Abstract
Although many studies have found psychological depression associated with higher circulating levels of C-reactive protein (CRP), not all findings are consistent. Since DNA sequence variation in the CRP gene has also been shown to predict plasma CRP levels, we hypothesized that plasma CRP may covary with depressive symptomatology as a function of allelic variation in the CRP gene. We tested this hypothesis in 868 healthy community volunteers of European ancestry. Depressive symptomatology was measured using the Center for Epidemiological Studies – Depression (CESD) scale, and plasma CRP was assayed from whole blood. Three polymorphisms [rs1417938 (A/T), rs1800947 (C/G) and rs1205 (C/T)] were genotyped and three-locus haplotypes were generated. Regression models adjusting for demographic and lifestyle-related covariates showed no direct association of CESD depression scores with CRP. In regression models adjusting for age, gender, education, smoking status and statin use, one CRP haplotype (T-G-C) was associated with CRP level (p = 0.014) and a second haplotype (A-G-T) showed marginal association (p=0.064 respectively). Neither haplotype was related to depressive symptoms. However, plasma CRP was predicted by the interaction of A-G-T haplotype with depressive symptomatology (p = 0.009). Higher CESD scores were associated positively with CRP levels among individuals with the A-G-T haplotype (p = 0.004). In secondary analyses, body mass index was found to partially account for the moderating effects of the A-G-T haplotype on the association of depression with circulating CRP. In conclusion, we found that haplotypic variation in the CRP locus moderates an association of depressive symptoms with circulating CRP, which is partially mediated by BMI.
Keywords: CRP, depression, inflammation, genotypes, haplotypes, interaction
Introduction
C-reactive protein (CRP) is a circulating marker of systemic inflammation that has been shown to predict cardiovascular pathology and mortality in epidemiological investigations (Ridker et al., 2006; Ridker and Cook, 2007; Sabatine et al., 2007). A growing literature suggests that clinical depression and self reported depressive symptomatology covary positively with circulating CRP levels, even after adjusting for traditional risk factors, including age, sex, race, smoking status, body mass index (BMI) and HDL cholesterol concentrations (Danner et al., 2003; Ford et al., 2004; Penninx et al., 2003; Kop et al., 2002). However, other studies have either failed to observe an association between depression and circulating CRP (Bremmer et al., 2008; Hemingway et al., 2003; Janszky et al., 2005; Ladwig et al. 2003; Schins et al., 2005) or have observed an inverse relationship (Whooley et al., 2007). These inconsistencies may stem from differences in study sample (patient versus community-based samples), index of depression (clinical diagnosis or symptom report), or other differences of methodology or analysis (e.g. range of covariate adjustments). It is also possible that unexamined genetic factors moderate associations between affect and CRP.
Biometric studies show genetic variation to account for a significant portion of interindividual variability in circulating levels of CRP, with estimated heritability of about 0.4 (Pankow et al., 2001; Vickers et al., 2002). Additionally, DNA sequence variation in the gene encoding CRP (the CRP gene) has been associated with levels of circulating CRP (Carlson et al., 2005; Crawford et al., 2006; Kathiresan et al., 2006; Lange et al., 2006; Miller et al. 2005; Russell et al., 2004; Szalai et al., 2005; Wang et al., 2006). Moreover, one recent study has shown that common genetic variation partially accounted for covariation of depression with the inflammatory marker IL6 (Su et al., 2009). Because polymorphisms of a number of regulatory genes have been shown to predict phenotypes of interest in interaction with predisposing behavioral or biological factors (Caspi et al. 2002, 2003; Covault et al., 2007; Manuck et al., 2004), we hypothesized that CRP-related genetic variation might analogously moderate an association of depressive symptoms with circulating CRP. Accordingly, here we examine whether depressive symptomatology covaries with plasma CRP as a function of polymorphic variation previously shown to predict circulating CRP levels.
To date, genetically moderated associations between psychological and biological variables have tended to focus on single polymorphisms (Caspi et al. 2002, 2003; Covault et al., 2005; Manuck et al., 2004). A limitation of this approach is failure to consider other variation in the gene of interest that may contribute to phenotypic differences. The recently completed Human Haplotype Mapping project (HapMap; www.hapmap.org) has identified numerous single nucleotide polymorphisms (SNPs) within individual genes. With respect to such variation, genetic transmission from parent to offspring is characterized by lack of recombination between homologous regions on chromosomes. This process leads to non-random association, or linkage disequilibrium (LD), between variants (alleles) of closely spaced polymorphisms, whereby alleles at different loci are transmitted together on a chromosome more often than expected by chance. The term “haplotype” is used to define a chromosomal segment within which alleles are in high LD and are therefore transmitted together through generations. Each haplotype can be “tagged” by a SNP that acts as a marker of aggregate variation within the haplotype. Consequently, typing the allele at the tag SNP locus provides information about groups of linked variation in a gene. In practice, typing a few tag SNPs, instead of sequencing the entire gene, offers a relatively inexpensive way to examine the association between overall variation within a gene and a measured phenotype. In addition, association between a tag SNP of no known function and a phenotype may indicate presence of a functional locus within the haplotype characterized by the tag SNP. Refined genetic mapping can then be implemented to identify the functional variation within the haplotype. Additionally, investigating the association of a multilocus haplotype (comprised of any combination of tag SNPs and non-tagging SNPs of known functionality) with a particular phenotype may provide information (over and above single locus effects) regarding the aggregate influence of the gene on the outcome of interest. Accordingly, here we studied multiple polymorphisms and their constituent haplotypes to better characterize potential moderating effects of variation in the CRP gene on the association between depression and circulating CRP.
Methods
2.1. Participants
This investigation was based on data derived from 1295 adults who participated in the University of Pittsburgh Adult Health and Behavior (AHAB) project between 2001 and 2005. The AHAB registry is a compendium of behavioral and biological measurements collected on mid-life community volunteers who were recruited via mass-mail solicitation from Southwestern Pennsylvania (primarily Allegheny County). Registry data include socio-demographic measurements; indices of personality, temperament, and psychopathology; aspects of social and cognitive functioning; health-impairing attributes of habit and lifestyle; biological measurements germane to cardiovascular, metabolic, endocrine, autonomic, immune and central nervous system functioning; and DNA for the study of genetic variation associated with registry phenotypes (e.g. Forbes et al., 2007; Halder et al., 2007; MacDonald et al., 2007; Neumann et al., 2006). Exclusions from AHAB participation included: age <30 or >54 years; a reported history of atherosclerotic cardiovascular disease, chronic kidney or liver disease, cancer treatment in the preceding year, and major neurological disorders, schizophrenia or other psychotic illness. Other exclusions included pregnancy and the use of insulin, glucocorticoid, antiarrhythmic, psychotropic, or prescription weight-loss medications. Data collections occurred over multiple laboratory sessions and informed consent was obtained in accordance with approved protocols and guidelines of the University of Pittsburgh Institutional Review Board.
Previous studies have shown that African Americans exhibit wide variation in individual bio-geographical ancestry that can confound genetic associations (Halder et al., 2003, 2008). Although ancestry also varies in European Americans and Europeans, it does so to a lesser extent. For this reason, we excluded African Americans to prevent confounding due to admixture, focusing on the 1099 European Americans in the AHAB sample. Because AHAB exclusions did not include common acute illnesses, such as recent colds or allergies, we also excluded the data of 213 participants having circulating CRP levels >10 mg/L. Finally, 18 individuals with missing genotype data were excluded, yielding a final sample of 868 individuals (436 males). These subjects did not differ in age, income, education or gender from European American AHAB participants who were excluded from the present analyses.
2.2. Procedure
Prior to the laboratory visit, participants were asked to fast for 8 hours and avoid exercise for 12 hours, alcohol for 24 hours and nicotine for 1 hour. All laboratory sessions were scheduled in the morning. Upon subjects' arrival, the project nurse completed a medical history and medication use interview, obtained measurements of height and weight for determination of BMI (Kg/m2), and drew a 40 cc blood sample. A portion of the blood sample collected in citrate-treated tubes was spun down and plasma collected and stored at -80°C until batched- analysis of CRP levels. A second portion of the blood sample was collected in EDTA treated tubes and stored at -80° C for DNA isolation.
2.3. Variable Measures
Circulating CRP
Plasma CRP was assayed using the BN II nephelometer (Dade-Behring Inc., Deerfield, Illinois, USA) utilizing a particle enhanced immunonephelometric assay. In this procedure, polystyrene particles are coated with monoclonal antibodies to CRP, which agglutinate in the presence of appropriate antigen, thereby increasing the intensity of scattered light. The light intensity is proportional to the amount of CRP in the sample. The assay has a detection range of 0.175-1100 mg/L. Intra-assay coefficients of variation (CV) range from 2.3-4.4% and inter-assay CV range from 2.1- 5.7%. Logarithmic transformation was applied to normalize raw score distributions of the CRP values.
Depressive Symptoms
Depressive symptoms were measured using the Center for Epidemiological Studies-Depression (CESD) scale (Radloff, 1977). This 20-item measure assesses how frequently subjects experienced a range of psychological and physical symptoms of depression during the past week. Responses are on a 4-point scale ranging from 0 (rarely or none of the time [<1 day]) to 3 (most or all of the time [5 to 7 days]). Higher scores indicate more severe depressive symptomatology, with a maximum score of 60. The CESD has excellent internal consistency (0.87) and reasonable test-retest reliability (average 0.57) (Radloff, 1977). Summed CESD scores were log transformed and standardized when used as a continuous variable in statistical models. CESD scores ≥ 16 was used as a criterion value designating clinically significant depression (Beekman et al., 1997).
DNA isolation and Genotyping
DNA was isolated from frozen whole blood samples following a previously described protocol (Miller et al., 1988). The CRP gene on chromosome one (1q21-23) has two exons, which are joined by a 280 base pair intron, and encode a 204 amino acid protein. Three common polymorphisms in the CRP gene, all designated as tag SNPs in European Americans in the HapMap, were used in the current study: rs1417938 (in the first intron), rs1800947 (synonymous SNP in exon 2, Leu184Leu) and rs1205 (in the 3′ UTR). We selected these markers using the Tagger algorithm (deBakker et al., 2005) and the HapMap genotype data in Europeans using an R2 cut off of 0.8 and a minor allele frequency cut off of 0.05. These SNPs have also been shown to predict circulating CRP levels and to be in strong LD with other polymorphisms in the CRP gene (Carlson et al., 2005; Crawford et al., 2006; Kathiresan et al., 2006; Lange et al., 2006; Miller et al. 2005; Russell et al., 2004; Szalai et al., 2005; Wang et al., 2006). Together, these SNPs capture 100% of alleles with R2 > 0.8 and physically cover 84% of the gene. Subjects were genotyped using Taqman Validated SNP genotyping assays (Applied Biosystems, Foster City, CA) following standard protocol, and genotypes were scored by allelic discrimination using the ABI 7900HT Fast Real-Time PCR system and the SDS 2.2 software (Applied Biosystems, Foster City, CA).
Covariate Measures
Several other variables that might explain an association between depressive symptomatology and CRP levels were assessed and used as standard covariates in the multivariate models. These included age, gender, BMI, smoking status, education levels and statin use. Smoking status was defined by participants' self-report and coded as a binary variable [current cigarette smoking vs. all other categories of tobacco use (which includes non-smokers, ex-smokers and those using other forms of tobacco)]. Education levels were measured as participants' self-report of cumulative years of schooling.
2.4. Genetic analyses
Allele frequencies and Hardy Weinberg equilibrium were ascertained using the Genepop software (Rousset and Raymond, 1995). Pairwise linkage disequilibrium (LD) was ascertained using the Linkage Disequilibrium Analyzer 1.0 software (Ding et al., 2003). Multilocus haplotypes (combinations of alleles of selected loci on each chromosome) were ascertained using PHASE v 2.0 (Stephens et al., 2001). The haplotypes obtained using PHASE were used in standard statistical tests as described in the following section. We used the program EHAP (Seltman et al., 2001, 2003; http://wpicr.wpic.pitt.edu/WPICCompGen/ehap_v1.htm) to obtain a global test of association of haplotypes, as described in the following section. The program STRUCTURE (Falush et al., 2003; Pritchard et al., 2000) was used to evaluate presence of genetic substructure in the sample, using 15 genome-spanning SNPs (rs1022106, rs1335995, rs1439564, rs1502812, rs1860300, rs548146, rs705388, rs715994, rs720517, rs722743, rs730899, rs734204, rs9059966, rs1328994, rs1485405). A model with admixture, uncorrelated allele frequencies, individual alpha parameters, and independent Fst for all subpopulations was used and run separately assuming 1, 2, or 3 subpopulations. For each model we used a burnin of 40,000 followed by 80,000 repetitions and compared the likelihoods of models fitting the data. Evidence of stratification was inferred if the likelihood of data fitting a model with ≥2 subpopulations was greater than the likelihood of data fitting a model with 1 population.
2.5. Statistical Analyses
All statistical analyses were performed using SPSS v15 (SPSS Inc. Chicago, IL). Bivariate correlations among variables were examined using Pearson correlation coefficients or point biserial correlations. Multiple regression analysis was used to examine the proportion of variation in circulating CRP levels explained by depressive symptomatology. In this model, the standard covariates were entered in step 1 and log standardized CESD scores were entered in step 2. A similar regression model was used to investigate the proportion of variation in depressive symptomatology explained by circulating CRP, with CESD scores as the outcome and log transformed CRP as the predictor.
Hierarchical regression models were also used to investigate the association of CRP polymorphisms and haplotypes with CESD scores. For these analyses haplotype status was dichotomized, comparing individuals with one or two copies of a haplotype to all others. Then, the standard covariates were entered in step one, and PHASE-estimated haplotypes and genotypes were entered in step 2 of models predicting CESD score.
Next, we examined the association of the haplotypes with circulating CRP. For these analyses, we first used the EHAP program to test for an overall association of haplotypes with CRP. EHAP estimates individual haplotypes while incorporating uncertainty in the data and permits the consideration of covariates. For this analysis, CESD score and all standard covariates except BMI were entered as predictors of CRP. Genotypes at the three loci were included in the model. All other modeling parameters were set at default values. In a parallel set of analyses, we included BMI as an additional covariate in the model. In addition to the EHAP analyses, we also used hierarchical regression models to examine whether PHASE-estimated haplotypes and their constituent polymorphisms predicted levels of CRP alone or in interaction with depressive symptoms. Here, age, gender, education, smoking status and statin use were entered as covariates in step one, log standardized CESD scores and haplotype status in step 2, and the interaction of “haplotype X CESD” was entered in the final step. Finally, because BMI has been shown to predict both depression and CRP in prior literature (de Wit et al., 2009, Ford et al., 2001, Goodman et al., 2002, Herva et al., 2006, Howren et al., 2009, Panagiotakos et al., 2005, Simon et al., 2006, Visser et al., 1999, Williams et al., 2004, Zhao et al., 2009), we examined the possibility that BMI would partially account for any haplotype-dependent covariation of depression symptomatology with CRP levels. Here, BMI was entered along with the other covariates in the first step of a similar regression model predicting CRP. In the event of a reduction in the magnitude of interaction between depression scores and CRP haplotype when including BMI in the model, mediational analyses were conducted following the method described by Freedman & Schatzkin, 1992. To examine the validity of study results with respect to a clinically meaningful level of depressive symptomatology, we further subjected CRP levels to an analysis of covariance (ANCOVA) with two between subjects factors, CESD score (≥ 16 vs. < 16) and haplotype carrying status.
To control for multiple testing, we used the false discovery rate (FDR) (Benjamini and Hochberg, 1992) method and report FDR-adjusted P values. Since EHAP provides a Bonferroni adjusted P value, we report those for the EHAP results.
Results
3.1. Descriptive Statistics
Sample characteristics and bivariate correlations are presented in Table 1. Consistent with prior literature, higher levels of CRP were associated with higher BMI and fewer years in school and more symptoms of depression were associated with younger age, female gender, and higher BMI. Circulating CRP did not covary with CESD scores in bivariate analyses.
Table 1.
Sample Characteristics (mean and standard deviations) and bivariate correlations
| Mean (SD) | CRP r |
CESD r |
||
|---|---|---|---|---|
| Age (years) | 44.8 (6.7) | -0.047 | -0.087* | |
| # Gender (%Female) | 50 | 0.034# | -0.082* | |
| BMI (kg/m2) | 27.1 (5.2) | 0.44** | 0.095** | |
| #% using statins | >1 | -0.015# | 0.043 | |
| #% current smoker | 16 | 0.009# | 0.056 | |
| Years in School | 15.9 (2.8) | -0.088** | -0.055 | |
| Range | 6 – 24 | |||
| CRP (μg/ml) | 1.6 (1.8) | 0.051 | ||
| Range | 0.2 – 9.9 | |||
| CESD | 7.4 (7.5) | 0.051 | ||
| Range | 0-42 |
Correlation Coefficients are shown.
P < 0.05
P < 0.01
Point biserial correlations
BMI: Body Mass Index. Gender coded as 1 = Male, 2 = Female, Smoking status (0 = not current smokers, 1 = current smokers), Statin Use (0 = not using statins, 1 = currently using statins); correlations were conducted on log transformed CRP and CESD variables.
Allele frequencies were 69% (A) and 31% (T) for rs1417938, 94% (G) and 6% (C) for rs1800947, and 67% (C) and 33% (T) for rs1205, which are similar to corresponding allele frequencies in epidemiological studies of European Americans (Carlson et al., 2005; Crawford et al., 2006; Lange et al., 2006; Miller et al. 2005). Proportions of missing genotype data were 6% (rs1417938), 3% (1800947) and 4% (rs1205), respectively. Distributions of genotypes conformed to Hardy-Weinberg equilibrium for all loci {rs1417938 (A/T) [AA = 374; AT = 375; TT = 68 (p > 0.05)]; rs1800947 (C/G) [CC = 750; CG = 88; GG = 6 (p > 0.05)] and rs1205(C/T): CC = 376, CT = 367; TT = 88 (p > 0.05)]}. Pairwise LD (r2) values were 0.13 (rs1205-rs1800947: D′ = 1), 0.19 (rs1205-rs1417938, D′ = 1) and 0.03 (rs1800947-rs1417983, D′ = 0.93), respectively. STRUCTURE analyses showed no evidence of genetic stratification in the sample and no further adjustments were made to control for stratification.
Using PHASE, four different haplotypes (designated as H1 through H4 for ease of identification) were ascertained in the sample (see Table 2). In the absence of parent genotypes, individuals' haplotypes were inferred probabilistically. Only those individuals for whom haplotypes were ascertained with > 95% confidence were included in the analyses. These haplotypes were confirmed with the EHAP program, which estimated six haplotypes in the sample of which two were very rare (less than 0.01 frequency). The four remaining haplotypes were identical to the ones obtained with PHASE; however, as EHAP does not provide a list of haplotypes for each individual, like the PHASE output, direct comparisons were not possible. Frequency of EHAP and PHASE estimated haplotypes are shown in Table 2.
Table 2.
Observed CRP Haplotypes and circulating CRP levels associated with each haplotype
| Haplotype | Sequence | PHASE Frequency |
EHAP Frequency |
Mean CRP (μg/ml) |
|---|---|---|---|---|
| H1 | A-G-C | 0.36 | 0.43 | 1.7 |
| H2 | T-G-C | 0.31 | 0.31 | 1.8 |
| H3 | A-G-T | 0.27 | 0.2 | 1.5 |
| H4 | A-C-T | 0.06 | 0.06 | 1.6 |
Order of SNPs: rs1417938 (A>T); rs1800947 (G>C); rs1205 (C>T)
PHASE Frequency = Frequency of haplotypes obtained with PHASE and represents proportion of individuals who carry at least one copy of a specific haplotype to the total number of haplotypes in all subjects.
EHAP Frequency = Haplotype Frequencies obtained with EHAP program and represents Mean CRP values (not adjusted for covariates) for each haplotype group is shown.
3.2. Association between depressive symptomatology and circulating CRP levels
In initial regression analyses controlling for age, gender, education, smoking status, statin use, and BMI, there was no independent association of depressive symptoms with CRP levels (B = 0, SE = 0.016, t = 0.155, p > 0.05). Together, the standard covariates accounted for 21% of the variance in circulating CRP (R = 0.463). Similarly, CRP as a predictor did not explain variability in depressive symptoms after standard covariates were included in the model (B = 0.002, SE = 0.068, t = 0.029, P = 0.977).
3.3 Main effects of genotypes and haplotypes on depression symptoms
In regression models controlling for age, gender, education, smoking status and statin use, neither individual genotypes nor haplotypes showed an association with CESD scores (P>0.05 for all for both FDR-adjusted and unadjusted values).
3.4. Main effects of genotypes and haplotypes on circulating CRP
In regression models controlling for age, gender, education, smoking status and statin use, circulating CRP was predicted by allelic variation at rs1417938 (B = -0.079, SE = 0.029, t = 2.773, p = 0.0.024) and rs1205 (B = -0.074, SE = 0.027, t = -2.749, p = 0.024), but not at rs1800947 (B = -0.055, SE = 0.053, t = -1.023, p = 0.136). Based on previous reports (Carlson et al., 2005; Crawford et al., 2006; Kathiresan et al., 2006; Lange et al., 2006; Miller et al. 2005; Wang et al., 2006), an additive model was used for these individual SNP analyses. Individuals carrying either the T allele of rs1417983 or the C allele of rs1205 (the alleles present in haplotype H2) had significantly higher mean CRP levels than other genotypes (rs1417983: TT+AT [untransformed CRP= 1.8 μg/ml] vs. AA [untransformed CRP = 1.5 μg/ml], t = -2.773, p = 0.02; rs1205: CC+CT [untransformed CRP = 1.7 μg/ml] vs. TT [untransformed CRP = 1.3 μg/ml]; t = 2.749, p = 0.03). Similarly adjusted regression models showed a significant positive association of haplotype H2 with CRP (untransformed CRP for carriers and non-carriers =1.8 μg/ml and 1.5 μg/ml, respectively; B = 0.153, SE = 0.066, t = 2.313, p = 0.021), and a marginal inverse association for haplotype H3 (untransformed CRP for carriers and non-carriers =1.5 μg/ml and 1.7 μg/ml, respectively; B = -0.116, SE = 0.064, t = -1.751, p = 0.064). Haplotypes H1 and H4 were not significantly associated with CRP (p's > 0.1).
In addition to the above analyses, we also used the program EHAP to obtain a global test of association of haplotypes with CRP. EHAP accounts for the haplotype uncertainty that is present when parental genotypes are not available and permits the consideration of covariates. It uses a maximum likelihood based method for inferring haplotypes. In a model that included age, gender, smoking status, education and statin use as covariates, no global association was detected between haplotypes and circulating CRP (Bonferroni adjusted P = 0.11).
3.4. Do CRP genotypes or haplotypes moderate an association between depressive symptoms and circulating CRP levels?
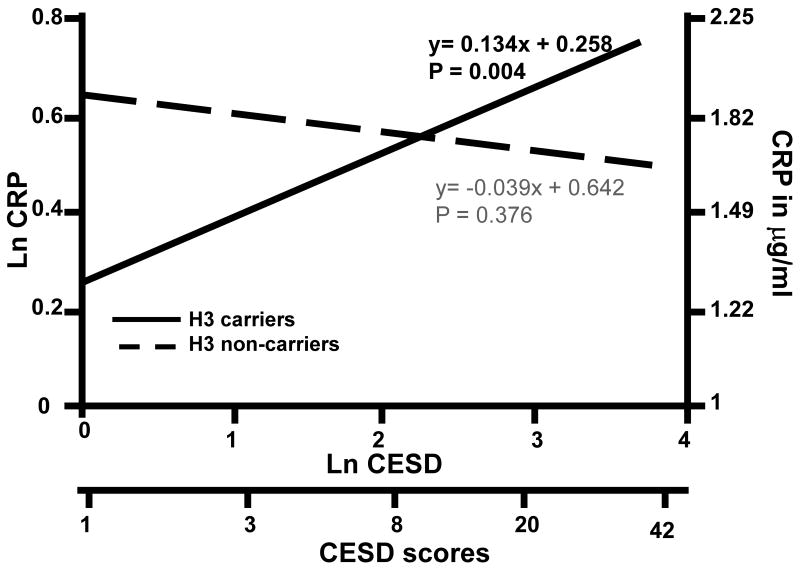
To test whether the CRP genotypes or haplotypes moderate an association between depressive symptoms and circulating CRP, we next entered the interaction term (genotype X CESD or haplotype status X CESD) in a third step of the regression models. Results revealed a significant interaction between haplotype H3 and CESD score in the prediction of CRP (B = 0.173, SE = 0.064, t = 2.697, p = 0.009). As shown in Figure 1, CESD scores were positively associated with CRP among H3 carriers (B = 0.133, SE = 0.047, t = 2.848, p = 0.004) but not in non-carriers (B = -0.039, SE = 0.044, t = -0.886, p = 0.376). Neither individual SNPs nor haplotypes H1, H2 and H4 showed an interaction with depressive symptoms in the prediction of CRP (Ps>0.05).
Figure 1.
Interaction of depressive symptomatology and haplotype H3 status predicts circulating CRP, after adjustment for age, gender, education, smoking status and statin use. Log-transformed CESD scores, which were used as predictor in the regression model, are represented on the X-axis, along with a corresponding scale of untransformed CESD scores shown at the bottom (for comparison purposes). The Y-axis displays log transformed circulating CRP levels on the left axis. For reference, comparison values of CRP, back-transformed to the unit of CRP measurement (μg/ml), are presented on the right ordinate. Solid line represents significant association between depression and circulating CRP in haplotype H3 carriers (P = 0.004). Broken line represents association between depressive symptoms and circulating CRP in non-carriers of haplotype H3 (P = 0.376).
In a subsequent ANCOVA, we examined effects of clinically elevated CESD scores (≥ 16 vs. < 16) and haplotype H3 carrying status on CRP levels, after controlling for the standard covariates. Results confirmed a significant interaction between depression status and haplotype H3 (F = 4.27, df = 3, p = 0.01). Tukey HSD post-hoc comparison revealed a significant difference in covariate-adjusted mean CRP levels between depressed and non-depressed subjects in H3 carriers (1.9 μg/ml vs. 1.3 μg/ml; P = 0.014). CRP levels did not differ between depressed and non-depressed H3 non-carriers (1.3 μg/ml vs. 1.6 μg/ml, p>0.05)
3.5 Might BMI account for the H3-moderated association of depressive symptomatology with CRP level?
Since BMI correlated significantly with both CESD scores and circulating CRP levels (Table 1), we next investigated whether the main and interaction effects observed in the preceding analyses might be accounted for by BMI. Including BMI as a covariate in the EHAP analysis yielded a significant result (p=0.005) indicating an overall association of haplotypes with circulating CRP. We then tested main effects of PHASE-estimated haplotypes H2 and H3 on circulating CRP levels by including BMI as a covariate in the model (in addition to age, gender, education, smoking status and statin use). When BMI was included as a covariate in the models, the main effect of both H2 and H3 increased in significance (H2: B = 0.195, SE = 0.059, t = 3.324, p = 0.006; H3: B = -0.136, SE = 0.059, t = -2.312, p = 0.032).
Next, we entered the interaction term (haplotype H3 X CESD) in the third step of the regression model for H3. The H3 X CESD interaction also remained a significant predictor of circulating CRP (B = 0.115, SE = 0.057, t = 2.007, p = 0.045), although attenuated in strength (from B = 0.173, p = 0.009 to B = 0.115, p = 0.045), implying possible partial mediation by BMI.
For mediation analyses we first explored whether haplotypes alone or in combination with depressive symptoms predict BMI. Here, age, gender, education, smoking status and statin use were entered in step 1, haplotype and CESD score in step 2 and the interaction of haplotype and CESD score in the final step of the model predicting BMI. Results showed no independent association of haplotype H2 or H3 with BMI. Haplotype H3 showed a marginal interaction with CESD scores in the prediction of BMI (B = 0.707, SE = 0.339, t = 2.087, p = 0.07). We then investigated the independent association of BMI with circulating CRP levels. After adjusting for age, gender, education, smoking status and statin use, BMI showed a significant positive association with circulating CRP in this sample (B = 0.088, SE = 0.006, t = 15.413, p < 0.0001).
Finally, we tested whether the haplotype H3 X CESD interaction was partially mediated by BMI, using the method of Freedman and Schatzkin (1992). This analysis showed BMI to be a significant mediator of the H3 X CESD interaction (t = 2.9, p = 0.0038) in this sample. Because the H3 X CESD interaction term remained significant (though weakened) in the regression model with BMI as a covariate, correlated variation in BMI does not fully account for the H3-dependent association of CESD scores with CRP.
Discussion
This investigation aimed to examine whether sequence variation in the CRP gene, which has been associated with circulating levels of CRP (Carlson et al., 2005; Crawford et al., 2006; Kathiresan et al., 2006; Lange et al., 2006; Miller et al. 2005; Russell et al., 2004; Szalai et al., 2005; Wang et al., 2006), moderates an association between depressive symptomatology and circulating CRP levels. Consistent with existing evidence (Carlson et al., 2005; Crawford et al., 2006; Kathiresan et al., 2006; Lange et al., 2006; Miller et al. 2005; Wang et al., 2006), we observed an association of genetic variation at two individual loci and two CRP haplotypes with circulating levels of CRP in our community sample of 868 European American, mid-life adult volunteers. In single locus tests we confirmed that the T allele of rs1417983 and the C allele of rs1205 (which are present in haplotype H2) were associated with higher levels of circulating CRP, similar to previously reported single-locus tests (Carlson et al., 2005; Lange et al., 2006; Miller et al., 2005, Russell et al., 2004). Associations between the third tag SNP, rs1800947, and CRP have been reported inconsistently in the literature (Carlson et al., 2005; Kathiresan et al., 2006; Lange et al., 2006; Miller et al., 2005; Wang et al., 2006) and the current findings showed no significant relationship with circulating CRP. In multivariate regression analyses we found that the T-G-C haplotype (H2) was associated with higher CRP levels, and conversely, the A-G-T haplotype (H3) was associated with lower CRP levels in this sample. Interestingly, neither single loci nor haplotypes were associated with depressive symptoms in this study.
CRP is an acute phase protein that increases during systemic inflammation and is a well-established predictor for CVD, even in apparently healthy individuals. Depression and depressive symptoms are also risk factors for CVD and often covary with inflammation. However, the relationship between depression and inflammation is itself uncertain. Two opposing mechanistic hypotheses have been postulated to explain the covariation of depression and inflammation. One hypothesis postulates a pathway of cytokine-induced depression. An alternate hypothesis suggests that depression dysregulates immune system pathways in ways that promote inflammation. If inflammation leads to depression, then variation in inflammatory genes, which are known to be associated with levels of the inflammatory biomarker, might also predict depressive symptoms. This we did not see in the present study, and would not be expected if instead depression leads to inflammation. If anything, one might expect that depressive symptoms, acting as a stimulus for inflammatory response might interact with genetic variation to predict the inflammatory marker. Moreover, this pattern is consistent with the finding here, though the cross-sectional nature of these data precludes any causal interpretation. Thus, while genetics may explain some of the relationship between depression and inflammation, a longitudinal design is best suited to study the temporal relationship between depression and inflammation. A regression model such as one we have used can only indicate a statistical association between variables at a given time point and for our analysis such an association would not provide direct evidence of a pathway from inflammation to depression, since both are complex multigenic outcomes.
The interaction between depression and haplotype H3 that we have observed indicates that depressive symptoms covary positively with CRP levels among carriers of the H3 haplotype, but not in its absence. These relationships were independent of demographic and lifestyle health risk factors, including age, sex, years of education, smoking, and statin use. An examination of this statistical interaction, as illustrated in Figure 1, indicates that the lower CRP level otherwise attributable to Haplotype H3 is mitigated at higher levels of depressive symptoms, though it remains unclear how depression or its behavioral, neuroendocrine or other physiologic correlates might preferentially augment CRP production in H3 carriers. Overall, these results suggest that the association of depressive symptomatology with circulating CRP levels, which has been observed inconsistently in previous studies (Bremmer et al., 2008; Hemingway et al., 2003; Janszky et al., 2005; Ladwig et al. 2003; Schins et al., 2005; Whooley et al., 2007), may be moderated partly by variation in the CRP gene.
It is also possible that inconsistencies across previous studies reflect differences in sample characteristics. In the current study, we found no overall association of depressive symptomatology with CRP levels among relatively-healthy mid-life adults. It is possible that our participants are younger and healthier than in previous studies that showed a positive association of depressive symptoms with circulating CRP levels across all subjects (Kop et al., 2002; Lesperance et al., 2004; Liukkonen et al., 2006; Penninx et al., 2003; Vaccarino et al., 2007). Depressive symptomatology was also generally lower in the current sample than in prior studies, with mean CESD scores of 7.4 and only 12% of individuals having clinically relevant CESD score (≥ 16) (Beekman et al., 1997). Further, subjects with clinically meaningful CESD scores did not vary significantly in age, gender, education, smoking status and statin use from those with lower CESD scores. Only BMI was slightly, but significantly higher in subjects with CESD scores ≥ 16 (Mean BMI 27 vs. 26), suggesting that BMI may influence any observed association. As our subsequent analyses have shown, BMI does partially mediate the haplotype-dependent association of depressive symptomatology with circulating CRP. In sum, our findings suggest that stronger and more consistent associations between depression and levels of CRP may be found if CRP-related genetic variation is considered.
The current study also contributes to an understanding of the role of BMI in relationships between genetic variation, depressive symptoms, and levels of CRP. Consistent with the extant literature, we found positive associations of BMI with circulating levels of CRP (Visser et al., 1999, Ford et al., 2001, Williams et al., 2004, Panagiotakos et al., 2005) and depression (Goodman et al., 2002, Herva et al., 2006, Simon et al., 2006, de Wit et al., 2009, Zhao et al., 2009). Furthermore, BMI partially accounted for associations between depression and CRP observed among haplotype H3 carriers. Even after adjustment for BMI, however, we still observed residual interaction of H3 haplotype with CESD scores. This pattern is consistent with a recent meta analysis finding that BMI contributes to the variance in CRP associated with symptoms of depression, but does not fully account for this relationship (Howren et al., 2009). The current results suggest that genetic variation at the CRP locus contributes to associations between depression and CRP by pathways both related and unrelated to BMI. However, the study design and findings do not clarify the temporal relationship between depression and inflammation.
Although previous studies of CRP – related genetic variation and circulating CRP levels have examined the moderating effects of age, gender, race, medication use, socioeconomic status and the metabolic health of individuals, we believe ours' is the first to consider potential interaction with a psychological variable. Of course, the cross sectional nature of the current study precludes causal interpretation. It is possible that depression is associated with activation of multiple physiological and/or behavioral pathways that influence immune function among vulnerable individuals. Alternatively, higher levels of systemic inflammation may differentially affect the central nervous system among A-G-T haplotype carriers. In this regard, a growing literature supports immune-to-brain communication, with activation of peripheral inflammatory processes influencing neural activity involved in the regulation of affect (Maier and Watkins, 1998), raising the possibility that systemic inflammation results in increased symptoms of depression.
The SNPs genotyped here are all identified in HapMap as tag SNPs in European Americans, marking variations within a linked chromosomal segment. It is likely that these segments harbor other functional variants that influence CRP levels. By using tag SNPs, we aimed to extend our examination of specific loci to a much larger segment of the gene. The loci studied here are either functional [e.g. rs1205 (Miller et al., 2005; Crawford et al., 2006)] or are closely associated with other functional loci in the gene. For instance, the SNP rs1417983 is in strong LD with a promoter SNP, rs301244, which is known to regulate CRP production by modulating transcription factor binding (Miller et al., 2005). Most importantly, although we used tag SNPs, which themselves are markers for larger chromosomal segments, we also generated three-locus haplotypes in order to identify the combination of tag SNP alleles present on each chromosome. Since parental genotypes were not available, subjects' haplotypes were inferred probabilistically and are thus subject to computational bias. Hence, we restricted our analyses to those haplotypes for which confidence levels were >95%.
The STRUCTURE analyses did not identify subpopulations within the sample, which we interpreted as evidence of absence of potential confounding by population substructure. Empirical evidence suggests that genetic stratification is far less common in populations of European ancestry, relative to African American or Hispanic populations. Although recent reports indicate that genetic stratification may not be entirely absent in populations of primarily European ancestry (Douglas et al., 2004; Lesperance et al., 2004), so far only one phenotype, height, has been shown to covary with ancestry within continental Europe (Campbell et al., 2005) and it is as yet unclear to what extent differences in genetic ancestry may influence candidate gene association studies in European Americans.
Limiting our analysis to individuals of European-American descent, however, also limits the generalizability of our findings to non-European American populations. Given evidence that circulating levels of CRP are higher in African Americans than European Americans (Carlson et al., 2005; Lange et al., 2006), further research is warranted, using African American samples implementing appropriate controls for genetic admixture using ancestry informative markers (e.g. Halder et al., 2003, 2008)
Despite these limitations, our findings provide initial evidence that CRP-related genetic variation moderates an association of depressive symptomatology with circulating levels of CRP, a marker of inflammation thought to play a role in the pathogenesis of cardiovascular and other inflammatory diseases. In the future, longitudinal investigations will be needed to determine whether depression and the A-G-T CRP haplotype interact similarly in the chronic regulation of CRP levels and to better elucidate how depression may shape the physical health of individuals.
Acknowledgments
Preparation of the article was supported by NIH grants P01 HL040962 and R01 HL065137 (SBM) and R01 HL065137 (ALM) and HL076852/076858 (IH). Expert Technical assistance of Janet Lower is gratefully acknowledged. We thank Dr. Bernie Devlin and Shawn Wood for help with EHAP analyses.
Footnotes
Conflict of Interest Statement: All authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beekman AT, Deeg DJ, Van Limbeek J, Braam AW, De Vries MZ, Van Tilburg W. Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): results from a community-based sample of older subjects in The Netherlands. Psychol Med. 1997;27:231–235. doi: 10.1017/s0033291796003510. [DOI] [PubMed] [Google Scholar]
- Bremmer MA, Beekman AT, Deeg DJ, Penninx BW, Dik MG, Hack CE, Hoogendijk WJ. Inflammatory markers in late-life depression: results from a population-based study. J Affect Disord. 2008;106:249–255. doi: 10.1016/j.jad.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Campbell CD, Ogburn EL, Lunetta KL, Lyon HN, Freedman ML, Groop LC, Altshuler D, Ardlie KG, Hirschhorn JN. Demonstrating stratification in a European American population. Nat Genet. 2005;37:868–872. doi: 10.1038/ng1607. [DOI] [PubMed] [Google Scholar]
- Carlson CS, Aldred SF, Lee PK, Tracy RP, Schwartz SM, Rieder M, Liu K, Williams OD, Iribarren C, Lewis EC, Fornage M, Boerwinkle E, Gross M, Jaquish C, Nickerson DA, Myers RM, Siscovick DS, Reiner AP. Polymorphisms within the C-reactive protein (CRP) promoter region are associated with plasma CRP levels. Am J Hum Genet. 2005;77:64–77. doi: 10.1086/431366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Covault J, Tennen H, Armeli S, Conner TS, Herman AI, Cillessen AH, Kranzler HR. Interactive effects of the serotonin transporter 5-HTTLPR polymorphism and stressful life events on college student drinking and drug use. Biol Psychiatry. 2007;61:609–616. doi: 10.1016/j.biopsych.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Crawford DC, Sanders CL, Qin X, Smith JD, Shephard C, Wong M, Witrak L, Rieder MJ, Nickerson DA. Genetic variation is associated with C-reactive protein levels in the Third National Health and Nutrition Examination Survey. Circulation. 2006;114:2458–2465. doi: 10.1161/CIRCULATIONAHA.106.615740. [DOI] [PubMed] [Google Scholar]
- Danner M, Kasl SV, Abramson JL, Vaccarino V. Association between depression and elevated C-reactive protein. Psychosom Med. 2003;65:347–356. doi: 10.1097/01.psy.0000041542.29808.01. [DOI] [PubMed] [Google Scholar]
- de Bakker PI, Yelensky R, Pe'er I, Gabriel SB, Daly MJ, Altshuler D. Efficiency and power in genetic association studies. Nat Genet. 2005;37:1217–1223. doi: 10.1038/ng1669. [DOI] [PubMed] [Google Scholar]
- de Wit LM, van Straten A, van Herten M, Penninx BW, Cuijpers P. Depression and body mass index, a u-shaped association. BMC Public Health. 2009;9 doi: 10.1186/1471-2458-9-14. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding K, Zhou K, He F, Shen Y. LDA--a java-based linkage disequilibrium analyzer. Bioinformatics. 2003;19:2147–2148. doi: 10.1093/bioinformatics/btg276. [DOI] [PubMed] [Google Scholar]
- Douglas KM, Taylor AJ, O'Malley PG. Relationship between depression and C-reactive protein in a screening population. Psychosom Med. 2004;66:679–683. doi: 10.1097/01.psy.0000138132.66332.85. [DOI] [PubMed] [Google Scholar]
- Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164:1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford DE, Erlinger TP. Depression and C-reactive protein in US adults: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2004;164:1010–1014. doi: 10.1001/archinte.164.9.1010. [DOI] [PubMed] [Google Scholar]
- Ford ES, Galuska DA, Gillespie C, Will JC, Giles WH, Dietz WH. C-reactive protein and body mass index in children: findings from the Third National Health and Nutrition Examination Survey, 1988-1994. J Pediatr. 2001;138(4):486–92. doi: 10.1067/mpd.2001.112898. [DOI] [PubMed] [Google Scholar]
- Freedman LS, Schatzkin A. Sample size for studying intermediate endpoints within intervention trails or observational studies. Am J Epid. 1992;136(9):1148–59. doi: 10.1093/oxfordjournals.aje.a116581. [DOI] [PubMed] [Google Scholar]
- Goodman E, Whitaker RC. A prospective study of the role of depression in the development and persistence of adolescent obesity. Pediatrics. 2002;110(3):497–504. doi: 10.1542/peds.110.3.497. [DOI] [PubMed] [Google Scholar]
- Halder I, Shriver MD. Measuring and using admixture to study the genetics of complex diseases. Hum Genomics. 2003;1:52–62. doi: 10.1186/1479-7364-1-1-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder I, Muldoon MF, Ferrell RE, Manuck SB. Serotonin Receptor 2A (HTR2A) Gene Polymorphisms Are Associated with Blood Pressure, Central Adiposity, and the Metabolic Syndrome. Metab Syndr Relat Disord. 2007;5:323–330. doi: 10.1089/met.2007.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder I, Shriver M, Thomas M, Fernandez JR, Frudakis T. A panel of ancestry informative markers for estimating individual biogeographical ancestry and admixture from four continents: utility and applications. Hum Mutat. 2008;29:648–658. doi: 10.1002/humu.20695. [DOI] [PubMed] [Google Scholar]
- Hemingway H, Shipley M, Mullen MJ, Kumari M, Brunner E, Taylor M, Donald AE, Deanfield JE, Marmot M. Social and psychosocial influences on inflammatory markers and vascular function in civil servants (the Whitehall II study) Am J Cardiol. 2003;92:984–987. doi: 10.1016/s0002-9149(03)00985-8. [DOI] [PubMed] [Google Scholar]
- Herva A, Laitinen J, Miettunen J, Veijola J, Karvonen JT, Laksy K, Joukamaa M. Obesity and depression: results from the longitudinal Northern Finland 1966 Birth Cohort Study. Int J Obes (Lond) 2006;30(3):520–7. doi: 10.1038/sj.ijo.0803174. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71(2):171–86. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Janszky I, Lekander M, Blom M, Georgiades A, Ahnve S. Self-rated health and vital exhaustion, but not depression, is related to inflammation in women with coronary heart disease. Brain Behav Immun. 2005;19(6):555–63. doi: 10.1016/j.bbi.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Kathiresan S, Larson MG, Vasan RS, Guo CY, Gona P, Keaney JF, Jr, Wilson PW, Newton-Cheh C, Musone SL, Camargo AL, Drake JA, Levy D, O'Donnell CJ, Hirschhorn JN, Benjamin EJ. Contribution of clinical correlates and 13 C-reactive protein gene polymorphisms to interindividual variability in serum C-reactive protein level. Circulation. 2006;113:1415–1423. doi: 10.1161/CIRCULATIONAHA.105.591271. [DOI] [PubMed] [Google Scholar]
- Kop WJ, Gottdiener JS, Tangen CM, Fried LP, McBurnie MA, Walston J, Newman A, Hirsch C, Tracy RP. Inflammation and coagulation factors in persons > 65 years of age with symptoms of depression but without evidence of myocardial ischemia. Am J Cardiol. 2002;89:419–424. doi: 10.1016/s0002-9149(01)02264-0. [DOI] [PubMed] [Google Scholar]
- Ladwig KH, Marten-Mittag B, Lowel H, Doring A, Koenig W. Influence of depressive mood on the association of CRP and obesity in 3205 middle aged healthy men. Brain Behav Immun. 2003;17:268–275. doi: 10.1016/s0889-1591(03)00056-4. [DOI] [PubMed] [Google Scholar]
- Lange LA, Carlson CS, Hindorff LA, Lange EM, Walston J, Durda JP, Cushman M, Bis JC, Zeng D, Lin D, Kuller LH, Nickerson DA, Psaty BM, Tracy RP, Reiner AP. Association of polymorphisms in the CRP gene with circulating C-reactive protein levels and cardiovascular events. JAMA. 2006;296:2703–2711. doi: 10.1001/jama.296.22.2703. [DOI] [PubMed] [Google Scholar]
- Lesperance F, Frasure-Smith N, Theroux P, Irwin M. The association between major depression and levels of soluble intercellular adhesion molecule 1, interleukin-6, and C-reactive protein in patients with recent acute coronary syndromes. Am J Psychiatry. 2004;161:271–277. doi: 10.1176/appi.ajp.161.2.271. [DOI] [PubMed] [Google Scholar]
- Liukkonen T, Silvennoinen-Kassinen S, Jokelainen J, Rasanen P, Leinonen M, Meyer-Rochow VB, Timonen M. The association between C-reactive protein levels and depression: Results from the northern Finland 1966 birth cohort study. Biol Psychiatry. 2006;60:825–830. doi: 10.1016/j.biopsych.2006.02.016. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Carter CS, Flory JD, Ferrell RE, Manuck SB. COMT val158Met and executive control: a test of the benefit of specific deficits to translational research. J Abnorm Psychol. 2007;116:306–312. doi: 10.1037/0021-843X.116.2.306. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, Ferrell RE, Muldoon MF. Socio-economic status covaries with central nervous system serotonergic responsivity as a function of allelic variation in the serotonin transporter gene-linked polymorphic region. Psychoneuroendocrinology. 2004;29:651–668. doi: 10.1016/S0306-4530(03)00094-5. [DOI] [PubMed] [Google Scholar]
- Miller DT, Zee RY, Suk Danik J, Kozlowski P, Chasman DI, Lazarus R, Cook NR, Ridker PM, Kwiatkowski DJ. Association of common CRP gene variants with CRP levels and cardiovascular events. Ann Hum Genet. 2005;69:623–638. doi: 10.1111/j.1529-8817.2005.00210.x. [DOI] [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann SA, Brown SM, Ferrell RE, Flory JD, Manuck SB, Hariri AR. Human choline transporter gene variation is associated with corticolimbic reactivity and autonomic-cholinergic function. Biol Psychiatry. 2006;60:1155–1162. doi: 10.1016/j.biopsych.2006.03.059. [DOI] [PubMed] [Google Scholar]
- Panagiotakos DB, Pitsavos C, Yannakoulia M, Chrysohoou C, Stefanadis C. The implication of obesity and central fat on markers of chronic inflammation: The ATTICA study. Atherosclerosis. 2005;183(2):308–15. doi: 10.1016/j.atherosclerosis.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Pankow JS, Folsom AR, Cushman M, Borecki IB, Hopkins PN, Eckfeldt JH, Tracy RP. Familial and genetic determinants of systemic markers of inflammation: the NHLBI family heart study. Atherosclerosis. 2001;154:681–689. doi: 10.1016/s0021-9150(00)00586-4. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Kritchevsky SB, Yaffe K, Newman AB, Simonsick EM, Rubin S, Ferrucci L, Harris T, Pahor M. Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study. Biol Psychiatry. 2003;54:566–572. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. App Psych Meas. 1977;1:385–401. [Google Scholar]
- Ridker P, Rifai N, Koenig W, Blumenthal RS. C-reactive protein and cardiovascular risk in the Framingham Study. Arch Intern Med. 2006;166:1327–1328. doi: 10.1001/archinte.166.12.1327-b. author reply 1328. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cook NR. Biomarkers for prediction of cardiovascular events. N Engl J Med. 2007;356:1472–1473. [PubMed] [Google Scholar]
- Rousset F, Raymond M. Testing heterozygote excess and deficiency. Genetics. 1995;140:1413–1419. doi: 10.1093/genetics/140.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AI, Cunninghame Graham DS, Shepherd C, Roberton CA, Whittaker J, Meeks J, Powell RJ, Isenberg DA, Walport MJ, Vyse TJ. Polymorphism at the C-reactive protein locus influences gene expression and predisposes to systemic lupus erythematosus. Hum Mol Genet. 2004;13:137–147. doi: 10.1093/hmg/ddh021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatine MS, Morrow DA, Jablonski KA, Rice MM, Warnica JW, Domanski MJ, Hsia J, Gersh BJ, Rifai N, Ridker PM, Pfeffer MA, Braunwald E. Prognostic significance of the Centers for Disease Control/American Heart Association high-sensitivity C-reactive protein cut points for cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation. 2007;115:1528–1536. doi: 10.1161/CIRCULATIONAHA.106.649939. [DOI] [PubMed] [Google Scholar]
- Schins A, Tulner D, Lousberg R, Kenis G, Delanghe J, Crijns HJ, Grauls G, Stassen F, Maes M, Honig A. Inflammatory markers in depressed post-myocardial infarction patients. J Psychiatr Res. 2005;39:137–144. doi: 10.1016/j.jpsychires.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Seltman H, Roeder K, Devlin B. TDT meets MHA: Family-based association analysis guided by the evolution of haplotypes. Am J Hum Genet. 2001;68:1250–1263. doi: 10.1086/320110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltman H, Roeder K, Devlin B. Evolutionary-based association analysis using haplotype data. Genet Epidemiol. 2003;25:48–58. doi: 10.1002/gepi.10246. [DOI] [PubMed] [Google Scholar]
- Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, Kessler RC. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63(7):824–30. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalai AJ, Wu J, Lange EM, McCrory MA, Langefeld CD, Williams A, Zakharkin SO, George V, Allison DB, Cooper GS, Xie F, Fan Z, Edberg JC, Kimberly RP. Single-nucleotide polymorphisms in the C-reactive protein (CRP) gene promoter that affect transcription factor binding, alter transcriptional activity, and associate with differences in baseline serum CRP level. J Mol Med. 2005;83:440–447. doi: 10.1007/s00109-005-0658-0. [DOI] [PubMed] [Google Scholar]
- Su S, Miller AH, Snieder H, Bremner JD, Ritchie J, Maisano C, Jones L, Murrah NV, Goldberg J, Vaccarino V. Common genetic contributions to depressive symptoms and inflammatory markers in middle-aged men: the Twins Heart Study. Psychosom Med. 2009;71:152–158. doi: 10.1097/PSY.0b013e31819082ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Johnson BD, Sheps DS, Reis SE, Kelsey SF, Bittner V, Rutledge T, Shaw LJ, Sopko G, Bairey Merz CN. Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia: the National Heart, Lung, and Blood Institute-sponsored WISE study. J Am Coll Cardiol. 2007;50:2044–2050. doi: 10.1016/j.jacc.2007.07.069. [DOI] [PubMed] [Google Scholar]
- Vickers MA, Green FR, Terry C, Mayosi BM, Julier C, Lathrop M, Ratcliffe PJ, Watkins HC, Keavney B. Genotype at a promoter polymorphism of the interleukin-6 gene is associated with baseline levels of plasma C-reactive protein. Cardiovasc Res. 2002;53:1029–1034. doi: 10.1016/s0008-6363(01)00534-x. [DOI] [PubMed] [Google Scholar]
- Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282(22):2131–5. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- Wang Q, Hunt SC, Xu Q, Chen YE, Province MA, Eckfeldt JH, Pankow JS, Song Q. Association study of CRP gene polymorphisms with serum CRP level and cardiovascular risk in the NHLBI Family Heart Study. Am J Physiol Heart Circ Physiol. 2006;291:H2752–2757. doi: 10.1152/ajpheart.01164.2005. [DOI] [PubMed] [Google Scholar]
- Williams MJ, Williams SM, Milne BJ, Hancox RJ, Poulton R. Association between C-reactive protein, metabolic cardiovascular risk factors, obesity and oral contraceptive use in young adults. Int J Obes Relat Metab Disord. 2004;28(8):998–1003. doi: 10.1038/sj.ijo.0802713. [DOI] [PubMed] [Google Scholar]
- Whooley MA, Caska CM, Hendrickson BE, Rourke MA, Ho J, Ali S. Depression and inflammation in patients with coronary heart disease: findings from the Heart and Soul Study. Biol Psychiatry. 2007;62:314–320. doi: 10.1016/j.biopsych.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Ford ES, Dhingra S, Li C, Strine TW, Mokdad AH. Depression and anxiety among US adults: associations with body mass index. Int J Obes (Lond) 2009 doi: 10.1038/ijo.2008.268. [Epub ahead of print]. PMID: 19125163. [DOI] [PubMed] [Google Scholar]