Abstract
The prevalence of type 2 diabetes is higher among African Americans (AA) vs European Americans (EA), is highest at middle age, and is related to obesity. This study was conducted to test the hypothesis that the association of adiposity (percent body fat; %fat) with indices of insulin sensitivity and β-cell function would differ with ethnicity and age. Subjects were 168 healthy, normoglycemic AA and EA girls and women aged 7–12 yr, 18–32 yr, and 40–70 yr. An intravenous glucose tolerance test was used to assess indices of insulin secretion and action: Insulin sensitivity (SI), acute C-peptide secretion (X0); basal, first-phase, second-phase, and total β-cell responsivity to glucose (PhiB, Phi1, Phi2, and PhiTOT, respectively); and the disposition index (DI = SI × PhiTOT). %fat was assessed with dual-energy X-ray absorptiometry. Adiposity was significantly associated with insulin sensitivity among EA (−0.57 P<0.001) but not AA (−0.20, P=0.09). Adiposity appeared stimulatory to β-cell function in the two groups of younger subjects and in EA, but inhibitory in postmenopausal women, particularly AA postmenopausal women. Among AA postmenopausal women, %fat was inversely associated with Phi1 (r = −0.57, P<0.05) and PhiTOT (r = −0.68, P<0.01). These results suggest that the impact of adiposity on insulin secretion and action differs with age and ethnicity.
Introduction
Type 2 diabetes, a disease that results from declines in insulin sensitivity and secretion, is closely associated with obesity (1), and generally occurs at middle age (2). Nonetheless, the potential interplay between adiposity and age on insulin sensitivity and secretion has not been widely examined. The strong association between obesity and type 2 diabetes suggests that adipose tissue has an adverse effect on β-cell function. However, most research to date has documented a positive association between body fat and insulin secretion. This positive association likely occurs secondary to an obesity-mediated reduction in insulin sensitivity, and a compensatory increase in circulating insulin (3–5). This compensatory increase in insulin secretion may be due to an increase in exposure of the β-cell to free fatty acids (FFA), the flux of which increases as adipose tissue insulin sensitivity declines (6;7). However in order for type 2 diabetes to develop, insulin secretion ultimately must become inadequate to compensate for the degree of insulin resistance (8), suggesting that with prolonged obesity the association between adiposity and β-cell function may shift from positive to inverse among individuals susceptible to type 2 diabetes. Such associations have not been examined, particularly among populations at high risk for type 2 diabetes such as African Americans (AA) (9).
The prevalence of type 2 diabetes is higher among AA than European Americans (EA) (9) for reasons that are not clear. Insulin sensitivity is lower among AA vs EA independent of obesity (10–13), and likely plays a role in the greater propensity for type 2 diabetes among AA. Although obesity is more prevalent among AA, particularly women (14), greater obesity does not explain greater risk for type 2 diabetes among AA (15). Potential ethnic differences in the effect of adiposity on aspects of insulin secretion or action have not been examined.
Risk for type 2 diabetes increases up until mid-life, with 64% of adult incident cases occurring between ages 40 and 64 yr (2). Although part of this increased risk may be attributed to adiposity, it is likely that the physiological changes that occur with aging per se contribute to a decline in β-cell function (16). Among women, the decline in estrogen at menopause also may result in decreased insulin secretion (17). The potential interactive effects of age and adiposity on β-cell function have not been carefully sorted out. Further, the possibility that β-cell function in AA may be uniquely sensitive to adverse effects of age and adiposity has not been examined.
This study was conducted to identify associations between adiposity and β-cell function, and to determine the extent to which these associations were independent of insulin sensitivity. We tested the specific hypothesis that the association of adiposity (percent body fat; %fat) with indices of insulin sensitivity and β-cell function differs with ethnicity and age. Further, we hypothesized that adiposity would have a more detrimental effect on β-cell function among postmenopausal AA women than among postmenopausal EA women. This study is unique in examining the association of adiposity with insulin sensitivity and β-cell function using robust measures of body composition, insulin sensitivity, and β-cell function. Further, this study is the first to report on the influence of ethnicity and age on these relationships.
Methods and Procedures
Participants
Participants were AA and EA girls and women, recruited as three age groups: 7–12 years (prepubertal), 18–32 years (premenopausal), and 40–70 years (postmenopausal). Exclusion criteria were type 1 or type 2 diabetes, polycystic ovary disease, disorders of glucose or lipid metabolism, use of medication that could affect body composition or glucose metabolism (including anti-hypertensive medication, oral contraceptives, and postmenopausal hormone replacement therapy), use of tobacco, alcohol consumption in excess of 400 grams per week, history of hypoglycemic episodes, and a medical history that counter-indicated inclusion in the study. The minimum weight for children was 20 kg to minimize risks associated with blood sampling. All subjects had normal glucose tolerance (18). Children were evaluated for pubertal status using the criteria of Marshall and Tanner (19). Women were queried regarding their menstrual cycles, and were classified as postmenopausal if they had not had a cycle in the past 12 months. Serum FSH was used to verify postmenopausal status (FSH>35 IU/ml). Because many postmenopausal AA women used anti-hypertensive medication, this group was difficult to recruit, and ultimately had a lower sample size. Participants were informed of the experimental design, and written consent was obtained. The study was approved by the Institutional Review Board for Human Use at the University of Alabama at Birmingham (UAB).
Protocol
All testing was done on an in-patient basis at UAB’s General Clinical Research Center (GCRC). Adult participants were asked to consume at least 250 grams carbohydrates for 3 days prior to admission, and were provided with a list of common foods and their carbohydrate content. Child participants were asked to consume an amount of carbohydrate that was proportional to their smaller body size and energy requirements. Subjects came to the GCRC the evening prior to testing. While at the GCRC, participants were given a standard meal consisting of 50% energy from carbohydrate, 30% energy from fat, and 20% energy from protein. No food was consumed for 12 hours prior to intravenous glucose tolerance testing, which was performed at 7:00 a.m. the following morning. After completion of the glucose tolerance test, subjects were given a late breakfast/lunch. All children were accompanied by one parent during testing.
Intravenous glucose tolerance test (IVGTT)
Insulin sensitivity and β-cell dynamics were determined during an intravenous glucose tolerance test (IVGTT). Flexible catheters were placed in the antecubital spaces of both arms. Three blood samples were taken over a 15 min period to determine basal glucose and insulin (the average of the values was used for basal concentrations). At time zero, glucose (50% dextrose, 300 mg/kg) was given intravenously. Insulin (0.02 Units/kg) was infused over a 5-min period from 20–25 min post glucose injection. For adults, blood samples (2.0 ml) were collected at the following times (min) relative to glucose administration: 2, 3, 4, 5, 6, 8, 10, 12, 15, 19, 20, 21, 22, 24, 26, 28, 30, 35, 40, 45, 50, 55, 60, 70, 80, 100, 120, 140, 180, 210, 240, 300. For children, a reduced sampling protocol was used, where blood was drawn at baseline (two samples) and at 2, 3, 4, 5, 6, 8, 10, 12, 15, 19, 20, 21, 22, 24, 26, 28, 30, 35, 40, 50, 60, 70, 180, and 240 min post glucose injection. Serum was stored at −85°C until analysis.
Laboratory analyses
Concentrations of glucose, insulin, and C-peptide were analyzed in the Metabolism Core Laboratory of the GCRC and Clinical Nutrition Research Center (CNRC). Glucose was measured in 10 μl of sera using an Ektachem DT II System (Johnson and Johnson Clinical Diagnostics). This analysis had a mean intra-assay coefficient of variation (CV) of 0.61%, and a mean inter-assay CV of 1.45%. Insulin was assayed in duplicate 100 μl aliquots with reagents from Linco Research Inc. (St. Charles, MO); assay sensitivity was 3.35 μIU/ml; mean intra-assay CV was 3.49%; and mean interassay CV was 5.57%. C-peptide was assayed in duplicate 25 ul aliquots with double-antibody radioimmunoassay reagents (Diagnostic Products Corporation, Los Angeles, CA); assay sensitivity was 0.318 ng/mL; mean intraassay CV was 3.57%; and mean interassay CV was 5.59%.
Estimates of insulin sensitivity and β-cell responsivity to glucose
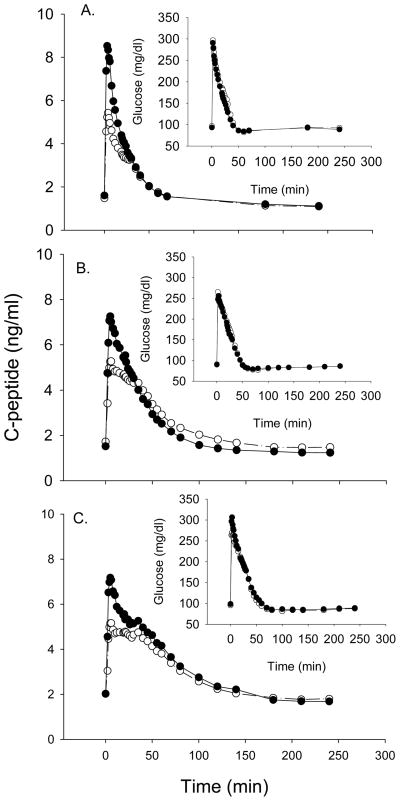
Insulin sensitivity (SI) was derived from glucose and insulin values using minimal modeling (20). Assessment of β-cell function requires measurement of C-peptide to isolate insulin secretion from insulin clearance (21–23). C-peptide and glucose data obtained in conjunction with the intravenous glucose tolerance test are shown in Fig. 1. Mathematical modeling of these data was used to derive indices of β-cell function specific to basal, first-, and second-phase insulin secretion (22). The major outcome variables of interest were the amount of C-peptide released immediately following glucose administration (X0); and basal (PhiB), first-phase (Phi1), second-phase (Phi2), and total (PhiTOT) β-cell responsivity to glucose. Because β-cell function needs to be interpreted in light of the prevailing insulin sensitivity, we also used the Disposition Index (DI = SI × PhiTOT). If an individual’s β-cells respond to a decrease in insulin sensitivity by adequately increasing insulin secretion, the product of β-cell function and insulin sensitivity (the disposition index) is unchanged, and normal glucose tolerance is retained. In contrast, if there is not an adequate compensatory increase in β-cell function to the decreased insulin sensitivity, the individual develops glucose intolerance. Because among glucose-tolerant individuals β-cell function is inversely related to insulin sensitivity, DI is almost constant within a healthy homogeneous population (24;25).
Fig. 1.
C-peptide and glucose (inset) during the IVGTT by age group in AA (●) and EA (○). A. Prepubertal girls; B. Premenopausal women; C. Postmenopausal women.
Body composition and fat distribution
Body composition (%fat) was determined by dual-energy X-ray absorptiometry (Lunar Prodigy; (GE Healthcare Lunar, Madison, WI) in the Department of Nutrition Sciences at UAB. Subjects were scanned in light clothing while lying flat on their backs with arms at their sides. Intra-abdominal adipose tissue (IAAT) was analyzed by computed tomography scanning (26;27) with a HiLight/Advantage Scanner (General Electric, Milwaukee) located in the UAB Department of Radiology. Subjects were scanned in the supine position with arms stretched above their heads. A 5mm scan at the level of the umbilicus (approximately the L4–L5 intervertebral space) was taken. Scans were analyzed for cross-sectional area (cm2) of adipose tissue using the density contour program with Hounsfield units for adipose tissue set at −190 to −30. All scans were analyzed by the same individual. The CV for repeat cross-section analysis of scans among 40 subjects in our laboratory is less than 2% (27).
Statistical Analysis
Subject characteristics were examined using two-way analysis of variance for effects of ethnicity and age group. Body composition, and outcomes of interest from the IVGTT (SI, X0, PhiB, Phi1, Phi2, PhiTOT, DI), were examined. SI was further examined using analysis of covariance to determine the influence of ethnicity after adjusting for age group, %fat, and IAAT.
Preliminary analyses indicated that in general all outcome measures of interest were associated more strongly with %fat than with any particular adipose tissue depot. Thus, all subsequent analyses were conducted using %fat as the independent variable.
Pearson simple and partial correlation analyses were used to explore potential differences based on ethnicity or age group on the relationship between adiposity and indices of insulin secretion-action (SI, X0, PhiB, Phi1, Phi2, PhiTOT, DI), adjusting for either age group or ethnicity as indicated. Further adjustment for SI was included to identify independent associations of adiposity with indices of β-cell function. Where potential differences were detected between ethnic or age groups on the association between %fat and outcomes of interest, analysis of covariance (ANCOVA; adjusting for age or ethnic group, as appropriate) was used to examine the significance of the (ethnic/age group × %fat) term. The POWCOR Program (28) was used to compare the strength of the associations between ethnic groups and among age groups. Within postmenopausal women, simple correlation analysis was used to examine the association of %fat with outcomes of interest by ethnic group.
Additional correlation analyses were conducted to examine associations among abdominal adipose depots (IAAT, SAAT) and outcomes of interest within each ethnicity-age subgroup. Analyses were conducted both unadjusted, and after adjusting for SI.
Values for SI, X0, PhiB, Phi1, Phi2, PhiTOT, and DI were log10 transformed prior to statistical analysis to ensure a normal distribution. All statistical tests were two-sided and were performed using a Type I error rate of 0.05. All statistical analyses were performed using SAS (version 9.2; SAS Institute, Inc., Cary, NC).
Results
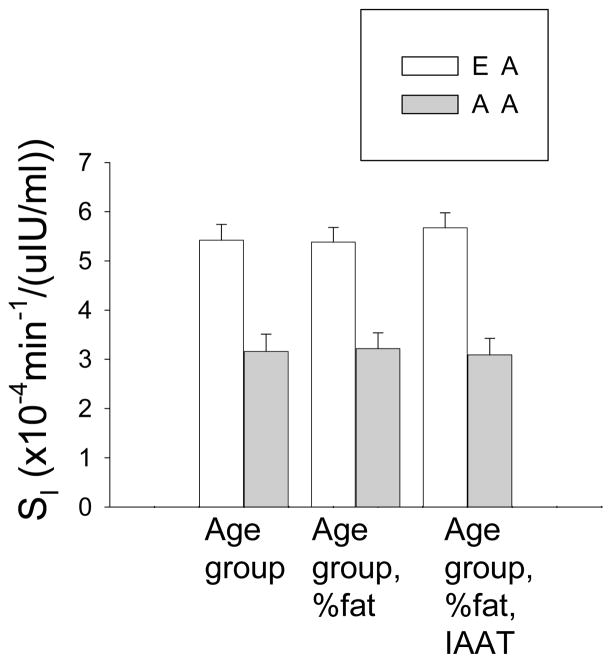
Two-way ANOVA indicated significant main effects of ethnicity for SI, X0, Phi1, and PhiTOT, and of age group for %fat, PhiB and Phi2 (Table 1). AA had lower SI and Phi2, and higher X0 and Phi1. Women had greater %fat, PhiB, and Phi2 than children. Lower SI in AA remained after adjusting for %fat and IAAT (Fig. 2).
Table 1.
Subject characteristics and outcome measures by ethnicity and age group (mean ±SD)a.
| Pre-pubertal | Pre-menopausal | Post-menopausal | |
|---|---|---|---|
| EA | n=29 | n=32 | n=31 |
| Age (yr) | 10.3 ±1.5 | 25.9 ±3.4 | 55.7 ±4.2 |
| %fat | 26.0 ±7.7 | 36.5 ±8.5 | 38.8 ±8.7 |
| IAAT (cm2) | 32.8 ±13.0 | 68.4 ±44.8 | 117.9 ±58.8 |
| SAAT (cm2) | 91.7 ±49.4 | 286.1 ±167.4 | 263.8 ±159.8 |
| SI [x10−4 min−1/(μIU/ml)] | 5.71 ±2.11 | 4.91 ±2.67 | 5.79 ±5.30 |
| X0 (pmol/L) | 1938 ±917 | 2185 ±1158 | 2057 ±941 |
| Phi Basal (109/min) | 4.7 ±1.5 | 6.4 ±3.2 | 6.5 ±3.1 |
| Phi 1 (109) | 174 ±82 | 213 ±111 | 203 ±134 |
| Phi 2 (109/min) | 7.6 ±3.0 | 11.5 ±4.5 | 11.0 ±4.6 |
| PhiTOT (109/min) | 22.9 ±12.4 | 24.7 ±12.4 | 24.1 ±14.1 |
| DI [10−13 min−2/(μIU/ml)] | 120 ±52 | 118 ±82 | 161 ±245 |
| AA | n=33 | n=25 | n=18 |
| Age (yr) | 9.8 ±1.6 | 25.0 ±3.3 | 55.5 ±6.3 |
| %fat | 24.4 ±9.9 | 36.0 ±10.9 | 44.6 ±7.3 |
| IAAT (cm2) | 27.7 ±17.9 | 41.6 ±22.9 | 122.4 ±40.8 |
| SAAT (cm2) | 88.7 ±75.1 | 252.3 ±179.5 | 417.0 ±172.6 |
| SI [x10−4 min−1/(μIU/ml)] | 3.07 ±1.52 | 3.54 ±3.58 | 2.81 ±2.02 |
| X0 (pmol/L) | 3419 ±1438 | 3500 ±1649 | 3017 ±888 |
| Phi Basal (109/min) | 5.3 ±3.3 | 5.3 ±1.7 | 7.1 ±2.7 |
| Phi 1 (109) | 312 ±130 | 345 ±202 | 241 ±103 |
| Phi 2 (109/min) | 5.7 ±3.7 | 10.0 ±5.4 | 11.4 ±5.4 |
| PhiTOT (109/min) | 51.3 ±56.3 | 40.5 ±29.6 | 28.1 ±13.8 |
| DI [10−13 min−2/(μIU/ml)] | 152 ±181 | 137 ±141 | 80 ±69 |
Significant main effects (P<0.05) were observed for ethnicity (SI, X0, Phi1, and PhiTOT) and age group (%fat, PhiB and Phi2).
Fig. 2.
Insulin sensitivity by ethnicity. Data adjusted for age group (left), age group and %fat (center), and age group, %fat, and IAAT (right). SI was lower among AA vs EA (P<0.001 for all).
Results from Pearson partial correlation analysis revealed that the associations of %fat with SI and indices of β-cell function differed with ethnicity and age group. %fat was inversely associated with SI among EA but not AA, and positively associated with PhiB and Phi2 among EA but not AA (Table 2). These relationships were attenuated, but not eliminated, by further adjustment for SI. POWCOR analysis indicated that the r values for SI (P<0.01) and Phi2 (P<0.05) differed with ethnicity, such that %fat was more strongly associated with both SI and Phi2 among EA. ANCOVA indicated that the (ethnicity × %fat) term was significant for SI (P<0.001) and PhiB (P<0.05).
Table 2. Percent body fat vs. SI and β-cell measures.
Values are partial correlation coefficients (r) adjusted for ethnicity and age group (all combined) or age group (EA, AA)a.
| All | All Adjusted for SI | EA | EA Adjusted for SI | AA | AA Adjusted for SI | |
|---|---|---|---|---|---|---|
| SI | −0.40b | --- | −0.57b | --- | −0.20 | --- |
| X0 | 0.14 | 0.03 | 0.18 | 0.05 | 0.11 | 0.05 |
| PhiB | 0.31 b | 0.18d | 0.43 b | 0.23d | 0.20 | 0.15 |
| Phi1 | −0.12 | −0.17d | −0.06 | −0.11 | −0.16 | −0.21 |
| Phi2 | 0.36 b | 0.23c | 0.48 b | 0.26d | 0.20 | 0.17 |
| PhiTOT | −0.04 | −0.06 | 0.08 | 0.07 | −0.12 | −0.12 |
| DI | −0.39 b | −0.18d | −0.44 b | −0.03 | −0.34 c | −0.28d |
r values differed with ethnicity for SI (P<0.01) and Phi2 (P<0.05). P from ANCOVA for (ethnicity × %fat): SI (P<0.001), PhiB (P<0.05).
P<0.001;
P<0.01;
P<0.05
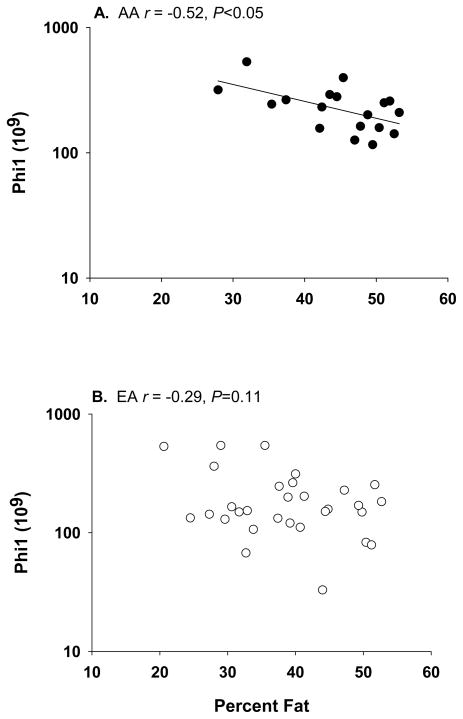
Looking across age groups, %fat was positively associated with X0 among children, an association that was attenuated by adjustment for SI (Table 3). %fat was inversely associated with DI among adults. %fat was inversely associated with Phi1 among postmenopausal women; this relationship remained unaltered by adjustment for SI. POWCOR analysis indicated differences among the strength of the r values for X0 (prepubertal vs postmenopausal, P<0.05), Phi1 (prepubertal vs postmenopausal, P<0.05), Phi2 (prepubertal vs premenopausal, P<0.01), PhiTOT (prepubertal vs postmenopausal, P<0.05), and DI (prepubertal vs adults, P<0.05). ANCOVA indicated that the (age group × %fat) interaction term was significant or borderline significant for X0 (P=0.0559), Phi1 (P<0.01), and DI (P<0.001). Pearson simple correlation analysis within postmenopausal women by ethnic group indicated that adiposity was adversely related to SI among EA, but to Phi1 and PhiTOT among AA (Table 4, Fig. 3). The associations within AA, but not EA, remained significant after adjustment for SI.
Table 3. Percent body fat vs. SI and β-cell measures.
Values are partial correlation coefficients (r) adjusted for ethnicitya.
| Pre-pubertal | Prepub. adjusted for SI | Premeno-pausal | Premeno. adjusted for SI | Postmeno-pausal | Postmeno. adjusted for SI | |
|---|---|---|---|---|---|---|
| SI | −0.40b | --- | −0.32c | --- | −0.55d | --- |
| X0 | 0.33 c | 0.21 | 0.15 | 0.04 | −0.09 | −0.25 |
| PhiB | 0.18 | 0.07 | 0.42 b | 0.34c | 0.35 c | 0.13 |
| Phi1 | 0.02 | −0.13 | −0.07 | −0.15 | −0.36 c | −0.36c |
| Phi2 | 0.05 | −0.12 | 0.51 d | 0.46d | 0.33 c | 0.03 |
| PhiTOT | 0.12 | 0.06 | −0.07 | −0.11 | −0.27 | −0.24 |
| DI | −0.15 | 0.02 | −0.49 d | −0.39b | −0.56 d | −0.23 |
r values differed with age group for X0 (prepubertal vs postmenopausal, P<0.05), Phi1
(prepubertal vs postmenopausal, P<0.05), Phi2 (prepubertal vs premenopausal, P<0.01), PhiTOT (prepubertal vs postmenopausal, P<0.05), and DI (prepubertal vs both adult groups, P<0.05). P from ANCOVA for (age group × %fat): X0 (P=0.0559), Phi1 (P<0.01), DI (P<0.001).
P<0.05;
P<0.01;
P<0.001
Table 4. Percent body fat vs. SI and β-cell measures within postmenopausal women.
Values are simple Pearson correlation coefficients (r), and coefficients adjusted by SI, shown by ethnic group.
| EA | EA Adjusted for SI | AA | AA Adjusted for SI | |
|---|---|---|---|---|
| SI | −0.64a | --- | −0.24 | --- |
| X0 | −0.01 | −0.16 | −0.37 | −0.49 |
| PhiB | 0.43b | 0.22 | 0.21 | 0.06 |
| Phi1 | −0.29 | −0.27 | −0.57 b | −0.57b |
| Phi2 | 0.55c | 0.24 | −0.06 | −0.28 |
| PhiTOT | −0.14 | −0.01 | −0.68 c | −0.68c |
| DI | −0.56c | −0.02 | −0.58 b | −0.63b |
P<0.001,
P<0.05,
P<0.01
Fig.3.
Within postmenopausal women, %fat was related to Phi1 among A) AA (r = −0.57, P<0.05) but not B) EA (r = −0.29, P=0.11).
Examination of abdominal fat depots revealed several patterns that differed across age and ethnic groups. In general, among children, similar patterns of associations were observed with SAAT as with generalized adiposity (%fat), and no associations were observed with IAAT. Among adults, SAAT and IAAT were associated with SI among EA (inversely), and with β-cell measures among both EA (positive association) and AA (inverse associations). Within postmenopausal AA women, SAAT was inversely associated with X0 (−0.54, P<0.05), Phi1 (−0.52, P=0.05), and PhiTOT (−0.58, P<0.05) after adjusting for SI, and IAAT was inversely associated with X0 (−0.56, P<0.05) and Phi1(−0.52, P=0.05) after adjusting for SI.
Discussion
Type 2 diabetes occurs when insulin secretion is insufficient for the degree of insulin resistance. Disease prevalence increases with adiposity, is highest at mid-life, and is greater among AA vs EA. This study is the first to examine whether adiposity differentially affects aspects of β-cell function among AA vs EA, and among children vs adults. Results suggested that increased adiposity was more closely associated with reduced SI among EA vs AA. Adiposity appeared stimulatory to β-cell function in younger subjects and EA, but inhibitory in postmenopausal women, particularly AA postmenopausal women. These observations indicate that the process through which adiposity impacts metabolic disease changes with age, and differs with ethnic background.
It has been widely reported that greater adiposity is associated with lower insulin sensitivity (e.g., 29;30). However, in this population, the association of adiposity with insulin sensitivity was significant among EA but not AA. This observation suggests that the factors that determine SI differ between EA and AA, with adiposity playing a greater role among EA. The insulin sensitivity measure used in this study reflects whole-body insulin sensitivity, capturing both insulin stimulation of glucose uptake at skeletal muscle, and insulin inhibition of hepatic glucose production. It has been suggested that whole-body SI derived from IVGTT reflects a larger hepatic component among AA than EA (31). Studies using tracers to quantify hepatic and peripheral SI are needed to determine if these measures, or their association with adipose tissue, differ with ethnicity.
Ethnic differences also were observed in the association between adiposity and β-cell function. In all subjects combined, increased adiposity was reflected in increased PhiB and Phi2. However, subsequent analyses showed that these relationships were only significant among EA. These responses likely compensate for reduced insulin sensitivity, which occurs secondary to adiposity. In fact, among EA, adjustment for insulin sensitivity indicated that a portion of the variance ascribed to adiposity appeared due to a reduction in insulin sensitivity. It is possible that AA do not compensate for adiposity with increased insulin secretion because adiposity has a minimal impact on insulin sensitivity in this group.
Age also affected the associations among adiposity and β-cell function. Our data indicated that children with more body fat secreted more insulin immediately following the glucose injection (X0, pmol/L), an effect that appeared due in part to an adiposity-mediated reduction insulin sensitivity. Similar associations were not observed in adults. Thus, obesity and insulin resistance in children may be associated with an increase in the amount of insulin stored in the immediately releasable pool (32). This accommodation may decline with age or chronic obesity. In adults, PhiB and Phi2 were positively associated with %fat. In contrast, Phi1either was not associated with adiposity (premenopausal women), or was inversely associated with adiposity (postmenopausal women). An inadequate first-phase insulin response is considered an early event in the etiology of type 2 diabetes (33). Our results here are provocative in that the inverse association between adiposity and Phi1 appeared to develop with age, being significant only among postmenopausal women. Further, the association was independent of SI, suggesting a direct effect of adipose tissue on the β-cell, or an effect mediated by a mechanism separate from SI. Importantly, our results suggest that even among healthy glucose tolerant women, age and adiposity interact to adversely affect first-phase insulin secretion. Postmenopausal women may be at greater risk for lipotoxicity than premenopausal women due to the loss of the protective effect of estrogen on the β-cell (17).
Possible mechanisms for a direct effect of adiposity on the β-cell include FFA and adipokines. The positive association between adiposity and β-cell function observed in AA premenopausal women may reflect the reported stimulatory effect of FFA on insulin secretion. Research in rat islets and humans indicates that acute or periodic exposure to elevated free fatty acids, the flux of which may be elevated in obesity (34), results in an increased glucose-stimulated insulin secretion (7). However, chronic exposure to elevated free fatty acids decreases glucose-stimulated insulin secretion, perhaps explaining the inverse association observed in this study between adiposity and β-cell function among postmenopausal AA women. Alternatively, obesity-mediated depression in β-cell function could be mediated by the adipocyte-derived hormone leptin, serum concentrations of which are elevated in obesity. Data from mouse models indicate that the adipocyte-derived hormone leptin tonically inhibits insulin secretion (35–38). Leptin may inhibit insulin secretion directly by action on the pancreas (35;39), or indirectly via suppression of bioactivity of the bone-derived hormone osteocalcin (40).
The prevalence of type 2 diabetes is greater among AA vs EA, and increases with age and adiposity. Thus, a primary objective of this study was to determine if the association of adiposity with measures of insulin secretion and action differed specifically among postmenopausal AA and EA women. We found that within postmenopausal women, adiposity was adversely related to SI among EA, but to insulin secretion among AA. This observation may suggest that the process through which adiposity facilitates metabolic disease differs with ethnic background. It is possible that adiposity primarily leads to a deterioration of SI among EA, with subsequent metabolic derangement deriving from both insulin resistance and the associated hyperinsulinemia. In contrast among older AA women, adiposity may primarily impact the β-cell, resulting in decreased insulin secretion followed by impaired glucose tolerance and type 2 diabetes.
We also examined whether abdominal fat distribution was associated with SI and β-cell function, and whether these associations differed with age or ethnicity. In general, among children, similar patterns of associations were observed with SAAT as with generalized adiposity (%fat), and no associations were observed with IAAT, which appeared relevant only among adults. The lack of association of IAAT with SI among children may be due to the fact that children had minimal IAAT. Among adults, SAAT and IAAT were inversely associated with SI among EA but not among AA, paralleling results with %fat. Thus, the absence of association of adiposity with SI among AA persisted even when examining specific depots known to be associated with insulin resistance. In adults SAAT and IAAT were associated with β-cell function among both EA (positive association) and AA (inverse associations). The inverse association of abdominal fat with β-cell function among AA is provocative. It would be of interest to explore the possibility that adipokine production, or tissue sensitivity to adipokines, differs with ethnicity.
Strengths of the study include the robust measures of insulin sensitivity, β-cell function, and body composition in prepubertal girls, premenopausal women, and postmenopausal women. Weaknesses are the relatively small sample size in each group, the inclusion of only women, and the observational nature of the study. Further, use of only healthy girls and women may limit the generalizability of the findings. The use of participants with normal glucose tolerance, particularly among the postmenopausal women, may have selected against those at risk for type 2 diabetes.
In conclusion, results from this study suggest that adiposity was more closely associated with insulin sensitivity among EA vs AA. Differences in the association of adiposity with SI may yield insight into ethnicity-specific physiologic determinants of insulin sensitivity. Adiposity appeared stimulatory to β-cell function in younger subjects, but inhibitory in postmenopausal women, particularly AA postmenopausal women. These observations suggest that the process through which adiposity impacts metabolic disease changes with age, and differs with ethnic background. Future studies are warranted to determine whether adipocyte-derived factors such as FFA or leptin mediate the observed inverse association between adiposity and β-cell function.
Acknowledgments
This work was supported by R01DK58278, R01DK067426, M01-RR-00032, P30-DK56336, and P60DK079626. Maryellen Williams and Cindy Zeng conducted laboratory analyses; Tena Hilario served as project coordinator; Crystal Douglas and Jeannine Lawrence provided support with subject recruitment and data entry.
Footnotes
Disclosure Statement
The authors have no conflicts of interest.
Reference List
- 1.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–9. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Distribution of age at diagnosis of diabetes among adult incident cases aged 18–79 years, United States, 2007. Department of Health and Human Services; 2009. pp. 1–2. CDC’s Diabetes Program - Data and Trends. Ref Type: Report. [Google Scholar]
- 3.Polonsky KS, Given E, Carter V. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J Clin Invest. 1988;81:442–8. doi: 10.1172/JCI113339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuster DP, Kien CL, Osei K. Differential impact of obesity on glucose metabolism in black and white American adolescents. American Journal of Medical Sciences. 1998;316:361–7. doi: 10.1097/00000441-199812000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Casazza K, Phadke R, Fernandez JR, Watanabe RM, Goran MI, Gower BA. Obesity attenuates the contribution of African admixture to the insulin secretory profile in peripubertal children: a longitudinal analysis. Obesity. 2009;17:1318–25. doi: 10.1038/oby.2008.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergman RN, Kim SP, Hsu IR, et al. Abdominal obesity: role in the pathophysiology of metabolic disease and cardovascular risk. The American Journal of Medicine. 2007;120 (suppl 2A):S3–S8. doi: 10.1016/j.amjmed.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 7.McGarry JD, Dobbins RL. Fatty acids, lipotoxicity and insulin secretion. Diabetologia. 1999;42:128–38. doi: 10.1007/s001250051130. [DOI] [PubMed] [Google Scholar]
- 8.DeFronzo R, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. A balance overview. Diabetes Care. 1992;15:318–68. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- 9.National Center for Health Statistics and Centers for Disease Control and Prevention. Age-adjusted prevalence of diagnosed diabetes by race/ethnicity and sex, United States, 1980–2005. 2005 http://www.cdc.gov/diabetes/statistics/prev/national/figraceethsex.htm. Ref Type: Report.
- 10.Osei K, Schuster DP. Ethnic differences in secretion, sensitivity, and hepatic extraction of insulin in black and white Americans. Diabetic Medicine. 1994;11:755–62. doi: 10.1111/j.1464-5491.1994.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 11.Gower BA, Nagy TR, Goran MI. Visceral fat, insulin sensitivity, and lipids in prepubertal children. Diabetes. 1999;48:1515–21. doi: 10.2337/diabetes.48.8.1515. [DOI] [PubMed] [Google Scholar]
- 12.Haffner SM, D’Agostino R, Jr, Saad MF, et al. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes. 1996;45:742–8. doi: 10.2337/diab.45.6.742. [DOI] [PubMed] [Google Scholar]
- 13.Arslanian S, Suprasongsin C. Differences in the in vivo insulin secretion and sensitivity of healthy black versus white adolescents. J Pediatr. 1996;129:440–3. doi: 10.1016/s0022-3476(96)70078-1. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Differences in prevalence of obesity among Black, White, and Hispanic adults --- United States, 2006--2008. Morbidity and Mortality Weekly Report. 2009;58:740–4. [PubMed] [Google Scholar]
- 15.Shai I, Jiang R, Manson JE, et al. Ethnicity, obesity, and risk of type 2 diabetes in women. Diabetes Care. 2006;29:1585–90. doi: 10.2337/dc06-0057. [DOI] [PubMed] [Google Scholar]
- 16.Chang AM, Halter JB. Aging and insulin secretion. American Journal of Physiology (Endocrinology and Metabolism) 2003;284:E7–E12. doi: 10.1152/ajpendo.00366.2002. [DOI] [PubMed] [Google Scholar]
- 17.Nadal A, Alonso-Magdalena P, Soriano S, Ropero AB, Quesada I. The role of estrogens in the adaptation of islets to insulin resistance. Journal of Physiology. 2009 doi: 10.1113/jphysiol.2009.177188. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Diabetes Association. Screening for type 2 diabetes. Diabetes Care. 2003;26 (suppl 1):S21–S24. doi: 10.2337/diacare.26.2007.s21. [DOI] [PubMed] [Google Scholar]
- 19.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. American Journal of Physiology (Endocrinology and Metabolism) 1979;236:E667–E677. doi: 10.1152/ajpendo.1979.236.6.E667. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe RM, Steil GM, Bergman RN. Critical evaluation of the combined model approach for estimation of prehepatic insulin secretion. American Journal of Physiology (Endocrinology and Metabolism) 1998;274:E172–E183. doi: 10.1152/ajpendo.1998.274.1.E172. [DOI] [PubMed] [Google Scholar]
- 22.Toffolo G, De Grandi F, Cobelli C. Estimation of beta-cell sensitivity from intravenous glucose tolerance test C-peptide data. Knowledge of the kinetics avoids errors in modeling the secretion. Diabetes. 1995;44:845–54. doi: 10.2337/diab.44.7.845. [DOI] [PubMed] [Google Scholar]
- 23.Polonsky KS, Rubenstein AH. C-Peptide as a measure of the secretion and hepatic extraction of insulin. Pitfalls and limitations. Diabetes. 1984;33:486–94. doi: 10.2337/diab.33.5.486. [DOI] [PubMed] [Google Scholar]
- 24.Bergman RN, Ader M, Huecking K, Van CG. Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes. 2002;51(Suppl 1):S212–S220. doi: 10.2337/diabetes.51.2007.s212. [DOI] [PubMed] [Google Scholar]
- 25.Cobelli C, Toffolo G, Dalla Man C, et al. Assessment of beta-cell function in humans, simultaneously with insulin senstivity and hepatic extraction, from intravenous and oral glucose tests. American Journal of Physiology (Endocrinology and Metabolism) 2007;293:E1–E15. doi: 10.1152/ajpendo.00421.2006. [DOI] [PubMed] [Google Scholar]
- 26.Kekes-Szabo T, Hunter GR, Nyikos I, Nicholson C, Snyder S, Berland L. Development and validation of computed tomography derived anthropometric regression equations for estimating abdominal adipose tissue distribution. Obesity Res. 1994;2:450–7. doi: 10.1002/j.1550-8528.1994.tb00092.x. [DOI] [PubMed] [Google Scholar]
- 27.Goran M, Kaskoun MC, Shuman WP. Intra-abdominal adipose tissue in young children. Int J Obesity. 1995;19:279–83. [PubMed] [Google Scholar]
- 28.Allison DB, Gorman BS. POWCOR: A power analysis and sample size program for testing differences between dependent and independent correlations. Educational and Psychological Measurements. 1993;53:133–7. [Google Scholar]
- 29.Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. American Journal of Physiology (Endocrinology and Metabolism) 2000;278:E941–E948. doi: 10.1152/ajpendo.2000.278.5.E941. [DOI] [PubMed] [Google Scholar]
- 30.Abbasi F, Brown BW, Lamendola C, McLaughlin T, Reaven GM. Relationship between obesity, insulin resistance, and coronary heart disease risk. Journal of the American College of Nutrition. 2002;40:937–43. doi: 10.1016/s0735-1097(02)02051-x. [DOI] [PubMed] [Google Scholar]
- 31.Alvarez JA, Bush N, Hunter GR, Brock DW, Gower BA. Ethnicity and weight status impact the accuracy of proxy indices of insulin sensitivity. Obesity. 2009;16:2739–44. doi: 10.1038/oby.2008.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barg S, Lindqvist A, Obermuller S. Granule docking and cargo release in pancreatic β cells. Biochemical Society Transactions. 2008;36:294–9. doi: 10.1042/BST0360294. [DOI] [PubMed] [Google Scholar]
- 33.Del Prato S, Tiengo A. The importance of first-phase insulin secretion: implications for the therapy of type 2 diabetes mellitus. Diabetes Metab Res Rev. 2001;17:164–74. doi: 10.1002/dmrr.198. [DOI] [PubMed] [Google Scholar]
- 34.Shadid S, Kanaley JA, Sheehan MT, Jensen MD. Basal and insulin-regulated free fatty acid and glucose metabolism in humans. American Journal of Physiology (Endocrinology and Metabolism) 2007;292:E1770–E1774. doi: 10.1152/ajpendo.00655.2006. [DOI] [PubMed] [Google Scholar]
- 35.Morioka T, Asilmaz E, Hu J, et al. Disruption of leptin receptor expression in the pancreas directly affects B cell growth and function in mice. J Clin Invest. 2007;117:2868. doi: 10.1172/JCI30910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kieffer TJ, Habener JF. The adipoinsular axis: effects of leptin on pancreatic β-cells. American Journal of Physiology (Endocrinology and Metabolism) 2000;278:E1–E14. doi: 10.1152/ajpendo.2000.278.1.E1. [DOI] [PubMed] [Google Scholar]
- 37.Seufert J. Leptin effects on pancreatic beta-cell gene expression and function. Diabetes. 2004;53 (Suppl 1):S152–S158. doi: 10.2337/diabetes.53.2007.s152. [DOI] [PubMed] [Google Scholar]
- 38.Kulkarni RN, Wang Z-L, Wang R-M, et al. Leptin rapidly suppresses insulin release from insulinoma cells, rat and human islets, and, in vivo in mice. J Clin Invest. 1997;100:2729–36. doi: 10.1172/JCI119818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Covey SD, Wideman RD, McDonald C, et al. The pancreatic B cell is a key site for mediating the effects of leptin on glucose homeostasis. Cell Metabolism. 2006;429:291–302. doi: 10.1016/j.cmet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 40.Hinoi E, Gao N, Jung DY, et al. The sympathetic tone mediates leptin’s inhibition of insulin secretion by modulating osteocalcin bioactivity. Journal of Cell Biology. 2008;183:1235–42. doi: 10.1083/jcb.200809113. [DOI] [PMC free article] [PubMed] [Google Scholar]