Abstract
Sterile protection against infection with Plasmodium sporozoites requires high numbers of memory CD8 T-cells. However, infections with unrelated pathogens, as may occur in malaria endemic areas, dramatically decrease pre-existing memory CD8 T-cells. It remains unknown whether unrelated infections will compromise numbers of Plasmodium-specific memory CD8 T-cells and thus limit the duration of anti-malarial immunity generated by subunit vaccination. We show that P. berghei circumsporozoite-specific memory CD8 T-cells underwent significant attrition in numbers in mice subjected to unrelated infections. Attrition was associated with preferential loss of effector memory CD8 T-cells and reduced immunity to P. berghei sporozoite challenge. However, and of relevance to deployment of Plasmodium vaccines in malaria endemic areas, attrition of memory CD8 T-cells was reversed by booster immunization, which restored protection. These data suggest that regular booster immunizations may be required to sustain protective vaccine-induced Plasmodium-specific memory CD8 T-cells in the face of attrition caused by unrelated infections.
Introduction
Malaria causing Plasmodium species exert an enormous global health burden resulting in ~250 million infections and 900,000 deaths in 2008 (1). The mammalian life-cycle of Plasmodium begins when infected mosquitoes deposit sporozoites into the dermal tissue, which then travel to the liver (2), differentiate in infected hepatocytes and release merozoites that initiate red blood cell infection and malaria disease (3, 4). Both radiation-attenuated sporozoites (RAS) (5) and subunit vaccines (6) provide some protection of humans against sporozoite challenge, however, achieving long-term sterilizing immunity remains difficult. Importantly, protection mediated by these vaccines is CD8 T cell-dependent in rodent and primate models of malaria (6, 7), although this has not been definitely proven in humans. However, CD4 T cells, antibodies, and NK cells also contribute to protective immunity in rodents following RAS-vaccination (7–13).
Memory CD8 T cells can remain stable in numbers for essentially the life of laboratory mice (14). However, memory CD8 T cells undergo attrition in frequency amongst total CD8 T cells and in some cases total numbers when mice are infected with unrelated pathogens or exposed to inflammation (15–22). One study showed that antigen-specific CD8 T cell numbers can increase dramatically upon repeated infection of mice, which resulted in a modest (~33%) but statistically significant decrease in pre-existing memory CD8 T cell numbers (19). This suggested that it may be possible to generate large CD8 T cell responses to protect against highly successful pathogens, without negatively impacting pre-existing memory CD8 T cell protection to other pathogens. However, a recent study showed that attrition of infection-induced memory CD8 T cells compromised immunity to a very high dose, but not low dose, challenge with Listeria monocytogenes (LM) (21). Furthermore, in scenarios where large numbers of memory CD8 T cells are required for protection, as is the case for resistance to Plasmodium infection (23, 24), attrition of vaccine-induced memory CD8 T cells may compromise protection. Here we addressed the impact of subsequent infections on the maintenance of pre-existing Plasmodium-specific memory CD8 T cell numbers, subset composition, protective capacity, and ability to respond to booster immunizations.
Materials and Methods
Mice, Immunizations, and Infections
BALB/c mice (NCI, Frederick, MD) were housed at the University of Iowa. Mice were primed with 0.4–1×106 dendritic cells (DC) coated with circumsporozoite protein (CS)252-260 (DC-CS) i.v. and boosted 7 days later with 1×107 recombinant actA- inlB-deficient LM expressing CS252-260 (LM-CS) i.v., as described (23). Naïve and (DC-CS+LM-CS) mice were infected with 2×105 pfu lymphocytic choriomeningitis virus (LCMV) Armstrong i.p., 5×106 actA-deficient LM (DP-L1942) (LM) i.v., 1×104 pfu vaccinia virus-western reserve (VacV) i.p., and 2×105 pfu mouse hepatitis virus-1 (MHV-1) i.p. Memory CS252-specific CD8 T cells were boosted with 2×107 LM-CS i.v. Mouse experiments were approved by the IACUC at the University of Iowa.
Quantification and phenotypic analysis of CS252-specific CD8 T cells
Spleens were disrupted into single cell suspensions. Livers were perfused with cold HBSS through the hepatic portal vein and made into single cell suspensions. Liver mononuclear cells were collected by centrifugation in 35% Percoll/HBSS. Spleen and liver mononuclear cells were treated with Tris-ammonium chloride to lyse red blood cells. PBL were obtained by treating blood with Tris-ammonium chloride.
Spleen CS252-specific CD8 T cells were identified by intracellular cytokine stain for IFN-γ after a 5 hour incubation in brefeldin A (Biolegend, San Diego, CA), in the presence or absence of 200 nM CS252-260 peptide. P815 antigen-presenting cells were added to the stimulation to identify CS252-specific CD8 T cells in PBL and livers. After stimulation, cells were fixed, permeabilized and stained for intracellular IFN-γ, TNF, and IL-2 as recommended by the manufacturer (BD Biosciences). Cell surface expression of CD62L was detected by incubating cells with 0.1 mM TAPI-2 (Peptides International Inc., Louisville, KY) for 30 minutes prior to and during stimulation with CS252-260 peptide (25).
Antibodies
The following antibodies were used from eBioscience (San Diego, CA) CD4-APC (RM4-5), CD8-FITC/PE/PE-Cy7 (53–6.7), CD11a-FITC (M17/4), CD27-PE (LG.7F9), CD127-APC (A7R34), KLRG1-APC (2F1), Golden Syrian Hamster IgG-APC, IFN-γ-APC/PE-Cy7 (XMG1.2), TNF-APC (MP6-XT22), IL-2-PE (JES6-5H4), rat IgG2a-PE/APC (eBR2a). The following antibodies were used from BD Pharmingen CD62L-PE (MEL-14).
Sporozoite challenge
P. berghei (Pb) ANKA clone 234 sporozoites were isolated from the salivary glands of infected Anopheles stephensi mosquitoes. Mice were challenged with 1000 Pb sporozoites i.v. Parasitized red blood cells were identified by Giemsa stain 10 days post challenge. Protection is defined as the absence of blood stage parasites. At least 10 fields were examined for each mouse designated as protected.
Statistical Analysis
Data were analyzed using Prism4 software.
Results and Discussion
Memory CS252-specific CD8 T cells decrease in frequency and total numbers following infection with unrelated pathogens
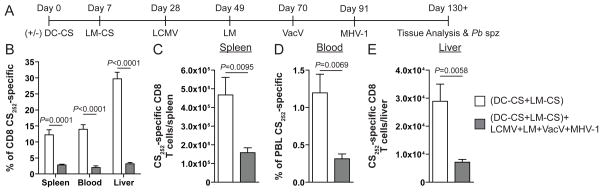
To address the impact of unrelated infections on maintenance and protection by epitope-specific vaccine-induced memory CD8 T cells, we employed a quantitative model of sterilizing immunity to Plasmodium sporozoite infection (23). BALB/c mice were immunized with DC coated with CS252-260 (DC-CS) and boosted one week later with attenuated L. monocytogenes (LM) expressing the CS252-260 epitope (LM-CS). This vaccination approach provides essentially life-long protection to BALB/c mice in a CD8 T cell-dependent manner against repeated challenge with Pb sporozoites (23, 26). Following the LM-CS boost mice were left alone or infected at three week intervals with; LCMV, LM that do not express the CS252-260 peptide, VacV, and finally MHV-1 (Fig. 1A). Controls were age matched naïve mice and mice were only infected with LCMV, LM, VacV, and MHV-1 to control for any non-specific resistance against sporozoite challenge. T cell analyses and Plasmodium challenge infections were undertaken ~6 weeks after the last unrelated infection (Fig. 1A). DC-CS+LM-CS immunization and infection with multiple pathogens had no impact on the frequency of CD4 T cells amongst the PBL compared to naïve mice (Supplemental Fig. 1A and B). However, the frequency of CD8 T cells in the PBL was higher in mice infected with multiple pathogens compared to naïve mice (Supplemental Fig. 1A and C). The increase in circulating CD8 T cells tracked with increased frequency of antigen-experienced CD8 T cells (Supplemental Fig. 1A, D, and E), which were detected by down-regulation of CD8α and up-regulation of CD11a (27). Consistent with a previous report (19), these results show that as the number of infections increased so did the frequency of total CD8 T cells and antigen-experienced CD8 T cells amongst the PBL.
FIGURE 1.
CS252-specific CD8 T cells undergo attrition in multiple tissues. A, Experimental design. B, Frequency of CS252-specific of all CD8 T cells as detected by intracellular cytokine staining (ICS) for IFN-γ. C, Total number of CS252-specific CD8 T cells per spleen. D, Percent of PBL that are CS252-specific CD8 T cells. E, Total number of CS252-specific CD8 T cells per liver. B–E, Cumulative data (mean±S.E.M.) from 6 mice from two experiments. Data were analyzed by unpaired two-tailed t-test.
Increases in total CD8 T cell numbers after repeated infections correlate with decreased frequencies of infection-induced pre-existing memory CD8 T cells (19). To address this issue for epitope-specific vaccine-induced CD8 T cell responses we analyzed memory CS252-specific CD8 T cells in the spleen, blood, and liver of control immune mice and those subjected to unrelated infection. Consistent with previous reports that demonstrate varying degrees of attrition (15–22), we observed a substantial decrease in the frequency of CD8 T cells that were CS252-specific in multiple tissues of DC-CS+LM-CS immunized mice subsequently infected with multiple pathogens (Supplemental Fig. 1F and Fig. 1B). Importantly, we also observed decreases in the total number of memory CS252-specific CD8 T cells in the spleen and liver and frequency of total PBL in DC-CS+LM-CS immunized mice subjected to unrelated infections (Fig. 1C–E). These data demonstrate that pre-existing epitope-specific vaccine-induced memory CD8 T cells undergo substantial attrition in total cell numbers in multiple tissues after encounter with unrelated pathogens.
Multiple infections change the subset composition of memory CD8 T cells
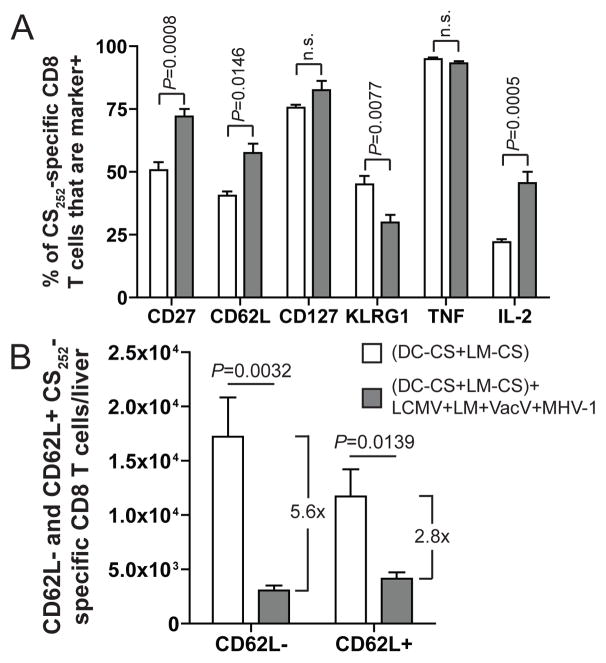
Previous work has suggested that effector memory CD8 T cells (Tem, CD62L−, CD27lo, IL-2−), in contrast to central memory CD8 T cells (Tcm, CD62L+, CD27hi, IL- 2+), are critical for immunity to liver-stage Plasmodium infection (24). However, it is unclear how attrition impacts the composition of pre-existing memory CD8 T cell populations. LM-specific memory CD8 T cells that had or had not undergone attrition in response to modified vaccinia virus Ankara exhibited no differences in CD62L, CD127, IL-2 or TNF production (21). To address this issue for epitope-specific vaccine-induced memory CD8 T cells we analyzed the phenotype and polyfunctionality of IFN-γ producing memory CS252-specific CD8 T cells that had or had not undergone infection-induced attrition. Attrition had no impact on the frequency of memory CS252-specific CD8 T cells that were CD127+ or TNF+ (Fig. 2A and Supplemental Fig. 1G–I). Conversely, attrition increased the percent of memory CS252-specific CD8 T cell populations that were CD27hi, CD62L+, and IL-2+ (canonical Tcm markers (28)) (Fig. 2A and Supplemental Fig. 1G–I). Additionally, attrition decreased the percent of memory CS252-spcecific CD8 T cells that were KLRG1+ (Fig. 2A and Supplemental Fig. 1G–I). These results show that attrition can decrease the fraction of pre-existing memory CD8 T cells that are Tem.
FIGURE 2.
Infection with multiple pathogens alters the phenotype and subset composition of memory CS252-specific CD8 T cells. Mice were treated as shown in Fig. 1A. A, Percent of CS252-specific CD8 T cells as detected ICS for IFN-γ that are marker positive in the liver. B, Number of CD62L negative and CD62L positive CS252-specific CD8 T cells in the liver. The number to the right of each group represents the fold difference between the means. A and B, Data (mean±S.E.M.) from 6 mice from two experiments. Data were analyzed by unpaired two-tailed t-test.
Although reproducible, the attrition-induced decrease in the percent CS252-specific Tem was relatively modest. However, coupling the changes in percent Tem with the attrition-induced decrease in total CS252-specific memory CD8 T cells revealed a preferential loss of the Tem subset. Specifically, we observed that Tem (CD62L−) CS252-specific CD8 T cells decreased in numbers approximately twice as much as Tcm (CD62L+) CS252-specific CD8 T cells in the spleen (4.3-fold versus 2.3-fold reduction) and liver (5.6-fold versus 2.8-fold reduction) (Supplemental Fig. 1J and Fig. 2B respectively). This change in subset composition is likely to have functional consequences since Tem and Tcm CD8 T cells provide different levels of protection for specific pathogens. For example, Tem CD8 T cells may be more effective at providing protection against Plasmodium sporozoite challenge than Tcm CD8 T cells (24, 29). On the other hand, Tcm CD8 T cells provide increased protection against infection with LCMV clone 13 and vaccinia virus (28). Thus, the combined impact of infection-induced attrition in memory CD8 T cell numbers and memory subsets on protective immunity may be pathogen-dependent, with serious consequences for infections like Plasmodium, where protection may be tightly linked to numbers of Tem CD8 T cells. Furthermore, future studies should determine if the characteristics of the CD8 T cells (i.e. ratios of Tem and Tcm) induced using specific vaccine delivery vectors and prime-boost regimens could also influence the impact of attrition on vaccine-induced memory CD8 T cell numbers and protective immunity.
Attrition of memory CS252-specific CD8 T cells results in decreased protection against Plasmodium sporozoites that can be rescued by booster immunization
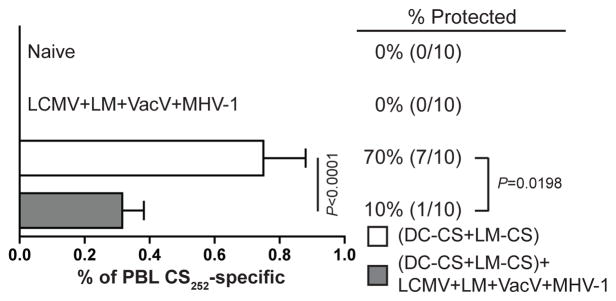
We next determined if multiple unrelated infections decrease CS252-specific CD8 T cell mediated protection against sporozoite challenge. To address the degree of attrition in the same mice used for challenge studies, we quantified the fraction of PBL that were memory CS252-specific CD8 T cells. Consistent with our previous analysis (Fig. 1D), there was a decrease in the percent of PBL that were memory CS252-specific CD8 T cells in DC-CS+LM-CS immunized mice infected with multiple pathogens compared to DC-CS+LM-CS immunized mice (Fig. 3). We then challenged those same mice and age-matched naïve mice with a stringent dose of 1000 Pb sporozoites where 100% of the naïve mice developed blood stage parasitemia (Fig. 3). Consistent with our previous results (23), a high proportion (70%) of DC-CS+LM-CS immunized mice were protected (Fig. 3). However, only 10% of DC-CS+LM-CS immunized mice infected with multiple pathogens were protected (Fig. 3). Finally, 100% of the LCMV+LM+VacV+MHV-1 infected mice that did not contain CS-specific memory CD8 T cells also developed blood stage parasitemia (Fig. 3), showing that the unrelated infections do not induce non-specific resistance to Pb sporozoite infection. Thus, infection-induced attrition and/or phenotypic changes of pre-existing Plasmodium-specific memory CD8 T cells profoundly decrease protection against Plasmodium sporozoite challenge.
FIGURE 3.
Attrition of CS252-specific CD8 T cells results in decreased protection against P. berghei sporozoite challenge. Mice were treated as shown in Fig. 1A. Percent of PBL that are CS252-specific CD8 T cells as detected ICS for IFN-γ. Data (mean±S.D.) are from 10 mice per group. Data were analyzed by unpaired two-tailed t-test. Mice were challenged with 1000 Pb sporozoites. Numbers to right of bar graph represents the percent of mice protected against the development of blood stage parasitemia (number protected/number challenged). Data are representative of two experiments. Protection results were analyzed by Fisher’s exact test.
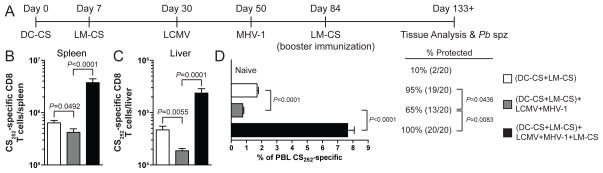
A well defined characteristic of memory CD8 T cells is their ability to expand in numbers upon antigen re-encounter and provide increased protection (30). However, it is not known if attrition impacts these attributes of memory CD8 T cells. To determine if booster immunization could rescue CD8 T cell numbers and protection we immunized a cohort of mice with DC-CS+LM-CS and subsequently infected a subset of animals with LCMV and MHV-1 to induce attrition (Fig. 4A). We chose to use only these two pathogens because single infections with LCMV and MHV-1 induced the greatest attrition of CS252-specific memory CD8 T cells (data not shown). CS252-specific CD8 T cells were reduced in the blood, spleen, and liver (Supplemental Fig. 2A and Fig. 4B–C) in mice subjected to unrelated infections. In addition to decreases in cell numbers, CS252-specific CD8 T cells in mice infected with LCMV and MHV-1 also exhibited changes in the expression of CD27, CD62L, and KLRG1 and production of IL-2 (Supplemental Fig. 2B), consistent with previous results (Fig. 2). Finally, DC-CS+LM-CS immunized mice that had undergone infection-induced attrition were less resistant to Pb sporozoite challenge (Fig. 4D). However, booster immunization with LM-CS induced substantial expansion of memory CS252-specific CD8 T cells that had undergone attrition (Supplemental Fig. 2A and Fig. 4B–C). Additionally, phenotypic and functional analyses revealed that the resulting memory CS252-specific CD8 T cells in the boosted mice exhibited an Tem phenotype (CD62L−, CD27lo, IL-2−) (Supplemental Fig. 2B). Strikingly, booster immunizations completely rescued protection against Pb sporozoites challenge (Fig. 4D). These data demonstrate that a population of memory CS252-specific CD8 T cells that has undergone attrition retains its capacity to be boosted in numbers, which in turn rescues protection against sporozoite challenge. It is also possible that immunization to generate extremely large memory CS252-specific CD8 T cells, perhaps by additional booster immunizations would ameliorate the impact of attrition due to unrelated infections and preserve long-term protection from Plasmodium challenge.
FIGURE 4.
Booster immunization increases memory CS252-specific CD8 T cell numbers and rescues protection against P. berghei sporozoite challenge. A, Experimental design. B, Total number of CS252-specific CD8 T cells per spleen as detected ICS for IFN-γ. C, Total number of CS252-specific CD8 T cells per liver. B and C, Cumulative data (mean±S.E.M.) from 5–8 mice from three experiments. D, Percent of PBL that are CS252-specific CD8 T cells. Data (mean±S.E.M.) are from 20 mice per group from two experiments. B–D, Data was analyzed by unpaired two-tailed t-test. Mice were challenged with 1000 Pb sporozoites. Numbers to right of bar graph represents the percent of mice protected against the development of blood stage parasitemia (number protected/number challenged). Protection results were analyzed by Fisher’s exact test.
Vaccination with RAS has long been viewed as the “gold” standard in malaria vaccine development. RAS vaccination elicits a multi-factorial immune response including CD8 T cells, CD4 T cells, antibodies, and NK cells that contribute to protective immunity (7–13). Of note, it has been shown in both rodent and non-human primates that protection is CD8 T cell-dependent (6, 7). It will be of great interest to determine if RAS-induced protective immunity also decreases following infection with multiple pathogens. However, these experiments are complicated by fact that, due to the paucity of defined epitopes, RAS-induced CD8 T cells can only be tracked using a surrogate activation marker approach (24), which will not be informative once mice are subsequently infected with different pathogens that will induce T cell populations specific for the unrelated pathogens but exhibiting the same activation markers as the Plasmodium specific T cells (27).
Given that multiple pathogens are endemic to the same regions of the world as Plasmodia, it is likely that infection-induced attrition of vaccine generated Plasmodium-specific memory CD8 T cells will be an important issue to overcome for long-term protection in malaria endemic areas. We demonstrate Plasmodium-specific memory CD8 T cells that have undergone infection-induced attrition retain their capacity to respond to booster immunizations, which rescues protection against challenge with Plasmodium sporozoites. Therefore, booster immunizations provided at determined intervals will likely be required to maintain the large number of memory CD8 T cells required for protection against Plasmodium sporozoites.
Supplementary Material
Acknowledgments
We thank Stanley Perlman and Vladimir Badovinac for comments and Noah Butler for help with experiments. We thank Jemmie Hoang, Lecia Epping, Brendan Dunphy, and Lyric Bartholomay for Pb infected mosquitoes.
Abbreviations used in this paper
- DC
dendritic cells
- CS
circumsporozoite protein
- LM
Listeria monocytogenes
- LCMV
lymphocytic choriomeningitis virus
- VacV
vaccinia virus-western reserve
- MHV-1
mouse hepatitis virus-1
- Pb
P. berghei
Footnotes
This work was supported by National Institutes of Health grants 1-F32-AI084329 (N.W.S.) and AI085515 (J.T.H.).
References
- 1.WHO. World Malaria Report 2008. World Health Organization; 2009. [Google Scholar]
- 2.Aly AS, Vaughan AM, Kappe SH. Malaria parasite development in the mosquito and infection of the mammalian host. Annu Rev Microbiol. 2009;63:195–221. doi: 10.1146/annurev.micro.091208.073403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menard R, Heussler V, Yuda M, Nussenzweig V. Plasmodium pre-erythrocytic stages: what’s new? Trends Parasitol. 2008;24:564–569. doi: 10.1016/j.pt.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Kappe SH, Vaughan AM, Boddey JA, Cowman AF. That was then but this is now: malaria research in the time of an eradication agenda. Science. 2010;328:862–866. doi: 10.1126/science.1184785. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, Doolan DL, Sacci J, de la Vega P, Dowler M, Paul C, Gordon DM, Stoute JA, Church LW, Sedegah M, Heppner DG, Ballou WR, Richie TL. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185:1155–1164. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 6.Hill AV. Pre-erythrocytic malaria vaccines: towards greater efficacy. Nat Rev Immunol. 2006;6:21–32. doi: 10.1038/nri1746. [DOI] [PubMed] [Google Scholar]
- 7.Doolan DL, Hoffman SL. The complexity of protective immunity against liver-stage malaria. J Immunol. 2000;165:1453–1462. doi: 10.4049/jimmunol.165.3.1453. [DOI] [PubMed] [Google Scholar]
- 8.Schofield L, Villaquiran J, Ferreira A, Schellekens H, Nussenzweig R, Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987;330:664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- 9.Weiss WR, Sedegah M, Beaudoin RL, Miller LH, Good MF. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc Natl Acad Sci U S A. 1988;85:573–576. doi: 10.1073/pnas.85.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss WR, Sedegah M, Berzofsky JA, Hoffman SL. The role of CD4+ T cells in immunity to malaria sporozoites. J Immunol. 1993;151:2690–2698. [PubMed] [Google Scholar]
- 11.Oliveira GA, Kumar KA, Calvo-Calle JM, Othoro C, Altszuler D, Nussenzweig V, Nardin EH. Class II-restricted protective immunity induced by malaria sporozoites. Infect Immun. 2008;76:1200–1206. doi: 10.1128/IAI.00566-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar KA, Sano G, Boscardin S, Nussenzweig RS, Nussenzweig MC, Zavala F, Nussenzweig V. The circumsporozoite protein is an immunodominant protective antigen in irradiated sporozoites. Nature. 2006;444:937–940. doi: 10.1038/nature05361. [DOI] [PubMed] [Google Scholar]
- 13.Doolan DL, Hoffman SL. IL-12 and NK cells are required for antigen-specific adaptive immunity against malaria initiated by CD8+ T cells in the Plasmodium yoelii model. J Immunol. 1999;163:884–892. [PubMed] [Google Scholar]
- 14.Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat Med. 2001;7:913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- 15.Selin LK, Vergilis K, Welsh RM, Nahill SR. Reduction of otherwise remarkably stable virus-specific cytotoxic T lymphocyte memory by heterologous viral infections. J Exp Med. 1996;183:2489–2499. doi: 10.1084/jem.183.6.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selin LK, Lin MY, Kraemer KA, Pardoll DM, Schneck JP, Varga SM, Santolucito PA, Pinto AK, Welsh RM. Attrition of T cell memory: selective loss of LCMV epitope-specific memory CD8 T cells following infections with heterologous viruses. Immunity. 1999;11:733–742. doi: 10.1016/s1074-7613(00)80147-8. [DOI] [PubMed] [Google Scholar]
- 17.Smith DK, Dudani R, Pedras-Vasconcelos JA, Chapdelaine Y, van Faassen H, Sad S. Cross-reactive antigen is required to prevent erosion of established T cell memory and tumor immunity: a heterologous bacterial model of attrition. J Immunol. 2002;169:1197–1206. doi: 10.4049/jimmunol.169.3.1197. [DOI] [PubMed] [Google Scholar]
- 18.Kim SK, Welsh RM. Comprehensive early and lasting loss of memory CD8 T cells and functional memory during acute and persistent viral infections. J Immunol. 2004;172:3139–3150. doi: 10.4049/jimmunol.172.5.3139. [DOI] [PubMed] [Google Scholar]
- 19.Vezys V, Yates A, Casey KA, Lanier G, Ahmed R, Antia R, Masopust D. Memory CD8 T-cell compartment grows in size with immunological experience. Nature. 2009;457:196–199. doi: 10.1038/nature07486. [DOI] [PubMed] [Google Scholar]
- 20.Welsh RM, Selin LK. Attrition of memory CD8 T cells. Nature. 2009;459:E3–4. doi: 10.1038/nature08091. discussion E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huster KM, Stemberger C, Gasteiger G, Kastenmuller W, Drexler I, Busch DH. Cutting edge: memory CD8 T cell compartment grows in size with immunological experience but nevertheless can lose function. J Immunol. 2009;183:6898–6902. doi: 10.4049/jimmunol.0902454. [DOI] [PubMed] [Google Scholar]
- 22.Bahl K, Huebner A, Davis RJ, Welsh RM. Analysis of apoptosis of memory T cells and dendritic cells during the early stages of viral infection or exposure to toll-like receptor agonists. J Virol. 2010;84:4866–4877. doi: 10.1128/JVI.02571-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt NW, Podyminogin RL, Butler NS, Badovinac VP, Tucker BJ, Bahjat KS, Lauer P, Reyes-Sandoval A, Hutchings CL, Moore AC, Gilbert SC, Hill AV, Bartholomay LC, Harty JT. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proc Natl Acad Sci U S A. 2008;105:14017–14022. doi: 10.1073/pnas.0805452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt NW, Butler NS, Badovinac VP, Harty JT. Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites. PLoS Pathog. 2010;6:e1000998. doi: 10.1371/journal.ppat.1000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jabbari A, Harty JT. Simultaneous assessment of antigen-stimulated cytokine production and memory subset composition of memory CD8 T cells. J Immunol Methods. 2006;313:161–168. doi: 10.1016/j.jim.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt NW, Butler NS, Harty JT. CD8 T cell immunity to Plasmodium permits generation of protective antibodies after repeated sporozoite challenge. Vaccine. 2009;27:6103–6106. doi: 10.1016/j.vaccine.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rai D, Pham NL, Harty JT, Badovinac VP. Tracking the total CD8 T cell response to infection reveals substantial discordance in magnitude and kinetics between inbred and outbred hosts. J Immunol. 2009;183:7672–7681. doi: 10.4049/jimmunol.0902874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 29.Berenzon D, Schwenk RJ, Letellier L, Guebre-Xabier M, Williams J, Krzych U. Protracted protection to Plasmodium berghei malaria is linked to functionally and phenotypically heterogeneous liver memory CD8+ T cells. J Immunol. 2003;171:2024–2034. doi: 10.4049/jimmunol.171.4.2024. [DOI] [PubMed] [Google Scholar]
- 30.Harty JT, V, Badovinac P. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.