology & Behavior. 1975;14:297–304. doi: 10.1016/0031-9384(75)90037-2. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, De Leon RD, Tillakaratne N, Recktenwald MR, Hodgson JA, Roy RR. Use-dependent plasticity in spinal stepping and standing. Advances in Neurology. 1997;72:233–247. [PubMed] [Google Scholar]
- Ferguson AR, Bolding KA, Huie JR, Hook MA, Santillano DR, Miranda RC, Grau JW. Group I metabotropic glutamate receptors control metaplasticity of spinal cord learning through a protein kinase C-dependent mechanism. Journal of Neuroscience. 2008;28:11939–11949. doi: 10.1523/JNEUROSCI.3098-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson AR, Crown ED, Grau JW. Nociceptive plasticity inhibits adaptive learning in the spinal cord. Neuroscience. 2006;141:421–431. doi: 10.1016/j.neuroscience.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Gómez -Pinilla F, Huie JR, Ying Z, Ferguson AR, Crown ED, Baumbauer KM, Edgerton VR, Grau JW. BDNF and learning: Evidence that instrumental training promotes learning within the spinal cord by up-regulating BDNF expression. Neuroscience. 2007;148:893–906. doi: 10.1016/j.neuroscience.2007.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau JW, Barstow DG, Joynes RL. Instrumental learning within the spinal cord: I. Behavioral properties. Behavioral Neuroscience. 1998;112:1366–1386. doi: 10.1037//0735-7044.112.6.1366. [DOI] [PubMed] [Google Scholar]
- Grau JW, Crown ED, Ferguson AR, Washburn SN, Hook MA, Miranda RC. Instrumental learning within the spinal cord: underlying mechanisms and implications for recovery after injury. Behavioral and Cognitive Neuroscience Reviews. 2006;5:191–239. doi: 10.1177/1534582306289738. [DOI] [PubMed] [Google Scholar]
- Groves PM, Thompson RF. Habituation: A dual-process theory. Psychological Review. 1970;77:419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Heng C, de Leon RD. The rodent lumbar spinal cord learns to correct errors in hindlimb coordination caused by viscous force perturbations during stepping. Journal of Neuroscience. 2007;27:8558–8562. doi: 10.1523/JNEUROSCI.1635-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero JF, Laird JM, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Progress in Neurobiology. 2000;61:169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- Hodgson JA, Roy RR, de Leon R, Dobkin B, Edgerton VR. Can the mammalian lumbar spinal cord learn a motor task? Medicine and Ccience in Sports and Exercise. 1994;26:1491–1497. [PubMed] [Google Scholar]
- Hook MA, Huie JR, Grau JW. Peripheral inflammation undermines the plasticity of the isolated spinal cord. Behavioral Neuroscience. 2008;122:233–249. doi: 10.1037/0735-7044.122.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huie JR, Baumbauer KM, Hughes AJ, Grau JW. 2008 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2008. Evidence of timing in the absence of supraspinal input: Fixed space stimulation enables future instrumental learning. [Google Scholar]
- Ikeda H, Stark J, Fischer H, Wagner M, Drdla R, Jager T, Sandkuhler J. Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science. 2006;312:1659–1662. doi: 10.1126/science.1127233. [DOI] [PubMed] [Google Scholar]
- Joynes RL, Grau JW. Mechanisms of Pavlovian conditioning: Role of protection from habituation in spinal conditioning. Behavioral Neuroscience. 1996;110:1375–1387. doi: 10.1037//0735-7044.110.6.1375. [DOI] [PubMed] [Google Scholar]
- Joynes RL, Grau JW. Instrumental learning within the spinal cord: III. Prior exposure to noncontingent shock induces a behavioral deficit that is blocked by an opioid antagonist. Neurobiology of Learning and Memory. 2004;82:35–51. doi: 10.1016/j.nlm.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Joynes RL, Janjua K, Grau JW. Instrumental learning within the spinal cord: VI. The NMDA receptor antagonist, AP5, disrupts the acquisition and maintenance of an acquired flexion response. Behavioural Brain Research. 2004;154:431–438. doi: 10.1016/j.bbr.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annual Review of Neuroscience. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- Konorski JA. Conditioned reflexes and neuron organization. New York: Hafner; 1948. [Google Scholar]
- Lemoine AP, Armando I, Brun JC, Barontini M, Segura ET. Stressor predictability influences open field behavior, pain sensitivity and brain MAO inhibitory activity (tribulin) in the rat. Behavioural Brain Research. 1994;61:91–95. doi: 10.1016/0166-4328(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Maier SF, Seligman ME. Learned helplessness: Theory and evidence. Journal of Experimental Psychology: General. 1976;105:3–46. [Google Scholar]
- Malan TP, Ossipov MH, Gardell LR, Ibrahim M, Bian D, Lai J, Porreca F. Extraterritorial neuropathic pain correlates with multisegmental elevation of spinal dynorphin in nerve-injured rats. Pain. 2000;86:185–194. doi: 10.1016/s0304-3959(00)00243-8. [DOI] [PubMed] [Google Scholar]
- McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Research Reviews. 2008;57:134–146. doi: 10.1016/j.brainresrev.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmier JB, Seligman ME. Effects of inescapable shock upon subsequent escape and avoidance responding. Journal of Comparative and Physiological Psychology. 1967;63:28–33. doi: 10.1037/h0024166. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes. New York: Oxford University Press; 1927. [Google Scholar]
- Perrett SP, Dudek SM, Eagleman D, Montague PR, Friedlander MJ. LTD induction in adult visual cortex: Role of stimulus timing and inhibition. Journal of Neuroscience. 2001;21:2308–2319. doi: 10.1523/JNEUROSCI.21-07-02308.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Rashid MH, Millecamps M, Sanoja R, Entrena JM, Cervero F. Decreased nociceptive sensitization in mice lacking the Fragile X mental retardation protein: Role of mGluR1/5 and mTOR. Journal of Neuroscience. 2007;27:13958–13967. doi: 10.1523/JNEUROSCI.4383-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy RR, Hutchinson DL, Pierotti DJ, Hodgson JA, Edgerton VR. EMG patterns of rat ankle extensors and flexors during treadmill locomotion and swimming. Journal of Applied Physiology. 1991;70:2522–2529. doi: 10.1152/jappl.1991.70.6.2522. [DOI] [PubMed] [Google Scholar]
- Schwartz G, Harris R, Shrom D, Berry MJ. Detection and prediction of periodic patterns by the retina. Nature Neuroscience. 2007;10:552–554. doi: 10.1038/nn1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer WA, Thompson RF, Neilson DRJ. Response decrement of the flexion reflex in the acute spinal cat and transient restoration by strong stimuli. Journal of Neurophysiology. 1966;29:221–239. doi: 10.1152/jn.1966.29.2.221. [DOI] [PubMed] [Google Scholar]
- Timberlake W. Biological behaviorism. In: O’Donohue W, Kitchener R, editors. Handboook of Behaviorism. New York: Academic Press; 1999. pp. 243–285. [Google Scholar]
- Timberlake W, Lucas GA. Behavior systems and learning: From misbehavior to general principles. In: Klein SB, Mowrer RR, editors. Contemporary Learning Theories: Instrumental Conditioning Theory and the Impact of Biological Constraints on Learning. Hillsdale: Erlbaum; 1989. pp. 237–275. [Google Scholar]
- Traub RJ. Spinal modulation of the induction of central sensitization. Brain Research. 1997;778:34–42. doi: 10.1016/s0006-8993(97)00946-3. [DOI] [PubMed] [Google Scholar]
- Urban L, Thompson SW, Dray A. Modulation of spinal excitability: co-operation between neurokinin and excitatory amino acid neurotransmitters. Trends in Neurosciences. 1994;17:432–438. doi: 10.1016/0166-2236(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Urban MO, Gebhart GF. Supraspinal contributions to hyperalgesia. Proceedings of the National Academy of Sciences. 1999;96:7687–7692. doi: 10.1073/pnas.96.14.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ. Windup and central sensitization are not equivalent. Pain. 1996;66:105–108. [PubMed] [Google Scholar]
- Woolf CJ, Thompson SN. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of posti-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Lee CL, Carp JS. Operantly conditioned plasticity in spinal cord. Proceedings of the National Academy of Sciences. 1991;627:338–348. doi: 10.1111/j.1749-6632.1991.tb25936.x. [DOI] [PubMed] [Google Scholar]
- Young EE, Baumbauer KM, Hillyer J, Joynes RL. Local anesthetic treatment significantly attenuates acute pain responding but does not prevent the neonatal injury-induced reduction in adult spinal behavioral plasticity. Behavioral Neuroscience. 2007;121:1073–1081. doi: 10.1037/0735-7044.121.5.1073. [DOI] [PubMed] [Google Scholar]
- Young EE, Baumbauer KM, Hillyer JE, Patterson AM, Hoy KC, Jr, Mintz EM, Joynes RL. The neonatal injury-induced spinal learning deficit in adult rats: central mechanisms. Behavioral Neuroscience. 2008;122:589–600. doi: 10.1037/0735-7044.122.3.589. [DOI] [PubMed] [Google Scholar]