Abstract
Purpose
Acute myelogenous leukemia (AML) does not have a high cure rate, particularly in patients with poor-risk features. Such patients might benefit from additional therapy in complete remission (CR). Tipifarnib is an oral farnesyltransferase inhibitor with activity in AML. We conducted a phase II trial of maintenance tipifarnib monotherapy for 48 adults with poor-risk AML in first CR.
Experimental Design
Tipifarnib 400 mg twice daily for 14 of 21 days was initiated after recovery from consolidation chemotherapy, for a maximum of 16 cycles (48 weeks).
Results
Twenty (42%) patients completed 16 cycles, 24 (50%) were removed from study for relapse, and 4 (8%) discontinued drug prematurely for intolerance. Nonhematologic toxicities were rare, but tipifarnib dose was reduced in 58% for myelosuppression. Median disease-free survival (DFS) was 13.5 months (range, 3.5–59+ months), with 30% having DFS >2 years. Comparison of CR durations for 25 patients who received two-cycle timed sequential therapy followed by tipifarnib maintenance with 23 historically similar patients who did not receive tipifarnib showed that tipifarnib was associated with DFS prolongation for patients with secondary AML and adverse cytogenetics.
Conclusions
This study suggests that some patients with poor-risk AML, including patients with secondary AML and adverse cytogenetics, may benefit from tipifarnib maintenance therapy. Future studies are warranted to examine alternative tipifarnib dosing and continuation beyond 16 cycles.
Cure rates for adults with acute myelogenous leukemia (AML) remain inadequate. Poor-risk features such as age >60 years, secondary AML including prior myelodysplasia and treatment-related AML, adverse cytogenetics, and hyperleukocytosis with extramedullary disease in the absence of “favorable” cytogenetics identify patients who are not only less likely to achieve complete remission (CR) with cytotoxic chemotherapy but who are also predisposed to shorter disease-free survival (DFS) despite intensive induction and postremission therapies (1 – 5). In the group of adults with one or more of these poor-risk features, median CR duration is <9 months, the 1-year DFS is 20%, and achievement of second CR is <40% (6 – 13). Such patients might benefit from additional treatment in the so-called minimal residual disease state (i.e., maintenance therapy) that might suppress relapse by exploiting previously untargeted pathways and might not predispose to resistance to other chemotherapy agents. To date, however, maintenance chemotherapies given in CR following induction and consolidation regimens have not resulted in major prolongations of DFS or overall survival in a consistent fashion (2, 12 – 14).
Tipifarnib is an orally available, nonpeptidomimetic farnesyltransferase inhibitor that has clinical activity in myeloid malignancies including elderly adults with AML who are not candidates for traditional cytotoxic therapy (15 – 18), high-risk myelodysplasia (19 – 21), and myeloproliferative disorders including agnogenic myeloid metaplasia (22) and imatinib-resistant chronic myelogenous leukemia (23). In the phase II setting, tipifarnib was well tolerated as a single agent at 600 mg twice daily for 3 of 4 to 6 weeks in elderly adults with newly diagnosed AML (15, 17). In the setting of myelodysplasia, however, 600 mg twice daily given for 4 of 6 weeks was associated with myelosuppression and neurotoxicity that required drug discontinuation (20). In contrast, 300 mg twice daily given for 21 of 28 days (19) or 600 mg twice daily on an alternate week schedule (24) seems to be well tolerated in myelodysplasia patients and associated with achievement of durable responses in roughly 30% of high-risk myelodysplasia patients, including patients with chronic myelomonocytic leukemia in transformation.
On the basis of the activity of tipifarnib against myeloid malignancies and its ease of administration, we designed a phase II trial of tipifarnib monotherapy as maintenance therapy for adults with poor-risk AML in first CR following cytotoxic induction and consolidation therapies. We selected an intermediate dose (400 mg twice daily) and duration (14 of 21 days for a total of 16 cycles) of tipifarnib in an attempt to maximize tolerability and at the same time provide a dose known to induce maximal inhibition of farnesyltransferase in marrow blasts cells, as determined in the clinical-laboratory correlative phase I study in adults with refractory acute leukemias (16).
Patients, Materials, and Methods
Patient eligibility and selection
Between September 2002 and March 2006, 48 adults of ages >18 y and with newly diagnosed AML with poor-risk features were entered on study if they met one or more of the following eligibility criteria: age >60 y in the absence of favorable cytogenetics (t[8;21], t[15;17], t[16;16], or inv[16]) or adults of any age with multilineage dysplasia (25 – 27), secondary AML (myelodysplasia/AML or treatment-related AML), adverse cytogenetics (e.g., -5q/-5, -7q/-7, 11q23 abnormalities, 20q-, complex), FLT3 positivity, or hyperleukocytosis >100,000 blasts/µL and/or extensive extramedullary disease in the absence of favorable cytogenetics. Patients were not eligible if they had acute promyelocytic leukemia; if t(8;21), t(16;16), or inv (16) was present as the sole cytogenetic abnormality; if they were younger than 60 y and did not have any poor-risk features; or if >120 d had elapsed from the start of the last consolidation cycle to the start of tipifarnib maintenance therapy. All patients had achieved CR following induction therapy, and CR was confirmed morphologically, immunophenotypically, and cytogenetically by bone marrow aspirate and biopsy within 14 d before beginning tipifarnib maintenance.
Twenty-three patients received induction therapy with 7-d continuous infusion of cytosine arabinoside (ara-C) plus 3 d of anthracycline (“7 + 3”), followed by consolidation therapy consisting of 1 to 4 cycles of moderate-dose ara-C (400 mg/m2/d continuously for 5 d) or high-dose ara-C (2–3 g/m2 every 12 h × 6 doses total). Twenty-five patients received two-cycle timed sequential therapy (7, 9) consisting of induction with ara-C 2 g/m2 given by 72-h continuous infusion beginning on day 1, daunorubicin 45 mg/m2/d given days 1 through 3, and etoposide 400 mg/m2/d given days 8 through 10, and followed by a second cycle of timed sequential ara-C and anthracycline (ara-C 2 g/m2 given by 72-h continuous infusion beginning on day 1 and again on day 10, plus daunorubicin 45 mg/m2/d given days 1–3).
Treatment schema
Tipifarnib was administered orally at a dose of 400 mg twice daily for 14 of every 21 d (1 cycle) beginning 28 ± 7 following marrow recovery for consolidation chemotherapy, for a maximum of 16 cycles total (1 y). Tipifarnib was withheld for any grade >2 nonhematologic toxicity (excluding alopecia, nausea, and vomiting that is controlled with appropriate antiemetic therapy) or for grade 3 neutropenia (>100/µL, <500/µL) or thrombocytopenia (>10,000/µL, <20,000/µL; lasting >3 wk after completion of each 21-d cycle). For nonhematologic toxicities, tipifarnib was resumed at 300 mg twice daily for 14 of 21 d following resolution to grade 1 toxicity or better within 14 d after the first occurrence of the above drug-related toxicity, and was resumed at 200 mg twice daily after resolution of a second occurrence of a drug-related toxicity. For hematologic toxicities, absolute neutrophil count had to recover to >500/µL, and platelets to > 20,000/µL, unsupported to restart tipifarnib maintenance therapy. Tipifarnib dose was reduced to 300 mg twice daily for patients requiring a treatment delay to allow count recovery to minimal acceptable levels. Tipifarnib was reduced to 200 mg twice daily after a second occurrence of a drug-related toxicity. Therapy was discontinued for any grade 4 nonhematologic toxicity, grade 2 to 3 nonhematologic toxicity that did not resolve to grade 1 or better within 14 d of occurrence, completion of all 16 cycles of tipifarnib, bone marrow relapse, or development of extramedullary leukemia.
Response and toxicity evaluation
To assess response to therapy and document ongoing remission status, blood counts and bone marrow aspirate/biopsy were done before beginning tipifarnib (within 14 d pretreatment); following completion of cycles 3, 6, 9, 13, and 16; or at any time that leukemia regrowth is suspected. Continuing CR was defined as bone marrow showing <5% myeloblasts with normal maturation of all cell lines evidenced by absolute neutrophil count >1,000/µL, platelets ≥100,000/µL, hematocrit ≥33% and/or hemoglobin ≥11 g/dL, absence of blasts in peripheral blood, absence of identifiable leukemic cells in the bone marrow by multiparameter flow cytometry, clearance of disease-associated cytogenetic abnormalities, and clearance of any previously existing extramedullary disease. Relapsed AML was defined as any of the following: reappearance of marrow and/or peripheral blood blasts by morphologic, immunophenotypic, and/or cytogenetic measurements; recurrence of trilineage dysplasia; or development or recurrence of extramedullary leukemia. DFS was measured from the time at which criteria were met for CR following initial induction therapy until the first date that recurrent disease was objectively documented, or death from any cause occurred. Adverse events used the descriptions and grading scales found in the revised National Cancer Institute Common Toxicity Criteria and used the Common Toxicity Criteria version 2.0 for adverse event reporting.
Statistical methods
Kaplan-Meier curves were used to describe DFS. Cox proportional hazards models were used to determine associations between clinical characteristics and DFS in simple and multiple regression settings. Main effects and interactions were considered between treatment and clinical characteristics. The proportionality assumption was checked by inspection of hazard functions. Statistical significance was set at α = 0.10 for this nonrandomized study with small sample size. In addition, we compared DFS for the 25 patients receiving two-cycle timed sequential therapy followed by tipifarnib maintenance with the DFS for 23 poor-risk AML patients treated by Bolanos-Meade et al. (7) with two-cycle timed sequential therapy without tipifarnib maintenance. In the “historically comparable” study, induction consisted of all-trans retinoic acid 45 mg/m2 administered orally days 1 to 6, ara-C 2 g/m2 given by 72-h continuous infusion beginning on day 2, idarubicin 12 mg/m2/d given days 2 to 4 (8 mg/m2/d for age ≥60 y), and etoposide 300 mg/m2 given by 72-h continuous infusion beginning day 9; consolidation consisted of all-trans retinoic acid 45 mg/m2 administered orally days 1 to 6, ara-C 2 g/m2 given by 72-h continuous infusion beginning days 2 and 11, and idarubicin 12 mg/m2/d given days 2 to 4 (8 mg/m2/d for age ≥60 y).
Results
Patient characteristics
Between October 2002 and March 2006, 48 adults with poor-risk AML in first CR were entered on study after completing induction and consolidation chemotherapies. Median follow-up as of July 1, 2007 was 31 months. As detailed in Table 1, roughly 2 of 3 of the patients were of ages ≥60 years, more than half had adverse cytogenetics, and 2 of 3 had at least two poor-risk factors. With regard to types of induction and consolidation therapies, 25 (52%) received two-cycle timed sequential therapy (7, 9) and 23 (48%) received “7 + 3” – based induction therapy followed by 1 to 4 cycles of consolidation with moderate-dose to high-dose ara-C (median, 2 cycles; range, 1–4 cycles). The median time from achievement of CR to the start of tipifarnib was 3.5 months (range, 2–6 months). The median time from the start of the patient’s last cycle of consolidation to the start of tipifarnib was 2 months (range, 1–4 months).
Table 1.
Characteristics of 48 adults with AML with poor-risk features in first CR
| n (%) | |
|---|---|
| Gender | |
| Male | 28 (58) |
| Female | 20 (42) |
| Median age (range), y | 63 (27–83) |
| Types of induction/consolidation therapy | |
| 2 cycles of timed sequential therapy | 25 (52) |
| “7 + 3”/moderate- or high-dose ara-C | 23 (48) |
| Poor-risk features | |
| Age ≥60 y | 31 (65) |
| Secondary AML | 16 (33) |
| Adverse cytogenetics | 25 (52) |
| Leukostasis/extramedullary AML | 16 (33) |
| ≥2 risk factors | 32 (67) |
Toxicities
A total of 562 cycles were given to the 48 patients, with the median number of cycles per patient being 11 (range, 0.5–16). Four (8%) patients, ages 59 to 77 years and with secondary AML (2) and adverse cytogenetics (3), required premature drug discontinuation after 0.5 to 4 cycles due to exfoliative rash (1) or drug intolerance due to gastrointestinal symptoms (3), without evidence for recurrent AML at the time of discontinuation. Hospitalizations were infrequent during tipifarnib administration, occurring in 4 (8%) patients during 5 (1%) cycles of therapy as a result of infection (2 patients, in setting of grade 3 neutropenia: line sepsis, influenza), bowel resection for obstruction (prior history of colon cancer) with postoperative pancreatitis unrelated to tipifarnib (1 patient, two hospitalizations), and non – tipifarnib-related lumbar back pain (1 patient). As detailed in Table 2, nonhematologic toxicities were fatigue, ataxia, and sensory peripheral neuropathy, most of which were grade 1 in intensity and all of which resolved after tipifarnib therapy was completed. The majority of patients (28 of 48; 58%) required at least one reduction in tipifarnib dose, with 27 of those 28 having grade ≥3 myelosuppression including neutropenia (4), thrombocytopenia (13), or both (10). The occurrence of grade 3 thrombocytopenia with or without grade 3 neutropenia heralded relapsing disease in 3 patients. One patient required dose reduction for grade 3 fatigue. Eight patients required two dose reductions. However, blood product support was required in only 3 (6%) patients during a total of 5 (0.6%) cycles, with 1 patient requiring RBC transfusion and 2 patients requiring both red cells and platelets. Likewise, the incidence of fever with or without documented infection was low [20 of 562 (3.6%) episodes], with 2 febrile episodes associated with neutropenia and 5 documented infections (3 catheter based, 1 bacterial pharyngitis, and 1 influenza).
Table 2.
Toxicities occurring during tipifarnib maintenance
| Nonhematologic toxicities |
| Fatigue in 20 (42%): 15 grade 1, 4 grade 2, 1 grade 3 |
| Central nervous system in 4 (8%): grade 1 ataxia (2), grade 1 memory loss (2) |
| Sensory neuropathy in 7 (12%): all grade 1 (exacerbation of prior neuropathy in 4 of 7) |
| Hematologic toxicities |
| Dose reduction for myelosuppression in 31 (65%) |
| Blood product support in 3 (6%) patients, 5 (0.6%) cycles |
| Fever/infection: 20 (3.6%) cycles |
| Nonneutropenic fever in 18, neutropenic fever in 2 |
| Documented infection in 5 (10.4%) patients: catheter 3, pharyngitis 1, influenza 1 |
Clinical outcome
Of the 48 patients enrolled on study, 20 (42%) completed all 16 cycles of tipifarnib maintenance therapy and 24 (50%) were removed from study before completing 16 cycles because of relapse of AML. The median number of cycles completed for the 48 patients was 11 (range, 0.5–16). For the 20 patients completing all 16 cycles, median age was 59 years (range, 27–80 years), 10 (50%) had adverse cytogenetics, and 10 (50%) had ≥2 poor-risk factors. As of July 1, 2007, 15 (31%) remain in continuous CR for a median of 28+ months (range, 16+ to 59+ months) after completing 16 cycles of tipifarnib maintenance therapy. The 15 in continuous CR have a median age of 61 years (range, 27–80 years), 8 (53%) have adverse cytogenetics, and 8 (53%) have ≥2 poor-risk factors.
As depicted in Fig. 1, the median duration of CR for the 48 patients is 13.5 months (range, 3.5–59+ months), with 30% of all patients enjoying a CR of at least 2 years. For the cohort of 25 patients who received tipifarnib maintenance following two-cycle timed sequential therapy induction and consolidation, the percentage of patients achieving CR ≥12 months duration was 56% (14 of 25), whereas 5 of 16 (31%) receiving <2 cycles of moderate-dose or high-dose ara-C consolidation and 5 of 7 (71%) receiving ≥3 cycles of moderate-dose or high-dose ara-C consolidation remain in CR for ≥12 months. When measured from the time of tipifarnib initiation, median CR duration is 11 months (range, 0.75–57+ months), with 22 of 48 (46%) remaining in CR for 12 months and 10 of 42 (24%) remaining in CR for 24 months (6 patients continue in CR at 13+ to 23+ months). The proportions of patients with DFS >12 months and those remaining in continuous CR were similar for those who began tipifarnib within 3.5 months of achieving CR versus those who began tipifarnib after 3.5 months [10 of 24 (42%) for each group], and for those who began tipifarnib within 2 months of completing consolidation therapy versus those who began tipifarnib more than 2 months after completing consolidation therapy [7 of 24 (29%) versus 8 of 24 (33%), respectively].
Fig. 1.
DFS for 48 patients receiving tipifarnib maintenance following induction and consolidation therapies.
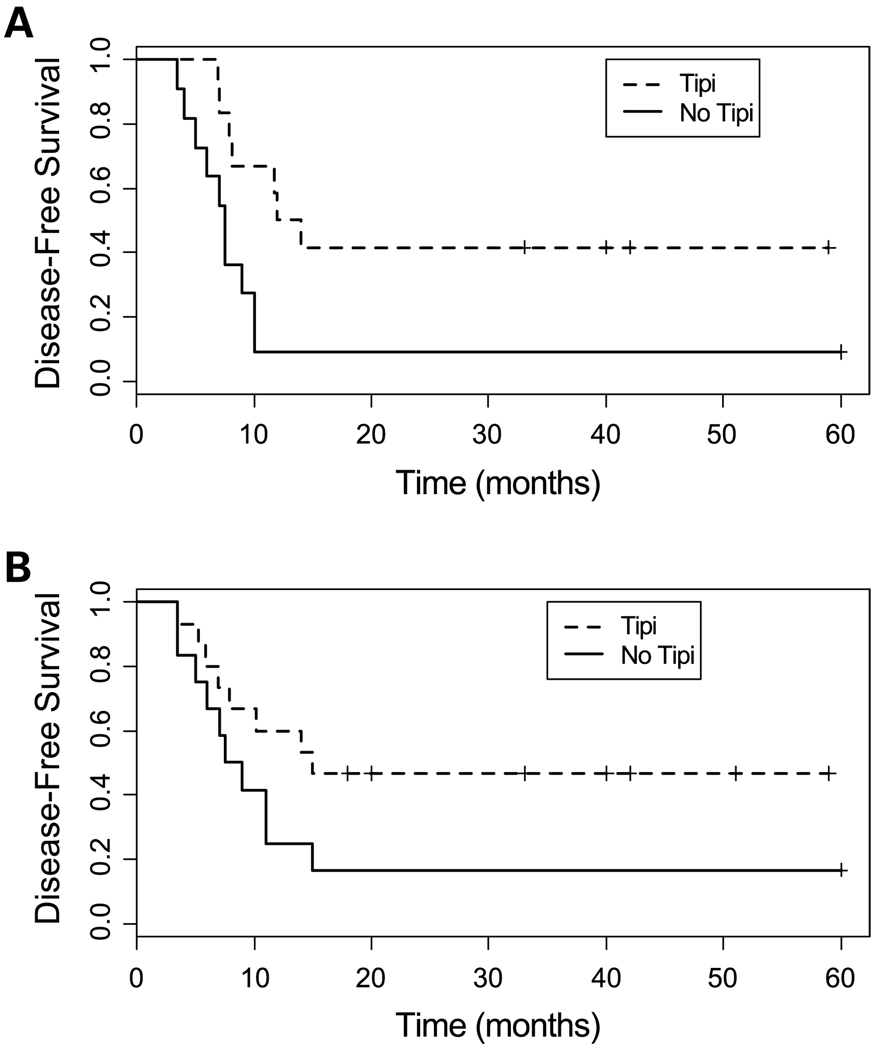
In Tables 3 and 4, we compare the outcomes for the 25 patients receiving two-cycle timed sequential therapy followed by tipifarnib maintenance with 23 patients who received a similar two-cycle timed sequential therapy regimen (and therefore a comparable dose and schedule of intensive chemotherapy) without tipifarnib maintenance (7). In a univariate analysis, the hazard ratios (HR) comparing CR durations between the two groups showed that tipifarnib exerts a strong protective effect on patients with secondary AML (HR, 0.28; P = 0.01) and patients with adverse cytogenetics (HR, 0.42; P = 0.07; Table 3; Fig. 2A and B). Similarly, tipifarnib maintenance following two-cycle timed sequential therapy was associated with a significantly greater proportion of patients with secondary AML or adverse cytogenetics whose DFS is at least 12 months. Although they were not statistically significant, the estimated HRs for tipifarnib in patients of ages ≥60 years and those with adverse cytogenetics were <1, showing a trend toward a protective effect from treatment with tipifarnib.
Table 3.
Duration of CR for adults receiving tipifarnib following two-cycle timed sequential therapy: comparison with two-cycle timed sequential therapy without subsequent maintenance therapy
| Two-cycle TST plus tipifarnib | Two-cycle TST unmaintained | HR (P) | |
|---|---|---|---|
| CR duration [median (range)], mo | |||
| All patients | 14.5 (3.5–59+) | 10 (3–60+) | 0.68 (0.26) |
| Age ≥60 y | 10.2 (3.5–51+) | 7.7 (4–60+) | 0.73 (0.50) |
| Secondary AML | 14.5 (5.2–59+) | 8.7 (3–60+) | 0.28 (0.01) |
| Adverse cytogenetics | 16 (5.1–59+) | 8.3 (3–60+) | 0.42 (0.07) |
| DFS ≥12 mo, n (%) | |||
| All patients | 14/25 (56) | 7/23 (30) | |
| Age ≥60 y | 5/13 (38) | 3/13 (19) | |
| Secondary AML | 7/12 (58) | 2/12 (17) | |
| Adverse cytogenetics | 9/15 (60) | 4/14 (29) |
Abbreviation: TST, timed sequential therapy.
Table 4.
Multiple regression analysis of the age-adjusted effect of tipifarnib following two-cycle timed sequential therapy on duration of CR
| Secondary AML |
Adverse cytogenetics |
HR (95% CI) | P |
|---|---|---|---|
| Yes | Yes | 0.11 (0.03–0.46) | 0.02 |
| Yes | No | 0.42 (0.12–1.40) | 0.16 |
| No | Yes | 0.69 (0.21–2.24) | 0.53 |
| No | No | 2.54 (0.75–8.56) | 0.13 |
NOTE: Adjusted for age <65 versus >65 y (HR, 0.67; P = 0.09).
Abbreviation: 95% CI, 95% confidence interval.
Fig. 2.
A, comparison of DFS for patients with secondary AML who received two-cycle timed sequential therapy with (12 patients; dotted line) and without (12 patients; solid line) tipifarnib maintenance therapy. B, comparison of DFS for patients with adverse cytogenetics who received two-cycle timed sequential therapy with (15 patients; dotted line) and without (14 patients; solid line) tipifarnib maintenance therapy.
A Cox multiple regression analysis was done including treatment (tipifarnib versus no tipifarnib), age ≥60 years, adverse cytogenetics, and secondary AML. All interactions with age were found not to be significant, but the other two-way interactions were included in the model. As delineated in Table 4, tipifarnib was significantly associated with longer DFS in patients who exhibit both secondary AML and adverse cytogenetics (HR, 0.11; P = 0.02) and, to a lesser extent, in patients with secondary AML without adverse cytogenetics (HR, 0.42; P = 0.16), but not for patients without secondary AML with or without adverse cytogenetics [HR, 0.69 (P = 0.53) and 2.54 (P = 0.13), respectively].
Effect of tipifarnib maintenance therapy on the outcome of treatment at relapse
Eighteen patients who relapsed during or after completing tipifarnib maintenance therapy underwent reinduction chemotherapy (16) or allogeneic stem cell transplant (2) at the time of relapse. The median age of these patients at the time of relapse was 50 years (range, 27–68 years), and the median CR duration was 11.7 months (range, 5.1–28 months). Twelve (67%) achieved a second CR including both patients who underwent allogeneic stem cell transplant in early relapse. The median age of those achieving second CR was 49 years (range, 28–67 years) and the median CR duration before relapse was 14 months (range, 5.8–28 months). As best as can be determined, tipifarnib maintenance therapy did not interfere with patients receiving reinduction chemotherapy or stem cell transplantation.
Discussion
Maintenance chemotherapy in CR following multiple cytotoxic regimens is effective in the setting of acute lymphoblastic leukemia but has not yet been proved to lengthen CR in AML, particularly in older patients or those with poor-risk features (1, 2, 12 – 14). Nonetheless, the concept of maintenance therapy after intensive therapy is appealing if indeed such therapy could prolong CR or prevent relapse without incurring serious toxicities or clonal evolution of remaining leukemic cells. It is becoming increasingly possible to identify AML patients whose CR durations are predicted to be short, based on clinical and biological features that reflect inherent drug resistance (1, 3, 25 – 30). Indeed, in adults with one or more poor-risk features, the median CR duration is <9 months and the 1-year DFS is 20% (1 – 9). In such patients, the so-called “minimal residual disease” state should provide a fertile testing ground for new approaches aimed at prolonging CR and preventing or deterring relapse.
An important characteristic for any “maintenance” regimen is low toxicity, tolerability, and an ability to permit patients to pursue active lives without requiring frequent trips to the hospital or hospitalizations. In this regard, tipifarnib was well tolerated with a low incidence of adverse effects. We selected an intermediate tipifarnib dose for this study (400 mg twice daily for 14 of every 21 days) relative to the dose and schedule used for induction therapy for older adults with AML (16, 17) to ameliorate risks and complications of significant marrow suppression, as had previously been detected in patients with high-risk myelodysplasia (19 – 21). Whereas moderate myelo-suppression occurred in more than half of our patients, it was rarely accompanied by infection or the need for hospitalization or blood product transfusion, and was readily circumvented by subsequent tipifarnib dose reduction to a dose (300 mg twice daily) that inhibits the farnesyltransferase enzyme reproducibly and potently (16). Perhaps most importantly, tipifarnib maintenance therapy did not seem to exert a negative effect on the ability of patients to achieve a second CR after first relapse because 67% of patients achieved a second CR.
Our phase II trial shows that oral tipifarnib maintenance therapy in first CR following cytotoxic induction and consolidation chemotherapies for adults with poor-risk AML was associated with a median CR duration of 13.5 months, 1-year DFS 52%, and 2-year DFS 30%, especially in patients with secondary AML, adverse cytogenetics, or both poor-risk features. The salutary effect of tipifarnib for patients with secondary AML and/or adverse cytogenetics is of particular interest and is consistent with previous demonstrations of tipifarnib activity in patients with high-risk myelodysplasia (19) and in elderly patients with myelodysplasia/AML including those with adverse cytogenetics (15, 17). On the other hand, our historical comparison for patients receiving two-cycle timed sequential therapy suggests that tipifarnib maintenance may not prolong DFS in patients whose sole risk factor was age ≥60 years and whose AML did not exhibit poor-risk biology. One future goal is to determine who will benefit from the addition of tipifarnib in the minimal residual disease setting. This may be particularly challenging in the case of patients with normal cytogenetics, where clinical outcomes and cure rates can vary dramatically, reflecting heterogeneity on the molecular level (28 – 30). In this regard, recent studies have defined single-gene mutations and gene expression signatures that may help to discriminate prognostic subgroups in cytogenetically “normal” AML (28 – 30).
In sum, the results of this limited phase II study suggest that some patients with poor-risk AML, including those with secondary AML and/or adverse cytogenetics, may benefit from tipifarnib maintenance therapy without incurring clinically or biologically significant risks. Future studies of this approach should examine alternative tipifarnib dosing (e.g., reduced dosing, different schedules) and continuation of therapy beyond 16 cycles, as well as a randomized, placebo-controlled trial of tipifarnib maintenance therapy. Finally, stratification based on molecular features may refine our ability to target the subset of patients who stand to derive the greatest benefit from the tipifarnib maintenance approach.
Acknowledgments
Grant support: National Cancer Institute Cooperative Agreements U01CA70095 (J.E. Karp) and CA 69854 (J.E. Karp and I. Gojo).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Estey E. Acute myeloid leukemia and myelodysplastic syndromes in older patients. J Clin Oncol. 2007;25:1908–1915. doi: 10.1200/JCO.2006.10.2731. [DOI] [PubMed] [Google Scholar]
- 2.Goldstone AH, Burnett AK, Wheatley K, et al. Attempts to improve treatment outcomes in acute myeloid leukemia (AML) in older patients: the results of the United Kingdom Medical Research Council AML 11 trial. Blood. 2001;98:1302–1311. doi: 10.1182/blood.v98.5.1302. [DOI] [PubMed] [Google Scholar]
- 3.Grimwade D, Walker H, Harrison G, et al. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML 11 trial. Blood. 2001;98:1312–1320. doi: 10.1182/blood.v98.5.1312. [DOI] [PubMed] [Google Scholar]
- 4.Lancet JE, Willman CL, Bennett JM. Acute myelogenous and aging: clinical interactions. Hematol Oncol Clin North Am. 2000;14:251–267. doi: 10.1016/s0889-8588(05)70287-2. [DOI] [PubMed] [Google Scholar]
- 5.Stone RM, Berg DT, George SL, et al. Postremission therapy in older patients with de novo acute myeloid leukemia: a randomized trial comparing mitoxantrone and intermediate-dose cytarabine with standard-dose cytarabine. Blood. 2001;98:548–553. doi: 10.1182/blood.v98.3.548. [DOI] [PubMed] [Google Scholar]
- 6.Archimbaud E, Jehn U, Thomas X, et al. Multicenter randomized phase II trial of idarubicin vs mitoxantrone, combined with VP16 and cytarabine for induction/consolidation therapy, followed by a feasibility study of autologous peripheral blood stem cell transplantation in elderly patients with acute myeloid leukemia. Leukemia. 1999;13:843–849. doi: 10.1038/sj.leu.2401445. [DOI] [PubMed] [Google Scholar]
- 7.Bolanos-Meade J, Karp JE, Guo C, et al. Timed sequential therapy of acute myelogenous leukemia in adults: a phase II study of retinoids in combination with the sequential administration of cytosine arabinoside, idarubicin and etoposide. Leuk Res. 2003;27:313–321. doi: 10.1016/s0145-2126(02)00177-7. [DOI] [PubMed] [Google Scholar]
- 8.Breems DA, Van Putten WLJ, Huijgens PC, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol. 2005;23:1969–1978. doi: 10.1200/JCO.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 9.Geller RB, Burke PJ, Karp JE, et al. A two-step timed sequential treatment for acute myelocytic leukemia. Blood. 1989;74:1499–1506. [PubMed] [Google Scholar]
- 10.Litzow M. The therapy of relapsed acute leukemia in adults. Blood Rev. 2004;18:39–63. doi: 10.1016/s0268-960x(03)00036-5. [DOI] [PubMed] [Google Scholar]
- 11.Lowenberg B, Suici S, Archimbaud E, et al. Use of recombinant GM-CSF during and after remission induction chemotherapy in patients aged 61 years and older with acute myeloid leukemia: final report of AML-11, a phase III randomized study of the Leukemia Cooperative Group of European Organisation for the Research and Treatment of Cancer and the Dutch Belgian Hemato-Oncology Cooperative Group. Blood. 1997;90:2952–2961. [PubMed] [Google Scholar]
- 12.Lowenberg B, Suici S, Archimbaud E, et al. Mitoxantrone versus daunorubicin in induction-consolidation chemotherapy—the value of low-dose cytarabine for maintenance of remission, and an assessment of prognostic factors in acute myeloid leukemia in the elderly: final report of the Leukemia Cooperative Group of the European Organization for the Research and Treatment of Cancer and the Dutch-Belgian Hemato-Oncology Cooperative Hovon Group randomized phase III study AML-9. J Clin Oncol. 1998;16:872–881. doi: 10.1200/JCO.1998.16.3.872. [DOI] [PubMed] [Google Scholar]
- 13.Buchner T, Berdel WE, Schoch C, et al. Double induction containing either two courses or one course of high-dose cytarabine plus mitoxantrone and postremission therapy by either autologous stem-cell transplantation or by prolonged maintenance for acute myeloid leukemia. J Clin Oncol. 2006;24:2480–2489. doi: 10.1200/JCO.2005.04.5013. [DOI] [PubMed] [Google Scholar]
- 14.Cassileth PA, Lynch E, Hines JD, et al. Varying intensity of postremission therapy in acute myeloid leukemia. Blood. 1992;79:1924–1930. [PubMed] [Google Scholar]
- 15.Erba HP, Kopecky KJ, Kirschbaum MH, et al. Phase II studies of different schedules and doses of the farnesyltransferase inhibitor tipifarnib (R115777, Zarnestra, NSC 702818) for patients of age 70 or older with previously untreated acute myeloid leukemia (AML): a North American Intergroup study (S0432) [abstract 440] Blood. 2007;110:136a. [Google Scholar]
- 16.Karp JE, Lancet JE, Kaufmann SH, et al. Clinical and biologic activity of the farnesyltransferase inhibitor R115777 in adults with refractory and relapsed acute leuekmias: a phase I clinical-laboratory correlative trial. Blood. 2001;97:3361–3369. doi: 10.1182/blood.v97.11.3361. [DOI] [PubMed] [Google Scholar]
- 17.Lancet JE, Gojo I, Gotlib J, et al. A phase II study of the Farnesyltransferase inhibitor tipifarnib in elderly patients with previously untreated poor-risk acute myelogenous leukemia. Blood. 2007;109:1387–1394. doi: 10.1182/blood-2006-04-014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lancet JE, Karp JE. Farnesyltransferase inhibitors in hematologic malignancies: new horizons in therapy. Blood. 2003;102:3880–3889. doi: 10.1182/blood-2003-02-0633. [DOI] [PubMed] [Google Scholar]
- 19.Fenaux P, Raza A, Mufti G, et al. A multicenter phase 2 study of the farnesyltransferase inhibitor tipifarnib in intermediate- to high-risk myelodysplastic syndrome. Blood. 2007;109:4158–4163. doi: 10.1182/blood-2006-07-035725. [DOI] [PubMed] [Google Scholar]
- 20.Kurzrock R, Albitar M, Cortes JE, et al. Phase II study of R115777, a farnesyl transferase inhibitor, in myelodysplastic syndrome. J Clin Oncol. 2004;22:1287–1292. doi: 10.1200/JCO.2004.08.082. [DOI] [PubMed] [Google Scholar]
- 21.Kurzrock R, Kantarjian HM, Cortes JE, et al. Farnesyltransferase inhibitor R115777 in myelodysplastic syndrome: clinical and biologic activities in the phase 1 setting. Blood. 2003;102:4527–4534. doi: 10.1182/blood-2002-11-3359. [DOI] [PubMed] [Google Scholar]
- 22.Mesa RA, Camoriano JK, Geyer SM, et al. A phase II trial of tipifarnib inmyelofibrosis: primary, post-polycythemia vera and post-essential thrombocythemia. Leukemia. 2007;21:1964–1970. doi: 10.1038/sj.leu.2404816. [DOI] [PubMed] [Google Scholar]
- 23.Cortes J, Albitar M, Thomas D, et al. Efficacy of the farnesyl transferase inhibitor R115777 in chronic myeloid leukemia and other hematologic malignancies. Blood. 2003;101:1692–1697. doi: 10.1182/blood-2002-07-1973. [DOI] [PubMed] [Google Scholar]
- 24.Kurzrock R, Verstovsek S, Wright JJ, et al. Alternate week administration of the farnesyltransferase inhibitor tipifarnib (Zarnestra, R115777) in patients with myelodysplastic syndrome: results of a phase I study [abstract 2521] Blood. 2005;106:7082. [Google Scholar]
- 25.Arber DA, Stein AS, Carter NH, Ikle D, Forman SJ, Slovak ML. Prognostic impact of acute myeloid leukemia classification. Importance of detection of recurring cytogenetic abnormalities and multilineage dysplasia on survival. Am J Clin Pathol. 2003;119:672–680. doi: 10.1309/EM7K-CQR4-GLMH-RCX4. [DOI] [PubMed] [Google Scholar]
- 26.Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the clinical advisory committee meeting—Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–3849. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 27.Yanada M, Suzuki M, Kawashima K, et al. Long-term outcomes for unselected patients with acute myeloid leukemia categorized according to the World Health Organization classification: a single center experience. Eur J Haematol. 2005;74:418–423. doi: 10.1111/j.1600-0609.2004.00397.x. [DOI] [PubMed] [Google Scholar]
- 28.Bullinger L, Dohner K, Bair E, et al. Use of gene expression profiling to identify prognostic subclasses in adult acute myeloid leukemia. N Engl J Med. 2004;350:1605–1616. doi: 10.1056/NEJMoa031046. [DOI] [PubMed] [Google Scholar]
- 29.Mrozek K, Marcucci G, Paschka P, Whitman SP, Bloomfield CD. Clinical relevance of mutations and gene-expression changes in adult acute myeloid leukemia with normal cytogenetics: are we ready for a prognostically prioritized molecular classification? Blood. 2007;109:431–448. doi: 10.1182/blood-2006-06-001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radmacher MD, Marcucci G, Ruppert AS, et al. Independent confirmation of a prognostic gene-expression signature in adult acute myeloid leukemia with a normal karyotype: a Cancer and Leukemia Group B study. Blood. 2006;108:1677–1683. doi: 10.1182/blood-2006-02-005538. [DOI] [PMC free article] [PubMed] [Google Scholar]