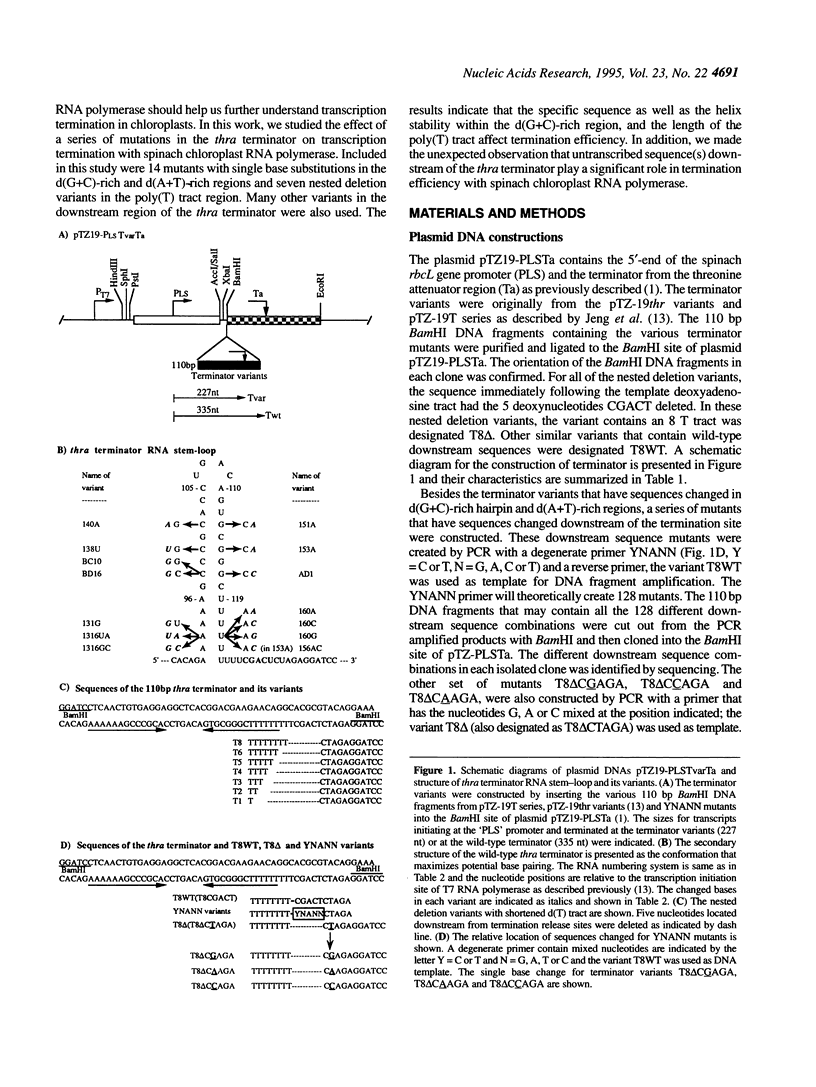
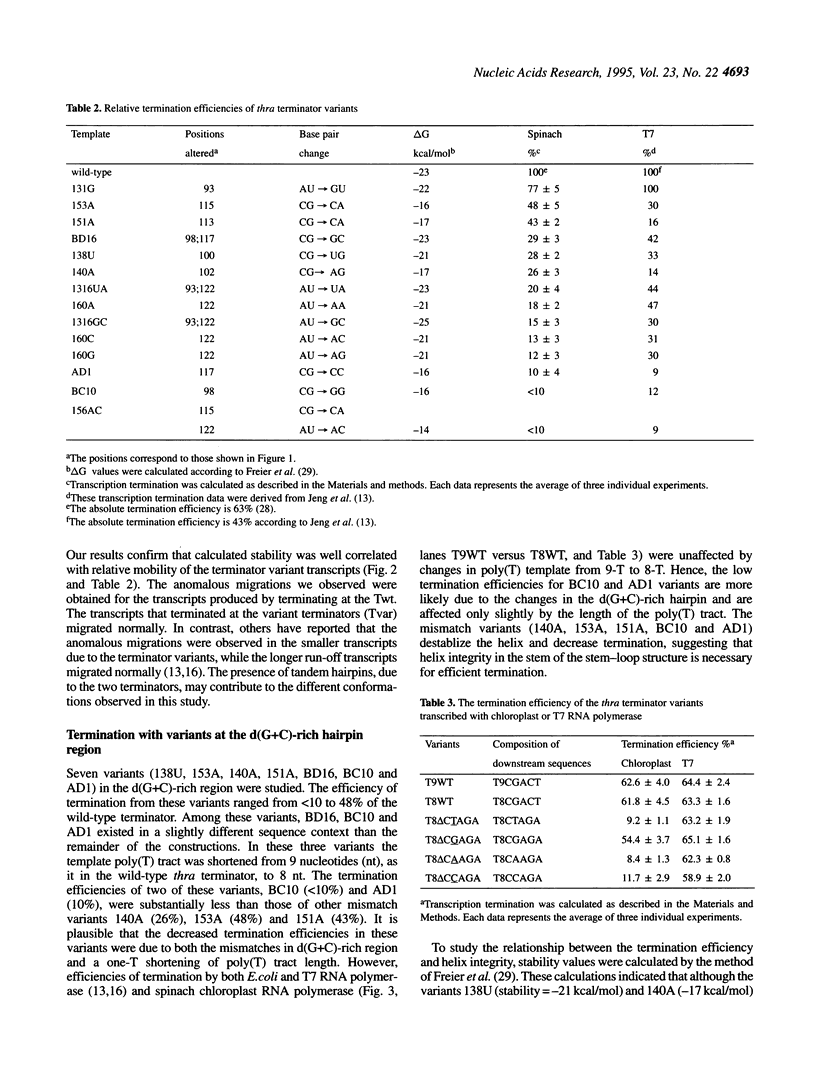
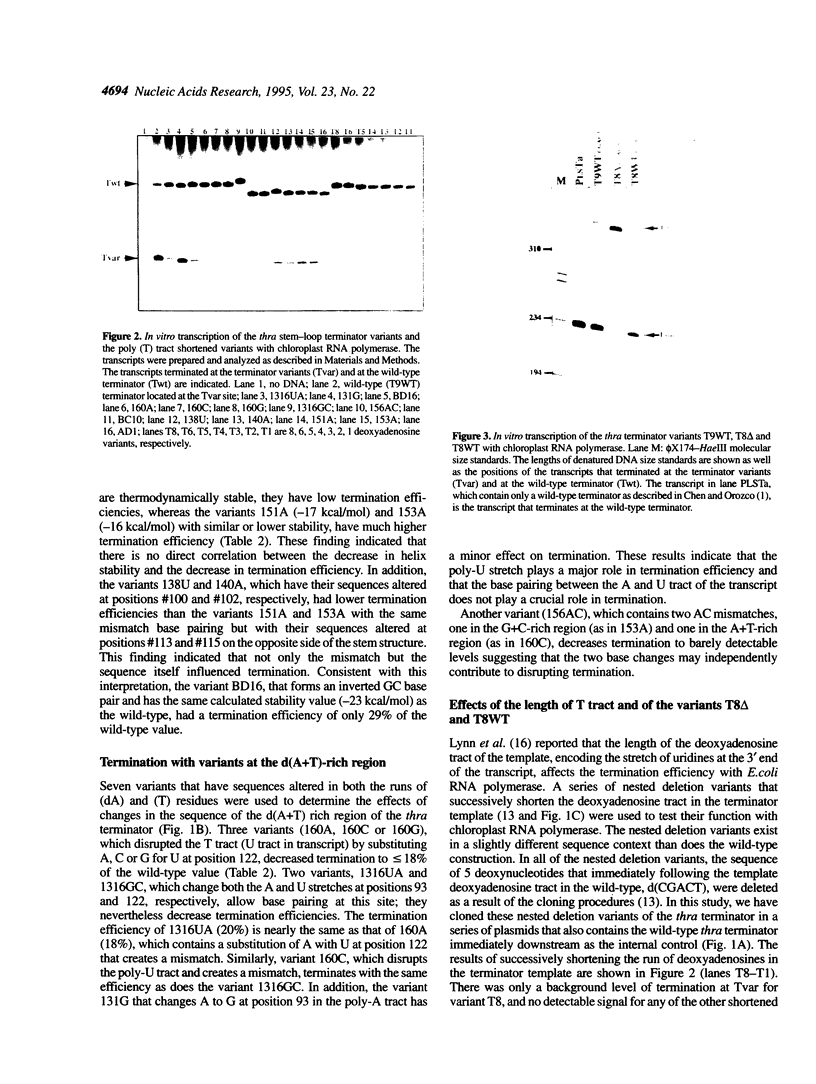
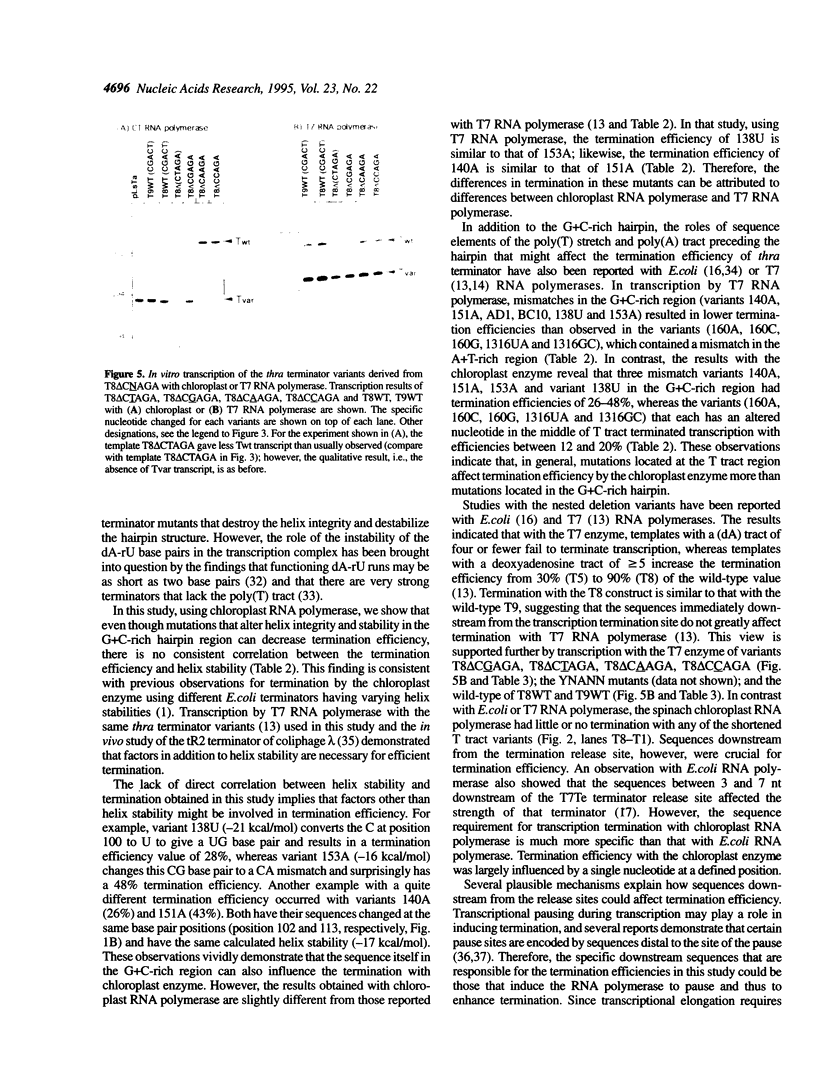
Abstract
We have investigated the mechanism of transcription termination in vitro by spinach chloroplast RNA polymerase using templates encoding variants of the transcription-termination structure (attenuator) of the regulatory region of the threonine (thr) operon of Escherichia coli. Fourteen sequence variants located within its d(G+C) stem-loop and d(A+T)-rich regions were studied. We found that the helix integrity in the stem-loop structure is necessary for termination but that its stability is not directly correlated with termination efficiency. The sequence of the G+C stem-loop itself also influences termination. Moreover, the dA template stretch at the 3' end of the terminator plays a major role in termination efficiency, but base pairing between the A and U tract of the transcript does not. From the studies using deletion variants and a series of mutants that alter the sequences immediately downstream from the transcription termination site, we found that termination of transcription by spinach chloroplast RNA polymerase was also modulated by downstream DNA sequences in a sequence-specific manner. The second base immediately following the poly(T) tract is crucial for determining the termination efficiency by chloroplast RNA polymerase, but not of the T7 or E.coli enzymes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M. Control of transcription termination. Annu Rev Biochem. 1978;47:967–996. doi: 10.1146/annurev.bi.47.070178.004535. [DOI] [PubMed] [Google Scholar]
- Altmann C. R., Solow-Cordero D. E., Chamberlin M. J. RNA cleavage and chain elongation by Escherichia coli DNA-dependent RNA polymerase in a binary enzyme.RNA complex. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3784–3788. doi: 10.1073/pnas.91.9.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. L., Koren R., Mildvan A. S. Magnetic resonance studies of the conformation of enzyme-bound adenylyl(3' leads to 5')uridine and adenosine 5'-triphosphate on RNA polymerase from Esherichia coli. Biochemistry. 1977 Jul 26;16(15):3322–3333. doi: 10.1021/bi00634a007. [DOI] [PubMed] [Google Scholar]
- Blowers A. D., Klein U., Ellmore G. S., Bogorad L. Functional in vivo analyses of the 3' flanking sequences of the Chlamydomonas chloroplast rbcL and psaB genes. Mol Gen Genet. 1993 Apr;238(3):339–349. doi: 10.1007/BF00291992. [DOI] [PubMed] [Google Scholar]
- Chamberlin M. J. New models for the mechanism of transcription elongation and its regulation. Harvey Lect. 1992 1993;88:1–21. [PubMed] [Google Scholar]
- Chen L. J., Orozco E. M., Jr Recognition of prokaryotic transcription terminators by spinach chloroplast RNA polymerase. Nucleic Acids Res. 1988 Sep 12;16(17):8411–8431. doi: 10.1093/nar/16.17.8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. J., Rogers S. A., Bennett D. C., Hu M. C., Orozco E. M., Jr An in vitro transcription termination system to analyze chloroplast promoters: identification of multiple promoters for the spinach atpB gene. Curr Genet. 1990 Jan;17(1):55–64. doi: 10.1007/BF00313249. [DOI] [PubMed] [Google Scholar]
- Cheng S. W., Lynch E. C., Leason K. R., Court D. L., Shapiro B. A., Friedman D. I. Functional importance of sequence in the stem-loop of a transcription terminator. Science. 1991 Nov 22;254(5035):1205–1207. doi: 10.1126/science.1835546. [DOI] [PubMed] [Google Scholar]
- Farnham P. J., Platt T. A model for transcription termination suggested by studies on the trp attenuator in vitro using base analogs. Cell. 1980 Jul;20(3):739–748. doi: 10.1016/0092-8674(80)90320-7. [DOI] [PubMed] [Google Scholar]
- Farnham P. J., Platt T. Rho-independent termination: dyad symmetry in DNA causes RNA polymerase to pause during transcription in vitro. Nucleic Acids Res. 1981 Feb 11;9(3):563–577. doi: 10.1093/nar/9.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freier S. M., Kierzek R., Jaeger J. A., Sugimoto N., Caruthers M. H., Neilson T., Turner D. H. Improved free-energy parameters for predictions of RNA duplex stability. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9373–9377. doi: 10.1073/pnas.83.24.9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner J. F. Initiation, pausing, and termination of transcription in the threonine operon regulatory region of Escherichia coli. J Biol Chem. 1982 Apr 10;257(7):3896–3904. [PubMed] [Google Scholar]
- Jeng S. T., Gardner J. F., Gumport R. I. Transcription termination by bacteriophage T7 RNA polymerase at rho-independent terminators. J Biol Chem. 1990 Mar 5;265(7):3823–3830. [PubMed] [Google Scholar]
- Jeng S. T., Gardner J. F., Gumport R. I. Transcription termination in vitro by bacteriophage T7 RNA polymerase. The role of sequence elements within and surrounding a rho-independent transcription terminator. J Biol Chem. 1992 Sep 25;267(27):19306–19312. [PubMed] [Google Scholar]
- Krummel B., Chamberlin M. J. Structural analysis of ternary complexes of Escherichia coli RNA polymerase. Deoxyribonuclease I footprinting of defined complexes. J Mol Biol. 1992 May 20;225(2):239–250. doi: 10.1016/0022-2836(92)90918-a. [DOI] [PubMed] [Google Scholar]
- Krummel B., Chamberlin M. J. Structural analysis of ternary complexes of Escherichia coli RNA polymerase. Individual complexes halted along different transcription units have distinct and unexpected biochemical properties. J Mol Biol. 1992 May 20;225(2):221–237. doi: 10.1016/0022-2836(92)90917-9. [DOI] [PubMed] [Google Scholar]
- Lee D. N., Phung L., Stewart J., Landick R. Transcription pausing by Escherichia coli RNA polymerase is modulated by downstream DNA sequences. J Biol Chem. 1990 Sep 5;265(25):15145–15153. [PubMed] [Google Scholar]
- Levin J. R., Chamberlin M. J. Mapping and characterization of transcriptional pause sites in the early genetic region of bacteriophage T7. J Mol Biol. 1987 Jul 5;196(1):61–84. doi: 10.1016/0022-2836(87)90511-0. [DOI] [PubMed] [Google Scholar]
- Lynn S. P., Gardner J. F., Reznikoff W. S. Attenuation regulation in the thr operon of Escherichia coli K-12: molecular cloning and transcription of the controlling region. J Bacteriol. 1982 Oct;152(1):363–371. doi: 10.1128/jb.152.1.363-371.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn S. P., Kasper L. M., Gardner J. F. Contributions of RNA secondary structure and length of the thymidine tract to transcription termination at the thr operon attenuator. J Biol Chem. 1988 Jan 5;263(1):472–479. [PubMed] [Google Scholar]
- Martin F. H., Tinoco I., Jr DNA-RNA hybrid duplexes containing oligo(dA:rU) sequences are exceptionally unstable and may facilitate termination of transcription. Nucleic Acids Res. 1980 May 24;8(10):2295–2299. doi: 10.1093/nar/8.10.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudler E., Goldfarb A., Kashlev M. Discontinuous mechanism of transcription elongation. Science. 1994 Aug 5;265(5173):793–796. doi: 10.1126/science.8047884. [DOI] [PubMed] [Google Scholar]
- Nudler E., Kashlev M., Nikiforov V., Goldfarb A. Coupling between transcription termination and RNA polymerase inchworming. Cell. 1995 May 5;81(3):351–357. doi: 10.1016/0092-8674(95)90388-7. [DOI] [PubMed] [Google Scholar]
- Orlova M., Newlands J., Das A., Goldfarb A., Borukhov S. Intrinsic transcript cleavage activity of RNA polymerase. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4596–4600. doi: 10.1073/pnas.92.10.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T. Transcription termination and the regulation of gene expression. Annu Rev Biochem. 1986;55:339–372. doi: 10.1146/annurev.bi.55.070186.002011. [DOI] [PubMed] [Google Scholar]
- Reynolds R., Bermúdez-Cruz R. M., Chamberlin M. J. Parameters affecting transcription termination by Escherichia coli RNA polymerase. I. Analysis of 13 rho-independent terminators. J Mol Biol. 1992 Mar 5;224(1):31–51. doi: 10.1016/0022-2836(92)90574-4. [DOI] [PubMed] [Google Scholar]
- Reynolds R., Chamberlin M. J. Parameters affecting transcription termination by Escherichia coli RNA. II. Construction and analysis of hybrid terminators. J Mol Biol. 1992 Mar 5;224(1):53–63. doi: 10.1016/0022-2836(92)90575-5. [DOI] [PubMed] [Google Scholar]
- Ryan T., Chamberlin M. J. Transcription analyses with heteroduplex trp attenuator templates indicate that the transcript stem and loop structure serves as the termination signal. J Biol Chem. 1983 Apr 25;258(8):4690–4693. [PubMed] [Google Scholar]
- Stern D. B., Gruissem W. Control of plastid gene expression: 3' inverted repeats act as mRNA processing and stabilizing elements, but do not terminate transcription. Cell. 1987 Dec 24;51(6):1145–1157. doi: 10.1016/0092-8674(87)90600-3. [DOI] [PubMed] [Google Scholar]
- Stern D. B., Jones H., Gruissem W. Function of plastid mRNA 3' inverted repeats. RNA stabilization and gene-specific protein binding. J Biol Chem. 1989 Nov 5;264(31):18742–18750. [PubMed] [Google Scholar]
- Surratt C. K., Milan S. C., Chamberlin M. J. Spontaneous cleavage of RNA in ternary complexes of Escherichia coli RNA polymerase and its significance for the mechanism of transcription. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7983–7987. doi: 10.1073/pnas.88.18.7983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telesnitsky A., Chamberlin M. J. Terminator-distal sequences determine the in vitro efficiency of the early terminators of bacteriophages T3 and T7. Biochemistry. 1989 Jun 13;28(12):5210–5218. doi: 10.1021/bi00438a044. [DOI] [PubMed] [Google Scholar]
- Wang D., Meier T. I., Chan C. L., Feng G., Lee D. N., Landick R. Discontinuous movements of DNA and RNA in RNA polymerase accompany formation of a paused transcription complex. Cell. 1995 May 5;81(3):341–350. doi: 10.1016/0092-8674(95)90387-9. [DOI] [PubMed] [Google Scholar]
- Yager T. D., von Hippel P. H. A thermodynamic analysis of RNA transcript elongation and termination in Escherichia coli. Biochemistry. 1991 Jan 29;30(4):1097–1118. doi: 10.1021/bi00218a032. [DOI] [PubMed] [Google Scholar]
- Yang M. T., Gardner J. F. Transcription termination directed by heteroduplex thr attenuator templates. Evidence that the transcript stem and loop structure is the termination signal. J Biol Chem. 1989 Feb 15;264(5):2634–2639. [PubMed] [Google Scholar]







