Demonstration of biocompatible quantum dots ten years ago[1,2] spurred interest in various nanomaterials with fascinating optical, electronic and magnetic properties, and their potential for medical applications.[3] Plasmon resonance, an optical phenomenon associated with metal nanoparticles, is currently investigated for biomedical imaging and intervention at the cellular level (Table 1). However, requirements of complete elimination from the body may limit the clinical utility of nanoparticles of these sizes.[18] Here we report preparation and testing of biodegradable plasmon resonant nanoshells of 63 nm diameter. Rather than a continuous metallic shell, this composite nanostructure is formed as a shell-shaped array of gold clusters supported on a spherical biodegradable core. This fundamentally new class of materials maintains optical tunability characteristic of solid metallic shells, yet upon degradation yields individual clusters of 5.7 nm diameter, compatible with the requirements of renal clearance. Biodegradable metallic nanoparticles may enable clinical translation of many research-stage technologies.
Table 1.
Examples of gold nanoparticles currently investigated for biomedical applications.
| Nanoparticle | Morphology | Characteristic Dimensiona, nm | Application | Ref | |
|---|---|---|---|---|---|
| nanospheres | solid spheres of gold | D | 35, 20 | dark field reflectance imaging | [4,5] |
| nanoshells | solid silica core coated with a concentric solid layer of gold | Ds | 143 | thermal therapy | [6] |
| 140 | OCTb contrast | [7] | |||
| 112 | SERSc substrate | [8] | |||
| nanocages | hollow cube of gold with truncated corners | Le | 36 | OCT contrast | [9] |
| 45 | thermal therapy | [10] | |||
| nanorods | solid cylinders of gold | La | 48.6 | luminescence imaging | [11] |
| 46.5, 57 | thermal therapy | [12,13] | |||
| 27 | SERS substrate | [14] | |||
| 50 | photoacoustic imaging | [15] | |||
| 70, 44 | OCT contrast | [16,17] | |||
(D - diameter, Ds - diameter including core and shell, Le - edge length, La - long axis
length.)
(OCT- Optical coherence tomography.)
(SERS – Surface enhanced Raman scattering.)
In the Rayleigh approximation of small non-interacting spherical particles, plasmon resonance occurs when ε= −2εm where ε is the dielectric function of the particles and εm is that of the medium, and results in intense absorption and scattering of electromagnetic radiation.[19] Gold is of particular interest to biomedical applications because, in addition to being bioinert, its refractive index satisfies the plasmon resonance condition in the visible range, around 530 nm. With a departure from restrictions of the Rayleigh approximation, particle shape and size further control the spectral position of the plasmon resonance, leading to the optical tunability of such particles throughout the visible to near-infrared range, with opportunities for imaging and therapeutic applications. However, concerns regarding unknown toxicities and elimination routes of nanoparticles[20] led to investigation of the hydrodynamic diameter as a critical parameter in design of diagnostic and therapeutic nanoparticles. Consequently proposed criteria of nanoparticles’ clinical utility include “degradability to clearable components”, i.e., to particles having hydrodynamic diameter of 5–6 nm or less, clearable by renal filtration.[18] Currently investigated plasmon resonant nanostructures do not meet these criteria (Table 1).
In response to this challenge we demonstrate preparation of plasmon resonant nanoshells comprised of an array of gold clusters that upon degradation yield components of a clearable size (Figure 1a) The optical properties of this composite shell are no longer that of solid gold, rather they are described by the effective medium theory. To provide both dimensional stability and degradability, this shell is formed on the surface of a spherical biodegradable core.
Figure 1.
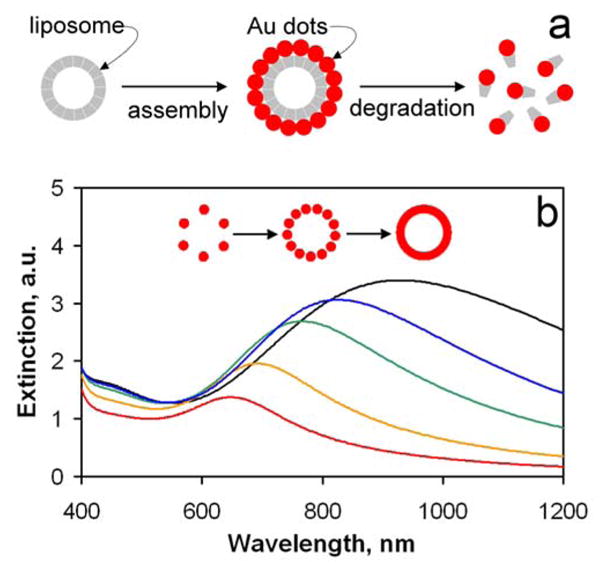
Model of biodegradable nanoshell and its tunable spectral characteristics. (a) Very small particles of gold, or nanodots, are assembled on the surface of biodegradable template to form a plasmon resonant structure equivalent of solid gold nanoshell, typically in the 50–200 nm range. An array of discrete particles, this structure is degradable to small clusters of clearable size. (b) Calculated extinction spectra of the biodegradable shell (see Supplementary Equations). Plasmon resonance maxima exhibit red-shift with increased fill factor, or density of gold nanodots in the shell, Φ = 0.7 (red), 0.8 (orange), 0.9 (green), 0.95 (blue), and 1 (black).
Optical properties of the composite nanoshell can be derived starting with the condition for the plasmon resonance in a solid spherical shell with a concentric dielectric core, approximated by Kerker:[21]
| (1) |
where q is the ratio of core diameter to that of the shell, and εc, εs and εm are the dielectric constants (generally, functions) of the core, the shell, and the surrounding medium, respectively. Since the dielectric constant of solid metallic shell εs varies with wavelength, adjusting the ratio q produces shells resonant at various wavelengths. Accordingly, increasing the amount of gold deposited on a dielectric core, resulting in a thicker shell, yields resonances at shorter wavelengths as demonstrated experimentally.[22] For the composite shell plasmon resonance condition can be obtained from (1) by substituting for εs the dielectric constant of the composite, obtained from the Maxwell Garnet effective medium theory:
| (2) |
where ε0 is the dielectric constant of the matrix, ε is the dielectric constant of gold[23] and Φ is the volume fraction of metal within the composite shell. Dielectric constant ε is furthermore corrected to account for the fact that the size of individual clusters is smaller than the electron mean free path in bulk gold.[24] In the limit Φ→1, dielectric constant of the composite equals that of metal. Therefore composite shells made of densely packed gold clusters have optical properties virtually indistinguishable from those of solid shells (Figure 1b). Unlike solid shells however, the composite shell has no inherent structural stability, a property that enables its controlled degradation. Dielectric constant of such composite is uniquely tunable by varying the metal volume fraction in the shell, Φ, whereby increasing Φ shifts the position of the plasmon resonance to longer wavelengths. This Φ-based tunability is also size-independent, in contrast to spectral tunability of, e.g., gold nanorods or quantum dots.
We prepared degradable plasmon resonant nanostructures in which liposomes made of dialkyl phosphatidylcholine served as the spherical template, and the composite shell was obtained by formation of gold-lipid complexes. Formation of noble metal-lipid complexes generally involves cationic ligands interacting with noble metal anions, such as choline and tetrachloroaurate, followed by reduction to zero-valent metals.[25, 26] In the past, soft templates were applied to direct synthesis of stable self-supporting inorganic structures on the nanometer scale.[27, 28] In the fundamentally new approach described here, the soft template is an integral part of the composite structure, and is required for optical resonances and degradability of this material.
Liposomes had a mean diameter of 63 nm, determined by dynamic light scattering (DLS). Addition of four fold excess ascorbic acid to liposome suspensions (20 mM lipids) containing 4–20 mM chloroauric acid produced an abrupt color change from the characteristic translucent white of liposomes to a blue, green, or grey color, depending on the quantity of gold reduced. A relatively broad extinction band appeared in the red to near-infrared spectral range, in addition to the short wavelength extinction due to the Rayleigh scattering of liposomes (Figure 2a). Spectral position of the newly formed band varied with the amount of gold added such that by increasing the quantity of gold, the peak of the resonance band shifted from 600 to 1300 nm in the manner consistent with the formation of plasmon resonance in composite shell. Energy Dispersive X-ray Spectroscopy (EDS) analysis indicates that gold is present on the surface of liposomes (Figures 2b and 2c), while not forming clusters of gold large enough to be individually observed in images with a pixel size of 0.12 nm (Supplemental Figure 1).
Figure 2.
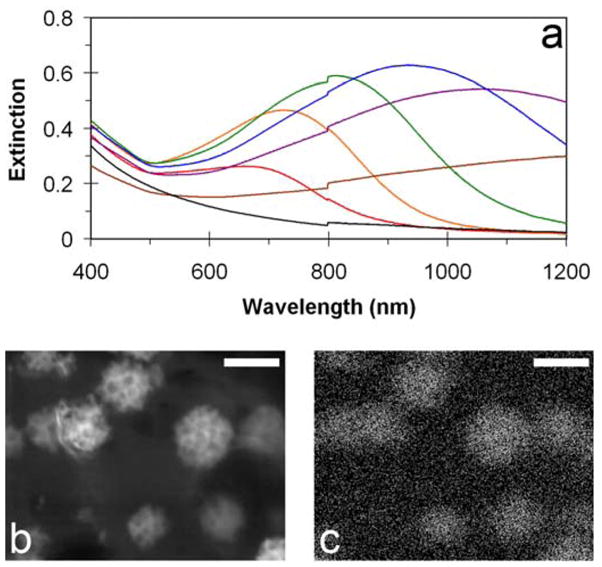
Extinction spectra of gold coated liposomes, electron micrograph and corresponding EDS map of gold. (a) Extinction spectra of 63 nm diameter liposomes with varying quantity of gold reduced: (red) 4mM, (orange) 8mM, (green) 10mM, (blue) 14mM, (purple) 16mM, (brown) 20mM, liposomes only (black). (b) High magnification electron micrograph of gold-coated liposomes (c) EDS map of gold of the same location. Marker bars represent 50nm.
Spectral tunability and EDS data suggest that the gold coating forms an assembly of small metallic clusters on the surface of the supporting template, rather than a solid metallic nanoshell. We therefore hypothesize that the plasmon resonance of this assembly is dependent on the stability of the liposomal scaffold. To test this hypothesis, optical resonances attributed to gold were monitored while the liposomal template was dissolved. A self-supporting solid metallic shell maintains its shape and associated optical properties even after the template is removed.[29] However, dissolution of gold-coated liposomes by incubation with Triton X-100, a non-ionic surfactant, resulted in the loss of plasmon resonance characteristic of gold nanoshells as well as the loss of the short-wavelength scattering characteristic of the liposomes (Figure 3). The lack of plasmon resonance in the visible or near infrared range indicates that gold is no longer organized in shell-like structures, and remaining gold nanoparticles are on the order of single nanometers in diameter. Indeed, sizing by DLS shows that following incubation with Triton, the remaining particles, presumably gold aggregated with surfactant and lipid molecules, have an average diameter of 5.7 nm.
Figure 3.
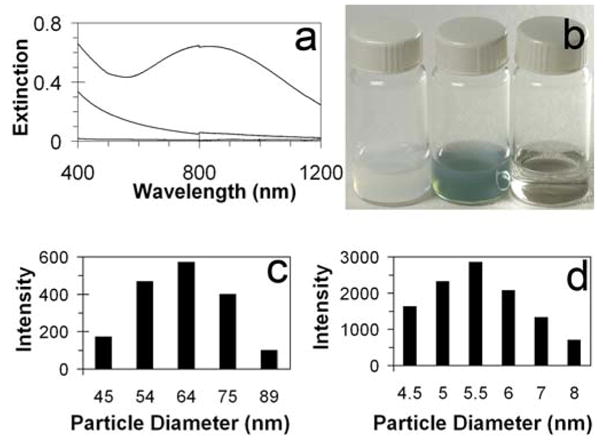
Liposome degradation by surfactant. (a) Extinction spectra: gold-coated liposome spectra (blue), bare liposome spectra (black) and gold-coated liposome spectra after incubation with Triton X-100 (red). (b) From left to right image of corresponding vials: Suspension of bare liposomes, gold-coated liposomes and gold-coated liposomes after incubation with Triton X-100. (c) Histograms depicting size distributions of bare liposomes (left) and degradation product (right).
To demonstrate degradation of composite nanoshells under conditions representing biologically relevant process, gold-coated liposomes were incubated with phopholipase A2 (PLA2). In the presence of calcium, PLA2 hydrolyzes the β-ester bond in glycerol-linked phospholipids, a major step in the physiologic pathway of lipid breakdown and recycling. Enzymatic degradation of gold-coated liposomes using bee venom PLA2 was monitored by the intensity of the plasmon resonance band. A suspension of gold-coated liposomes incubated overnight at 45°C with PLA2 and Ca2+, initially translucent with bluish color, developed into a cloudy white suspension. The loss of plasmon resonance (Supplemental Figure 2) indicates dissolution of the composite nanoshell, whereas the remaining cloudiness is attributed to agglomeration of hydrolyzed acyl tails. It is well established that the rate of hydrolysis of liposomes by PLA2 depends on the lipid composition;[30] it therefore follows that the rate of biodegradation of the plasmon resonant composite nanoshells could be similarly controlled by composition of the liposome scaffold.
Among several currently investigated biomedical applications of plasmon resonant structures, OCT imaging is of particular interest because it provides non-invasive imaging of living tissues, reasonable penetration depth (~2 mm) and spatial resolution (~10 μm) superior to present clinical methods of non-invasive imaging such as computed tomography, magnetic resonance imaging and ultrasound. Development of degradable plasmon resonant contrast agents may improve clinical OCT images, whereas further enhancements may include in vivo targeting and detection of molecular markers of diagnostic importance. We compared the OCT signal generated by gold-coated liposomes of 63 nm diameter having a plasmon resonance maximum at 912 nm to uncoated liposomes of the same diameter, each at lipid concentrations of 5 mM. In addition, gold-coated liposomes dissolved with Triton and PLA2 were tested. For these imaging experiments, small aliquots of suspensions were drawn into borosilicate glass capillary tubes of 0.85 mm ID. Using a time-domain OCT system operating at 890 nm center wavelength,[31] cross-sectional images of the tubes were collected (Figure 4) and intensity averages attained over the entire internal cross-section of each capillary tube. Gold coated liposomes produced a significant increase in image intensity, 7.2 dB relative to bare liposomes, 9.1 dB relative to gold-coated liposomes treated with Triton or PLA2, and 9.35 dB relative to PBS. This enhancement is comparable to that obtained with solid gold nanoparticles.[16]
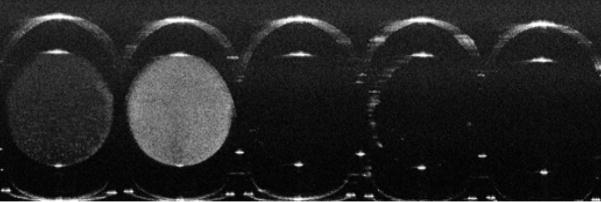
Figure 4.
OCT image of capillary tubes containing liposome suspensions and degradation products. From left: 63 nm diameter liposomes (5 mM lipid), 63 nm diameter liposomes coated with gold (5 mM lipid), Triton treated liposomes, PLA2 treated liposomes, PBS (control). Image size: 1.6 mm (vertical, in air) × 5.2 mm (horizontal). Capillary tubes and their contents appear elongated due to variance in refractive index of air, glass, and the liposome suspension.
We propose that clinical utility of plasmon resonant metallic nanoparticles can be increased by the use of biodegradable composite materials. Composite nanoshells introduced here demonstrate tunability of optical resonances and degradability to clusters of clearable size. Further advancement of this technology, including toxicity and elimination studies in animal models, may facilitate clinical applications of plasmon-based methods currently investigated for detection, diagnosis and treatment of diseases, most prominently cancer.
Experimental
Liposome Preparation
Dipalmitoylphosphatidylcholine (DPPC) was acquired from Avanti Polar Lipids (Alabaster, AL), either dissolved in chloroform or a dried powder. Chloroform-dissolved lipids were dried overnight under vacuum. Lipids were then resuspended in PBS (FisherBiotech, Fair Lawn, NJ) before freezing at −79 °C and thawing to 50 °C for ten cycles to distribute lipids into bilayer sheets. Extrusion followed for a total of ten cycles at 50 °C, above this lipids’ main phase transition temperature of 41°C, through perforated polycarbonate membranes, 50 nm pore size (Whatman, Florham Park, NJ) within a water-jacketed 10 mL LIPEX extruder (Northern Lipids Inc., Vancouver, BC) driven by compressed N2. Five minutes after reduction, vials of gold-coated liposomes were diluted as needed with PBS, to 1–5 mM lipid concentration. In buffered solutions, the liposome preparations were found to maintain their spectral characteristics and therefore structural stability for greater than 6 weeks when stored at 4 °C.
Dynamic Light Scattering
Dynamic light scattering was performed on a Brookhaven BI-200 SM goniometer with HeNe source, and BI8000 correlator (Brookhaven Instruments Corporation, Holtsville, NY) to measure liposome diameter as well as sizes of degradation products. Liposome preparations were diluted in PBS to 100 μM lipids before sizing.
Liposome Degradation
For degradation experiments, 10 mL quantities of 1 mM liposome suspensions were treated with 1 mL 10% Triton X-100 (t-octylphenoxypolyethoxyethanol, Sigma-Aldrich) and allowed to incubate at 50 °C for an hour before spectra were taken. In a control experiment incubation was performed with no surfactant present and no change of spectral characteristics of gold-coated liposome suspensions was observed. For phospholipase degradation experiments, liposome preparations were buffered in 0.1 M Tris-HCl, 127 mM NaCl, and 10 mM CaCl2. Gold was reduced onto the Tris-buffered liposomes in the same manner as used with PBS. Phospholipase degradation was performed by adding 10 μL of 1 mg bee venom phospholipase A2 (Sigma-Aldrich) dissolved in 1 mL filtered deionized H2O to 5 mL of 1 mM liposomes with 100 nm diameter. No degradation was observed in a control experiment where gold-coated liposomes and PLA2 were incubated without calcium present.
OCT imaging
OCT images were acquired using a time-domain system similar to one previously described [31]. The OCT system source is comprised of two superluminescent diodes which in combination have a center wavelength of 890 nm and a bandwidth of 150 nm. The resolution of the images produced by the system is 8 μm vertically and 14 μm horizontally. dB enhancement is based upon intensity averages attained over the entire internal cross-section of each capillary tube, an area of approximately 0.56 mm2, and was calculated as ten times the log of the quotient of these average intensities of compared samples.
Electron Microscopy
EDS imaged samples were prepared by evaporating a 4 μL drop of 2 mM lipid concentration liposome suspension onto a carbon sheet supported by 300-mesh copper grids. For high magnification SEM images, a 4 μL drop of 2 mM lipid concentration liposome suspension was frozen in nitrogen slush and freeze-dried overnight. All images were taken on a Hitachi (Schaumburg, IL) S-4800 Field Emission SEM.
Supplementary Material
Footnotes
This work was supported by the Arizona Biomedical Research Commission and by Grants K25CA120350 (MR) and R01CA109835 (JKB) from the National Cancer Institute of the National Institutes of Health. (Supporting Information is available online from Wiley InterScience or from the author).
References
- 1.Bruchez M, Jr, Moronne M, Gin P, Weiss S, Alivisatos AP. Science. 1998;281:2013. doi: 10.1126/science.281.5385.2013. [DOI] [PubMed] [Google Scholar]
- 2.Chan WCW, Nie S. Science. 1998;281:2016. doi: 10.1126/science.281.5385.2016. [DOI] [PubMed] [Google Scholar]
- 3.Alivisatos P. Nat Biotechnol. 2004;22:47. doi: 10.1038/nbt927. [DOI] [PubMed] [Google Scholar]
- 4.El Sayed IH, Huang X, El-Sayed MA. Nano Lett. 2005;5:829. doi: 10.1021/nl050074e. [DOI] [PubMed] [Google Scholar]
- 5.Kumar S, Harrison N, Richards-Kortum R, Sokolov K. Nano Lett. 2007;7:1338. doi: 10.1021/nl070365i. [DOI] [PubMed] [Google Scholar]
- 6.Gobin AM, Lee MH, Halas NJ, James WD, Drezek RA, West JL. Nano Lett. 2007;7:1929. doi: 10.1021/nl070610y. [DOI] [PubMed] [Google Scholar]
- 7.Barton JK, Halas NJ, West JL, Drezek RA. Proc SPIE. 2004;5316:99. [Google Scholar]
- 8.Jackson JB, Halas NJ. Proc Natl Acad Sci USA. 2004;101:17930. doi: 10.1073/pnas.0408319102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Saeki F, Wiley BJ, Cang H, Cobb MJ, Li Z, Au L, Zhang H, Kimmey MB, Li X, Xia Y. Nano Lett. 2005;5:473. doi: 10.1021/nl047950t. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Wang D, Xi J, Au L, Siekkinen A, Warsen A, Li Z, Zhang H, Xia Y, Li X. Nano Lett. 2007;7:1318. doi: 10.1021/nl070345g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, Huff TB, Zweifel DA, He W, Low PS, Wei A, Cheng J. Proc Natl Acad Sci USA. 2005;102:15752. doi: 10.1073/pnas.0504892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tong L, Zhao Y, Huff TB, Hansen MN, Wei A, Cheng J. Adv Mater. 2007;19:3136. doi: 10.1002/adma.200701974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Black KC, Kirkpatrick ND, Troutman TS, Xu L, Vagner J, Gillies RJ, Barton JK, Utzinger U, Romanowski M. Mol Imaging. 2008 in press. [PubMed] [Google Scholar]
- 14.Nikoobakht B, El-Sayed MA. J Phys Chem A. 2003;107:3372. [Google Scholar]
- 15.Eghtadari M, Oraevsky A, Copland JA, Kotov NA, Conjusteau A, Motamedi M. Nano Lett. 2007;7:1914. doi: 10.1021/nl070557d. [DOI] [PubMed] [Google Scholar]
- 16.Troutman T, Barton JK, Romanowski M. Opt Lett. 2007;32:1438. doi: 10.1364/ol.32.001438. [DOI] [PubMed] [Google Scholar]
- 17.Oldenburg A, Hansen M, Zweifel D, Wei A, Boppart S. Opt Express. 2006;14:6724. doi: 10.1364/oe.14.006724. [DOI] [PubMed] [Google Scholar]
- 18.Soo Choi H, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. Nat Biotechnol. 2007;25:1165. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bohren CF, Huffman DR. Absorption and Scattering of Light by Small Particles. Wiley; New York: 1983. [Google Scholar]
- 20.Oberdörster G, Oberdörster E, Oberdörster J. Environ Health Perspect. 2005;113:823. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerker M, Blatchford CG. Phys Rev B. 1982;26:4052. [Google Scholar]
- 22.Oldenburg SJ, Averitt RD, Westcott SL, Halas NJ. Chem Phys Lett. 1998;288:243. [Google Scholar]
- 23.Johnson PB, Christy RW. Phys Rev B. 1972;6:4370. [Google Scholar]
- 24.Kreibig U, Vollmer M. Optical Properties of Metal Clusters. Springer; Berlin: 1995. [Google Scholar]
- 25.Warshawsky A, Upson DA. J Poly Sci A. 1989;27:2963. [Google Scholar]
- 26.Ferrar WT, O’Brien DF, Warshawsky A, Voycheck CL. J Am Chem Soc. 1988;110:288. [Google Scholar]
- 27.Pileni MP. Nat Mater. 2003;2:145. doi: 10.1038/nmat817. [DOI] [PubMed] [Google Scholar]
- 28.Schnur JM. Science. 1993;262:1669. doi: 10.1126/science.262.5140.1669. [DOI] [PubMed] [Google Scholar]
- 29.Chah S, Fendler JH, Yi J. J Colloid Interface Sci. 2002;250:142. doi: 10.1006/jcis.2002.8328. [DOI] [PubMed] [Google Scholar]
- 30.Jørgensen K, Davidsen J, Mouritsen OG. FEBS Lett. 2002;531:23. doi: 10.1016/s0014-5793(02)03408-7. [DOI] [PubMed] [Google Scholar]
- 31.Barton JK, Guzman F, Tumlinson A. J Biomed Opt. 2004;9:618. doi: 10.1117/1.1695564. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.