Abstract
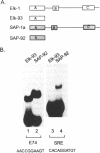
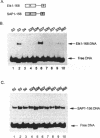
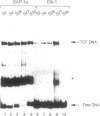
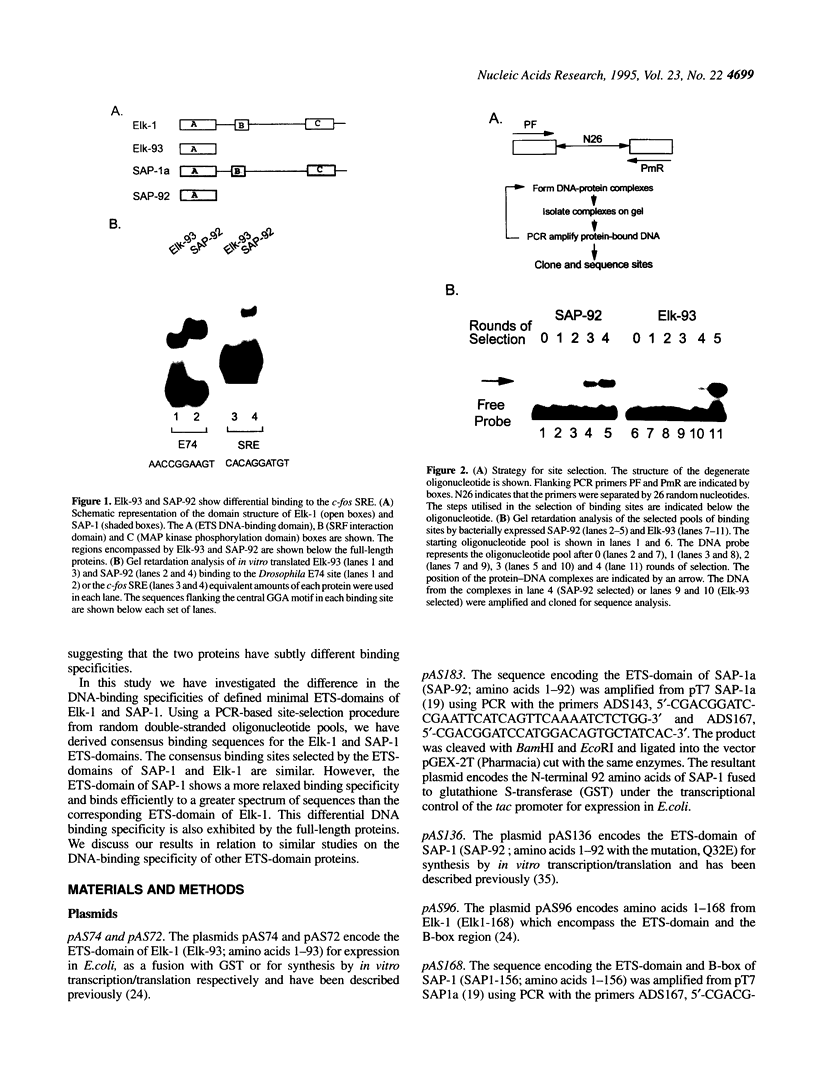
The ETS DNA-binding domain is conserved amongst many eukaryotic transcription factors. ETS-domains bind differentially to specific DNA sites containing a central GGA trinucleotide motif. The nucleotides flanking this motif define the binding specificity of individual proteins. In this study we have investigated binding specificity of the ETS-domains from two members of the ternary complex factor (TCF) subfamily, Elk-1 and SAP-1. The ETS DNA-binding domains of Elk-1 (Elk-93) and SAP-1 (SAP-92) select similar sites from random pools of double stranded oligonucleotides based on the consensus sequence ACCGGAAGTR. However, SAP-92 shows a more relaxed binding site selectivity and binds efficiently to a greater spectrum of sites than does Elk-93. This more relaxed DNA binding site selectivity is most pronounced in nucleotides located on the 3' side of the GGA motif. This differential DNA-binding specificity is also exhibited by longer TCF derivatives and, indeed by the full-length proteins. Our results suggest that the range of potential in vivo target sites for SAP-1 is likely to be greater than for Elk-1. We discuss our results in relation to other similar studies carried out with more divergent ETS-domains.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackwell T. K., Weintraub H. Differences and similarities in DNA-binding preferences of MyoD and E2A protein complexes revealed by binding site selection. Science. 1990 Nov 23;250(4984):1104–1110. doi: 10.1126/science.2174572. [DOI] [PubMed] [Google Scholar]
- Brown T. A., McKnight S. L. Specificities of protein-protein and protein-DNA interaction of GABP alpha and two newly defined ets-related proteins. Genes Dev. 1992 Dec;6(12B):2502–2512. doi: 10.1101/gad.6.12b.2502. [DOI] [PubMed] [Google Scholar]
- Dalton S., Treisman R. Characterization of SAP-1, a protein recruited by serum response factor to the c-fos serum response element. Cell. 1992 Feb 7;68(3):597–612. doi: 10.1016/0092-8674(92)90194-h. [DOI] [PubMed] [Google Scholar]
- Degnan B. M., Degnan S. M., Naganuma T., Morse D. E. The ets multigene family is conserved throughout the Metazoa. Nucleic Acids Res. 1993 Jul 25;21(15):3479–3484. doi: 10.1093/nar/21.15.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille H., Kortenjann M., Thomae O., Moomaw C., Slaughter C., Cobb M. H., Shaw P. E. ERK phosphorylation potentiates Elk-1-mediated ternary complex formation and transactivation. EMBO J. 1995 Mar 1;14(5):951–962. doi: 10.1002/j.1460-2075.1995.tb07076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille H., Sharrocks A. D., Shaw P. E. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature. 1992 Jul 30;358(6385):414–417. doi: 10.1038/358414a0. [DOI] [PubMed] [Google Scholar]
- Giovane A., Pintzas A., Maira S. M., Sobieszczuk P., Wasylyk B. Net, a new ets transcription factor that is activated by Ras. Genes Dev. 1994 Jul 1;8(13):1502–1513. doi: 10.1101/gad.8.13.1502. [DOI] [PubMed] [Google Scholar]
- Hill C. S., Marais R., John S., Wynne J., Dalton S., Treisman R. Functional analysis of a growth factor-responsive transcription factor complex. Cell. 1993 Apr 23;73(2):395–406. doi: 10.1016/0092-8674(93)90238-l. [DOI] [PubMed] [Google Scholar]
- Janknecht R., Ernst W. H., Nordheim A. SAP1a is a nuclear target of signaling cascades involving ERKs. Oncogene. 1995 Mar 16;10(6):1209–1216. [PubMed] [Google Scholar]
- Janknecht R., Ernst W. H., Pingoud V., Nordheim A. Activation of ternary complex factor Elk-1 by MAP kinases. EMBO J. 1993 Dec 15;12(13):5097–5104. doi: 10.1002/j.1460-2075.1993.tb06204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janknecht R., Nordheim A. Elk-1 protein domains required for direct and SRF-assisted DNA-binding. Nucleic Acids Res. 1992 Jul 11;20(13):3317–3324. doi: 10.1093/nar/20.13.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janknecht R., Nordheim A. Gene regulation by Ets proteins. Biochim Biophys Acta. 1993 Dec 23;1155(3):346–356. doi: 10.1016/0304-419x(93)90014-4. [DOI] [PubMed] [Google Scholar]
- Janknecht R., Zinck R., Ernst W. H., Nordheim A. Functional dissection of the transcription factor Elk-1. Oncogene. 1994 Apr;9(4):1273–1278. [PubMed] [Google Scholar]
- Karim F. D., Urness L. D., Thummel C. S., Klemsz M. J., McKercher S. R., Celada A., Van Beveren C., Maki R. A., Gunther C. V., Nye J. A. The ETS-domain: a new DNA-binding motif that recognizes a purine-rich core DNA sequence. Genes Dev. 1990 Sep;4(9):1451–1453. doi: 10.1101/gad.4.9.1451. [DOI] [PubMed] [Google Scholar]
- Kortenjann M., Thomae O., Shaw P. E. Inhibition of v-raf-dependent c-fos expression and transformation by a kinase-defective mutant of the mitogen-activated protein kinase Erk2. Mol Cell Biol. 1994 Jul;14(7):4815–4824. doi: 10.1128/mcb.14.7.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudet V., Niel C., Duterque-Coquillaud M., Leprince D., Stehelin D. Evolution of the ets gene family. Biochem Biophys Res Commun. 1993 Jan 15;190(1):8–14. doi: 10.1006/bbrc.1993.1002. [DOI] [PubMed] [Google Scholar]
- Lopez M., Oettgen P., Akbarali Y., Dendorfer U., Libermann T. A. ERP, a new member of the ets transcription factor/oncoprotein family: cloning, characterization, and differential expression during B-lymphocyte development. Mol Cell Biol. 1994 May;14(5):3292–3309. doi: 10.1128/mcb.14.5.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais R., Wynne J., Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993 Apr 23;73(2):381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- Nurrish S. J., Treisman R. DNA binding specificity determinants in MADS-box transcription factors. Mol Cell Biol. 1995 Aug;15(8):4076–4085. doi: 10.1128/mcb.15.8.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nye J. A., Petersen J. M., Gunther C. V., Jonsen M. D., Graves B. J. Interaction of murine ets-1 with GGA-binding sites establishes the ETS domain as a new DNA-binding motif. Genes Dev. 1992 Jun;6(6):975–990. doi: 10.1101/gad.6.6.975. [DOI] [PubMed] [Google Scholar]
- Pollock R., Treisman R. A sensitive method for the determination of protein-DNA binding specificities. Nucleic Acids Res. 1990 Nov 11;18(21):6197–6204. doi: 10.1093/nar/18.21.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price M. A., Rogers A. E., Treisman R. Comparative analysis of the ternary complex factors Elk-1, SAP-1a and SAP-2 (ERP/NET). EMBO J. 1995 Jun 1;14(11):2589–2601. doi: 10.1002/j.1460-2075.1995.tb07257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao V. N., Huebner K., Isobe M., ar-Rushdi A., Croce C. M., Reddy E. S. elk, tissue-specific ets-related genes on chromosomes X and 14 near translocation breakpoints. Science. 1989 Apr 7;244(4900):66–70. doi: 10.1126/science.2539641. [DOI] [PubMed] [Google Scholar]
- Rao V. N., Reddy E. S. A divergent ets-related protein, elk-1, recognizes similar c-ets-1 proto-oncogene target sequences and acts as a transcriptional activator. Oncogene. 1992 Jan;7(1):65–70. [PubMed] [Google Scholar]
- Sharrocks A. D. ERK2/p42 MAP kinase stimulates both autonomous and SRF-dependent DNA binding by Elk-1. FEBS Lett. 1995 Jul 10;368(1):77–80. doi: 10.1016/0014-5793(95)00604-8. [DOI] [PubMed] [Google Scholar]
- Sharrocks A. D., Gille H., Shaw P. E. Identification of amino acids essential for DNA binding and dimerization in p67SRF: implications for a novel DNA-binding motif. Mol Cell Biol. 1993 Jan;13(1):123–132. doi: 10.1128/mcb.13.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrocks A. D., Shore P. DNA bending in the ternary nucleoprotein complex at the c-fos promoter. Nucleic Acids Res. 1995 Jul 11;23(13):2442–2449. doi: 10.1093/nar/23.13.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrocks A. D., von Hesler F., Shaw P. E. The identification of elements determining the different DNA binding specificities of the MADS box proteins p67SRF and RSRFC4. Nucleic Acids Res. 1993 Jan 25;21(2):215–221. doi: 10.1093/nar/21.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore P., Bisset L., Lakey J., Waltho J. P., Virden R., Sharrocks A. D. Characterization of the Elk-1 ETS DNA-binding domain. J Biol Chem. 1995 Mar 17;270(11):5805–5811. doi: 10.1074/jbc.270.11.5805. [DOI] [PubMed] [Google Scholar]
- Shore P., Sharrocks A. D. The transcription factors Elk-1 and serum response factor interact by direct protein-protein contacts mediated by a short region of Elk-1. Mol Cell Biol. 1994 May;14(5):3283–3291. doi: 10.1128/mcb.14.5.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R., Marais R., Wynne J. Spatial flexibility in ternary complexes between SRF and its accessory proteins. EMBO J. 1992 Dec;11(12):4631–4640. doi: 10.1002/j.1460-2075.1992.tb05565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R. Ternary complex factors: growth factor regulated transcriptional activators. Curr Opin Genet Dev. 1994 Feb;4(1):96–101. doi: 10.1016/0959-437x(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Urness L. D., Thummel C. S. Molecular interactions within the ecdysone regulatory hierarchy: DNA binding properties of the Drosophila ecdysone-inducible E74A protein. Cell. 1990 Oct 5;63(1):47–61. doi: 10.1016/0092-8674(90)90287-o. [DOI] [PubMed] [Google Scholar]
- Wasylyk B., Hahn S. L., Giovane A. The Ets family of transcription factors. Eur J Biochem. 1993 Jan 15;211(1-2):7–18. doi: 10.1007/978-3-642-78757-7_2. [DOI] [PubMed] [Google Scholar]
- Whitmarsh A. J., Shore P., Sharrocks A. D., Davis R. J. Integration of MAP kinase signal transduction pathways at the serum response element. Science. 1995 Jul 21;269(5222):403–407. doi: 10.1126/science.7618106. [DOI] [PubMed] [Google Scholar]
- Williams G. T., Lau L. F. Activation of the inducible orphan receptor gene nur77 by serum growth factors: dissociation of immediate-early and delayed-early responses. Mol Cell Biol. 1993 Oct;13(10):6124–6136. doi: 10.1128/mcb.13.10.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. B., Ghysdael J., Owen M. J. Identification of nucleotide preferences in DNA sequences recognised specifically by c-Ets-1 protein. Nucleic Acids Res. 1992 Feb 25;20(4):699–704. doi: 10.1093/nar/20.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinck R., Hipskind R. A., Pingoud V., Nordheim A. c-fos transcriptional activation and repression correlate temporally with the phosphorylation status of TCF. EMBO J. 1993 Jun;12(6):2377–2387. doi: 10.1002/j.1460-2075.1993.tb05892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]