Abstract
Patients with temporal lobe seizures sometimes experience what John Hughlings Jackson described as “dreamy states” during seizure onset. These phenomena may be characterized by a re-experiencing of past events, feelings of familiarity (déjà vu), and hallucinations. In previous reports, patients have been aware of the illusory nature of their experiences. Here, however, the case of a patient with a documented 37-year history of temporal lobe epilepsy who is not aware is described. Fifteen years ago, the patient saw visions of traumatic autobiographical events that he had never previously recalled. He believed them to be veridical memories from his childhood, although evidence from his family suggests that they were not. The patient’s psychological reaction to the “recovery” of these traumatic “memories” was severe enough to qualify as posttraumatic stress disorder (PTSD). To our knowledge, this is the first report of PTSD caused by the misattribution of mental states that accompany a seizure.
Keywords: Temporal lobe epilepsy, Seizure, Experiential phenomena, Experiential responses, Posttraumatic stress disorder
1. Introduction
In 1880, Hughlings Jackson reported on the presentation of feelings of reminiscence and “dreamy states” that can occur at the onset of seizures [1]. These feelings have been described by patients as “dreamy feelings,” “dreams mixing with present thoughts,” “double consciousness,” “feeling of being elsewhere,” and “as if I went back to all that occurred in my childhood.” One patient recalled, “I feel as if all must be a dream, though well knowing at the same time it must be reality … through it all the fear of some impending catastrophe seems to be hanging over me” (p. 202). Psychical seizures, as they were called, often involve a sudden re-experiencing of the past or a sudden false interpretation of the present [2]. Today, these experiences are given many names depending on the sensory modality in which they present (e.g., visual, auditory) and the type of experience [3,4]. In the current discussion we use the term experiential response to refer to this phenomenon because it is the most commonly used and least specific.
Since the time of Hughlings Jackson, many accounts of these phenomena have been reported, and evidence from surgical stimulations and physiological recordings has provided insight, though not consensus, into the underlying processes [5]. Gloor [6] described the main features of these experiential responses: (1) there may be a vivid or intrusive recall of a past event; (2) there is a feeling of familiarity or reminiscence (i.e., déjà vu); (3) a sensation of dreaminess is characteristic; (4) the patient is said to be always aware of the incongruous and illusory nature of the experience; (5) affective states such as fear, sadness, guilt, anger, and sexual excitement are common; (6) these responses typically lack certain features such as forward motion in time. Sometimes, these experiences can be quite elaborate. Patient R.W., reported by Penfield and Perot [2], described fragments of (what the authors interpreted to be) a fantasy or dream. The authors described the patient’s responses on surgical stimulation of a portion of the right temporal–parietal–occipital junction:
“Oh gosh! There [the robbers] are, my brother is there. He is aiming an air rifle at me.” His eye moved slowly to the left. The figures seemed to disappear before the cessation of the stimulus. When asked, he said his brother was walking toward him, and the gun was loaded. When asked where he was, he said at his house, in the yard. His other little brother was there, that was all. When asked if he felt scared when he saw his brother, he said “Yes.” When asked if he always felt scared when he saw the robbers, he said, “Yes.” [2, p. 617]
Although not described in the account by these authors, it is reasonable to believe that the patient’s recollection of his brother and the yard was veridical and that the situation with the robbers and the impending assault was fictitious. This type of confabulation of fact and fiction is what we believe we observed in the patient described in this report.
2. Case report
Mr. F (an arbitrary pseudonym) was recently referred to our psychology clinic by his attending psychiatrist for evaluation and treatment of symptoms of severe anxiety and depression.
Mr. F is a 55-year-old right-handed Caucasian man with 13 years of formal education who was diagnosed with epilepsy in his early adulthood. He suffered a head trauma with loss of consciousness while a high school student, and had no prior history of meningitis, encephalitis, or febrile convulsions. By his report, his first seizure occurred when he was 17 years old. His developmental history is unremarkable. There is no known family history of epilepsy. His mother died 24 hours after his delivery, reportedly as a result of a blood clot. He reported having had a cyst removed from his left temporal lobe 1 year after his seizure onset, although other historical documents suggest it was a bone cyst and subsequently grafted. The CT scan is not consistent with a craniotomy. Medical records are not available from that time to confirm the details of his report. He has suffered multiple injuries because of his seizures, including rib fractures.
At the time of his initial presentation to our hospital in 2008, he was experiencing seizures twice weekly. A witnessed seizure in our psychiatric hospital (where he had been hospitalized for depression and overdosing on phenytoin and diazepam) was described as generalized shaking. He had no recall of this event, but could answer orientation questions during a subsequent complex partial seizure in the emergency room. The only significant findings on his neurological examination were an extensor plantar response on the left and atrophy of the muscles in the left C5 distribution. A CT scan of the head showed a small cyst in the mesial right temporal structures, most consistent with a choroidal fissure cyst (Fig. 1). His B12 level was 325 pg/mL; remaining labs were within normal limits except for an alkaline phosphatase of 177 U/L (likely a result of chronic administration of phenytoin). An EEG obtained the next day revealed bitemporal interictal spikes, more frequent on the right than the left. Nine years previously, his EEG had shown only right temporal spikes. Mr. F has refused MRI and video/EEG monitoring. Previously tried medications also included phenobarbital, carbamazepine, and gabapentin. After this hospital visit in 2008, he was changed from phenytoin to valproic acid.
Fig. 1.
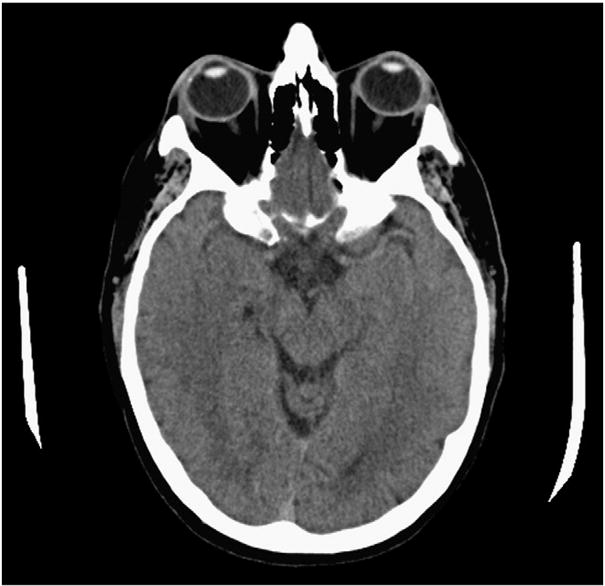
A CT scan of the head showed a small cyst in the mesial right temporal structures, most consistent with a choroidal fissure cyst.
Clinical interviews with Mr. F and his wife suggest that our patient experienced a detailed remembrance of what he believes to have been traumatic autobiographical experiences. However, evidence from his family suggests that these were not veridical memories, but rather experiential responses that occurred at the onset of his temporal lobe seizures, as have been described by Hughlings Jackson and others. Mr. F’s experiences are coherent with the description provided by Gloor [6], except that Mr. F does not perceive the incongruous and illusory nature of his experiences and he is, instead, insistent that these events occurred, noting the vivid details of the experiences. His misinterpretation of these seizure-related experiences as veridical, traumatic memories directly contributes to his present-day psychological distress.
During his assessment, Mr. F spoke freely about these memories, although they were clearly distressing for him. Mr. F agreed, and is presently in psychotherapy, to discuss the impact of these memories. Because of his strong emotional reaction to these memories and the strength of his focus on them, there was a possibility that prematurely addressing the falsehood of these memories might result in his terminating therapy. Unfortunately, Mr. F’s tendency to deny the impact of refractory epilepsy on his quality of life results in his focusing on these experiences rather than addressing day-to-day psychosocial functioning related to managing his epilepsy and his reactions to his actual limitations because of that diagnosis. He has discontinued follow-up with his neurologist and does not wish to undergo further evaluation (e.g., for epilepsy surgery) at this time.
Mr. F first recalled these traumatic childhood events 15 years ago when his wife purchased a pocket watch for him which, he notes, “triggered repressed memories.” Interviewed separately, his wife said that he did receive the watch a few weeks prior to the first recollection of the “memories,” but it was also precisely a week after experiencing a particularly dramatic seizure, during which he fell and hit his forehead on cement. Medical attention was not sought at that time; thus, no medical records of this event or of any resulting injury or follow-up are available. Mrs. F remembered that when Mr. F first reported his recollection of the events a week after his seizure and fall, he was “upset and confused” and has since continued to have intrusive recollections of the events and often insists on discussing them with family who routinely tell him they have no recollection of the events.
With the approval of our institutional review board, privacy office, and medical ethics lawyer, we describe his memories, but in a sufficiently vague manner so as to preserve his anonymity. The first recalled “memory” was estimated to have taken place when he was 4 or 5 years old, and comprised a series of related fragments involving hunting excursions in places actually frequented by his extended family in the southern United States. Prominent figures from then-contemporary American history participated in these hunts. During one incident, he “recalled” being frightened when one of these prominent U.S. historical figures stood up in a small boat with a shotgun, shot haphazardly, and rocked the boat. Later that same day, the same person impulsively shot their family’s prized farm animal, causing much turmoil in the family. Mr. F described that when he first recalled this memory 15 years ago, his brother “didn’t know what I was talking about,” and denied the incident had occurred. Interestingly, our own searches of historical literature indicated that these prominent figures were indeed visitors at these same locations at approximately the time Mr. F. actually had visited these places with his family. Mr. F may have known of these public figures frequenting the same vacation sites as his family and, as part of his experiential response, melded his autobiographical memories with his factual knowledge of the public figures’ visits. Multiple family members agree these events did not occur as Mr. F. recalls them.
The second of his recovered memories was presented as significantly more traumatic. In this memory, Mr. F was approximately 17 years old when a member of his rural community attempted to kill him in a “ritualistic” fashion with the encouragement of Mr. F.’s father. At the last possible second, before he was “sacrificed,” his church pastor came to rescue him. Mr. F recalled specific phrases from the ritual, but his recall of the events did not coalesce the way most veridical memories do; they were fragmented and lacked sequential order. Mr. F reported that there was no police report or arrest in this case. When asked why he thought the pocket watch was important in unlocking these “repressed memories,” Mr. F said that it was the same watch worn by the man who tried to ritualistically kill him. Mr. F’s wife reported that she had discussed the story with Mr. F’s family members, but they did not recall any such event or threat to Mr. F. when he was a teenager. Mrs. F reported that her husband has experienced recurrent symptoms of depression throughout their marriage, and this has added to his poor quality of life along with the impact of his epilepsy. She reported that in the 15 years following the fall and the recovery of these memories, his depression has progressively worsened and led him to attempt suicide several times in the past few years by overdosing on anticonvulsant medication. Prior to that, both Mrs. F and his wife note that he had had no significant history of psychological problems or a history of psychological or psychiatric diagnoses or treatment in his childhood, adolescence, or adulthood. Presently, Mr. F’s seizure activity is unpredictable, varying in occurrence from once every 3 weeks to two to three times per week. His wife reported that at the onset of a seizure, Mr. F will occasionally say that someone is hurting him and will command that person to stop. We have hypothesized that this may be a recurrence of his traumatic vision of being prepared for execution. Since the recovery of these memories, Mr. F reported recurrent, intrusive, and distressing thoughts involving these events. He continues to discuss the content of these memories with various family members, who are frustrated and distressed by his inability to accept that these events did not occur.
Mr. F’s psychological reaction to these memories/occurrences meets DSM-IV-TR criteria for Posttraumatic Stress Disorder (PTSD) [7]. Mr. F experienced an event, albeit of questionable veridicality, but perceived by the patient to be real, involving threatened death, and his response involved intense fear, helplessness, and horror (criterion A). Furthermore, he experiences recurrent and intrusive recollections of the ritual, as well as psychological and physiological reactivity to internal and external cues that symbolize and resemble the event (criterion B). Mr. F attempts to avoid activities, places, and people that arouse recollections of the trauma, experiences a feeling of detachment from others, demonstrates a restricted range of affect (criterion C), and reports difficulty falling asleep and concentrating (criterion D). These symptoms have endured for more than 1 month (criterion E) and cause significant distress and impairment in social functioning (criterion F). Indeed, his frequent discussions of the events with family members have caused significant strain in his relationships with the people to whom he recounts the stories repeatedly. There is no evidence to suggest that Mr. F’s experience would be better described and explained by another diagnosis, such as psychotic depression, and as reported, has had no history of delusions, hallucinations, or other psychotic-like symptoms or history. His responses are circumscribed to reactions to the specific memories in question; he exhibits no other current delusions or hallucinations. Furthermore, although his presentation may have initially been classified as Adjustment Disorder with Depressed Mood, he now qualifies for Major Depressive Disorder, Recurrent, along with Posttraumatic Stress Disorder.
Despite our strong recommendation, Mr. F declined our referral for a more current neurological evaluation, neuroimaging, and neuro-psychological evaluation. His most recent EEG appeared to demonstrate more extensive involvement (now bitemporal) than previously. Although routine interictal scalp EEG data are limited in their sensitivity and specificity, ictal EEG data might be more conclusive, but are unavailable. Furthermore, attempts to localize these occurrences to circumscribed regions may fail to appreciate the involvement of spatially distant networks [6] and deep limbic structures. It has been shown, for example, that repeated seizures or stimulation of a single cortical area, even within the same patient, can produce different responses, whereas stimulation of widely distinct areas within the same individual can produce similar phenomena [8].
3. Discussion
To our knowledge, this is the first report of a patient not being aware of the illusory nature of an experiential response that accompanied a seizure, thus producing a false memory. Furthermore, Mr. F’s experience is the first to demonstrate the magnitude of impairment that can result from such a misattribution. Mr. F’s experience is also unique because a particular seizure (accompanied by probable concussion) “unlocked” these memories, which were subsequently identified by Mr. F as having taken place at highly specific times and specific places in his life, a process that includes the integration of real and fictitious places, people, and events.
The influence of Mr. F’s probable concussion cannot be known definitively. If Mr. F sustained frontal lobe damage during the seizure in question, it is unlikely that this damage alone could cause the creation and then misattribution of autobiographical visions, and we have not found reports of this in the literature. If frontal lobe damage was sustained, a more likely possibility is that the temporal lobe seizure produced the vision, and co-occurring frontal lobe damage resulted in Mr. F’s inability to correctly connect the vision to its correct source (i.e., the seizure) and context (i.e., not in his childhood). This possibility is more consistent with previous reports of temporal lobe seizures [2,4,6] and the role of the frontal lobes in source memory [9].
It is possible that these memories are so intrusive and persistent in nature because they are re-experienced at the onset of his seizures (as suggested by his wife’s account) and are thus reinforced by frequent neural activity. Additionally, it is possible that, even though the memories are traumatic, they may be easier for Mr. F to focus on than the day-to-day psychological and social struggles he has managing an unpredictable course of epilepsy.
Our experience working with Mr. F illustrates several important points that extend beyond the idiosyncrasies of his particular presentation. To properly diagnose and plan treatment for patients with complicated and multifaceted difficulties, the practitioner must have broad knowledge of the range of problems and diagnoses involved and the skills and competencies required to work with the complex problems presented [10,11]. Furthermore, close communication between disciplines should be emphasized. Without careful assessment and consideration of all variables, this patient may have been prematurely diagnosed only with Major Depressive Disorder or Posttraumatic Stress Disorder or only with temporal lobe epilepsy, missing the complicated tension between these disorders.
Additionally, because of complexities in the reporting of this case, such as the de-identification of Mr. F’s unique memories, we consulted multiple sources to ethically report his case. Our institutional review board agreed that the proceedings described here officially constitute a case study and therefore do not require an institutional review board-approved protocol. Our institution’s privacy office and medical ethics lawyer were consulted to ensure our due diligence in protecting Mr. F’s interests. Mr. F provided formal written agreement to the writing and dissemination of this article. It should be noted that our obligation to protect his anonymity (despite his unique and perhaps identifiable memories), as well as a clinical responsibility to protect him from premature confrontation of the non-veridicality of his memories, complicated our ability to report this case. We recommend that others wishing to report on such clinically sensitive cases seek consultation from all available sources, such as ethics boards, institutional review boards, and privacy offices, as this was helpful to us.
Acknowledgments
We thank Mr. F for his candor and willingness to share his story with the psychological and medical communities. We also thank William Allen for his helpful comments and suggestions.
References
- 1.Jackson JH. On right or left sided spasm at the onset of epileptic paroxyms, and on crude sensation warnings, and elaborate mental states. Brain. 1880;2:192–206. [Google Scholar]
- 2.Penfield W, Perot P. The brain’s record of auditory and visual experience: a final summary and discussion. Brain. 1963;86:595–696. doi: 10.1093/brain/86.4.595. [DOI] [PubMed] [Google Scholar]
- 3.Barbeau E, Wendling F, Regis J, et al. Recollection of vivid memories after perirhinal region stimulations: synchronization in the theta range of spatially distributed brain areas. Neuropsychologia. 2005;43:1329–37. doi: 10.1016/j.neuropsychologia.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 4.Vignal JP, Maillard L, McGonigal A, Chauvel P. The dreamy state: hallucinations of autobiographic memory evoked by temporal lobe stimulations and seizures. Brain. 2007;130:88–99. doi: 10.1093/brain/awl329. [DOI] [PubMed] [Google Scholar]
- 5.Elliott B, Joyce E, Shorvon S. Delusions, illusions and hallucinations in epilepsy: 1. Elementary phenomena. Epilepsy Res. 2009;85:162–71. doi: 10.1016/j.eplepsyres.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Gloor P. Experiential phenomena of temporal lobe epilepsy: facts and hypotheses. Brain. 1990;113:1673–94. doi: 10.1093/brain/113.6.1673. [DOI] [PubMed] [Google Scholar]
- 7.text rev. 4. Washington, DC: American Psychiatric Assoc; 2000. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- 8.Horowitz MJ, Adams JE, Rutkins BB. Visual imagery on brain stimulation. Arch Gen Psychiatry. 1968;19:469–86. doi: 10.1001/archpsyc.1968.01740100085013. [DOI] [PubMed] [Google Scholar]
- 9.Janowsky JS, Shimamura AP, Squire LR. Source memory impairment in patients with frontal lobe lesions. Neuropsychologia. 1989;27:1043–56. doi: 10.1016/0028-3932(89)90184-x. [DOI] [PubMed] [Google Scholar]
- 10.Belar CD, Brown RA, Hersch LE, et al. Self-assessment in clinical health psychology: a model for ethical expansion of practice. Prof Psychol Res Pr. 2001;32:135–41. doi: 10.1037/0735-7028.32.2.135. [DOI] [PubMed] [Google Scholar]
- 11.France CR, Masters KS, Belar CD, et al. Application of the competency model to clinical health psychology. Prof Psychol Res Pr. 2008;39:573–80. [Google Scholar]


