Abstract
Development of the segmented central nerve cords of vertebrates and invertebrates requires connecting successive neuromeres. Here, we show both how a pathway is constructed to guide pioneer axons between segments of the Drosophila CNS, and how motility of the pioneers along that pathway is promoted. First, canonical Notch signaling in specialized glial cells causes nearby differentiating neurons to extrude a mesh of fine projections, and shapes that mesh into a continuous carpet that bridges from segment to segment, hugging the glial surface. This is the direct substratum that pioneer axons follow as they grow. Simultaneously, Notch uses an alternate, non-canonical signaling pathway in the pioneer growth cones themselves, promoting their motility by suppressing Abl signaling to stimulate filopodial growth while presumably reducing substratum adhesion. This propels the axons as they establish the connection between successive segments.
Keywords: Abl signaling, Axon guidance, Notch, Actin structure, Filopodia, Growth cone motility, Drosophila
INTRODUCTION
The axon scaffold of every bilaterian central nerve cord, whether in insects or mammals, is built along two major axes: lateral connections within a segment and longitudinal connections between segments. Although a great deal is known about lateral patterning in the CNS, the mechanisms that produce longitudinal connections have been elusive and controversial. In Drosophila, for example, longitudinal pioneer axons track along specialized glial cells, the interface glia (also called longitudinal glia) (Jacobs and Goodman, 1989a; Jacobs and Goodman, 1989b; Hidalgo et al., 1995; Hidalgo and Booth, 2000), so it has been speculated that those glia might provide the substratum for the pioneers. Perturbing the glia, however, produces only relatively mild defects in the initial extension of longitudinal pioneers (Hosoya et al., 1995; Jones et al., 1995; Hidalgo and Booth, 2000), arguing against this hypothesis. Genetic experiments implicate Drosophila DCC (Frazzled) and its ligand, Netrin, and also the receptor Notch with its ligand, Delta, in the development of CNS longitudinal axon tracts in the fly: mutation of any of these genes interferes with development of longitudinal tracts between segments (Giniger et al., 1993; Kolodziej et al., 1996; Mitchell et al., 1996). Frazzled is not expressed in the longitudinal pioneer neurons themselves, but rather in cells along the nascent longitudinal pathway. Frazzled marks the track the pioneers are destined to follow by capturing Netrin protein produced elsewhere in the CNS and presenting it for recognition by pioneer growth cones (using another, unidentified Netrin receptor) (Hiramoto et al., 2000). Notch promotes growth of longitudinal axons by regulating the Abl tyrosine kinase pathway (‘Notch/Abl signaling’) (Giniger, 1998; Crowner et al., 2003; Le Gall et al., 2008). The non-canonical Notch/Abl pathway stands in contrast to the usual mechanism by which Notch directly controls nuclear gene regulation: in most contexts, in response to ligand, the intracellular domain of Notch is released proteolytically from the membrane and transits to the nucleus to form a transcription complex that activates genes bearing binding sites for the Notch-associated DNA-binding protein, Su(H) (Lai, 2004). How modulation of Abl by Notch promotes growth cone motility is unknown.
In establishing longitudinal connections in the CNS two problems must be solved. The first is to mark the path for pioneer axons to follow, and the second is driving motility of their growth cones along that path. Whereas much is known about how late-growing axons follow an existing nerve, we do not understand what specifies the exact trajectories assumed by pioneer axons as they first establish a nerve pathway. Within the growth cone, although a host of signaling proteins have been identified in the cascades downstream of guidance receptors (Luo, 2000; Song and Poo, 2001), the logic that maps individual signaling molecules onto particular steps in morphogenesis remains obscure, as manipulating signaling molecules in vivo often yields contradictory results (Wills et al., 1999b; Wills et al., 1999a; Bashaw et al., 2000; Krause et al., 2002; Hsouna et al., 2003; Forsthoefel et al., 2005; Trichet et al., 2008). This has frustrated efforts to understand how any single receptor modulates the cytoskeleton to guide a specific growth cone in vivo.
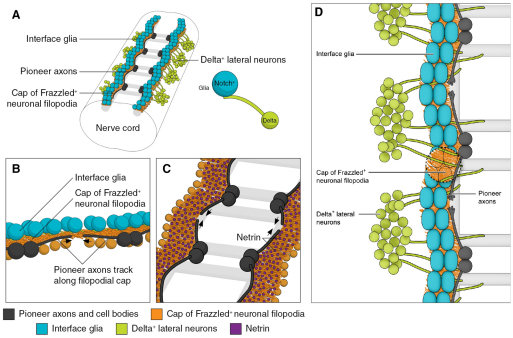
We now demonstrate how the interaction of four different CNS cell types specifies the pathway for Drosophila longitudinal pioneer axons, and how modulation of signal transduction in those pioneers drives their motility along that pathway. Ventrolateral neurons expressing the Notch ligand Delta project axons medially as they grow towards the commissures. These axons contact Notch-expressing interface glia, activating canonical, Su(H)-dependent Notch signaling in those glia. These glia, in turn, attract a cap of fine filopodial processes from surrounding neurons, and shapes that cap into a continuous track of neuronal membrane that bridges from one segment to the next. The neuronal cap, being enriched for the Drosophila DCC Frazzled, recruits Netrin protein, thus constructing a continuous Netrin domain that is the direct substratum for extending pioneer axons. Meanwhile, the Delta-positive commissural axons that stimulated canonical Notch signaling in the interface glia also act as stepping stones for the growing longitudinal axons. Delta on the commissural axons activates non-canonical Notch/Abl signaling in the longitudinal pioneers, stimulating growth cone motility by de-repressing the actin polymerization factor Enabled and suppressing activity of the Rac GTPases. By increasing filopodial development, and presumably also reducing substratum adhesion, this promotes the ability of advancing pioneer growth cones to cross the segment border and encounter one another, thereby establishing the first connection between successive segments of the fly CNS.
MATERIALS AND METHODS
Fly stocks
Flies were from the following sources: heartless-GAL4: Alan Michelson, NIH, USA; E(spl)mδ-lacZ: Jas Singh, Mt Sinai School of Medicine, NY, USA; UAS-Notch-lexA: Toby Lieber, Columbia University, NY, USA; GAL4-605: Gerd Technau, University of Mainz, Germany; frazzled−: Peter Kolodziej, Vanderbilt University, Nashville, TN, USA; Masaki Hiramoto and Yash Hiromi, NIG, Japan; GAL4-15J2: Andrea Brand, Welcome Trust, Cambridge, UK; UAS-mCD8-GFP, UAS-Rac1[N17], UAS-Rac1[V12] and repo-GAL4: Liqun Luo, Stanford University, Palo Alto, CA, USA; UAS-ena-GFP, UAS-FP4-mito, UAS-cpb: Julie Gates and Mark Peifer, University of North Carolina, Chapel Hill, NC, USA; UAS-trio[GEF1], Df(1)Net[A, B], NetB-myc: Barry Dickson, IMP, Vienna, Austria. Two copies of the modifying transgene were used for experiments using UAS-FP4-mito and UAS-Rac[N17]. All other stocks were from the Bloomington Drosophila Stock Center.
Temperature-shift protocols
For temperature-shift experiments we used Notchts1 (Nts1) or the heteroallelic combination Dl6B37/via1. To examine the mature axon pattern (stage 15/16), embryos were collected for 3 hours at 18°C, aged 8 hours at 18°C, shifted to restrictive temperature (32°C) for 6 hours and fixed. To quantify early-growing longitudinal axons (mid-stage 13), embryos were collected for 3 hours at 18°C, aged 7.5 hours at 18°C, shifted to restrictive temperature (32°C) for 4 hours 20 minutes and fixed. To visualize extending pioneer growth cones, embryos were collected for 3 hours at 18°C, aged 7 hours at 18°C, shifted to restrictive temperature (32°C) for 4 hours and fixed. Embryos for quantification of early CNS phenotypes were tightly staged based on embryo morphology: dorsal closure, head involution and gut morphogenesis.
Embryo preparation, antibodies and immunofluorescence
Embryos for most experiments were prepared, fixed and stained by standard methods (Bier et al., 1989). For Netrin-myc and actin-GFP we developed a modified hot triton fixation method (Miller et al., 1989). Embryos were collected, dechorionated and rinsed with 0.7% NaCl, 0.3% Triton X-100 (NaCl/Triton). Embryos were transferred to a 5 ml test tube in a small volume of NaCl/Triton, 3 ml of pre-warmed (90°C) NaCl/Triton was added and the tube placed immediately in a 90°C heat block for 5 seconds. Embryos were then vortexed briefly, poured into 20 ml ice-cold NaCl/Triton and held at 0°C for 10 minutes. Embryos were devitellinized with 1:1 heptane:95% methanol, 25 mM EGTA, by vortexing vigorously for 30 seconds, and rinsed with methanol/EGTA for at least 1 hour. Embryos were rehydrated through a methanol series into PBS, then postfixed with 4% methanol-free formaldehyde in 0.1 M NaPO4 pH 7.2 for 15 minutes at room temperature. Embryos were washed four times in PBT (0.1 M NaPO4 pH 7.2, 0.3% Triton X-100), then blocked and stained with antibodies.
Antibodies were from the following sources: rabbit anti-Frazzled: Y. N. Jan, UCSF, San Francisco, CA, USA; Laurie Lee and Peter Kolodziej, Vanderbilt University, Nashville, TN, USA; anti-Heartless: Alan Michelson; anti-Prospero: Chris Doe, University of Oregon, Eugene, OR, USA; Rb anti-Repo: Gerd Technau; rabbit anti-β-gal, mouse anti-β-gal and rabbit anti-HRP: Cappel Research Reagents, Cochranville, PA, USA; chick anti-GFP: Aves Lab, Tigard, OR, USA; mouse anti-GFP: Invitrogen. All other primary antibodies were from the Iowa Developmental Studies Hybridoma Bank. Secondary antibodies were from Jackson ImmunoResearch, except FITC-anti chicken IgY, which was from Aves Labs.
Peroxidase immunohistochemistry was performed using biotinylated secondary antibodies and VectaStain avidin-biotin-HRP (Vector Labs). Immunofluorescence was performed with fluorochrome-conjugated secondary antibodies, except detection of anti-Delta and NetB-myc, which were carried out using TSA-biotin (NEN) and FITC-streptavidin (Jackson ImmunoResearch), and some experiments employing TRITC-conjugated anti-HRP (Jackson ImmunoResearch). Immunofluorescent samples were examined by epifluorescence with a Zeiss AxioImager, using deconvolution or structured illumination (Apotome), or by confocal microscopy.
Scoring of phenotypes and statistical analysis
All embryos were examined in filet preparations. Embryos were mounted in 80% glycerol for 3,3′-diaminobenzidine (DAB) histochemistry, and in Fluorogard (BioRad) for immunofluorescence. In all experiments, embryo genotypes were scored unambiguously using gratuitous markers. For quantification of axonal phenotypes with mAb22C10, intersegment connections were scored at mid-stage 13. Left and right sides of a segment were scored independently. A connection was only scored as ‘absent’ if there were no axons detected connecting a pair of neuromeres; ‘thinning’ of a connection was not considered to be a mutant phenotype. n=200-450 hemisegments per dataset for all experiments. Statistical significance was assessed by ANOVA.
RESULTS
Longitudinal pioneers of the fly CNS
Intersegmental longitudinal connections in the fly CNS are pioneered by four interneurons, pCC, MP1, dMP2 and vMP2 (Fig. 1A) (Jacobs and Goodman, 1989a; Jacobs and Goodman, 1989b; Lin et al., 1995; Hidalgo and Brand, 1997). dMP2 and vMP2 are born in the middle of each segmental ganglion (‘neuromere’), and begin to extend axons during stage 12. The dMP2 axon grows posteriorly and vMP2 grows anteriorly, projecting towards the vMP2 and dMP2 axons pioneering from the next segment. Simultaneously, pCC sends its axon anteriorly in association with vMP2, while the MP1 axon wraps around dMP2 and extends posteriorly in association with it. The encounter of pioneers from successive segments establishes the first intersegment connection early in stage 13. The pioneer functions of these four axons are largely redundant as all must be ablated to produce a severe defect in the mature nerve (Lin et al., 1995; Hidalgo and Brand, 1997). Below, we will focus on dMP2 and vMP2.
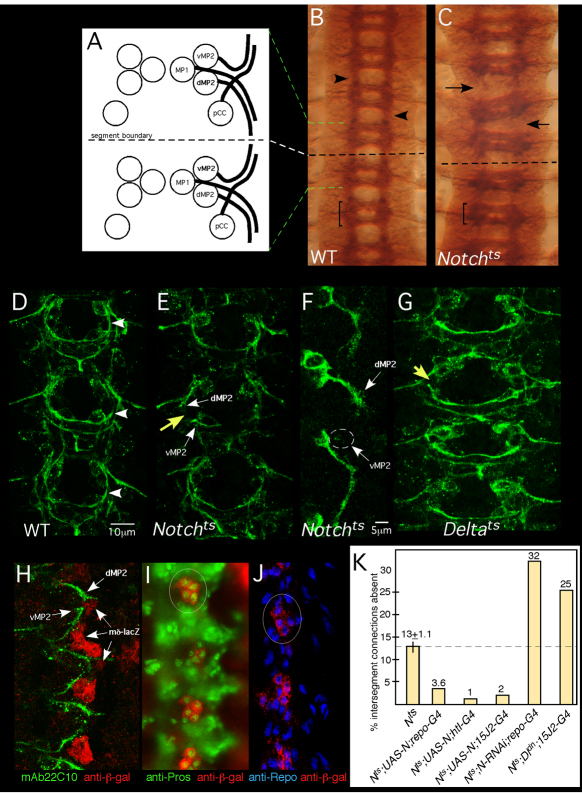
Fig. 1.
Notch is required for growth and guidance of longitudinal pioneers. (A) Four pioneer neurons establish intersegmental connections in the Drosophila CNS: pCC, MP1, dMP2 and vMP2. Two neuromeres and the intersegmental region between are shown. Green and white dashed lines indicate alignment of the schematic to the micrograph in B. Anterior is to the top. (B,C) Axon patterning in the mature embryonic nerve cord. Wild-type (WT; B) or Notchts (C) embryos were shifted to 32°C before pioneer axons extend, grown to stage 15/16, fixed and stained with mAb BP102. Arrows in C indicate gaps between adjacent segments in the mutant; compare with wild type (arrowheads in B). Bracket indicates a single neuromere. (D,E,G) Axon patterning in early CNS. WT (D), Notchts (E) or Deltats (G) embryos were shifted to 32°C then fixed at mid-stage 13 and stained with mAb 22C10 to reveal pioneer axons. Yellow arrows indicate missing longitudinal connections in the mutants; compare with wild type (arrowhead in D). White arrows highlight stalled dMP2 and vMP2 growth cones in Notchts. (F) dMP2 and vMP2 neurons in Notchts. A mid-stage 13 Notchts embryo expressing mCD8-GFP in dMP2 and vMP2 under control of GAL4-15J2 was prepared as described for D,E and visualized with anti-GFP. Arrows indicate stalled MP2 growth cones. Wisps of projections from a stalled vMP2 growth cone are encircled. (H) Wild-type early stage 13 embryo bearing a lacZ reporter for Notch signaling activity [E(spl)mδ-lacZ], labeled with anti-β-galactosidase (β-gal; red) and mAb22C10 (green). Arrows highlight the proximity of pioneer growth cones to cells with activated Notch signaling (lacZ+). (I,J) Stage 13 embryos bearing the E(spl)mδ-lacZ reporter, labeled with anti-β-gal (red) and markers for interface glia, anti-Prospero (I, green) or anti-Repo (J, blue). Circles highlight clusters of β-gal-positive cells. (K) Notchts embryos bearing the indicated transgenes were prepared as described for D,E and intersegmental connections that failed to form by mid-late stage 13 were quantified. All deviations from Notchts are statistically significant (P<0.05; ANOVA). The thin vertical line on the Notch bar shows s.e.m. None of the transgenes produced dominant defects in a wild-type background under these conditions (<2% of hemisegments).
Notch is required to form longitudinal connections in the CNS
Removing Notch midway through embryogenesis, using a temperature-sensitive mutation, prevented formation of mature longitudinal axon tracts (Fig. 1B,C) (Giniger et al., 1993; Giniger, 1998). Examining embryos at early stage 13 revealed that the Notch phenotype is apparent at the earliest stages of the pioneering of these tracts. In temperature-shifted embryos, the dMP2 and vMP2 axons grew in the appropriate direction, but stalled, failing to make contact even by late stage 13 (Fig. 1D-F). Expressivity of this phenotype depends on the timing of the temperature shift (Crowner et al., 2003). We adjusted conditions to give a very modest expressivity (13±1.1% of early hemisegmental connections missing) to minimize unrelated CNS defects. Failure to establish intersegment connections is not due to transformation of the identities of vMP2 and dMP2, as shown by the direction of axon growth and by staining for the molecular marker Odd skipped (Spana and Doe, 1996) (data not shown). Stalling of longitudinal pioneers was also observed in Deltats (Fig. 1G).
To ascertain where Notch is activated at this stage in wild type, we examined a transcriptional reporter for Notch activity, E(spl)mδ-lacZ (Cooper and Bray, 1999), and found β-galactosidase expression in a subset of the interface glia. These glia lined the pathway that the advancing longitudinal pioneer axons will follow and were present when those axons were growing (Fig. 1H) (Jacobs and Goodman, 1989a; Hidalgo and Booth, 2000). The β-galactosidase+ cells were identified as interface glia based on position and morphology (Fig. 1H), and by co-labeling for Prospero and Repo (Fig. 1I,J). The most prominent β-galactosidase expression is in glia within the neuromeres (Griffiths and Hidalgo, 2004), but at this stage there was also expression in interface glia between neuromeres, along the presumptive axon pathway (Fig. 1H, arrows).
Notch activation in interface glia was surprising because expression of Notch in neurons suppresses the late stage CNS axonal phenotype of Notchts (Giniger, 1998; Le Gall et al., 2008). Remarkably, we now found we could efficiently rescue the early axon phenotype of Notchts by expressing Notch either just in the glia [repo-GAL4 (all glia): 72% rescue; htl-GAL4 (interface glia): 92% rescue] or just in the dMP2 and vMP2 pioneers (15J2-GAL4: 85% rescue; P<0.01 in all cases; t-test) (Fig. 1K). We verified that in wild type Notch is expressed in interface glia and in both dMP2 and vMP2 (data not shown). We also verified that Notch activity in both cell types is physiologically relevant using loss-of-function experiments (Fig. 1K): RNAi against Notch in interface glia significantly enhanced the Notchts phenotype (2.5-fold; P<0.005), and cell-autonomous reduction of functional Notch in dMP2 and vMP2 by cis-inhibition (Sprinzak et al., 2010) using expression of Delta lacking its intracellular domain (Itoh et al., 2003) enhanced the Notchts phenotype by 92% (P<0.05). RNAi was not effective on the relevant timescale in dMP2 and vMP2 using 15J2-GAL4.
How can Notch rescue the growth of longitudinal pioneer axons both autonomously, acting in the pioneers, and non-autonomously, acting in the glia? We will first dissect the action of Notch in glia, and then turn to the Notch mechanism in pioneers.
Glial development in Notchts
Notch becomes activated in interface glia by contact en passant with the axons of Delta-expressing commissural interneurons (Thomas and van Meyel, 2007). Interface glia were contacted by Delta+ axons and displayed activated Notch signaling by early stage 13, in time to modulate growth of longitudinal pioneers (Fig. 2A). Moreover, expression in the glia of a Notch derivative lacking the ligand-binding domain could not rescue longitudinal axons (21% of intersegment connections were absent in Notchts; repo-GAL4; UAS-NotchΔEGF(10-12) versus 13% for Notchts, consistent with previous evidence showing a mild dominant-negative effect of this transgene) (Le Gall et al., 2008).
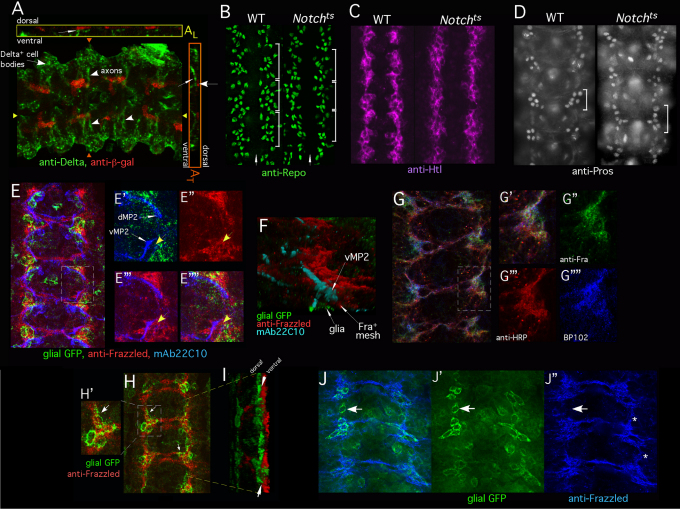
Fig. 2.
Interface glia recruit a cap of Frazzled+ neuronal processes. The CNS of stage 13 Drosophila embryos was analyzed by immunofluorescence. Embryos in E-G and J are very early stage 13, other panels are mid-stage 13. (A) Stage 13 embryos bearing E(spl)mδ-lacZ reporter, labeled with anti-β-gal (red) and anti-Delta (green). A z-projection is shown in A. White arrowheads indicate Delta-positive axons coursing medially from lateral neurons. Anterior is to the right. AL shows a longitudinal cross-section at the position of the yellow triangles. AT shows a transverse cross-section at the position of the orange triangles. Thin white arrows indicate Delta-positive axons (green) as they contact Notch signaling-positive glia (red). (B) Wild-type (WT) or Notchts embryos were temperature-shifted, fixed and labeled with anti-Repo. Each white bracket indicates one segment. White arrow indicates midline. Wild-type and Notchts embryos each have approximately ten Repo+ interface glia per hemisegment. A few non-interface glia are also visible in this section. (C) Wild-type or Notchts embryos prepared as described for B but visualized with anti-Htl. (D) Wild-type or Notchts embryos prepared as described for B but visualized with anti-Prospero. Five to six Pros+ cells are visible in each segment in wild type and in Notchts (bracket). Only one focal plane is shown so not all Pros+ cells are visible in all segments. (E-E″″) Wild-type embryo expressing mCD8-GFP in interface glia, under the control of htl-GAL4, labeled with anti-GFP (green), anti-Frazzled (red) and mAb 22C10 (blue). E shows the overlay of all channels. E′-E″″ are higher magnification views of the boxed region. Thin white arrows indicate the growth cones of dMP2 and vMP2. Glia (GFP, green) and growth cones (mAb 22C10, blue) are shown in E′. Anti-Frazzled (red) is shown in E″. Anti-Frazzled (red) and growth cones (mAb 22C10, blue) are shown in E‴. E″″ is the overlay of all markers. Yellow arrows highlight the gap between the vMP2 growth cone and the glial cell. Note close apposition of dMP2 and vMP2 growth cones with Frazzled. (F) Three-dimensional rendering of an image stack of an embryo prepared as described for E. vMP2 growth cone (mAb 22C10, cyan) migrates through a bed of Frazzled+ processes (red) that separate it from the associated interface glia (GFP; green). Image has been inverted to put glia beneath axon. (G-G″″) Wild-type embryo labeled with anti-Frazzled (green), anti-HRP (red) and mAb BP102 (blue). G shows overlay of all channels. A single optical section is shown (~0.7 μm). G′-G″″ show higher magnification views of the boxed region. G′ shows the overlay of channels; G″-G″″ show separate channels as indicated. (H-I) An embryo expressing mCD8-GFP in interface glia (htl-GAL4), labeled with anti-GFP (green) and anti-Frazzled (red). H is a z-projection. Arrows highlight the correspondence of glial position and Frazzled. H′ is a magnified view of the region indicated in H. I shows a three-dimensional rendering of H, viewed obliquely. Frazzled is ventral to the glia (arrows). (J-J″) z-projection of an early stage 13 wild-type embryo expressing mCD8-GFP in interface glia (GAL4-605), labeled with anti-GFP (green) and anti-Frazzled (blue). Frazzled+ cap is beginning to fill-in between successive segments (asterisks). J shows the overlay of channels. Arrows indicate an intersegmental region in which Frazzled is not yet detected but a glial cell is already present. J′ shows GFP signal in glia and J″ shows anti-Frazzled staining. At early stage 13 GAL4-605 is expressed primarily in interface glia; later it will also be in some neurons.
Notchts embryos had the normal number of interface glia, as assayed with anti-Repo (Fig. 2B). Moreover, the position and morphology of those glia appeared largely normal, and they expressed Htl and (where appropriate) Prospero (Fig. 2C,D). Thus, failure of overall glial development does not underlie the Notch axonal phenotype.
A neuronal ‘carpet’ separates interface glia from pioneer growth cones
We next found that longitudinal growth cones do not directly contact the interface glia, but rather a thin meshwork of neuronal tissue intervenes between. This meshwork stretches in an unbroken band from one segment to the next, potentially providing a pathway for longitudinal axons. Labeling late stage 12/early stage 13 wild-type embryos for longitudinal pioneer axons, interface glia and neuronal membrane revealed a small (~2-3 μm) gap between the glial surface and the advancing growth cones (Fig. 2E,F). This gap was filled by a fibrous-appearing meshwork that was labeled by four well-characterized markers for neuronal membranes: anti-HRP, mAb BP102, anti-Frazzled (DCC) and UAS-mCD8-GFP driven by neuron-specific GAL4 lines (Fig. 2E″-E″″,G). The advancing pioneer growth cones were invariably in intimate contact with this mesh, typically tracking along its edge (Fig. 2E,F, Fig. 3C). The substratum meshwork did not label with glial markers (anti-Htl or UAS-mCD8-GFP driven by glial-specific GAL4; Fig. 2H), but rather was positioned ventral to the glia. At this stage, the pioneer neurons themselves did not express Frazzled (Hiramoto et al., 2000), showing that the layer of neuronal membrane is provided by nearby differentiating neurons, and not by the pioneers. Moreover, previous serial section electron microscopy (Jacobs and Goodman, 1989a) showed that there are no other axons present in this region at the stage when the pioneers are extending, ruling out the possibility that the neuronal labeling in the meshwork derives from other axons. These observations are consistent with previous reports of a bed of unidentified filopodia closely apposed to the surface of the interface glia (Jacobs and Goodman, 1989a; Hidalgo and Booth, 2000). Development of the interface glia precedes morphogenesis of the neuronal meshwork (Fig. 2J), consistent with the glia positioning the meshwork rather than vice versa.
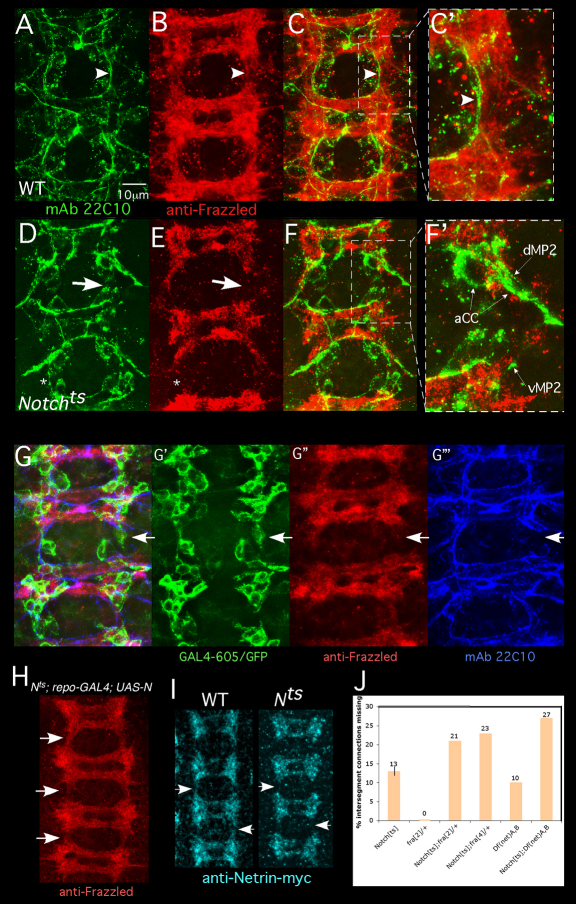
Fig. 3.
Notch is required for recruitment of a Frazzled- and Netrin-rich neuronal cap by interface glia. (A-F) Drosophila embryos were prepared as described in Fig. 1 legend and stained with mAb22C10 (green) and anti-Frazzled (red). A wild-type embryo is shown in A-C′. Boxed region in C is shown in C′. Arrowheads highlight correspondence between axon trajectory and Frazzled distribution. A Notchts is shown in D-F′ Boxed region in F is shown in F′. Thick arrows highlight a gap in the Frazzled pattern and a corresponding gap in the longitudinal tract. Thin arrows indicate stalled dMP2 and vMP2 growth cones. In some segments, axons cross between neuromeres despite a gap in Fra accumulation (asterisk). (G-G‴) Notchts embryos expressing mCD8-GFP in interface glia (GAL4-605) were fixed and labeled with anti-GFP (green), anti-Frazzled (red) and mAb 22C10 (blue). Overlay of all channels is shown in G. White arrows indicate an intersegmental connection that has failed to develop. G′-G‴ show separated channels, documenting a gap in the Frazzled pattern and a corresponding break in the early axon scaffold despite the presence of an interface glial cell in the gap. (H) A Notchts; repo-GAL4; UAS-Notch embryo was prepared as described for A-G and visualized with anti-Frazzled. Expression of Notch in glia rescues Frazzled distribution in nearby neurons (arrows; compare with B,E,G″). (I) Wild-type or Notchts embryos bearing a chromosomal NetrinB-myc ‘knock-in’ (Brankatschk and Dickson, 2006) were temperature-shifted, fixed and visualized with anti-myc. Netrin-myc spreads between segments in wild type, but gaps persist in Notchts (Nts; arrows). (J) Flies of the indicated genotype were temperature-shifted and intersegment connections were assayed with mAb22C10. The thin vertical line on the Notchts bar shows s.e.m.
Notch in interface glia induces formation of a continuous Fra+ Net+ neuronal domain
The essential glial function of Notch is to shape the associated neuronal meshwork into a continuous band that bridges between segments to provide the direct longitudinal substratum. In Notchts, the nearby differentiating neurons were labeled with the expected molecular markers, including anti-Frazzled (Fig. 3E), anti-Elav, mAb BP102 and anti-HRP (data not shown). In the mutant, however, although the neuronal meshwork formed within each segmental ganglion, it did not spread laterally between ganglia to make an unbroken band stretching from segment to segment. Instead, there were gaps in the mesh between segments (compare Fig. 3B,E), and pioneer axons often (but not always) stalled or misrouted when they encountered those gaps (compare Fig. 3C,F). Whether these gaps reflect a defect in the growth or orientation of the filopodia making up the meshwork has not been determined. Interface glia were still present in segments with stalled axons even though the neuronal meshwork was absent (Fig. 3G). Restoring Notch just to the glia (with repo-GAL4 or htl-GAL4) was sufficient to rescue the continuity of the neuronal meshwork (Fig. 3H), and thus, indirectly, the extension of the longitudinal pioneers. Temperature-shifts of Dlts produced gaps in the neuronal mesh, similar to those observed in Notchts (data not shown).
Pioneer longitudinal axons track along the edge of a band of Frazzled+ tissue, and this Frazzled+ domain immobilizes Netrin, presenting it for recognition by an alternate receptor on the pioneer growth cones (Hiramoto et al., 2000). Consistent with this, visualizing Netrin by immunostaining of Notchts revealed longitudinal gaps in the pattern of Netrin accumulation that mimicked the gaps in Frazzled (Fig. 3I). The Notch pioneer phenotype, moreover, was strongly enhanced by heterozygosity for a frazzled mutant (P<0.01), and the Notch Netrin double mutant had a significantly higher frequency of longitudinal defects than either mutation by itself (Fig. 3J). Thus, the crucial role of Notch in interface glia is to shape a continuous carpet of Fra- and Netrin-enriched neuronal tissue between segments, and the advancing longitudinal pioneers navigate by tracking along that carpet.
Non-canonical Notch/Abl signaling promotes motility of longitudinal pioneer growth cones
If Notch acts in glia to construct the longitudinal pathway, why does expression of Notch in pioneers rescue their axons? We expressed Notch selectively in the dMP2 and vMP2 pioneer neurons of Notchts and stained for early growing longitudinal axons and for Frazzled. Although restoration of Notch to the pioneers rescued growth of their axons, the continuity of the Frazzled+ neuronal meshwork was not restored: longitudinal gaps were still present (Fig. 4A-D). Evidently, restoration of Notch to the pioneers endowed them with the ability to extend across gaps in the Frazzled pathway. The ligand for Notch function in pioneers seems to be provided by the same Delta+ commissural axons that stimulate canonical Notch signaling in interface glia; after they passed under the glia, these Dl+ axons were perfectly positioned to act as stepping stones for advancing longitudinal pioneers (Fig. 4F, Fig. 7D). Consistent with this, expression in the pioneers of a Notch derivative lacking the ligand-binding repeats did not rescue the Notch axon phenotype (24% of intersegment connections absent in Notchts; 15J2-GAL4; UAS-NotchΔEGF(10-12) versus 14% for Notchts; 15J2-GAL4).
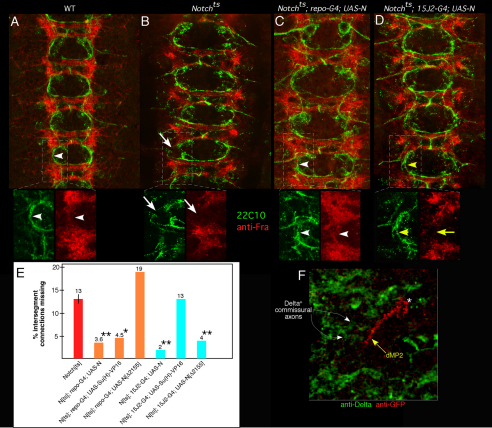
Fig. 4.
Different mechanisms for glial versus neuronal rescue of longitudinal pioneers by Notch. (A-D) Early stage 13 wild type (A), Notchts (B), Notchts; repo-GAL4; UAS-Notch (C; glial rescue) and Notchts;15J2-GAL4; UAS-Notch (D; pioneer-specific rescue) embryos were visualized with mAb 22C10 (green) and anti-Frazzled (red). Indicated regions in each panel are shown in insets beneath. Arrowheads in A and C highlight correspondence between Frazzled and pioneer axon trajectory; arrows in B indicates gap in Frazzled and corresponding break in longitudinal track in Notchts; yellow arrow and arrowheads in D indicate discordance between rescued axon growth but residual broken Frazzled pattern upon restoration of Notch to pioneers. (E) Embryos were prepared and scored as for the experiments shown in Figs 1, 2 and 3; bars indicate percentage of intersegment connections missing at mid-stage 13. Orange bars indicate rescue by expression of transgenes in glia; cyan bars indicate rescue by expression in dMP2 and vMP2. Statistical significance relative to Notchts is shown by asterisks: *P<0.05; **P<0.01 (ANOVA). (F) Wild-type embryo expressing mCD8-GFP in dMP2 visualized with anti-Delta (green) and anti-GFP (red). Yellow arrow indicates dMP2 growth cone as it contacts Delta+ commissural axons (white arrows). dMP2 cell body is marked with an asterisk.
Fig. 7.
Longitudinal axon pathway specification in the fly CNS. (A) Schematic showing four segments of the ventral nerve cord. Interface glia are in teal, underlying ‘cap‘ of neuronal filopodia in orange, lateral Delta+ cells in green and pioneer dMP2 and vMP2 cells and axons in black. Light gray shading marks the eventual locations of mature CNS axon tracts. Interface glia that contact incoming Delta+ axons turn on expression of Notch target genes; these glia recruit and shape a cap of Frazzled+ neuronal filopodia into a continuous substratum bridging from one segment to the next. (B) Higher resolution view of the longitudinal connective on one side of the nerve cord. Pale orange circles represent cell bodies of some of the differentiated neurons that provide filopodia to the cap underlying the interface glia. (C) Top view of the filopodial cap, with interface glia removed. Magenta speckling represents Netrin protein immobilized by binding to Frazzled in the filopodial cap. dMP2 and vMP2 pioneer growth cones track along the edge of the Frazzled+Netrin+ domain to establish the first intersegmental axonal connection (black arrows). (D) Top view of pioneer axons extending along the filopodial cap. Note Delta+ commissural axons projecting past the glial row, where they act as stepping stones for advancing longitudinal axons. For clarity, Netrin is not shown, and glia have been cut away to reveal the underlying filopodial cap.
In contrast to its action in glia, Notch action in pioneers does not employ canonical Su(H)-dependent signaling, but rather the non-canonical Notch/Abl signaling pathway (Crowner et al., 2003; Le Gall et al., 2008). We expressed two diagnostic reagents in either interface glia or in the dMP2 and vMP2 pioneers of Notchts embryos and assayed development of longitudinal axons at mid-stage 13. Su(H)-VP16 is a constitutively active, receptor-independent derivative of the canonical Notch effector Su(H), but has no influence on the Notch/Abl pathway (Crowner et al., 2003). NotchΔ2155 is a truncated derivative of full-length Notch that has no ability to stimulate canonical signaling but is almost fully active for the Notch/Abl interaction (Le Gall et al., 2008). Expression of Su(H)-VP16 in glia rescued construction of a continuous longitudinal track and thus fully rescued extension of longitudinal pioneers, whereas expression in the pioneers had no effect on the extension of their axons (Fig. 4E). Conversely, expression of NotchΔ2155 in the glia produced no rescue of either the Frazzled+ pathway or the pioneer axons, but in pioneers it was nearly as effective as wild-type Notch for rescuing axon extension (Fig. 4E). Moreover, we did not observe expression of the E(spl)mδ-lacZ canonical Notch reporter in any of the longitudinal pioneers at this stage (Le Gall et al., 2008). Finally, Notch interacted genetically with Abl to modify longitudinal axon development just as we have observed previously for pathfinding of the ISNb motonerve: removing one copy of the Abl gene by mutation suppressed the mature, stage 16 CNS axonal phenotype of Notch (Notchts: 29% of intersegmental connections defective; Notchts; Abl4/+: 13% defective; P<0.01; t-test).
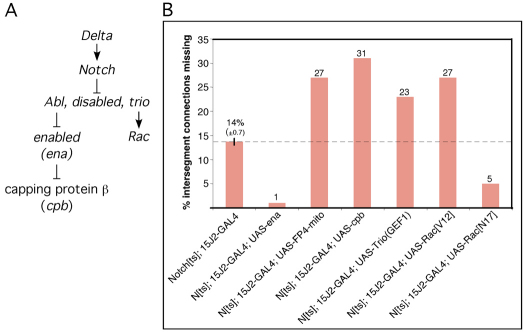
We verified further that the autonomous function of Notch in dMP2 and vMP2 axons is via the Notch/Abl interaction by selectively expressing constitutive or dominant-negative derivatives of various proteins of the Abl pathway in the pioneers and assaying enhancement or suppression of the Notch phenotype. (RNAi was ineffective for this experiment owing to the long half-lives of the proteins.) Previous experiments suggested that Notch suppresses the activity of the Abl signaling pathway (Fig. 5A) (Crowner et al., 2003). Consistent with this, expression of the Abl antagonist Enabled in dMP2 and vMP2 suppressed the early longitudinal axon phenotype of Notch, whereas expression of an Ena antagonist, FP4mito (Bear et al., 2000), enhanced it (Fig. 5B). Enabled, in turn, is an actin ‘anti-capping’ protein (Trichet et al., 2008), and overexpression of actin capping protein β (cpb) enhanced the Notch phenotype (Fig. 5B). The guanine nucleotide exchange factor Trio is a positive element of the Abl pathway (Liebl et al., 2000), and overexpression of the active, Rac-specific GEF1 domain of Trio or of Rac[V12] enhanced the Notch phenotype whereas expression of a dominant-negative Rac[N17] suppressed it, again consistent with Notch acting as a suppressor of Abl signaling (Fig. 5B) (Song and Giniger, 2011). None of these transgenes caused defects in longitudinal axons when expressed in a wild-type background with this driver (<2% of hemisegments defective). As indicated above (Fig. 4E), expression of Su(H)-VP16 in this paradigm had no effect on the early longitudinal axon phenotype. These data argue that Notch promotes motility of the dMP2 and vMP2 pioneer growth cones by suppressing Abl pathway signaling autonomously in these cells.
Fig. 5.
Notch interactions with Abl pathway in longitudinal pioneers. (A) Model for the Notch/Abl signaling module. Schematic representation of the non-canonical Notch/Abl signaling pathway. Arrows indicate positive regulation; ⊥ indicates negative regulation. In response to ligand, Notch antagonizes the activity of the Abl signaling module, which includes Abl tyrosine kinase, the adaptor protein Disabled, and Trio, a guanine exchange factor (GEF) for Rho GTPases. Abl, in turn, is a negative regulator of Enabled, a protein that inhibits capping of actin filaments, whereas Trio is a positive regulator of Rac GTPase. (B) Genetic interactions of Abl pathway components with Notch in dMP2 and vMP2. Embryos were prepared and early longitudinal connections quantified. Bars indicate percentage of intersegment connections missing. All genotypes shown were significantly different from Notchts (P<0.01; ANOVA). None of the Abl pathway reagents produced a dominant phenotype in a wild-type background by this assay (<2% of intersegment connections missing). The thin vertical line on the Notchts; 15J2-GAL4 bar shows s.e.m.
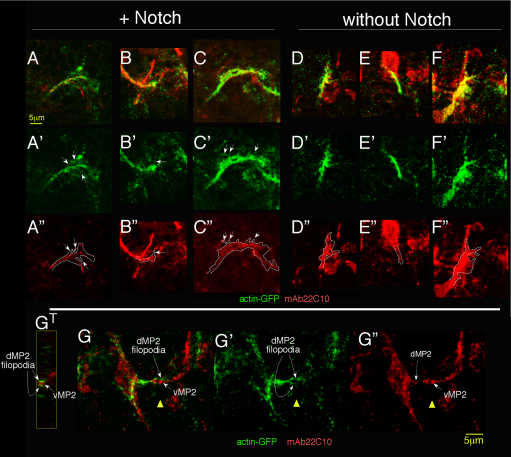
Visualizing actin in dMP2 and vMP2 growth cones revealed that Notch promotes the presence of filopodial structures on the growth cone. We expressed actin-GFP in the dMP2 and vMP2 growth cones of temperature-shifted Notchts mutants with or without wild-type Notch, and prepared embryos using a fixation that preserves actin structure (Miller et al., 1989). Embryos were double-labeled with anti-GFP and with a marker for the microtubule cytoskeleton (Futsch, the Drosophila MAP-1B). Filopodia were defined operationally as thin, whisker-like projections of uniform caliber that were actin-positive, but microtubule-negative, and were counted on dMP2 growth cones that were fixed while in transit between neuromeres (Fig. 6A-F). Filopodia were detected in the Notch mutant in 30% of growth cones, with an average total filopodial length of 1.2±0.4 μm/growth cone (n=33 dMP2 growth cones). By contrast, when Notch was restored to the growth cone, filopodia were detected on 93% of growth cones (n=41; P<10−7, χ2 test), with an average total filopodial length of 4.7±0.5 μm/growth cone (P<10−6, ANOVA). Clearly, Notch strongly promotes formation and/or stability of filopodia on dMP2 growth cones.
Fig. 6.
Notch promotes presence of filopodial structures in dMP2. (A-F″) Notchts; 15J2-GAL4 embryos expressing actin-GFP in dMP2 with (A-C″) or without (D-F″) wild-type Notch were temperature-shifted and fixed to preserve the actin cytoskeleton and visualized with anti-GFP (green) and a marker for the microtubule cytoskeleton, Futsch (mAb22C10, red). A gallery of single growth cones is shown. Middle row shows actin-GFP signal; arrows highlight whisker-like projections that label for actin-GFP but not Futsch. [Projections that co-labeled with Futsch (D,F) were interpreted as branches rather than filopodia and were not counted.] Bottom row shows Futsch signal with outline of actin signal superimposed. At this stage, 15J2-GAL4 expression is stronger in dMP2 than vMP2, allowing us to visualize this pioneer alone. Futsch expression in nearby cells accounts for unassigned red label. Mean length of single filopodia (2.2±0.1 μm) and maximum length (4.5 μm), were not significantly different with and without Notch, and were similar to dimensions observed in live imaging (Homem and Peifer, 2009). (G-G″) A GFP-labeled dMP2 growth cone (green, left) encounters the converging vMP2 (right) in an embryo prepared as described for A-F. Note the dMP2 filopodia embracing the oncoming vMP2 (G′). The prominent 22C10+ growth cone is vMP2; the central domain of the dMP2 growth cone is also indicated in mAb22C10 channel (G″; dashed arrow). GT shows a cross-section through the vMP2 growth cone (red) and dMP2 filopodia (green), at the position of the yellow triangle. Anterior is to the left.
DISCUSSION
The axons of the longitudinal pioneer interneurons of the Drosophila ventral nerve cord establish the initial connection between successive segments of the animal. The receptor Notch is crucial for making those first connections, performing two parallel, partially redundant but completely separate functions. Canonical Notch signaling in the interface glia constructs an unbroken track for longitudinal pioneer axons to follow by shaping a continuous band of neuronal membrane that bridges from segment to segment. Simultaneously, non-canonical Notch/Abl signaling in the pioneer neurons themselves promotes the motility of their growth cones, suppressing the activity of the Abl tyrosine kinase signaling module to stimulate filopodial development. Either signaling mechanism provides substantial rescue of a Notch mutant, but both are required for full activity in formation of longitudinal connections of the CNS.
The mechanism that guides the very first axon to establish the path of a nascent nerve is one of the most fundamental problems in neural development. For longitudinal pioneers of the Drosophila CNS, we now see that constructing their path requires coordinated contributions from four interacting cell types (Fig. 7). First, the axons of commissural interneurons bearing the Notch ligand Delta contact interface glia. The glia respond by activating canonical Notch signaling, enhancing expression of Notch target genes, including prospero (Griffiths and Hidalgo, 2004; Thomas and van Meyel, 2007) (I.K. and E.G., unpublished). The genetic program stimulated by Notch directly or indirectly allows the glial cells to attract a ‘cap’ of fine filopodial processes from nearby differentiating neurons, and shapes that cap into a continuous longitudinal band that bridges between segments. The neuronal cap atop the glia bears the Netrin receptor Frazzled (DCC), which in turn recruits soluble Netrin (Hiramoto et al., 2000), thus constructing a domain of accumulation of Netrin protein that hugs the surface of the associated glia. Finally, the pioneer growth cones advance along the edge of that domain of immobilized Netrin until they meet and fasciculate with their partners pioneering from the next segment. The consequence of this choreography is a nerve trajectory that follows, indirectly, the shape of the row of interface glia.
This view suggests plausible explanations for many aspects of longitudinal axon development that have, up to now, been confusing. Previous investigators have documented that the pioneer growth cones extend amidst a thicket of filopodia that cap the interface glia (Jacobs and Goodman, 1989a; Hidalgo and Booth, 2000). The provenance and significance of those filopodia were unknown, though it was clear that they did not derive from other axons (Jacobs and Goodman, 1989a). We now see that these filopodia come from surrounding, differentiating neurons, and that their function is to hold Frazzled, and therefore Netrin (Hiramoto et al., 2000), in a pattern dictated by the positions of the overlying glia, creating the Netrin domain along which the pioneers extend. This explains why the positions of the interface glia correlate so closely with the axon trajectory even though the glia are not the direct substratum. Our data, along with other recent results, also suggest why previous experiments investigating the guidance function of interface glia might have given such confusing results. Transformation of the glia into neurons in a gcm mutant would be predicted to place a row of DCC-expressing neurons in precisely the position of the wild-type filopodial carpet. Moreover, genetic experiments ablating or displacing the glia have relied on reagents targeting the progeny of the longitudinal glioblast (Jacobs et al., 1989; Hidalgo and Booth, 2000; von Hilchen et al., 2010), but we now know that only nine of the ten interface glia come from this precursor; the tenth, M-ISNG, is from a different lineage (Beckervordersandforth et al., 2008). M-ISNG is appropriately positioned to anchor the filopodial carpet in the absence of the other interface glia (Beckervordersandforth et al., 2008) and preliminary experiments suggest that it is sufficient for this (I.K. and E.G., unpublished). Moreover, von Hilchen et al. (von Hilchen et al., 2010) have argued that in those rare segments where pioneer axons stall owing solely to manipulations of the interface glia, all ten of them, including M-ISNG, tend to be absent or displaced. Finally, as in previous studies (Hiramoto et al., 2000), we find that the Netrin zygotic mutant has a mild, and genetically enhanceable phenotype, showing that the null for the gene is not null for the genetic pathway. It might be that there is a maternal contribution to Netrin, as there is for frazzled. Alternatively, because the receptor on longitudinal pioneers presumably recognizes a Netrin-Frazzled complex, it might be that this receptor has some affinity for Frazzled even in the absence of Netrin. Identification of the missing Netrin receptor will be necessary to clarify this point. It also seems likely that other neuronal components cooperate with the Netrin-Frazzled complex on the meshwork to provide substratum function, as expression of Fra in interface glia is not sufficient to rescue the Notch axonal phenotype (data not shown).
Once the pathway for an axon has been constructed, there remains the problem of driving the motility of the growth cone along that pathway. Somehow, the information encoded in a pattern of occupancy of cell surface receptors must be transformed into a pattern of cytoskeletal dynamics that drives growth cone motion. At the level of the axon, this is the bedrock problem in axon guidance, and it, too, has resisted analysis. Our data reveal how Notch modulates an elementary property of the actin cytoskeleton to promote motility of longitudinal pioneer growth cones. Through its antagonism of the Abl signaling network, Notch de-represses the Abl antagonist Enabled and suppresses the Rac GEF Trio. Enabled directly promotes filopodial growth (Krause et al., 2002; Trichet et al., 2008); suppressing Rac indirectly promotes filopodia, probably by redirecting various factors away from lamellipodia (P. Bradley and E.G., unpublished observations). Stimulating filopodial development probably promotes longitudinal axon growth in at least two ways. First, converging pioneer growth cones from successive segments need to encounter one another and fasciculate to establish the connection between segments (Fig. 6G). Extension of filopodia increases the area searched by an advancing growth cone, increasing the probability that it will encounter its partners advancing from the adjacent segment. Second, filopodia promote neurite growth by promoting microtubule invasion of the leading edge (Dent et al., 2007).
In parallel with stimulating filopodia, suppression of Trio, and thus of Rac, is expected to reduce substratum adhesion (Kaufmann et al., 1998; Symons, 2000; Crowner et al., 2003). When the pioneers are growing towards the segment border, small gaps in the Frazzled-Netrin pattern are not uncommon, so release of the advancing growth cone from the substratum is likely to aid its forward motion. Moreover, initially there is more Frazzled-bound Netrin within the neuromeres than there is at the segment border (Fig. 3I), requiring advancing pioneers to go down a gradient of Netrin, towards a region with less Netrin. It is possible that both of these properties make it helpful to limit substratum adhesion of the growth cone via reduction of Rac signaling by Notch.
It might seem paradoxical that Notch promotes axon growth by suppressing Abl signaling when Abl has been the archetype of a motility-promoting signaling pathway (Gertler et al., 1993). Indeed, genetic studies of Abl in axon guidance have often appeared to be confusing and contradictory (Bashaw et al., 2000; Hsouna et al., 2003; Forsthoefel et al., 2005). In part, this reflects pleiotropy. Abl appears to act in the glia, and in the cells providing the filopodial carpet, in addition to the pioneers (I.K. and E.G., unpublished). As the phenotype in a whole-animal mutant reflects the sum of unrelated functions in different cells, seemingly similar experiments can produce contradictory results if different cellular processes become limiting. For longitudinal axons, for example, if pathway establishment is limiting (in Abl− or fra− animals), reduction of Notch interferes with axon growth synergistically (Giniger, 1998); if growth cone function is limiting (in Notch− animals), reduction of the Abl pathway restores axon growth (this work). It was therefore essential in the current work to control gene activity, and analyze phenotypes, in single, identified cells.
Beyond pleiotropy, however, complexity arises because the effect of signaling molecules in motility is profoundly context dependent. Ena promotes actin polymerization but often restricts cell motility (Bear et al., 2000; Krause et al., 2002; Trichet et al., 2008); cofilin severs actin filaments but can promote net actin polymerization and cell migration (Loisel et al., 1999; Blanchoin et al., 2000; Ng and Luo, 2004). As axon growth is achieved by throughput through a cycle of actomyosin dynamics, it requires a balance among the steps of that cycle (Sheetz et al., 1998; Suter and Forscher, 2000; Giniger, 2002). Excessive activity or inactivity of any single step in the process inhibits motility by impairing progression through the cycle (Luo et al., 1994; Song and Giniger, 2011). Our data now reveal that, for Drosophila longitudinal pioneers, an essential aspect of growth cone movement is restraint of Abl activity to allow filopodial development, and perhaps also to limit substratum adhesion.
The data reported here reveal that Notch promotes CNS longitudinal axon growth in two very different ways, constructing a pathway using its canonical signaling mechanism and promoting motility via the Notch/Abl interaction. This dual role bears striking parallels to the dual role of Notch in radial migration of neurons in the mammalian cortex. There, as in the fly, canonical signaling by Notch is essential for the development of glial cells that define a migration pathway (Ever and Gaiano, 2005), whereas interaction with the Abl pathway protein Disabled controls neuronal motility and adhesion (Hashimoto-Torii et al., 2008; Song et al., 2010). Further study will be required to assess whether these parallels between Notch function in the fly and vertebrate nervous systems reflect a deeper mechanistic similarity. Similarly, it will be interesting to see whether formation of longitudinal nerve tracts in the spinal cord uses machinery homologous to that which we have described in the fly.
Acknowledgements
We thank members of our laboratory for helpful advice and assistance. We are also grateful to Roger Jacobs, Don van Meyel, Gerd Technau, Ben Altenhein and Mark Peifer for valuable discussions and for sharing unpublished observations. For comments on the manuscript we are grateful to Ben Altenhein, Ajay Chitnis, Alicia Hidalgo, Maude Le Gall, Chi-Hon Lee, Don van Meyel and Gerd Technau. We thank all of the investigators who generously provided reagents for these studies, and the NHGRI microscopy core for expert assistance. These experiments were supported by the Basic Neuroscience Program of the NINDS Intramural Research Program (Z01 NS003013). Deposited in PMC for release after 12 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- Bashaw G. J., Kidd T., Murray D., Pawson T., Goodman C. S. (2000). Repulsive axon guidance: Abelson and Enabled play opposing roles downstream of the roundabout receptor. Cell 101, 703-715 [DOI] [PubMed] [Google Scholar]
- Bear J. E., Loureiro J. J., Libova I., Fassler R., Wehland J., Gertler F. B. (2000). Negative regulation of fibroblast motility by Ena/VASP proteins. Cell 101, 717-728 [DOI] [PubMed] [Google Scholar]
- Beckervordersandforth R. M., Rickert C., Altenhein B., Technau G. M. (2008). Subtypes of glial cells in the Drosophila embryonic ventral nerve cord as related to lineage and gene expression. Mech. Dev. 125, 542-557 [DOI] [PubMed] [Google Scholar]
- Bier E., Vaessin H., Shepherd S., Lee K., McCall K., Barbel S., Ackerman L., Carretto R., Uemura T., Grell E., et al. (1989). Searching for pattern and mutation in the Drosophila genome with a P-lacZ vector. Genes Dev. 3, 1273-1287 [DOI] [PubMed] [Google Scholar]
- Blanchoin L., Pollard T. D., Mullins R. D. (2000). Interactions of ADF/cofilin, Arp2/3 complex, capping protein and profilin in remodeling branched actin filament networks. Curr. Biol. 10, 1273-1282 [DOI] [PubMed] [Google Scholar]
- Brankatschk M., Dickson B. J. (2006). Netrins guide Drosophila commissural axons at short range. Nat. Neurosci. 9, 188-194 [DOI] [PubMed] [Google Scholar]
- Cooper M. T., Bray S. J. (1999). Frizzled regulation of Notch signalling polarizes cell fate in the Drosophila eye. Nature 397, 526-530 [DOI] [PubMed] [Google Scholar]
- Crowner D., Le Gall M., Gates M. A., Giniger E. (2003). Notch steers Drosophila ISNb motor axons by regulating the Abl signaling pathway. Curr. Biol. 13, 967-972 [DOI] [PubMed] [Google Scholar]
- Dent E. W., Kwiatkowski A. V., Mebane L. M., Philippar U., Barzik M., Rubinson D. A., Gupton S., Van Veen J. E., Furman C., Zhang J., et al. (2007). Filopodia are required for cortical neurite initiation. Nat. Cell Biol. 9, 1347-1359 [DOI] [PubMed] [Google Scholar]
- Ever L., Gaiano N. (2005). Radial ‘glial’ progenitors: neurogenesis and signaling. Curr. Opin. Neurobiol. 15, 29-33 [DOI] [PubMed] [Google Scholar]
- Forsthoefel D. J., Liebl E. C., Kolodziej P. A., Seeger M. A. (2005). The Abelson tyrosine kinase, the Trio GEF and Enabled interact with the Netrin receptor Frazzled in Drosophila. Development 132, 1983-1994 [DOI] [PubMed] [Google Scholar]
- Gertler F. B., Hill K. K., Clark M. J., Hoffmann F. M. (1993). Dosage-sensitive modifiers of Drosophila abl tyrosine kinase function: prospero, a regulator of axonal outgrowth, and disabled, a novel tyrosine kinase substrate. Genes Dev. 7, 441-453 [DOI] [PubMed] [Google Scholar]
- Giniger E. (1998). A role for abl in Notch signaling. Neuron 20, 667-681 [DOI] [PubMed] [Google Scholar]
- Giniger E. (2002). How do Rho family GTPases direct axon growth and guidance? A proposal relating signaling pathways to growth cone mechanics. Differentiation 70, 385-396 [DOI] [PubMed] [Google Scholar]
- Giniger E., Jan L. Y., Jan Y. N. (1993). Specifying the path of the intersegmental nerve of the Drosophila embryo: a role for Delta and Notch. Development 117, 431-440 [DOI] [PubMed] [Google Scholar]
- Griffiths R. L., Hidalgo A. (2004). Prospero maintains the mitotic potential of glial precursors enabling them to respond to neurons. EMBO J. 23, 2440-2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto-Torii K., Torii M., Sarkisian M. R., Bartley C. M., Shen J., Radtke F., Gridley T., Sestan N., Rakic P. (2008). Interaction between Reelin and Notch signaling regulates neuronal migration in the cerebral cortex. Neuron 60, 273-284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo A., Brand A. H. (1997). Targeted neuronal ablation: the role of pioneer neurons in guidance and fasciculation in the CNS of Drosophila. Development 124, 3253-3262 [DOI] [PubMed] [Google Scholar]
- Hidalgo A., Booth G. E. (2000). Glia dictate pioneer axon trajectories in the Drosophila embryonic CNS. Development 127, 393-402 [DOI] [PubMed] [Google Scholar]
- Hidalgo A., Urban J., Brand A. H. (1995). Targeted ablation of glia disrupts axon tract formation in the Drosophila CNS. Development 121, 3703-3712 [DOI] [PubMed] [Google Scholar]
- Hiramoto M., Hiromi Y., Giniger E., Hotta Y. (2000). A Drosophila Netrin receptor, Frazzled, guides axons by controlling the distribution of Netrin. Nature 406, 886-889 [DOI] [PubMed] [Google Scholar]
- Homem C. C., Peifer M. (2009). Exploring the roles of diaphanous and enabled activity in shaping the balance between filopodia and lamellipodia. Mol. Biol. Cell 20, 5138-5155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoya T., Takizawa K., Nitta K., Hotta Y. (1995). Glial cells missing: a binary switch between neuronal and glial determination in Drosophila. Cell 82, 1025-1036 [DOI] [PubMed] [Google Scholar]
- Hsouna A., Kim Y. S., VanBerkum M. F. (2003). Abelson tyrosine kinase is required to transduce midline repulsive cues. J. Neurobiol. 57, 15-30 [DOI] [PubMed] [Google Scholar]
- Itoh M., Kim C. H., Palardy G., Oda T., Jiang Y. J., Maust D., Yeo S. Y., Lorick K., Wright G. J., Ariza-McNaughton L., et al. (2003). Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev. Cell 4, 67-82 [DOI] [PubMed] [Google Scholar]
- Jacobs J. R., Goodman C. S. (1989a). Embryonic development of axon pathways in the Drosophila CNS. I. A glial scaffold appears before the first growth cones. J. Neurosci. 9, 2402-2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J. R., Goodman C. S. (1989b). Embryonic development of axon pathways in the Drosophila CNS. II. Behavior of pioneer growth cones. J. Neurosci. 9, 2412-2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J. R., Hiromi Y., Patel N. H., Goodman C. S. (1989). Lineage, migration, and morphogenesis of longitudinal glia in the Drosophila CNS as revealed by a molecular lineage marker. Neuron 2, 1625-1631 [DOI] [PubMed] [Google Scholar]
- Jones B. W., Fetter R. D., Tear G., Goodman C. S. (1995). Glial cells missing: a genetic switch that controls glial versus neuronal fate. Cell 82, 1013-1023 [DOI] [PubMed] [Google Scholar]
- Kaufmann N., Wills Z. P., Van Vactor D. (1998). Drosophila Rac1 controls motor axon guidance. Development 125, 453-461 [DOI] [PubMed] [Google Scholar]
- Kolodziej P. A., Timpe L. C., Mitchell K. J., Fried S. R., Goodman C. S., Jan L. Y., Jan Y. N. (1996). frazzled encodes a Drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell 87, 197-204 [DOI] [PubMed] [Google Scholar]
- Krause M., Bear J. E., Loureiro J. J., Gertler F. B. (2002). The Ena/VASP enigma. J. Cell Sci. 115, 4721-4726 [DOI] [PubMed] [Google Scholar]
- Lai E. C. (2004). Notch signaling: control of cell communication and cell fate. Development 131, 965-973 [DOI] [PubMed] [Google Scholar]
- Le Gall M., De Mattei C., Giniger E. (2008). Molecular separation of two signaling pathways for the receptor, Notch. Dev. Biol. 313, 556-567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebl E. C., Forsthoefel D. J., Franco L. S., Sample S. H., Hess J. E., Cowger J. A., Chandler M. P., Shupert A. M., Seeger M. A. (2000). Dosage-sensitive, reciprocal genetic interactions between the Abl tyrosine kinase and the putative GEF trio reveal trio's role in axon pathfinding. Neuron 26, 107-118 [DOI] [PubMed] [Google Scholar]
- Lin D. M., Auld V. J., Goodman C. S. (1995). Targeted neuronal cell ablation in the Drosophila embryo: pathfinding by follower growth cones in the absence of pioneers. Neuron 14, 707-715 [DOI] [PubMed] [Google Scholar]
- Loisel T. P., Boujemaa R., Pantaloni D., Carlier M.-F. (1999). Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature 401, 613-616 [DOI] [PubMed] [Google Scholar]
- Luo L. (2000). Rho GTPases in neuronal morphogenesis. Nat. Rev. Neurosci. 1, 173-180 [DOI] [PubMed] [Google Scholar]
- Luo L., Liao J., Jan L. Y., Jan Y. N. (1994). Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 8, 1787-1802 [DOI] [PubMed] [Google Scholar]
- Miller K. G., Field C. M., Alberts B. M. (1989). Actin-binding proteins from Drosophila embryos: a complex network of interacting proteins detected by F-actin affinity chromatography. J. Cell Biol. 109, 2963-2975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell K. J., Doyle J. L., Serafini T., Kennedy T. E., Tessier-Lavigne M., Goodman C. S., Dickson B. J. (1996). Genetic analysis of Netrin genes in Drosophila: netrins guide CNS commissural axons and peripheral motor axons. Neuron 17, 203-215 [DOI] [PubMed] [Google Scholar]
- Ng J., Luo L. (2004). Rho GTPases regulate axon growth through convergent and divergent signaling pathways. Neuron 44, 779-793 [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Felsenfeld D. P., Galbraith C. G. (1998). Cell migration: regulation of force on extracellular-matrix-integrin complexes. Trends Cell Biol. 8, 51-54 [DOI] [PubMed] [Google Scholar]
- Song H., Poo M. (2001). The cell biology of neuronal navigation. Nat. Cell Biol. 3, E81-E88 [DOI] [PubMed] [Google Scholar]
- Song J. K., Giniger E. (2011). Noncanonical Notch function in motor axon guidance is mediated by Rac GTPase and the GEF1 domain of Trio. Dev. Dyn. 240, 324-332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J. K., Kannan R., Merdes G., Singh J., Mlodzik M., Giniger E. (2010). Disabled is a bona fide component of the Abl signaling network. Development 137, 3719-3727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spana E. P., Doe C. Q. (1996). Numb antagonizes Notch signaling to specify sibling neuron cell fates. Neuron 17, 21-26 [DOI] [PubMed] [Google Scholar]
- Sprinzak D., Lakhanpal A., Lebon L., Santat L. A., Fontes M. E., Anderson G. A., Garcia-Ojalvo J., Elowitz M. B. (2010). Cis-interactions between Notch and Delta generate mutually exclusive signalling states. Nature 465, 86-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter D. M., Forscher P. (2000). Substrate-cytoskeletal coupling as a mechanism for the regulation of growth cone motility and guidance. J. Neurobiol. 44, 97-113 [PubMed] [Google Scholar]
- Symons M. (2000). Adhesion signaling: PAK meets Rac on solid ground. Curr. Biol. 10, R535-R537 [DOI] [PubMed] [Google Scholar]
- Thomas G. B., van Meyel D. J. (2007). The glycosyltransferase Fringe promotes Delta-Notch signaling between neurons and glia, and is required for subtype-specific glial gene expression. Development 134, 591-600 [DOI] [PubMed] [Google Scholar]
- Trichet L., Sykes C., Plastino J. (2008). Relaxing the actin cytoskeleton for adhesion and movement with Ena/VASP. J. Cell Biol. 181, 19-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hilchen C. M., Hein I., Technau G. M., Altenhein B. (2010). Netrins guide migration of distinct glial cells in the Drosophila embryo. Development 137, 1251-1262 [DOI] [PubMed] [Google Scholar]
- Wills Z., Marr L., Zinn K., Goodman C. S., Van Vactor D. (1999a). Profilin and the Abl tyrosine kinase are required for motor axon outgrowth in the Drosophila embryo. Neuron 22, 291-299 [DOI] [PubMed] [Google Scholar]
- Wills Z., Bateman J., Korey C. A., Comer A., Van Vactor D. (1999b). The tyrosine kinase Abl and its substrate enabled collaborate with the receptor phosphatase Dlar to control motor axon guidance. Neuron 22, 301-312 [DOI] [PubMed] [Google Scholar]