Abstract
Rationale
Two categories of cardiac stem cells (CSCs) with predominantly myogenic (mCSCs) and vasculogenic (vCSCs) properties have been characterized in the human heart. However, it is unknown whether functionally-competent CSCs of both classes are present in the myocardium of patients affected by end-stage cardiac failure, and whether these cells can be harvested from relatively small myocardial samples.
Objective
To establish whether a clinically relevant number of mCSCs and vCSCs can be isolated and expanded from endomyocardial biopsies of patients undergoing cardiac transplantation, or LVAD implantation.
Methods and Results
Endomyocardial biopsies were collected with a bioptome from the right side of the septum of explanted hearts or the apical LV core at the time of LVAD implantation. Two-five biopsies from each patient were enzymatically dissociated and, after expansion, cells were sorted for c-kit (mCSCs), or c-kit and KDR (vCSCs) and characterized. mCSCs and vCSCs constituted 97% and 3% of the c-kit population, respectively. Population doubling time averaged 27 hours in mCSCs and vCSCs; 5×106 mCSCs and vCSCs were obtained in 28 and 41 days, respectively. Both CSC classes possessed significant growth reserve as documented by high telomerase activity and relatively long telomeres. mCSCs formed mostly cardiomyocytes, and vCSCs endothelial and smooth muscle cells.
Conclusions
The growth properties of mCSCs and vCSCs isolated from endomyocardial biopsies from patients with advanced heart failure were comparable to those obtained previously from larger myocardial samples of patients undergoing elective cardiac surgery.
Keywords: Cell therapy, telomere telomerase axis, CSC senescence
The human heart possesses two classes of cardiac stem cells (CSCs), which have powerful but distinct vasculogenic and myogenic properties.1,2 One category of CSCs is nested in vascular niches distributed throughout the coronary circulation and is primarily responsible for the turnover of endothelial cells (ECs) and smooth muscle cells (SMCs), and vasculogenesis,2 i.e., vCSCs. The other CSC pool is clustered in niches located in the myocardial interstitium and regulates myocyte renewal and cardiomyogenesis,1 i.e., mCSCs. These CSCs can be isolated from samples, ∼75-130 mg, of atrial and ventricular myocardium of patients undergoing elective cardiac surgery.1,2 This method of CSC harvesting is currently employed in a phase 1 clinical trial, which includes patients with ischemic cardiomyopathy undergoing coronary bypass surgery.3 Here, we sought to determine whether functionally-competent CSCs can be obtained from patients with advanced heart failure and whether sufficient quantities can be collected from endomyocardial biopsies. Affirmative answers to these questions would support the possibility that non-thoracotomy biopsies could serve as a source of autologous CSCs for the potential treatment of patients with advanced cardiomyopathies.
Methods
Endomyocardial biopsies were obtained and CSCs were collected and expanded in vitro. Telomere length, telomerase activity, cellular senescence and apoptosis were determined to define the growth properties of these cells. An expanded Methods section is available in the Online Data Supplement.
Results
Human Samples
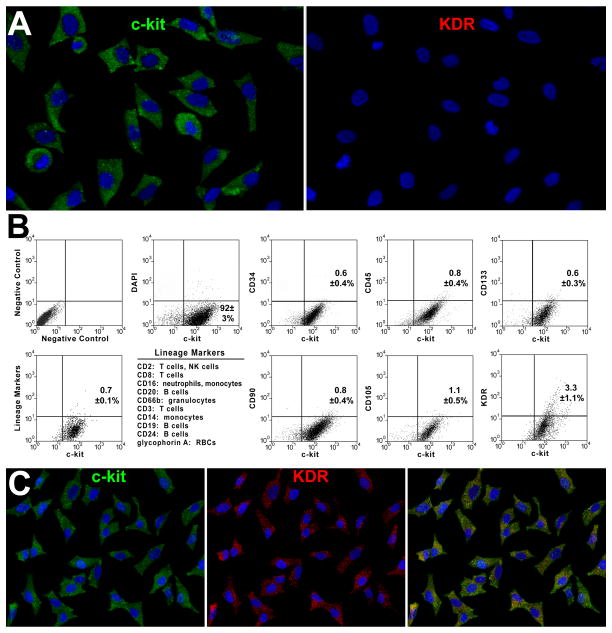
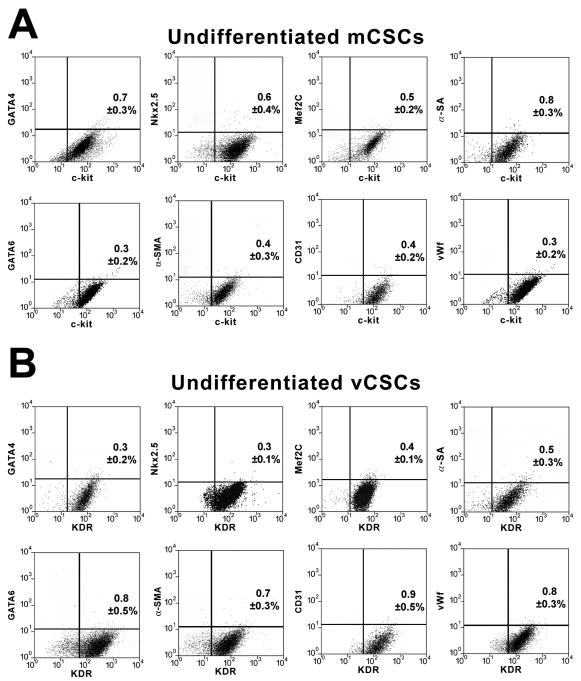
Biopsies of the right portion of the septum or apex of the left ventricle (LV) were harvested ex vivo from explanted hearts (n=13) or following removal of the LV apical region at the time of LVAD implantation (n=7). The characteristics of the patients are listed in Online Table I. Two-five biopsies were collected, yielding a total sample weight of 4.8±1.0 mg (Online Figure I). Based on previous results, <100 CSCs were present in each sample.4 Specimens were dissociated and the un-fractionated cell population was expanded for 17±2 days (Online Figure I). Cells were sorted for c-kit and characterized by FACS 11±2 days later (Online Figure I), when 5×106 cells were obtained (Figure 1A). C-kit-positive cells were negative for markers of hematopoietic and mesenchymal lineages; ∼3% expressed KDR (Figure 1B). C-kit-positive cells and cells positive for c-kit and KDR corresponded to mCSCs and vCSCs, respectively.2 vCSCs were amplified for an additional 13±3 days (Online Figure I) to obtain 5×106 cells (Figure 1C). Both classes of CSCs were obtained in all 20 cases studied.
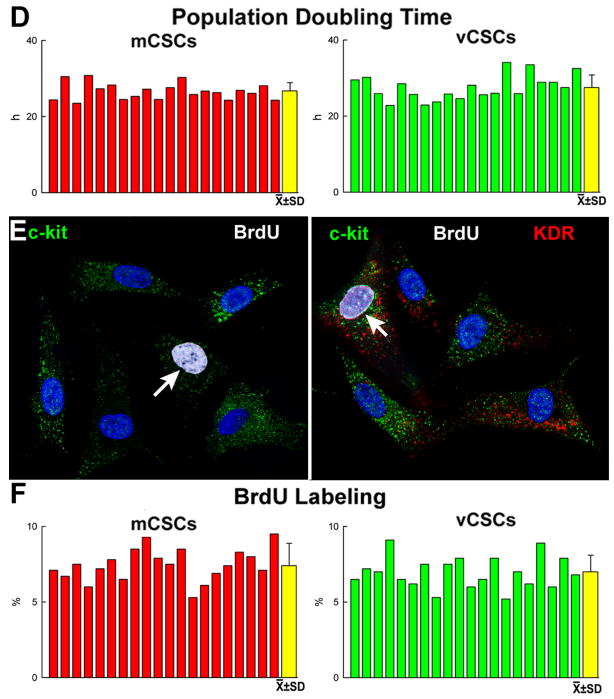
Figure 1. Isolation and expansion of mCSCs and vCSCs.
A, Sorted c-kit-positive (left, green) KDR-negative (right) cells in culture. B, Bivariate distribution of c-kit, markers of hematopoietic cells (CD34, CD45, CD133, cocktail of bone marrow cell lineages), mesenchymal cells (CD90, CD105), and KDR. C, Sorted c-kit-positive (left) KDR-positive (central, red) in culture. Right panel, merge. D, PDT in mCSCs and vCSCs: individual and average values. E, mCSCs and vCSCs labeled by BrdU (white, arrows). F, Percentage of BrdU-labeled mCSCs and vCSCs.
Growth Properties of mCSCs and vCSCs
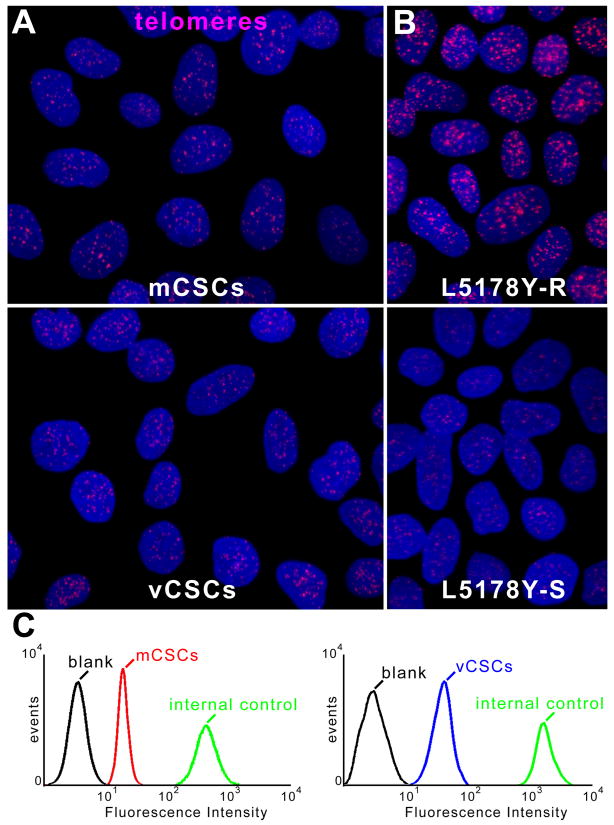
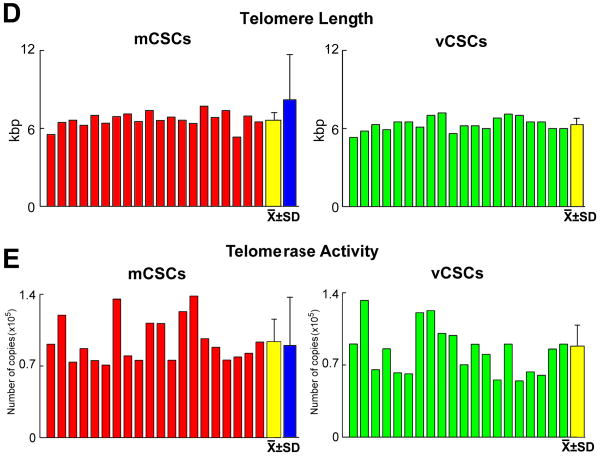
The growth of stem cells is regulated by the length of their telomeres and the level of telomerase activity.5 Thus, these variables were measured in CSCs together with population doubling time (PDT) and the fraction of cycling cells. PDT was comparable in mCSCs and vCSCs, averaging 27 and 28 hours, respectively (Figure 1D). Additionally, ∼7-8% mCSCs and vCSCs were positive for BrdU (Figure 1E and 1F). Telomere length, measured by flow-FISH,6 showed that mCSCs and vCSCs had telomeres varying from 5.3 to 7.7 kbp (Figure 2A-2D), far from telomere lengths of 1.5-2 kbp, which are associated with replicative senescence of human cells.5 The slightly shorter average telomere length in vCSCs was consistent with the higher number of divisions required for the expansion of this stem cell pool.
Figure 2. Telomere-telomerase axis.
A, Telomeres (magenta dots) in mCSCs (upper) and vCSCs (lower). B, Lymphoma cells with long (48 kbp, upper) and short (7.0 kbp, lower) telomeres. C, Flow-FISH of mCSCs, vCSCs and lymphoma cells (internal control); mCSCs or vCSCs were combined with lymphoma cells and incubated without (blank) or with PNA probe. Histogram represents intensity of PNA probe binding in gated mCSCs (red line), vCSCs (blue line), and control cells (green line) relative to blank (black line). D and E, Telomere length (D) and telomerase activity (E) in mCSCs and vCSCs: individual and average values. Blue bars: values in CSCs obtained from large myocardial samples (see ref. 1).
Telomerase activity was evaluated by quantitative PCR and was expressed as number of template copies normalized for the TSR8 oligonucleotide. The catalytic activity of the ribonucleoprotein was high in both mCSCs and vCSCs (Figure 2E). Importantly, enzyme activity was similar to that measured in mCSCs isolated from large surgical specimens of patients with modest alterations in ventricular function.1 These human mCSCs have been shown to regenerate extensive areas of infarcted myocardium, differentiating into functionally-competent cardiomyocytes and coronary vessels.1,2,7
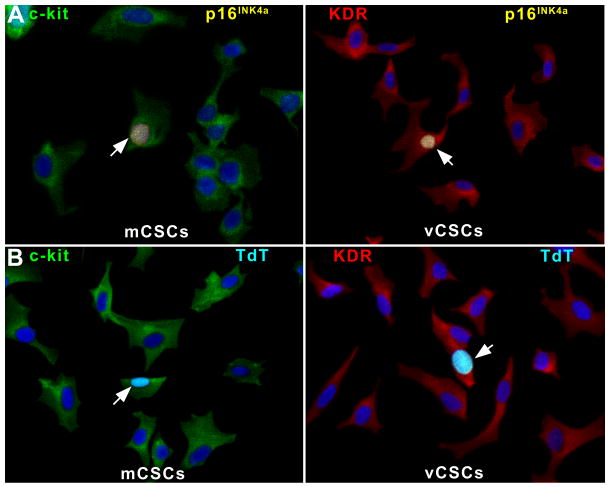
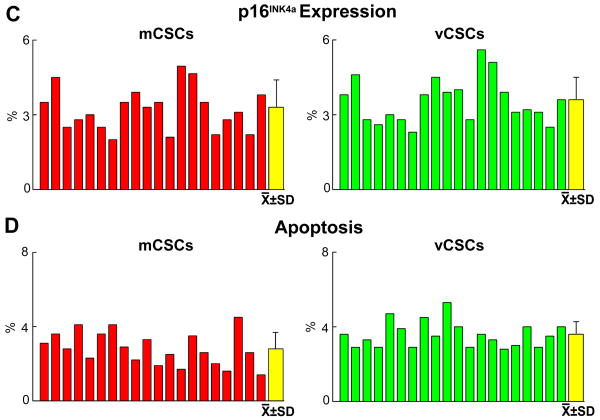
The well-preserved telomere-telomerase axis in mCSCs and vCSCs was consistent with the relatively low levels of cells positive for the senescence-associated protein p16INK4a, which prevents permanently reentry of stem cells into the cell cycle.1 Similarly, apoptosis was low (Figure 3A-3D). Thus, mCSCs and vCSCs expanded in culture to a clinically relevant number possess a significant capacity for further growth.
Figure 3. Senescence and apoptosis.
A, of p16INK4a in mCSCs and vCSCs (yellow, arrows). B, TdT-labeling of mCSCs and vCSCs (bright blue, arrows). C and D, Senescent (C) and apoptotic (D) mCSCs and vCSCs: individual and average values.
Differentiation of mCSCs and vCSCs
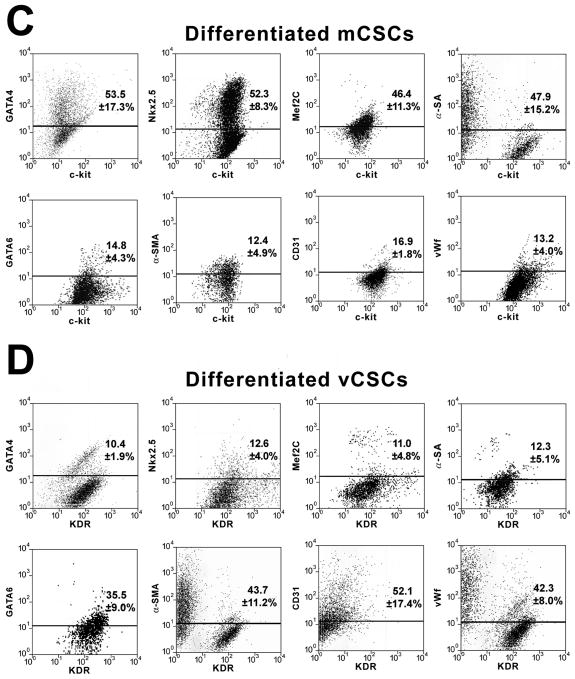
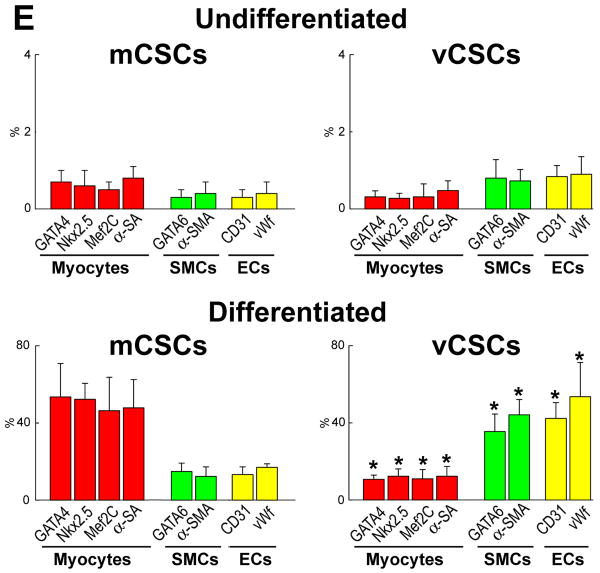
The commitment of mCSCs and vCSCs was assessed by FACS analysis. Prior to differentiation, mCSCs and vCSCs were negative for cardiac lineages (Figure 4A and 4B). However, mCSCs exposed to dexamethasone became predominantly c-kit-negative and expressed myocyte-specific transcription factors and contractile proteins. Small fractions of cells were positive for transcription factors, and membrane and cytoplasmic proteins specific of SMCs and ECs. Similarly, in the presence of dexamethasone, vCSCs lost largely c-kit and KDR and expressed transcription factors, and membrane and cytoplasmic proteins specific of SMCs and ECs. Additionally, some cells were positive for transcription factors and contractile proteins typical of cardiomyocytes (Figure 4C and 4D). Collectively, mCSCs formed 5-fold more myocytes than vCSCs, and vCSCs formed 4-fold more SMCs and 3.2-fold more ECs than mCSCs (Fig. 4E). Comparable differences in lineage specification of mCSCs and vCSCs were found when these stem cell classes were obtained from large surgical specimens.1,2
Figure 4. Differentiation of mCSCs and vCSCs.
A and B, Bivariate distribution of c-kit (A) or KDR (B) and cardiac markers in undifferentiated mCSCs (A) and vCSCs (B). C and D, Comparable analysis following cell differentiation. E, Quantitative results by FACS. *P<0.05 vs. mCSCs.
Discussion
The current study had two objectives: a) To determine whether patients with severe heart failure retain a pool of functionally-competent mCSCs and vCSCs in the diseased myocardium; and b) To establish whether in these patients with advanced heart failure, undergoing either cardiac transplantation or LVAD implantation, mCSCs and vCSCs can be isolated from small myocardial biopsies and expanded in vitro to a clinically relevant number for subsequent autologous delivery. Our results provide a positive answer to both questions, raising the possibility that autologous mCSC and vCSC therapy is feasible and can be considered for research evaluation in patients with advanced heart failure.
We have emphasized repeatedly that the telomere-telomerase axis is a major determinant of the growth behavior of mCSCs and vCSCs. Based on extensive characterization of mCSCs, we have shown that these cells undergo an average telomeric shortening of 130 bp per population doubling (PD).1 Critical telomere length associated with growth arrest and cellular senescence of mCSCs and human hematopoietic stem cells varies from 2.0 to 1.5 kbp.1,5 In this study, mCSCs and vCSCs at the end of expansion had telomere length of ∼6 kbp. It can be predicted that mCSCs and vCSCs can undergo 31 additional PDs (6-2 = 4 kbp /0.13 kbp = 31 PDs) before irreversible growth arrest is reached. Therefore, human CSCs can be extensively grown in vitro and implanted in vivo prior to a major loss in their proliferation potential.
Importantly, both classes of human CSCs, mCSCs and vCSCs, have been obtained from specimens of tissue weighing ∼5 mg and, following amplification, rapidly reached a value of 5×106 cells in ∼40 days. The time factor involved in the acquisition of a therapeutically applicable number of mCSCs and vCSCs constitutes a limitation for the management of acute events but does not preclude the treatment of chronic heart failure. Currently, one million mCSCs are delivered by intracoronary infusion to patients with post-infarction ischemic cardiomyopathy and impaired ventricular function.3 The number of mCSCs being used is significantly smaller than that of mononuclear bone marrow cells, 240×106, injected in an identical manner in subjects with acute myocardial infarction or chronic heart failure.8 Pools of 12.5 and 25 × 106 autologous cardiac cells, derived from the in vitro expansion of cardiospheres,9 are administrated to patients with recent myocardial infarction and depressed cardiac function (ClinicalTrials.gov Identifier: NCT00893360). The unproven hypothesis views cardiac progenitors as potentially more specific and effective in the direct restoration of damaged myocardium, or in the indirect activation of endogenous mCSCs, resulting in an identical tissue regenerative response.10 Both mechanisms may also be operative although, independently from the clinical outcome, it will be essentially impossible to define the actual role of mCSCs or cardiospheres in myocardial repair.
The recognition that hCSCs are active and widely distributed throughout the myocardium is at variance with their inefficient regenerative capacity following ischemic and non-ischemic myocardial injury. Although a direct explanation for this growth defect cannot be provided, hCSCs are properly equipped to regulate tissue homeostasis,11 but are largely inadequate to promote cardiac repair. By delivering 5×106 hCSCs, we may increase their frequency in the myocardium by several-fold.
Supplementary Material
Acknowledgments
Sources of Funding: This work was supported by NIH grants.
Non-Standard Abbreviations and Acronyms
- CSCs
Cardiac Stem Cells
- mCSCs
Myogenic Cardiac Stem Cells
- vCSCs
Vasculogenic Cardiac Stem Cells
- LVAD
Left Ventricular Assist Device
- PDT
Population Doubling Time
Footnotes
Disclosure: None
References
- 1.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D'Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bearzi C, Leri A, Lo Monaco F, Rota M, Gonzalez A, Hosoda T, Pepe M, Qanud K, Ojaimi C, Bardelli S, D'Amario D, D'Alessandro DA, Michler RE, Dimmeler S, Zeiher AM, Urbanek K, Hintze TH, Kajstura J, Anversa P. Identification of a coronary vascular progenitor cell in the human heart. Proc Natl Acad Sci USA. 2009;106:15885–15890. doi: 10.1073/pnas.0907622106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Bolli R, Chugh AR, D'Amario D, Stoddard MF, Ikram S, Wagner SG, Beache GM, Leri A, Hosoda T, Loughran JH, Goihberg P, Fiorini C, Solankhi NK, Fahsah I, Chatterjee A, Elmore JB, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Use of cardiac stem cells for the treatment of heart failure: translation from bench to the clinical setting. Circ Res. 2010:e33. Abstract. [Google Scholar]
- 4.Urbanek K, Torella D, Sheikh F, De Angelis A, Nurzynska D, Silvestri F, Beltrami CA, Bussani R, Beltrami AP, Quaini F, Bolli R, Leri A, Kajstura J, Anversa P. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci USA. 2005;102:8692–8697. doi: 10.1073/pnas.0500169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88:557–579. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- 6.Baerlocher GM, Vulto I, de Jong G, Lansdorp PM. Flow cytometry and FISH to measure the average length of telomeres (flow FISH) Nat Protoc. 2006;1:2365–2376. doi: 10.1038/nprot.2006.263. [DOI] [PubMed] [Google Scholar]
- 7.Hosoda T, D'Amario D, Cabral-Da-Silva MC, Zheng H, Padin-Iruegas ME, Ogorek B, Ferreira-Martins J, Yasuzawa-Amano S, Amano K, Ide-Iwata N, Cheng W, Rota M, Urbanek K, Kajstura J, Anversa P, Leri A. Clonality of mouse and human cardiomyogenesis in vivo. Proc Natl Acad Sci USA. 2009;106:17169–17174. doi: 10.1073/pnas.0903089106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Süselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM REPAIR-AMI Investigators. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 9.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marbán E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 10.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kajstura J, Gurusamy N, Ogórek B, Goichberg P, Clavo-Rondon C, Hosoda T, D'Amario D, Bardelli S, Beltrami AP, Cesselli D, Bussani R, del Monte F, Quaini F, Rota M, Beltrami CA, Buchholz BA, Leri A, Anversa P. Myocyte turnover in the aging human heart. Circ Res. 2010;107:1374–1386. doi: 10.1161/CIRCRESAHA.110.231498. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.